Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Domperidone and Naproxen in Tablet Dosage Form
Md. Shozan Mondal, Md. Ahsanul Haque, Mohammad Safiqul Islam, S.M. Ashraful Islam
Pages: 145-148

RP HPLC Method for the determination of Tamsulosin in bulk and Pharmaceutical Formulations
Manish Kumar Thimmaraju, Venkat Rao, Hemanth .K, P. Siddartha Kumar
Pages: 177-180

Simultaneous Estimation of Ibuprofen and Famotidine in Pure and Combination Dosage Form by RP-HPLC
Narendra Nyola, Govinda Samy Jeyabalan
DOI: 10.7324/JAPS.2012.2512Pages: 79-83

RP-HPLC Method Development for Estimation of Sildenafil Citrate in Tablets and in Seminal Fluid
Nitin Sharma, Praveen Rajput, Arti Thakkar, G. S. Sarma
DOI: 10.7324/JAPS.2012.2615Pages: 172-178

Simultaneous Estimation of Finasteride andTamsulosin Hydrochloride in Combined DosageForms by RP-HPLC-PDA Method
M. Sindhura, K. Raghavi, R. Prashanthi, Buchi N. Nalluri
DOI: 10.7324/JAPS.2012.2626Pages: 203-209

Development and Validation of RP-HPLC Method for Simultaneous Estimation of Enalapril Maleate and Amlodipine Besylate in Combined Dosage form
Bharat G. Chaudhari
DOI: 10.7324/JAPS.2012.2911Pages: 054-057

A RP-HPLC Method Development and Validation for the Estimation of Gliclazide in bulk and Pharmaceutical Dosage Forms
B.V.V Ravi kumar, A.K. Patnaik, Saroj Kumar Raul, Nagireddy Neelakanta Rao
DOI: 10.7324/JAPS.2013.3410Pages: 059-062

RP-HPLC Method Development and Validation of Gallic acid in Polyherbal Tablet Formulation
Kamal Kardani, Nilesh Gurav, Bhavna Solanki, Prateek Patel, Bhavna Patel
DOI: 10.7324/JAPS.2013.3508Pages: 037-042

Validation of Assay Indicating Method Development of Simvastatin in Bulk and its Tablet Dosage form by RP-HPLC
Nalini Kanta Sahoo, Madhusmita Sahu, P. Srinivasa Rao, R.S.Vineela, J.N.V. Indira Devi, N.Sandhya Rani, Goutam Ghosh
DOI: 10.7324/JAPS.2014.40120Pages: 117-122

Bioavailability of karanjin from Pongamia pinnata L. in Sprague dawley rats using validated RP-HPLC method
Naresh Shejawal, Sasikumar Menon, Sunita Shailajan
DOI: 10.7324/JAPS.2014.40303Pages: 010-014

Simultaneous estimation of Cefpodoxime proxetil and Ofloxacin In tablet dosage form using RP-HPLC
Annadi Chiranjeevi and Medidi Srinivas
DOI: 10.7324/JAPS.2014.40508Pages: 046-050

A comparative estimation of quercetin content from Cuscuta reflexa Roxb.using validated HPTLC and HPLC techniques
Sunita Shailajan, Harshvardhan Joshi, Bhavesh Tiwari
DOI: 10.7324/JAPS.2014.40721Pages: 123-128

An approach for validated RP-HPLC method for the analysis of paclitaxel in rat plasma
Nandhakumar Sathyamoorthy, Vijayalakshmi Rajendran, Naveena V.S.H, Magharla Dasaratha Dhanaraju
DOI: 10.7324/JAPS.2014.40913Pages: 073-076

Bioanalytical Method Development and Validation for the Determination of Levocetirizine in Pharmaceutical Dosage Form and Human Plasma by RP-HPLC
Nilesh Jain, Deepak Kumar Jain, Ruchi Jain, Vijay Kumar Patel, Preeti Patel, Surendra Kumar Jain
DOI: 10.7324/JAPS.2016.601008Pages: 063-067

Development and validation of a new RP-HPLC method for the estimation of dutasteride in bulk and pharmaceutical formulations
Poonguzhali Subramanian, P. S. Rajinikanth
DOI: 10.7324/JAPS.2016.601207Pages: 047-055

Application of modern RP-HPLC technique for the quantitation of betulinic acid from traditional drug Symplocos racemosa Roxb.
Sunita Shailajan, Sasikumar Menon, Dipti Singh, Gauri Swar, Suhina Bhosale
DOI: 10.7324/JAPS.2017.70321Pages: 129-134

Stability-indicating RP- HPLC -DAD method for the simultaneous estimation of Tramadol HCl and Diclofenac sodium
Ramalingam Peraman, D. Subba Rao, Rajesh Reddy Kadiri, Amaranatha Reddy Bommireddy
DOI: 10.7324/JAPS.2017.70912Pages: 085-093

Effect of Piperine on Pharmacokinetics of Rifampicin and Isoniazid: Development and Validation of High Performance Liquid Chromatography Method
Amit Singh, Smita Verma, Nouh M H Al Jarari, Ajay Pal Singh, Neeraj Kumar Fuloria, Shivkanya Fuloria, Pradeep Kumar Sharma, Chhotelal
DOI: 10.7324/JAPS.2018.8311Pages: 072-081

A new quantitative reverse phase high-performance liquid chromatographic method for the quantification of Rilpivirine hydrochloride in bulk and dosage form
Sonam Patel, Krishnaveni Nagappan, Gouru Santhosh Reddy
DOI: 10.7324/JAPS.2018.81122Pages: 157-162

RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation
Suhair S. Al-Nimry, Bashar M. Altaani, Razan H. Haddad
DOI: 10.7324/JAPS.2019.90211Pages: 079-086
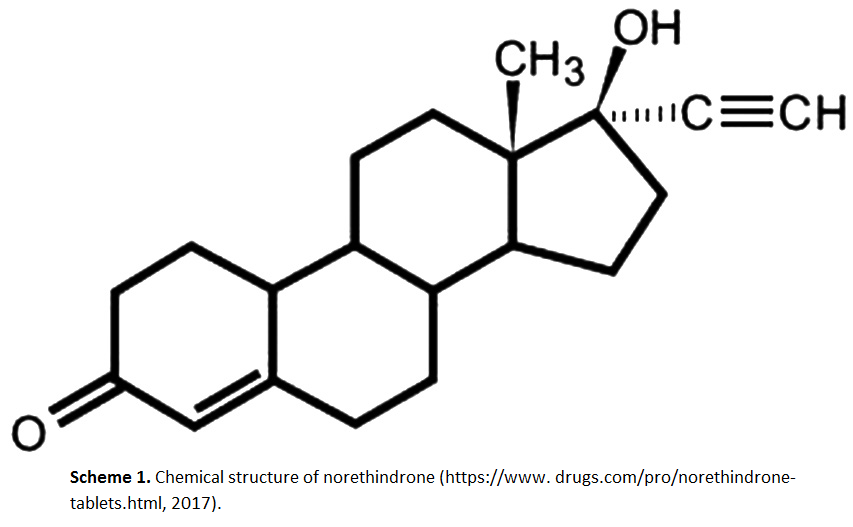
Validation and application of reversed-phase high-performance liquid chromatography for quantitative analysis of acid orange 7 and Sudan II in blusher products
Novalina B. R. Purba, Abdul Rohman, Sudibyo Martono
DOI: 10.7324/JAPS.2019.90714Pages: 100-105
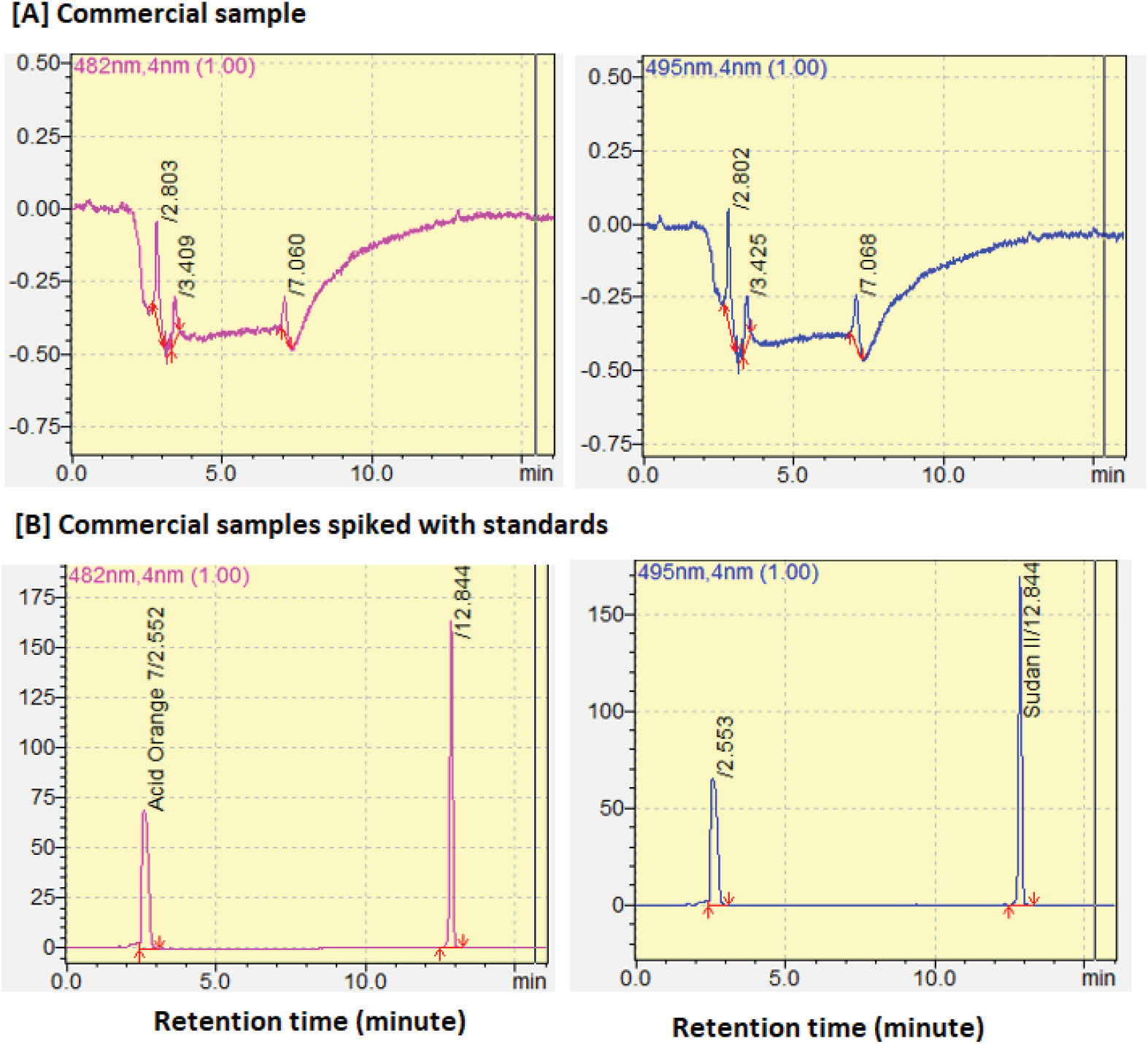
Development and validation of RP-HPLC method for pitavastatin calcium in bulk and formulation using experimental design
Vinodkumar D. Ramani, Girish K. Jani, Ashim Kumar Sen, Girish U. Sailor, Vijaykumar B. Sutariya
DOI: 10.7324/JAPS.2019.91010Pages: 075-083
Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138
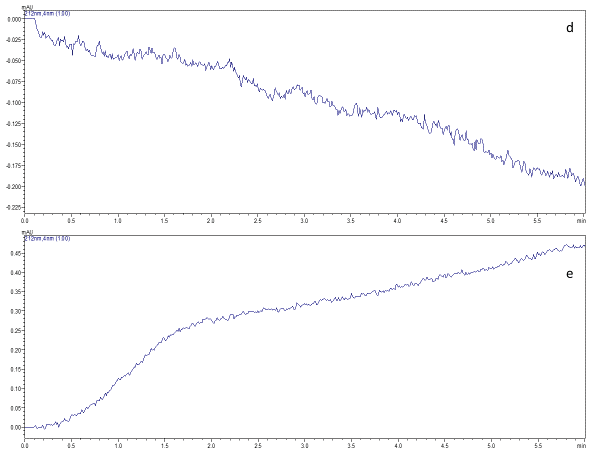
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
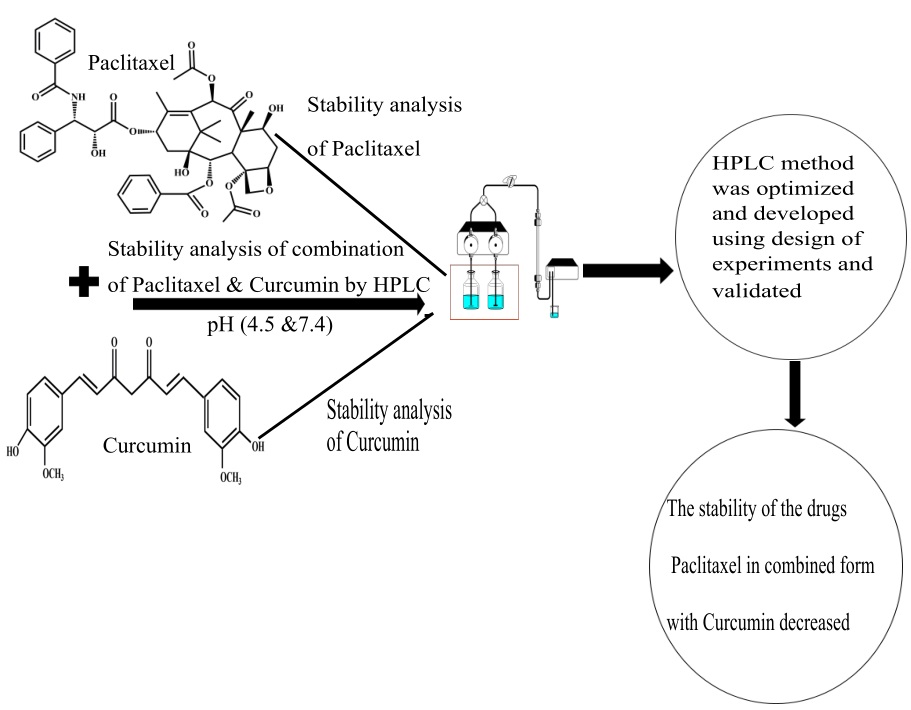
Strong cation exchange chromatography: a new option for concurrent quantification of saxagliptin and metformin
Pankaj Manohar Kharabe, Deepali Kailas Kadam
DOI: 10.7324/JAPS.2021.110913Pages: 110-117
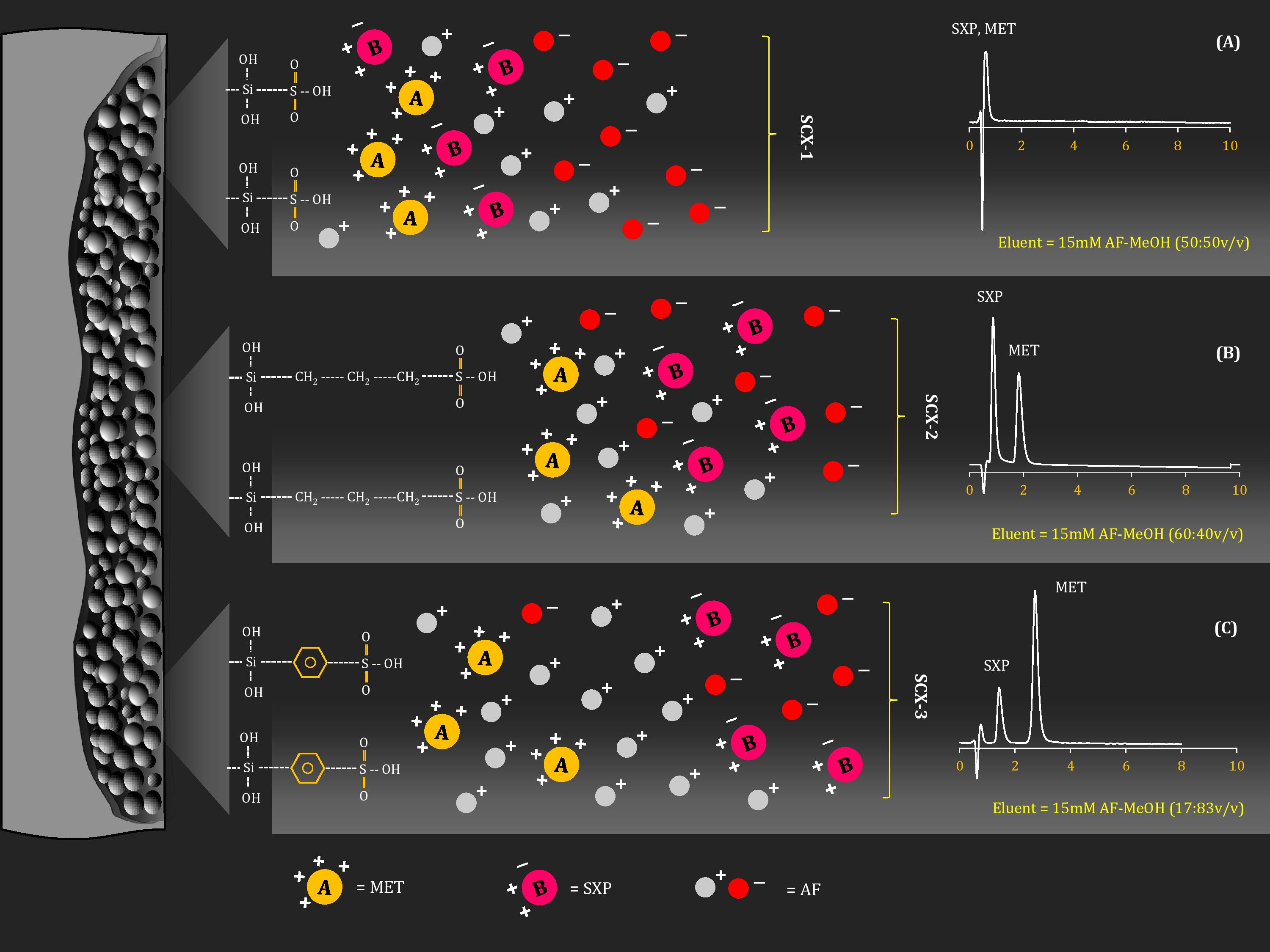
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
A new approach for evolution and quantification of Triamcinolone acetonide in medication shots by using RP-HPLC
Manikantha Naveen Vuddagiri, Veeraswami Boddu
DOI: 10.7324/JAPS.2021.1101216Pages: 169–174
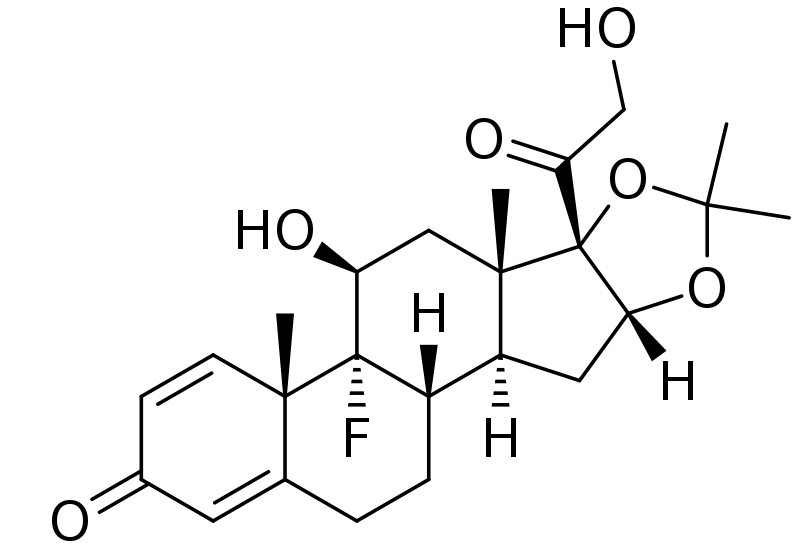
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form
Rameshwar Bhausaheb Gholve, Sanjay Sudhakar Pekamwar, Tukaram Mohanrao Kalyankar
DOI: 10.7324/JAPS.2021.1101211Pages: 121–134
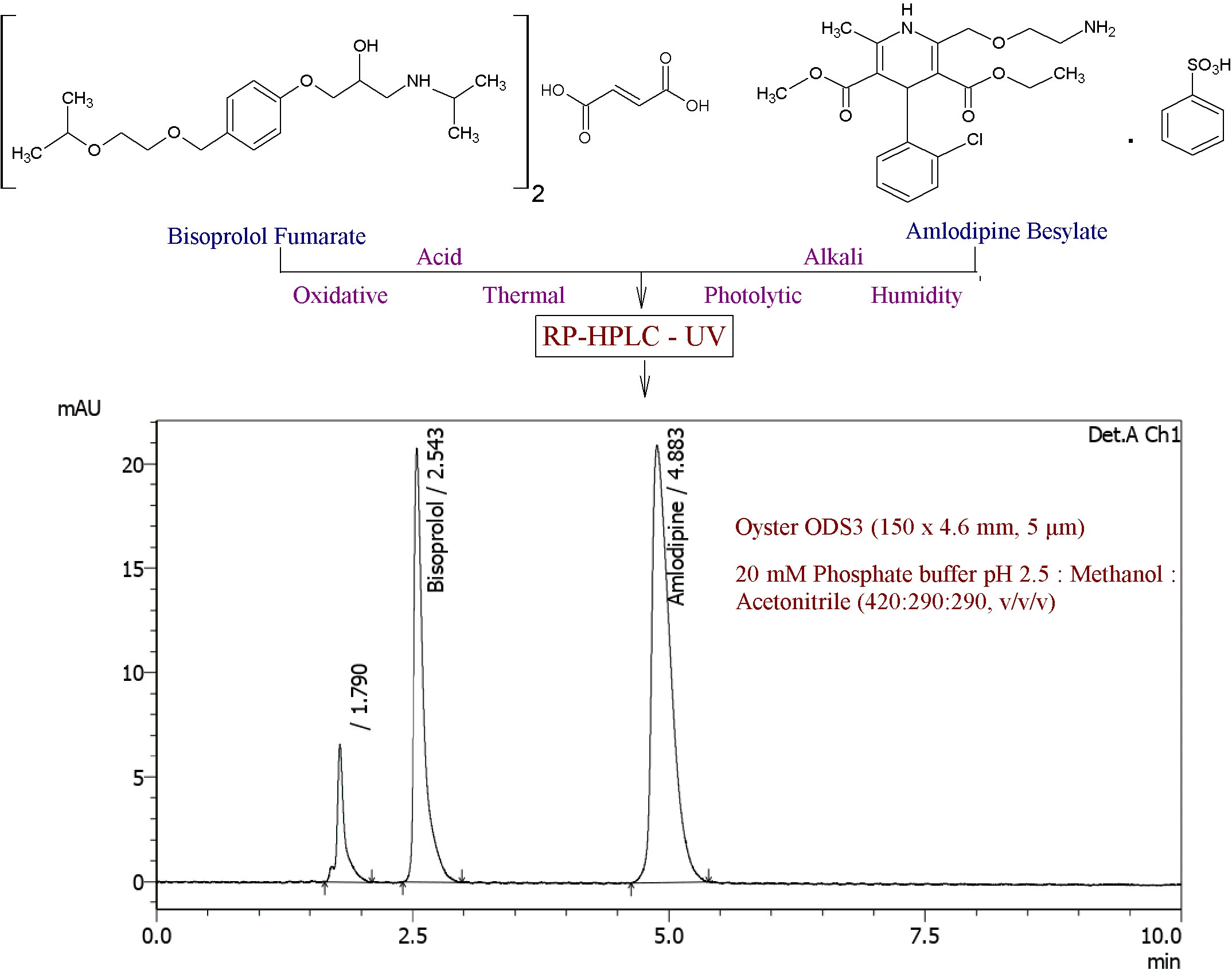
Assessment of pH-shift drug interactions of palbociclib by in vitro micro-dissolution in bio relevant media: An analytical QbD-driven RP-HPLC method optimization
Prajakta Harish Patil, Mrunal Desai, Rajat Radhakrishna Rao, Srinivas Mutalik, Gurupur Gautham Shenoy, Mahadev Rao, Puralae Channabasavaiah Jagadish
DOI: 10.7324/JAPS.2022.120505Pages: 078-087
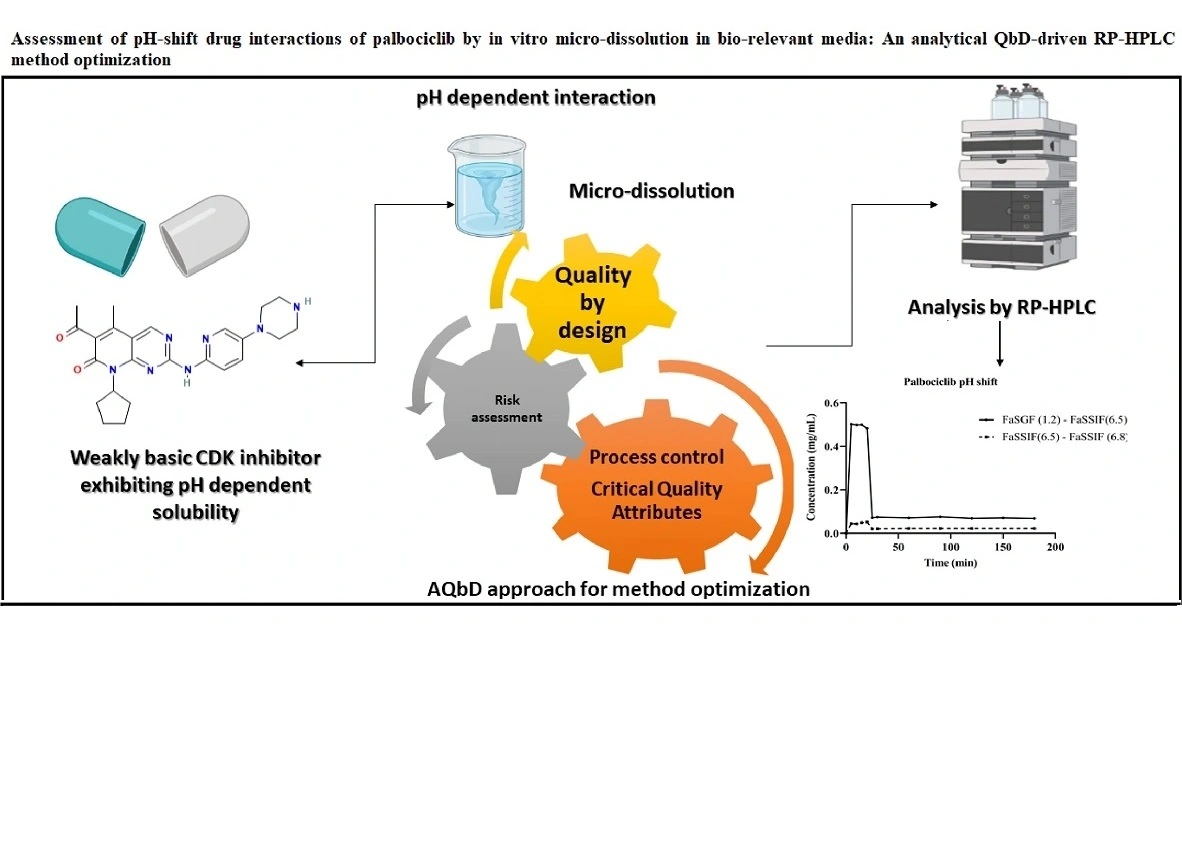
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155
QbD-based RP-HPLC method development for quantitative computation of phase III composition comprising apixaban and clopidogrel
Rashmi Shukla, Ankit Chaudhari, Pinak Patel, Krunal Detholia
DOI: 10.7324/JAPS.2024.181311Pages: 085-093

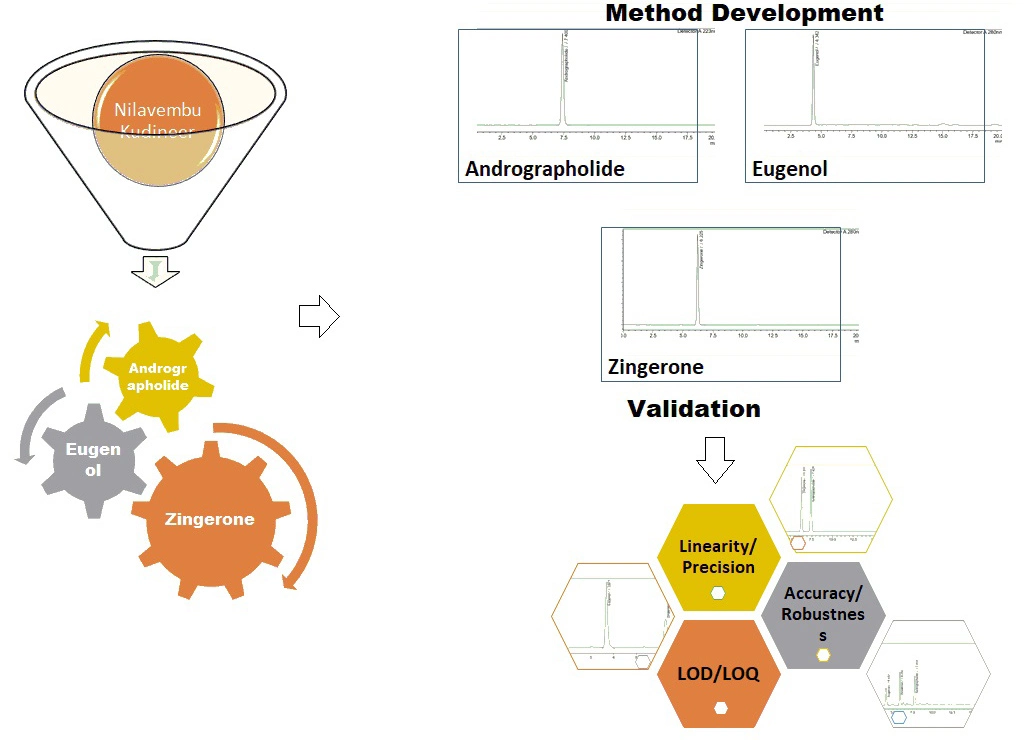
Determination of phytochemical markers andrographolide, eugenol and zingerone in nilavembu kudineer by RP-HPLC method
B. Sivagami, B. Sailaja
DOI: 10.7324/JAPS.2024.180359Pages: 128-134
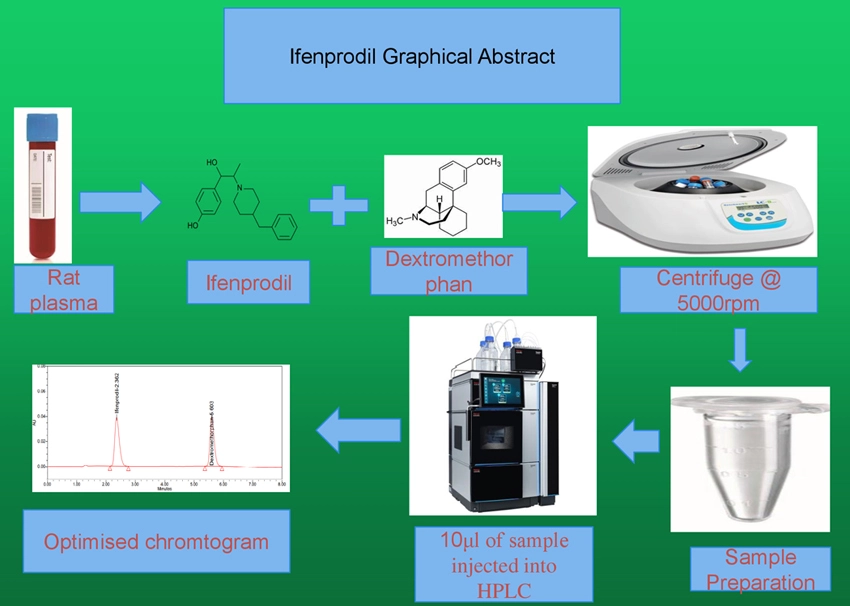
Development and validation of a bioanalytical RP-HPLC method for quantification of ifenprodil in rat plasma
Pavani Bonagiri, Narender Malothu, Srilakshmi Nallapaty, Chakravarthi Guntupalli
DOI: 10.7324/JAPS.2025.180823Pages: 189-196
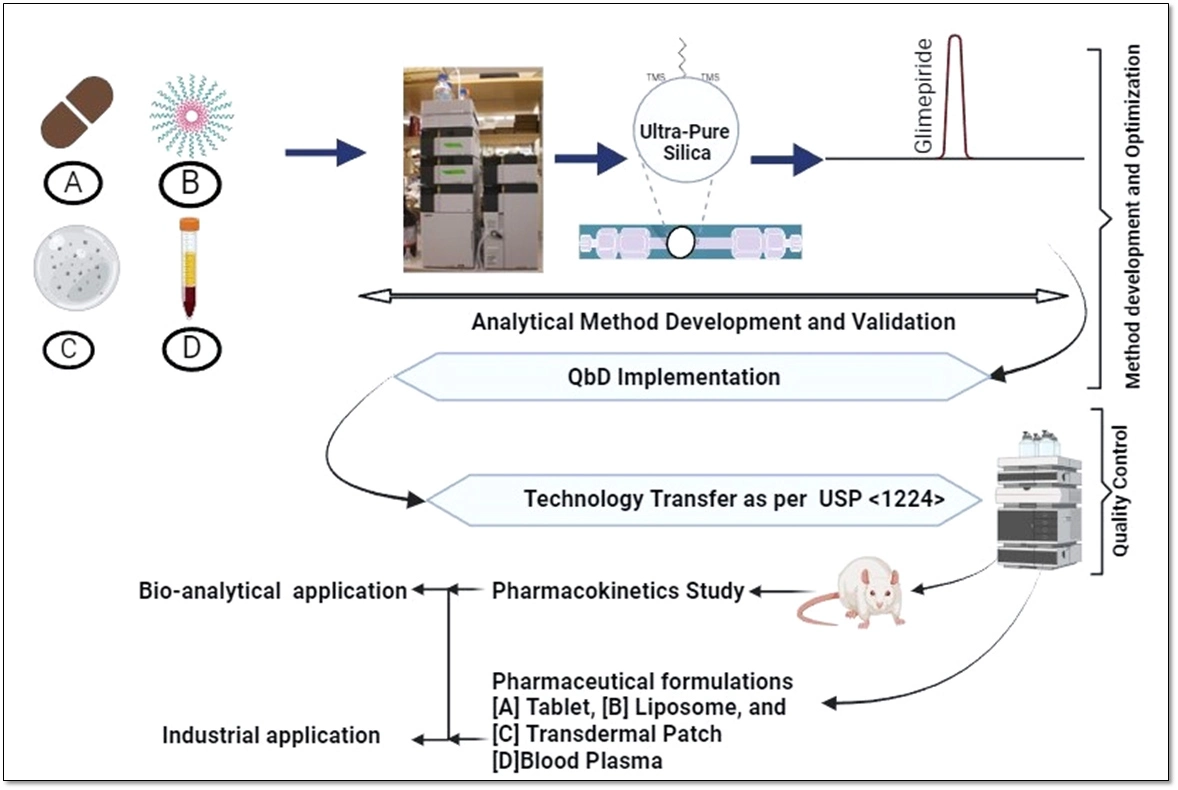
Development of a quality by design based hybrid RP-HPLC method for Glimepiride: Bioanalytical and industrial applications
Abhiram Kumar, Chhavi Dhiman, Madhaw Kumar, N. Kannappan, Deepak Kumar, Manish Kumar Chourasia, Kumar Pranav Narayan
DOI: 10.7324/JAPS.2025.214654Pages: 102-115
Supercritical fluid extraction, LC-MS profiling, and QbD-guided green HPLC method for standardization of Careya arborea Roxb. nanoemulsion
Abhijit S. Salokhe, Archana S. Patil, Yadishma Gaude, Pooja Rayanade, Rahul Koli, Namdeo S. Jadhav
DOI: 10.7324/JAPS.2025.266427Pages:
_.jpg)