INTRODUCTION
Acid orange 7 (AO7) is a class of synthetic azo dyes that are widely used as coloring agents in several products, including leather, textile, paper, food, and cosmetics products (Fang et al., 2013). It has been reported that most azo dyes, precursors, intermediates, and degradation products are carcinogenic, genetic toxic, and mutagenic (Hueper, 1970; Boeniger, 1980; Robens, 1980). AO7 [p-(2-hydroxy-1-naphtylazo)] is forbidden to be used in food products (Fang et al., 2013; GB 2760-2011, 2011), but it was allowed to be used in cosmetic products, except around eyes (Barot and Bahadur, 2015). According to the Scientific Committee on Consumer Safety (SCCS) of Cosmetics (2014), there is no regulation about the allowance limit of AO7 in cosmetic products except in non-oxidative hair dye products, in which AO7 was allowed in maximum levels of 0.5%. The synthetic Sudan dyes, including Sudan II (SII) [1-(phenylazo)-2-naphthol], are non-allowable and illegally used colorants either in the cosmetic industry or in the food products (Calbiani et al., 2004). SII leads to high incidence of bladder carcinomas in tested mice (Pielesz et al., 2002).
Many producers used both AO7 and SII as cheaper and more stable colorants than natural food colors. For the safety assurance of food and cosmetics products, many analytical methods, mainly based on chromatographic techniques, have been established for recording these colors (Purba et al., 2019).
The chemical structures of AO7 and SII were shown in Figure 1. Due to its selectivity and sensitivity, high-performance liquid chromatography (HPLC), especially ion pair-HPLC, using ultraviolet-visible detector, and photo-diode array (PDA) detector, and mass spectrometry (MS) is the common method for analysis of both AO7 and SII (Ma et al., 2006; Yang et al., 2019; Yoshioka and Ichihashi, 2008; Zatar, 2007), or using HPLC tandem with MS (LC-MS/MS) (Ding et al., 2009; Sun et al., 2007). The developed method was carried out using reverse chromatography for determination of Sudan dyes and para red in red chilli pepper using methanol: acetonitrile (20:80) as the mobile phase. The values of limit of detection (LOD) and limit of quantization (LOQ) reported for SII in this method were 3.9 and 13.0 μg/kg, respectively (Ertas et al., 2007). In this study, HPLC using PDA detector has been optimized using experimental design approach and validated according to the International Conference Harmonization (ICH).
 | Figure 1. The chemical structures of AO7 and SII. [Click here to view] |
MATERIALS AND METHODS
Materials
Reference standards of AO7 and SII were obtained from the National Agency of Drug and Food Control of Republic of Indonesia. All the solvents used for mobile phase were of HPLC grade and obtained from E. Merck (Darmstadt, Germany). Aquabidest was obtained from Ikapharmindo (Indonesia). Blush products were purchased from the local markets in Yogyakarta, namely, Viva Blush manufactured by PT. Vitapharm (Indonesia), Purbasari Blush manufactured by PT. Gloria Origita Cosmetics (Indonesia), and Ranee Blusher produced by PT. Multi Rona Anugrerah (Indonesia).
Preparation of reference standards
An approximately of 5.00 mg of each AO7 and SII was accurately weighed using analytical balance (Metler Toledo MX5) with sensitivity of 0.01 mg and was added into volumetric flask 5 ml. AO7 was dissolved in 3 ml methanol, sonicated using sonicator (Elma Ultrasonic, Germany) for 5 minutes, and made to volume with methanol (5 ml) to get solution with concentration of 1,000 μg/ml. SII was dissolved in 1 ml acetonitrile, added with 2 ml methanol, sonicated for 5 minutes and complete to 5 ml with methanol to get solution with concentration of 1,000 μg/ml (Purba et al., 2019).
Preparation of samples
An approximately of 100.0 mg of blush cosmetic products was accurately weighed using analytical balance (Metler Toledo MX5) with sensitivity of 0.1 mg, 1.0 ml of each standard solutions (AO7 and SII) and 1 ml acetonitrile were added, sonicated for 5 minutes, and complete to 5 ml with methanol. The solution was filtered with PTFE 0.45 μm. In HPLC vial, 125 μl of this solution was added with 875 μl of acetonitrile: methanol (1:1 v/v). The solution was injected into HPLC system (Purba et al., 2019).
HPLC instrumentation and condition
Separation of AO7 and SII was carried out using reversed-phase (RP)-HPLC condition, previously optimized using Box–Behnken Design (Purba et al., 2019). The factors responsible for HPLC separation, including column temperature, mobile phase composition, flow rate, were optimized using Box–Behnken Design, while the responses evaluated were peak area, retention time, and tailing factor. The chromatograph of Shimadzu LC 20AD chromatograph equipped with PDA (Shimadzu LC 20AD, M20A PDA Detector) at the wavelength of 300–650 nm. Separation of analytes was performed using C18 column (Thermo Synergy Gold 250 mm × 4.6 mm i.d., 5 μm). The mobile phase used was acetonitrile-water as a solvent (1:1 v/v) and delivered in gradient manner, with ACN 43% at 0–1 minute, and then increased into 90% ACN at 1.3–1.4 minute, and decreased at 43% ACN at 14.40–19. The mobile phase was delivered at flow rate of 0.9 ml/minute, using column temperature of 40°C.
System suitability test
System suitability testing (SST) is required to be performed by either the United States Pharmacopeia (USP) (2003) and Food and Drug Administration to check and ensure on-going performance of analytical systems and methods. SST, as an integral part of liquid chromatographic methods, is used to verify that the reproducibility of the liquid chromatographic system are adequate to perform quantitative analysis. SST is based upon the concept that the equipment, electronics, analytical operations, and samples to be analyzed constitute an integral system (Dolan, 2004). SST was carried out by injecting working standard solutions of AO7 and SII, each at concentrations of 25 μg/ml in six replicates. Parameters were evaluated include retention time, peak area, tailing factor, efficiency, and height equivalent to the theoretical plate (HETP). SST was assessed based on precision of HPLC condition for intended analysis. The % relative standard deviation (% RSD) of each set of parameters (retention time, peak area, tailing factor, efficiency, and HETP) should be less <2% (Snyder et al., 2010).
Validation of HPLC analysis
The validation of HPLC method was performed according to the guideline in International Conference on Harmonization (1996) by determining several performance characteristics, namely, selectivity, linearity and range, sensitivity, precision, accuracy, and robustness.
Selectivity evaluation
The selectivity assay was carried out by comparing retention times of working standard solutions of AO7 and SII, each at concentrations of 25 μg/ml. The resolution and spectral similarity index of analytes, as analyzed by PDA detector, were used for the selectivity evaluation.
Linearity and range
The linearity of HPLC method was performed by varying the concentration of each stock standards. The calibration curve was constructed by correlating the responses (peak area, y axis) and the concentration of each standards over certain range. The evaluation of linearity was carried out by the coefficient of determination (R2) along with percentage of intercept (%-y).
The sensitivity assay
For sensitivity evaluation, a series of standard solutions with low concentrations covering of 2–8 μg/ml was prepared, and the linear regression was constructed by correlating between peak area (y-axis) and its conconcentration at low levels. A-100 mg sample was accurately weighed, spiked with AO7 and SII each at concentration of 10 μg/ml and subjected to analysis using the validated method. The response (peak area) of spiked samples was measured such a that of RSD of 10 replicates falled at 17%–30%. The sensitivity of validated method was expressed as LOD and LOQ and calculated according to Eurachm [21].
yLOD = 3 × SD response of spiked samples
yLOQ = 3 × SD response of spiked samples
Precision evaluation
The precision of the developed method was assesed using repeatibility (intraday precision) and intermediate precision (interday precision) assays. During repeatibility test, 100-mg sample was accurately weighed, spiked with AO7 and SII each at concentration of 10 μg/ml and subjected to analysis using validated method in six replicates. The % RSD values of response were calculated. For intermediate precision, the response of spiked samples were analyzed in two different days.
The accuracy evaluation
The accuracy of HPLC method was done by standard addition method and expressed as a recovery percentage at three ranges, namely, 80%, 100%, and 120%, corresponding to 0.4%, 0.5%, and 0.6% of AO7 and SII, respectively.
Robutness assay
The robustness test was performed by varying parameters of column temperature at level 36°C and 44°C (optimum column temperature 40°C), as well as mobile phase composition, namely, the composition of ACN 1 at the levels of 39% and 47% (optimum composition of 43%), and the composition of ACN2 at levels of 85% and 95% (optimum composition of 90%).
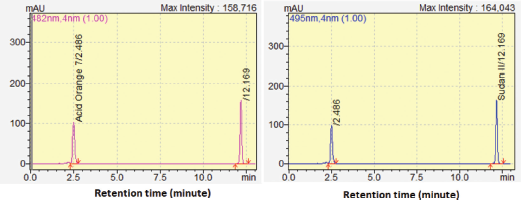 | Figure 2. The chromatogram of RP-HPLC for separation of AO7 and SII. [Click here to view] |
Data analysis
The linearity evaluation, the calculation of mean, standard deviation, RSD, and recovery percentage were performed using Excel software (Microsoft Inc., USA). The statistical test of one way analysis of variance (ANOVA) for robustness was performed using software SPSS version 22.
RESULTS AND DISCUSSION
System suitability test
SST was performed to adequate quantitative analysis by RP-HPLC system. Some parameters, namely, retention time, peak area, tailing factor, efficiency, and HETP were evaluated. The results showed that % RSD values of each set of parameters (retention time, peak area, tailing factor, efficiency, and HETP) were less <2% indicating the reproducibility of RP-HPLC system for quantitative analysis of AO7 and SII.
Validation of RP-HPLC
Validation of RP-HPLC for simultaneous determination of AO7 and SII was intended to reveal the performance characteristics, including selectivity, linearity and range, sensitivity, precision, accuracy, and robustness. The selectivity of RP-HPLC for analysis of AO7 and SII was evaluated by investigating the resolution value of peaks of AO7 and SII with adjacent peak. Both peaks have retention times of about 2.00 (AO7) and 12.85 (SII) with Rs value of 15 which is acceptable (Rs value > 2.0), with good separation was observed, as shown in Figure 2. Selectivity was observed by assessing the peak purity and similarity indexes either in the mixture of standard solutions or spiked samples. The purity index of AO7 and SII in the mixture of standard solutions was 0.999967 and 1.000000, respectively. In addition, the purity index for AO7 and SII in spiked samples was 0.999970 and 1.000000, respectively. The similarity for AO7 and SII in mixed standard solutions and in spiked samples to each standard was 1.000000 as well as 0.999999 for AO7 and 0.999998 for SII as shown in Figure 3. Therefore, it can be concluded that RP-HPLC was selective for the analysis of AO7 and SII.
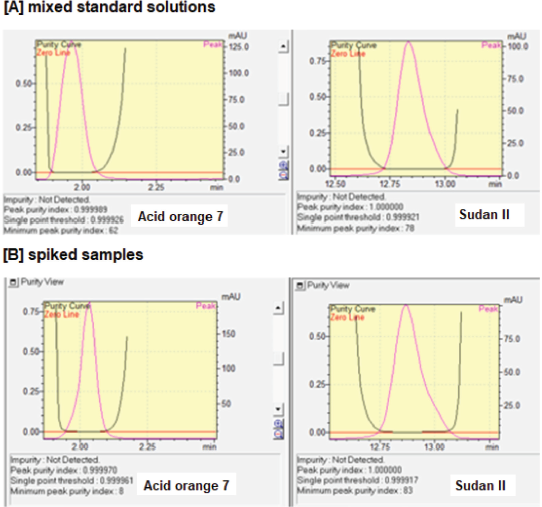 | Figure 3. The purity index of AO7 and SII in the mixed standard solutions (A) and in spiked samples (B). [Click here to view] |
The linearity of RP-HPLC method was assessed over the concentration ranges of 10.326–41.304 μg/ml and 9.967–39.869 μg/ml for AO7 and SII, respectively. The correlation between concentrations (x-axis) and peak area (y-axis) revealed the equations as included in Figure 4. The method was linear as indicated by high coefficient of correlation (r) values which are >0.999. ICH required that r values were ≥0.999. In addition, the % y-intercept for both analytes was <2.0% indicating that systematic errors were negligible (Bhawani et al., 2018; Magnusson and Örnemark, 2014).
Sensitivity of RP-HPLC method was expressed by LOD and LOQ values, based on calculation of responses of blank samples spiked with AO7 and SII such a that RSD values of responses fall within 17%–30%. The responses were then introduced to linear regression equations of AO7 and SII at low concentrations (covering of 2–8 μg/ml). LOD values obtained were 0.059 and 0.055 μg/ml for AO7 and SII, respectively. In addition, LOQ values were 0.179 and 0.167 μg/ml for AO7 and SII, respectively. LOD and LOQ values have been verified using blank samples spiked with AO7 and SII at concentrations around LOD and LOQ values, and the results showed that the method could detect and quantify the corresponding analytes.
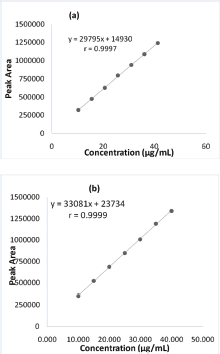 | Figure 4. The linear regression for relationship between concentrations of AO7 (a) and SII (b) (x-axes) with peak area. [Click here to view] |
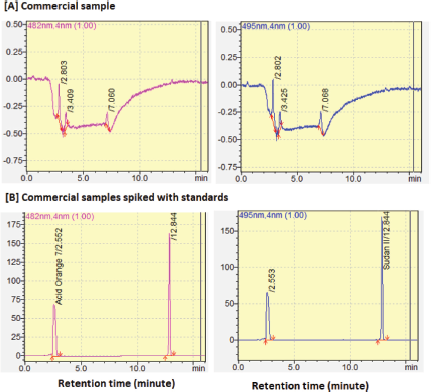 | Figure 5. RP-HPLC chromatogram of one commercial sample (A) with no detectable AO7 and Sudan III and commercial sample spiked with AO7 and SII (B). [Click here to view] |
The precision of RP-HPLC was studied using repeatability and intermediate precision using variation of day. The RSD values of responses (peak area) at six replicates during repeatability test were 0.674% (for AO7) and 0.931% (SII). In addition, RSD values during intermediate precision were 1.088% (day-1) and 1.039% (day-2) for AO7, and 0.434% (day-1) and 1.291% (day-2) for SII. All the RSD values obtained during repeatability and intermediate precision were lower than those required by RSD Horwitz at the corresponding concentration levels, which indicated that RP-HPLC method was precise enough (Miller and Miller, 2010).
The accuracy of RP-HPLC was evaluated by spiked blank method by spiking of analytes into blank samples, and the recovery of analytes was calculated. The levels of analytes spiked into blank samples were 80%, 100%, and 120% from target of analytes corresponding to 0.4%, 0.5%, and 0.6% of AO7 and SII, respectively. The mean recovery percentages obtained were 98.91%, 100.27%, and 99.73% for analyte targets of 80, 100, and 120, respectively. The acceptable recoveries for those levels are in the range 90%–110% (Gonzalez and Herrador, 2007). Therefore, it can be concluded that RP-HPLC method was accurate for the analysis of AO7 and SII in blusher product.
The Robustness of RP-HPLC was evaluated by observing retention time and peak area of AO7 and SII with variation of temperature at ±4°C from actual condition, variation in concentration of ACN 1 at ±4% and ACN 1 at ±5% (one way-ANOVA) showed that p < 0.05 which indicated that there were significant differences among retention time and peak area of AO7 and SII. Therefore, it should be noted that the optimum condition of RP-HPLC must be followed to get desired quantitative analysis of AO7 and SII.
Analysis of commercial samples
Analysis of three commercial samples from the local market in Yogyakarta showed that none of the samples contain AO7 and SII, as indicated by no peaks observed at corresponding retention time of AO7 and SII. This was confirmed by the presence of AO7 and SII due to spiking of samples with reference standards of AO7 and SII. The concentration of standard solutions in all spiked samples was 25.82 μg/ml for AO7 and 24.92 μg/ml for SII. The selection of this concentration because there are no regulation limits for blusher products yet. For this reason, to the concentration of two times of LOQ values was adopted in this method (Fig. 5).
CONCLUSION
RP-HPLC has been validated according to the ICH guideline intended for quantitative analysis of AO7 and SII. The characteristic of validation performance indicated that the developed RP-HPLC was valid and reliable and both AO7 and SII were not detected in the tested samples.
ACKNOWLEDGMENTS
The authors would like to thank the Indonesian National Agency of Drug and Food Control, Indonesian National Agency of Drug and Food Control in Denpasar, and Indonesian National Agency of Drug and Food Control in Yogyakarta for financial support and instrument facilities. The publication of this article is supported by the Ministry of Research and Higher Education through scheme "Penelitian Terapan Unggulan Perguruan Tinggi" with contract number 2717/UN1.DITLIT/DIT-LIT/LT/2019.
CONFLICT OF INTEREST
The authors have declared “no conflicts of interest with respect to the research, authorship, and/or publication of this article”.
AUTHORS’ CONTRIBUTION
N.B.R.P. performed research activity, compiled data, and prepared the manuscript. A.R. and S.M. designed research activities, prepared manuscript, and made critical thinking on the manuscript.
REFERENCES
Barot J, Bahadur A. Toxic impacts of C.I. acid orange 7 on behavioural, haematological and some biochemical parameters of labeo rohita fingerlings. Int J Sci Res Environm Sci, 2015; 3:284–90. CrossRef
Bhawani S, Nageshwari HG, Mamatha G, Venu M, Krishna SM, Krishna MKS. Analytical method development and validation of Bendamustine in bulk using RP-HPLC. Pharm Res, 2018; 2:000158. CrossRef
Boeniger M. Carcinogenicity and metabolism of azo dyes, especially those derived from benzidine. NIOSH (NTIS), Springfield, VA, 1980.
Calbiani F, Careri M, Elviri L, Mangia A, Pistarà L, ZagnoniI. Development and in-house validation of a liquid chromatography-electrospray-tandem mass spectrometry method for the simultaneous determination of Sudan I, Sudan II, Sudan III and Sudan IV in hot chilli products. J Chromatogr A, 2004; 1042:123–30. CrossRef
Ding Y, Sun C, Xu X. Simultaneous identification of nine carcinogenic dyes from textiles by liquid chromatography/electrospray ionization mass spectrometry via negative/positive ion switching mode. Eur J Mass Spectrom, 2009; 15:705–13. CrossRef
Dolan JW. System suitability. 2004; 17(6):328–32. Available via http://alfresco.ubm-us.net/alfresco_images/pharma/2014/08/22/351c1a16-183a-48dd-9241-4277b40c2168/article-98295.pdf (Accessed 16 July 2018).
Ertas E, Ozer H, Alasalvar CA. Rapid HPLC method for determination of sudan dyes and para red in red chilli pepper. Food Chem, 2007; 105:756–60. CrossRef
Fang G, Wu Y, Dong X, Liu C, He S, Wang S. Simultaneous determination of banned acid orange dyes and basic orange dyes in foodstuffs by liquid chromatography–tandem electrospray ionization mass spectrometry via negative/positive ion switching mode. J Agric Food Chem, 2013; 61:3834–41; doi:10.1021/jf400619y. CrossRef
Gonzalez AG, Herrador MA. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trends Anal Chem, 2007; 26(3):227–38. CrossRef
Hueper WC. Occupational and environmental cancers of the urinary system. Br J Surg, 1970; 57:940–5.
International Conference on Harmonization. Guidance for industry: Q2B validation of analytical procedures: methodology. p. 10, 1996. Available via www.fda.gov/cedr/guidance/index.htm. (Accessed 16 July 2018)
Ma M, Luo X, Chen B, Su S, Yao S. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high-performance liquid chromatography–diode array detection–electrospray mass spectrometry. J Chromatogr A, 2006; 1103:170–6. CrossRef
Magnusson B, Örnemark U. (eds.). Eurachem guide: The fitness for purpose of analytical methods—a laboratory guide to method validation and related topics. 2014. Available via http://www.eurachem.org. (Accessed 30 July 2018)
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. Prentice Hall, UK, 2010.
National Standard 2760-2011. Using standard of food additives. National Standard of People’s Republic of China, 2011.
Pielesz A, Baranowska I, Rybak A, WÅ‚ochowicz A. Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol Environ Safety, 2002; 53:42–7. CrossRef
Purba NBR, Rohman A, Martono S. The optimization of HPLC for quantitative analysis of acid orange 7 and Sudan II in cosmetic products using box behnken design. Int J Appl Pharm, 2019; 11:130–7. CrossRef
Robens JF. Thirteen-week subchronic toxicity studies of Direct Blue 6, Direct Black 38, and Direct Brown 95 dyes. Toxicol Appl Pharmacol, 1980; 54:431–42. CrossRef
SCCS, Scientific Committee on Consumer Safety Opinion On Acid Orange. Luxembourg, European Union. 2014. pp. 6–8. Available via https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_158.pdf. (Accessed 16 July 2018)
Snyder LR, Kirkland JJ, Dolan JW. Introduction to modern liquid chromatography. 3rd edition, John Wiley & Sons Inc. Publication, USA, 2010. CrossRef
Sun HW, Wang FC, Ai LF. Determination of banned 10 azo-dyes in hot chili products by gel permeation chromatography-liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr A, 2007; 1164:120–8. CrossRef
USP 27/NF 22. United States pharmacopoeial convention. Rockville, MD: United States Pharmacopeial Convention, p. 2281, 2003.
Yang Y, Zhang J, Yin J, Yang Y. Fast simultaneous determination of eight sudan dyes in chili oil by ultra-high-performance supercritical fluid chromatography. J Anal Methods Chem, 2019; 2019:3731028; doi:10.1155/2019/3731028. CrossRef
Yoshioka N, Ichihashi K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta, 2008; 74:1408–13. CrossRef
Zatar NA. Simultaneous determination of seven synthetic water-soluble food colorants by ion-pair reversed-phase high-performance liquid chromatography. J Food Technol, 2007; 5:220–4.