Formulation and evaluation of Salbutamol sulphate microspheres by solvent evaporation method
V.V Prasanth, Akashmoy Chakraborty, Sam T Mathew, Rinku Mathappan, V. Kamalakkannan
Pages: 133-137

Development of Oral Colon Specific PH Dependent Microcapsules of NSAID Drug Naproxen
J. K. Saboji, R. B. Gadve, S. M. Patil
DOI: 10.7324/JAPS.2012.2535Pages: 202-211

Design and evaluation of liposomal delivery system for L-Asparaginese
Anindita De, D. Nagaswamy Venkatesh
DOI: 10.7324/JAPS.2012.2818Pages: 112-117

Development and Characterization of Controlled Release Ketoprofen Microspheres
Marwa H. Abdallah, Omaima A. Sammour, Hanaa A. El-ghamry, Hanan M. El-nahas, Waleed Barakat
DOI: 10.7324/JAPS.2012.2310Pages: 60-67

Comparison between Microplate Spectrometry and LC/MS Chromato-graphy for Facile Pilot Pharmacokinetics and Biodistribution Studies of Doxorubicin-loaded Nanoparticle Drug Carriers
Pengxiao Cao and Younsoo Bae
DOI: 10.7324/JAPS.2012.2901Pages: 001-009

Studies on losartan novel extrudates design and evaluation to treat hypertension
Putta Rajesh Kumar, Jagannath M, Earshad Md, Gowtham K.K, Hafsa M, Shanta Kumar S.M.
DOI: 10.7324/JAPS.2012.21119Pages: 108-113

Atrazine degradation in liquid culture and soil by a novel yeast Pichia kudriavzevii strain Atz-EN-01 and its potential application for bioremediation
Evy Alice Abigail, Jaseetha Abdul Salam and Nilanjana Das
DOI: 10.7324/JAPS.2013.3606Pages: 035-043

An approach on microbial biosynthesis of L-glutaminase: a tumour inhibitor
Sunil Dutt P.L.N.S.N., Siddalingeshwara K.G, Karthic J, Mohsin S. Mushtaq, Naveen M Vishwanatha T and Prathiba K.S
DOI: 10.7324/JAPS.2013.3622Pages: 132-135

Evaluation of the MRSA Sensitivity to Essential Oils Obtained from four Algerian Medicinal Plants
KHADIR Abdelmounaïm, BENDAHOU Mourad, BENBELAID Fethi, BELLAHCENE Chafika, ABDELOUAHID Djamel-Eddine, MUSEILI Alin, PAOLLINI Julien, DESJOBER Jymy, COSTA Jean
DOI: 10.7324/JAPS.2013.3704Pages: 018-024

Bioavailability of karanjin from Pongamia pinnata L. in Sprague dawley rats using validated RP-HPLC method
Naresh Shejawal, Sasikumar Menon, Sunita Shailajan
DOI: 10.7324/JAPS.2014.40303Pages: 010-014

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Estimation of Pharmacokinetic Parameters Using Nonlinear Fixed Effects One Compartment Open Model
Mithun Kumar Acharjee, Uttom Kumar, Sitesh Chandra Bachar, Wasimul Bari
DOI: 10.7324/JAPS.2014.41210Pages: 056-059

Fabrication of Bucco-matrix tablets of Amoxicillin trihydrate on the basis of release and permeation kinetics
Gopa Roy Biswas, Subhasis Chakraborty, Nabarun Ghosh, Sutapa Biswas Majee
DOI: 10.7324/JAPS.2015.50408Pages: 048-052

A Five-Year Stability Study of Controlled-Release Diltiazem Hydrochloride Tablets Based on Poly(Ethylene Oxide)
Laila H. Emara, Ahmed A. El-Ashmawy, Nesrin F. Taha
DOI: 10.7324/JAPS.2015.50703Pages: 012-022

Development and validation of a stability indicating HPLC-diode array-fluorescence method for the determination of meclofenoxate hydrochloride and p-chlorophenoxyacetic acid
Marwa Said Moneeb, Feda Elgammal, Suzy Mohamed Sabry
DOI: 10.7324/JAPS.2016.60701Pages: 001-011

Fabrication and Characterization of chitosan based polymeric Escitalopram nanoparticles
Rashi Rajput, Sachin Kumar, Payal Nag, Manisha Singh
DOI: 10.7324/JAPS.2016.60725Pages: 171-177

Kinetics and mechanism of permanganate oxidation of nalidixic acid in aqueous alkaline medium
Ankita Jain, Gajala Tazwar, Vijay Devra
DOI: 10.7324/JAPS.2017.70118Pages: 135-143

Chromatogram profiles of andrographolide in A23187-induced New Zealand rabbit’s urine and faeces
Jutti Levita, Tanti Juwita, Selma Ramadhani, Nyi Mekar Saptarini, Mutakin Mutakin
DOI: 10.7324/JAPS.2017.70121Pages: 156-159

Pharmacokinetics of a new imidazoline receptor agonist in rat plasma after intragastric and intravenous administration
Kulikov Aleksandr, Avtina Tatyana, Pokrovsky Mikhail, Korokin Mikhail
DOI: 10.7324/JAPS.2017.70302Pages: 006-008

Pharmacokinetic and Pharmacodynamic evaluation of Camptothecin encapsulated Poly (methacylic acid-co-methyl methacrylate) nanoparticles
Mahalingam Manikandan, Krishnamoorthy Kannan
DOI: 10.7324/JAPS.2017.70303Pages: 009-016

The natural anti-tubercular agents: In silico study of physicochemical, pharmacokinetic and toxicological properties
Mohammad Firoz Khan, Md. Abdul Bari, Md. Kamrul Islam, Md. Shariful Islam, Md. Shahidulla Kayser, Nusrat Nahar, Md. Al Faruk, Mohammad A. Rashid
DOI: 10.7324/JAPS.2017.70506Pages: 034-038

Crushed Puffed Rice-HPMC-Chitosan based Single-Unit Hydro-dynamically Balanced System for the Sustained Stomach Specific Delivery of Metoprolol Succinate
Shashank Soni, Veerma Ram, Anurag Verma
DOI: 10.7324/JAPS.2017.71206Pages: 047-057

Degradation kinetics of anthocyanin extracted from roselle calyces (Hibiscus sabdariffa)
Maripillai Munusamy Pragalyaashree, Deivanayagame Tiroutchelvame, Siddant Sashikumar
DOI: 10.7324/JAPS.2018.81108Pages: 057-063

A validated LC-MS/MS method for the pharmacokinetic study of alogliptin in healthy rabbits
Yatha Ravi, Bigala B. Rajkamal
DOI: 10.7324/JAPS.2019.90204Pages: 029-037
Evaluation of synergism between Cefotaxime and Allium sativum against Extended-spectrum beta-lactamase and Ambler class C co-producers
Pratibha J. Shah, Manita T. Williamson
DOI: 10.7324/JAPS.2019.90314Pages: 098-104
Antioxidant biosensor based on superoxide dismutase from Indonesian microbes immobilized in Indonesian natural zeolite
Dyah Iswantini, Weniarti, Novik Nurhidayat, Zaenal Abidin, Trivadila
DOI: 10.7324/JAPS.2019.90413Pages: 104-109
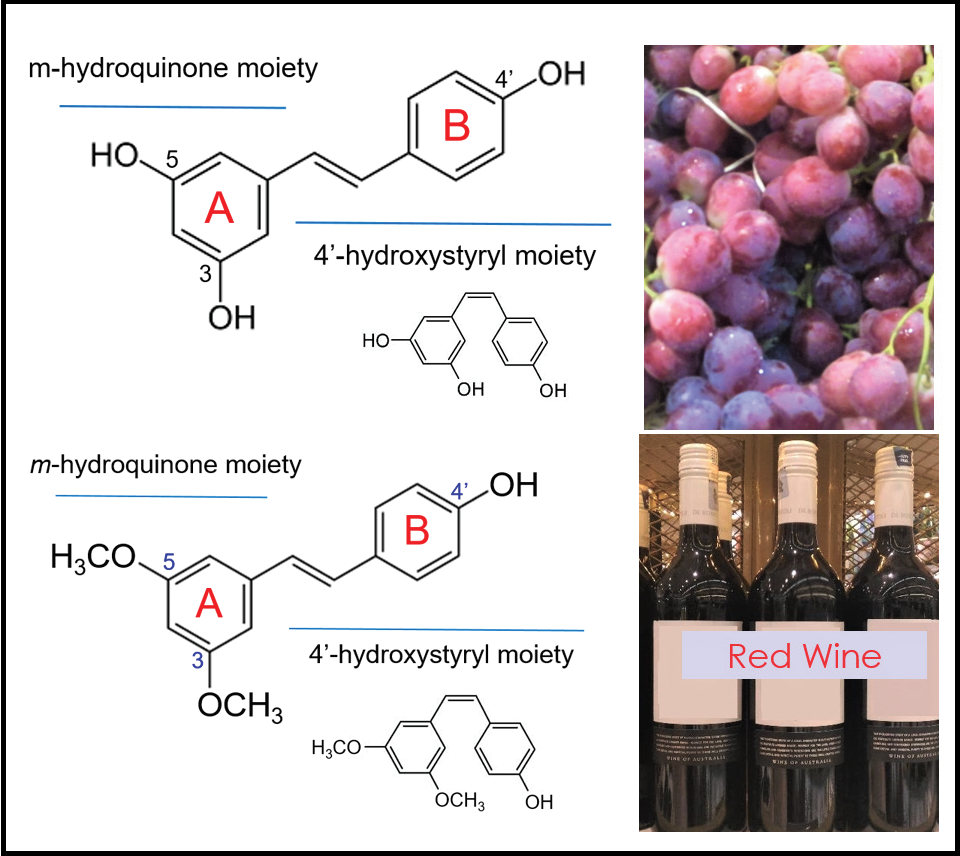
Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties
Eric Wei Chiang Chan, Chen Wai Wong, Yong Hui Tan, Jenny Pei Yan Foo, Siu Kuin Wong, Hung Tuck Chan
DOI: 10.7324/JAPS.2019.90717Pages: 124-129
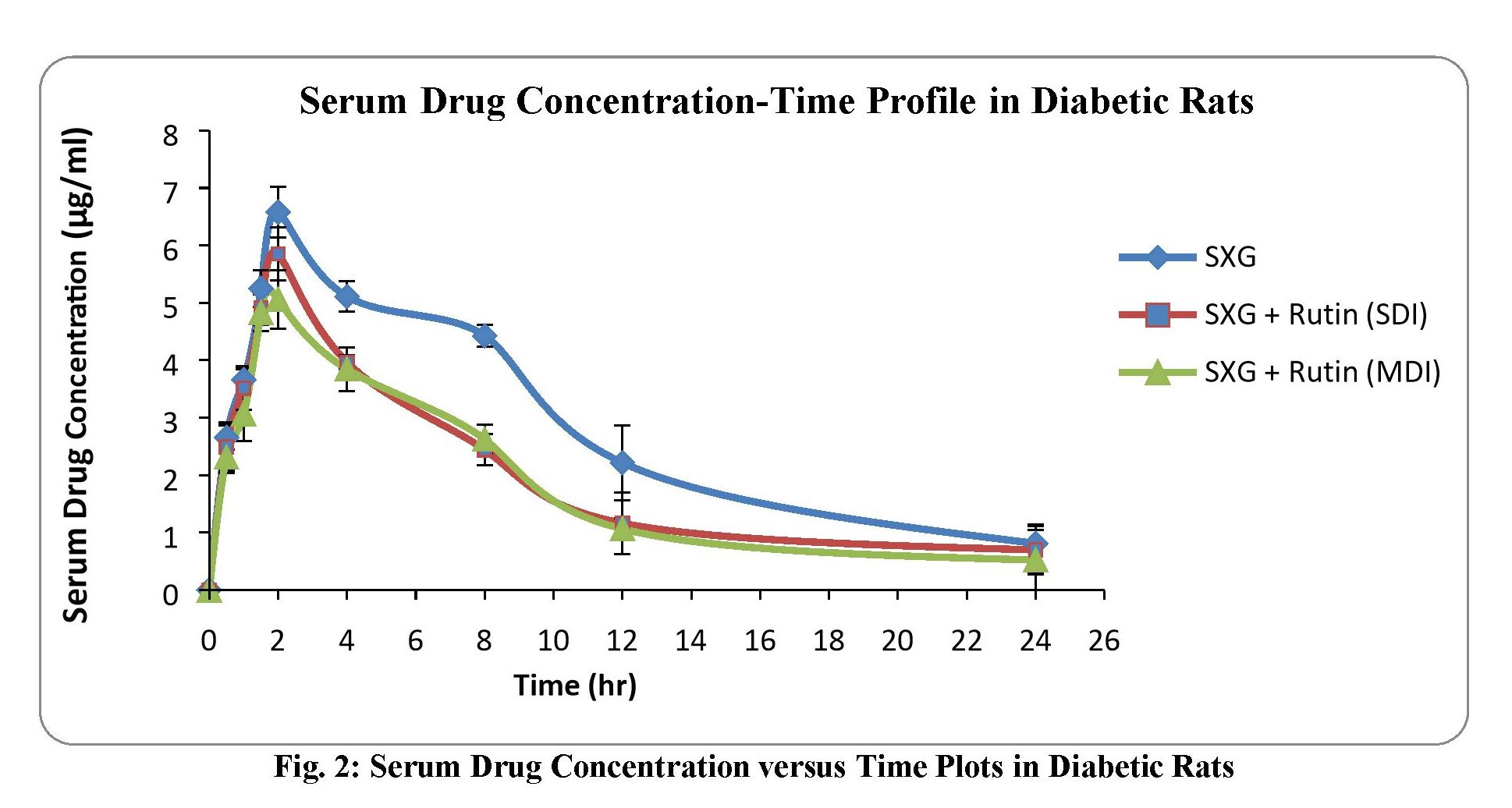
Study of alterations in pharmacokinetics and pharmacodynamics of Saxagliptin in the presence of Rutin: An interaction study in rats
Naga Raju Kandukoori, Pavani Uppu, Narsimha Reddy Yellu
DOI: 10.7324/JAPS.2020.101111Pages: 081-086
Effect of piperine and its analogs on pharmacokinetic properties of sorafenib tosylate: Bioanalytical method development and validation
Anshuly Tiwari, Kakasaheb R. Mahadik, Satish Y. Gabhe
DOI: 10.7324/JAPS.2020.101201Pages: 001-012
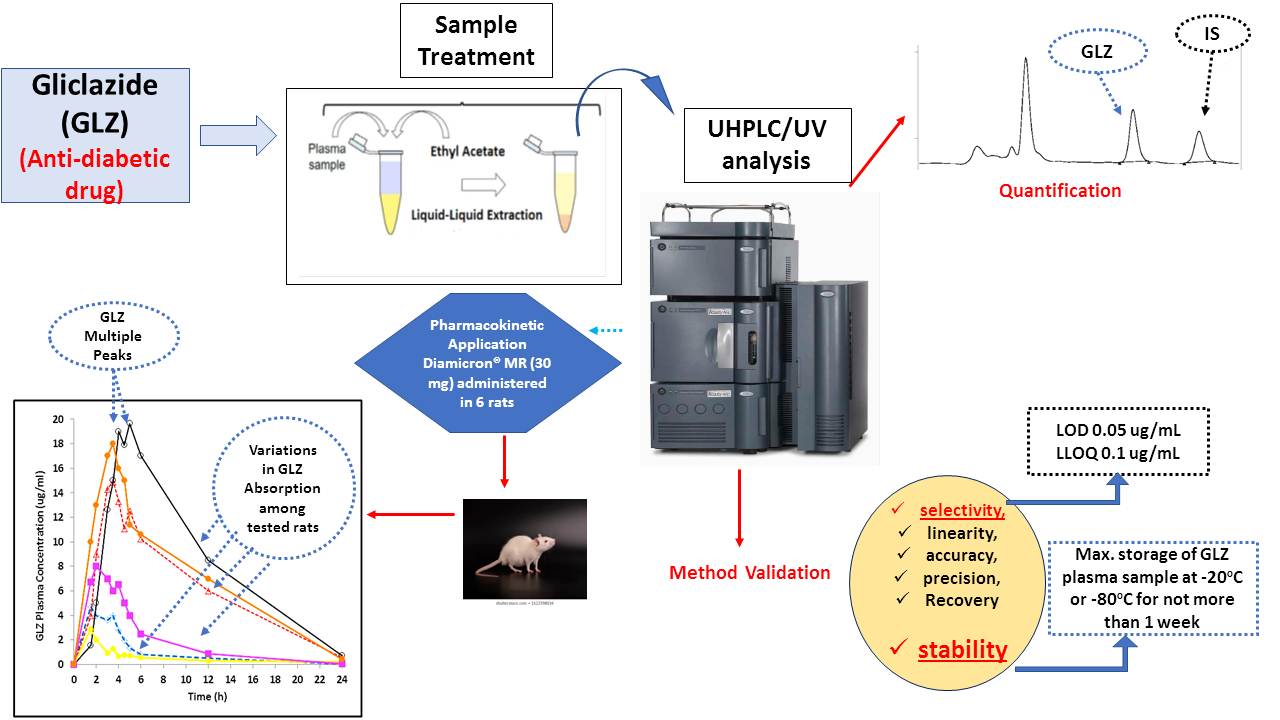
Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053
Method development and validation of LC–ESI–MS/MS method for the quantification of sonidegib in healthy rabbits
Vankayala Devendiran Sundar, Kumar Raja Jayavarapu, Parimala Krishnan
DOI: 10.7324/JAPS.2021.110510Pages: 071-078
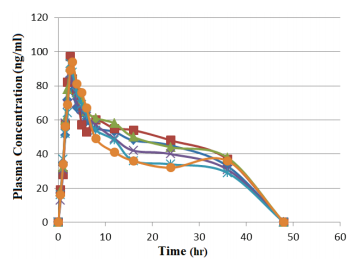
Hierarchical Bayesian approach applied to the formulation of sustained-release suppositories and dissolution profile modeling
Abdelhafid Benomar, Casimir Adade Adade, Yassir Elalaoui, Naoual cherkaoui, Younes Rahali, Abdelkader laatiris, Aicha Fahry
DOI: 10.7324/JAPS.2021.110705Pages: 055-062
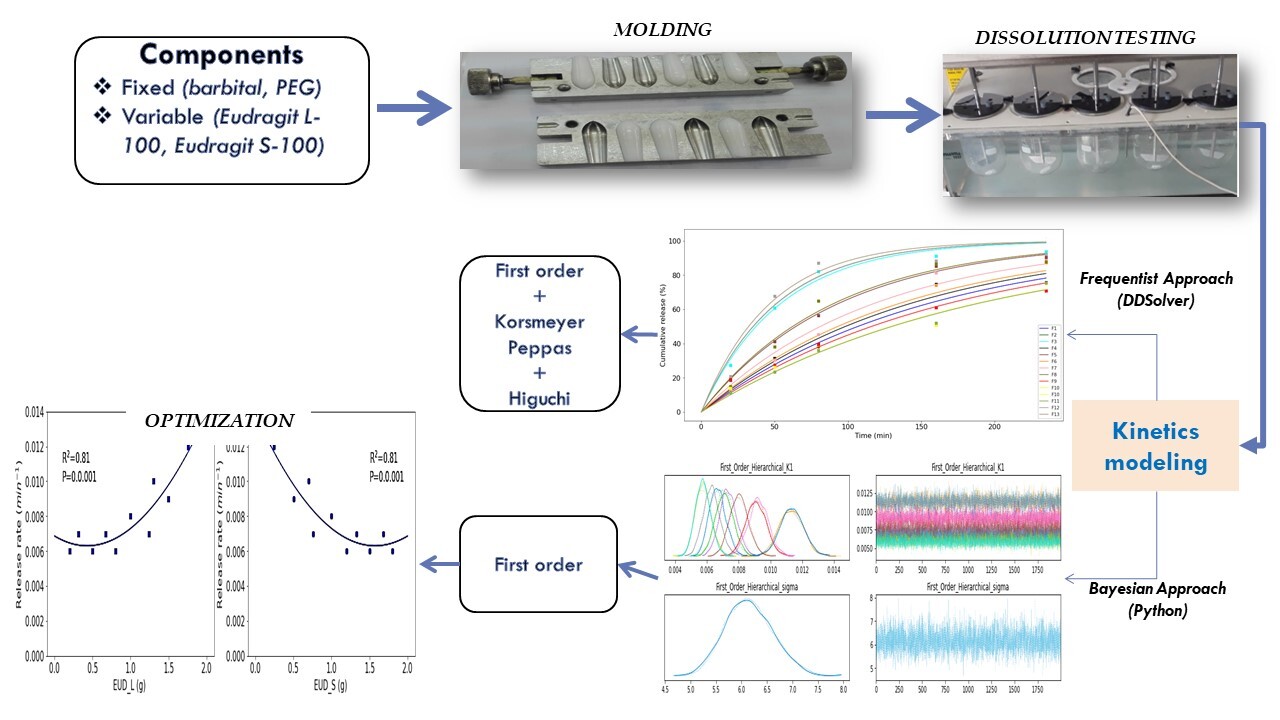
Novel glitazone attenuates rotenone-induced toxicity in mouse model of Parkinson’s disease
Ankith B. Shetty, Kamsagara Linganna Krishna, Bommenahally Ravanappa Prashantha Kumar, Hittanahalli Shivakumar Nandini
DOI: 10.7324/JAPS.2021.1101007Pages: 042-049
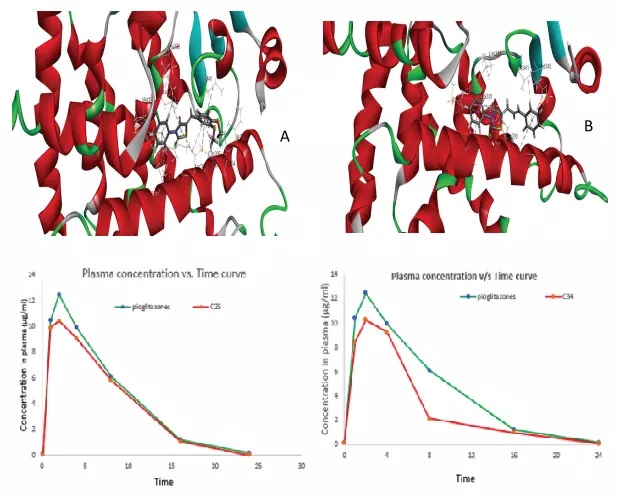
Pharmacokinetics and bioavailability of midazolam in rats following single dose administration of intravenous solution, intranasal dispersion, and in situ nasal gel
Elahehnaz Parhizkar, Saba Movaffagh, Shohreh Alipour
DOI: 10.7324/JAPS.2021.1101109Pages: 070–075
Clinical awareness of therapeutic drug monitoring among medical students—A descriptive cross-sectional study
Amberkar Mohanbabu Vittalrao, Aditya Kumar Adhikarla, Sadhana N. Holla, Meena Kumari Kamalkishore, Seema Kumari Kamal Kishore
DOI: 10.7324/JAPS.2021.1101105Pages: 034–045
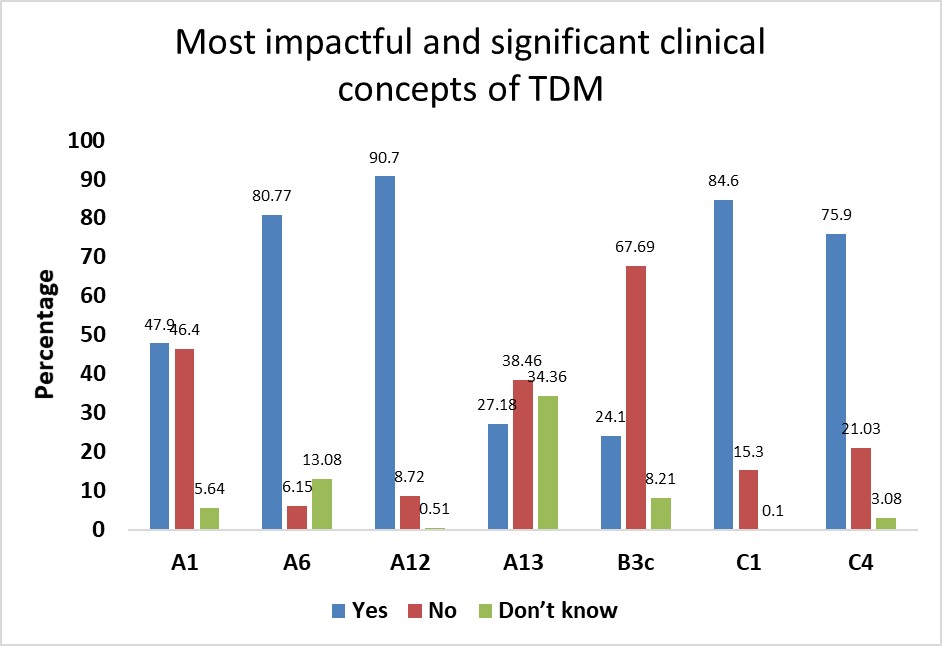
Gentamicin pharmacokinetics and pharmacodynamic correlation in pediatrics - A systematic review
Keerthana Chandrasekar, Vahini B, Vijay V, Shalini R, Arun KP
DOI: 10.7324/JAPS.2021.1101102Pages: 011-017
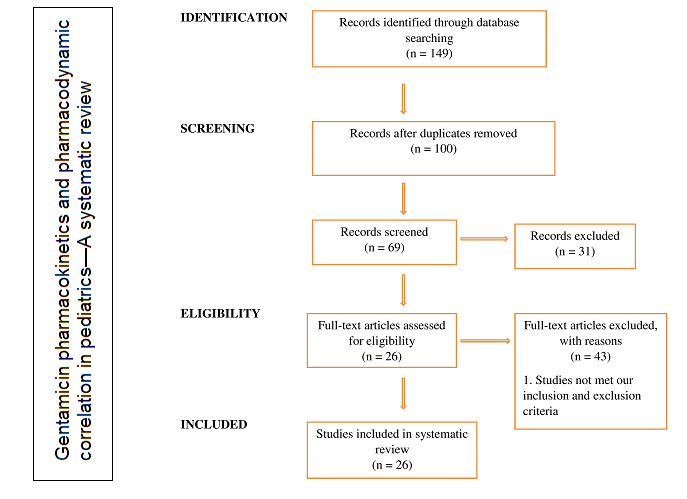
Chrysin or mannitol for treatment of acute kidney injury: Evidence for pharmacokinetic interaction
Heba M. I. Abdallah, Sally A. El Awdan, Salma A. El-Marasy, Omar A. Ahmed-Farid, Azza Hassan
DOI: 10.7324/JAPS.2021.1101213Pages: 139–150
Bioanalytical RP-HPLC method validation for resveratrol and its application to pharmacokinetic and drug distribution studies
Shivaprasad Gadag, Reema Narayan, Yogendra Nayak, Usha Yogendra Nayak
DOI: 10.7324/JAPS.2021.120216Pages: 158-164
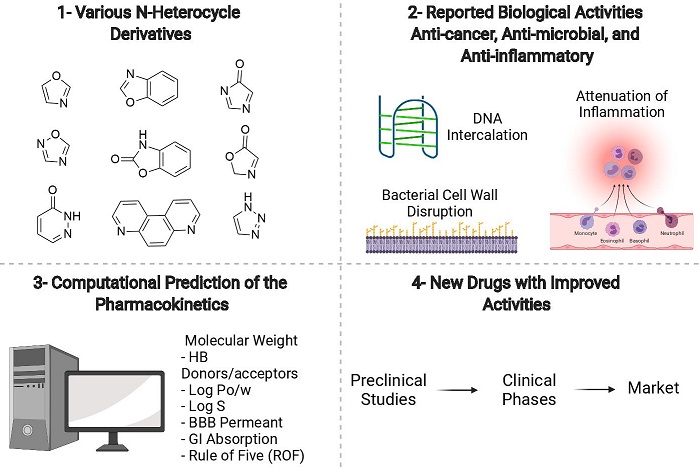
N-Heterocycle derivatives: An update on the biological activity in correlation with computational predictions
Sahar Saleh Alghamdi, Rasha Saad Suliman, Rawan Awadh Alshehri, Razan Suleiman Almahmoud, Renad Ibrahim Alhujirey
DOI: 10.7324/JAPS.2022.120504Pages: 059-077
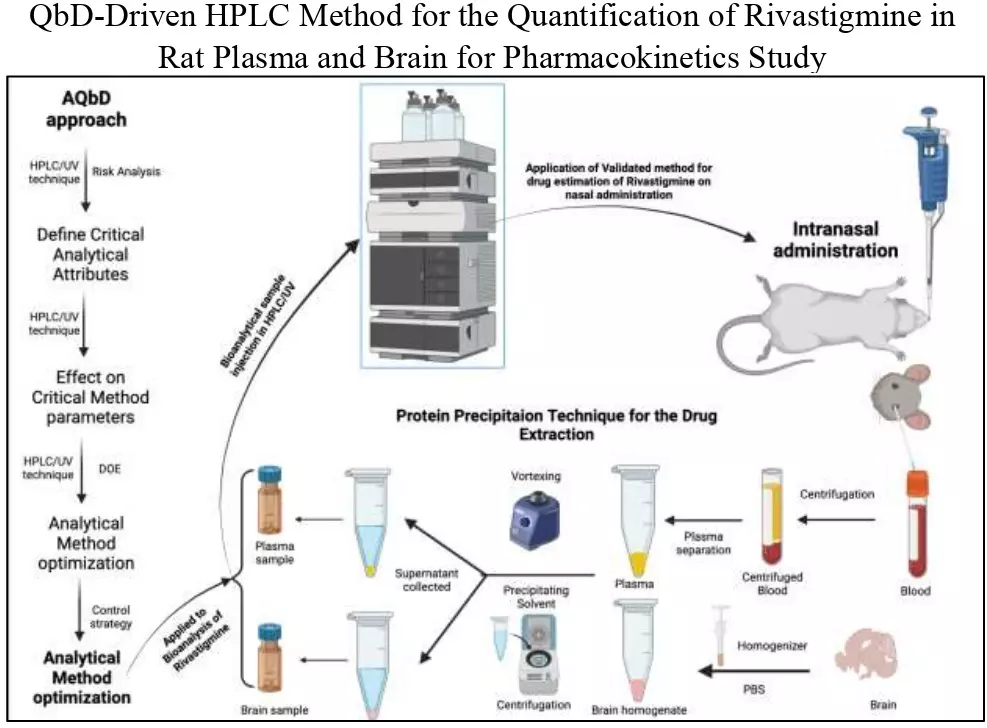
QbD-driven HPLC method for the quantification of rivastigmine in rat plasma and brain for pharmacokinetics study
Divya Gopalan, Prajakta H. Patil, Puralae Channabasavaiah Jagadish, Suvarna G. Kini, Angel Treasa Alex, Nayanabhirama Udupa, Srinivas Mutalik
DOI: 10.7324/JAPS.2022.120606Pages: 056-067
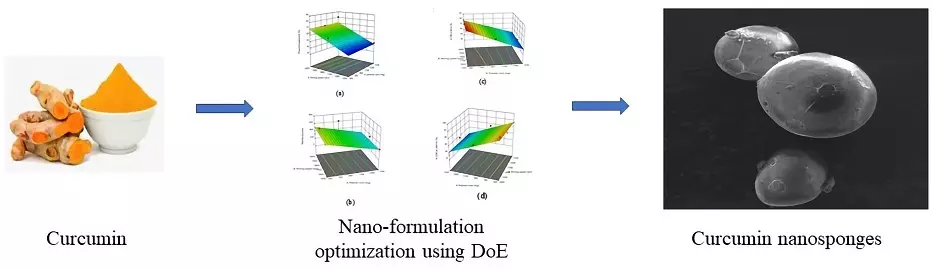
Design, formulation, and evaluation of curcumin-loaded nanosponges for the effective management of colitis
Praharsh Kumar Mandadhi Rajendra, Karthik Ganesan, Bala Sai Souith Nidamanuri, Jawahar Natarajan, Nirmala Puttaswamy
DOI: 10.7324/JAPS.2022.121207Pages: 059-071
Development and validation of LC-MS/MS method for alpelisib quantification in human plasma: Application to pharmacokinetics in healthy rabbits
Tandrima Majumder, Shiva Kumar Gubbiyappa
DOI: 10.7324/JAPS.2023.75269Pages: 089-096

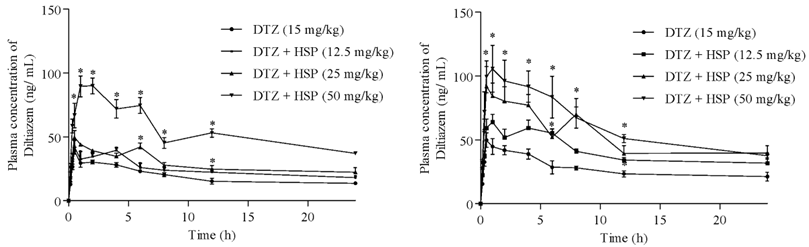
Influence of hesperetin on the pharmacokinetics of diltiazem in rats
Ravindra Babu Pingili, Surya Sandeep Mullapudi, Sridhar Vemulapalli, Naveen Babu Kilaru
DOI: 10.7324/JAPS.2023.118669Pages: 093-099
A validated LC-MS/MS method for simultaneous quantification of antitubercular drugs in rat plasma and its application for a pharmacokinetic interaction study with Immusante®
Lakavalli Mohankumar Sharath Kumar, Mohammed Mukhram Azeemuddin, Krishna Chaitanya Routhu, Keerthi Priya, Uddagiri Venkanna Babu, Sreedhara Ranganath Pai
DOI: 10.7324/JAPS.2023.118956Pages: 151-158
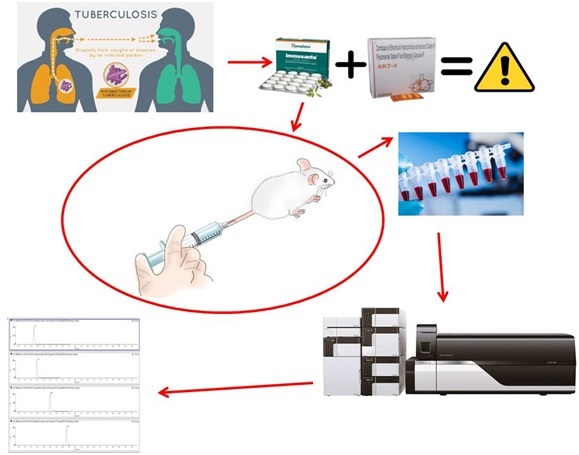
A validated LC-MS/MS method for studying the pharmacokinetic interaction of Immusante® and antiviral drugs in rats
Lakavalli Mohankumar Sharath Kumar, Mohammed Mukhram Azeemuddin, Tirumalai Ramanujam Tulasi, Madhavan Vijayakumar, Uddagiri Venkanna Babu, K Sreedhara Ranganath Pai
DOI: 10.7324/JAPS.2023.127117Pages: 125-131
_.jpg)
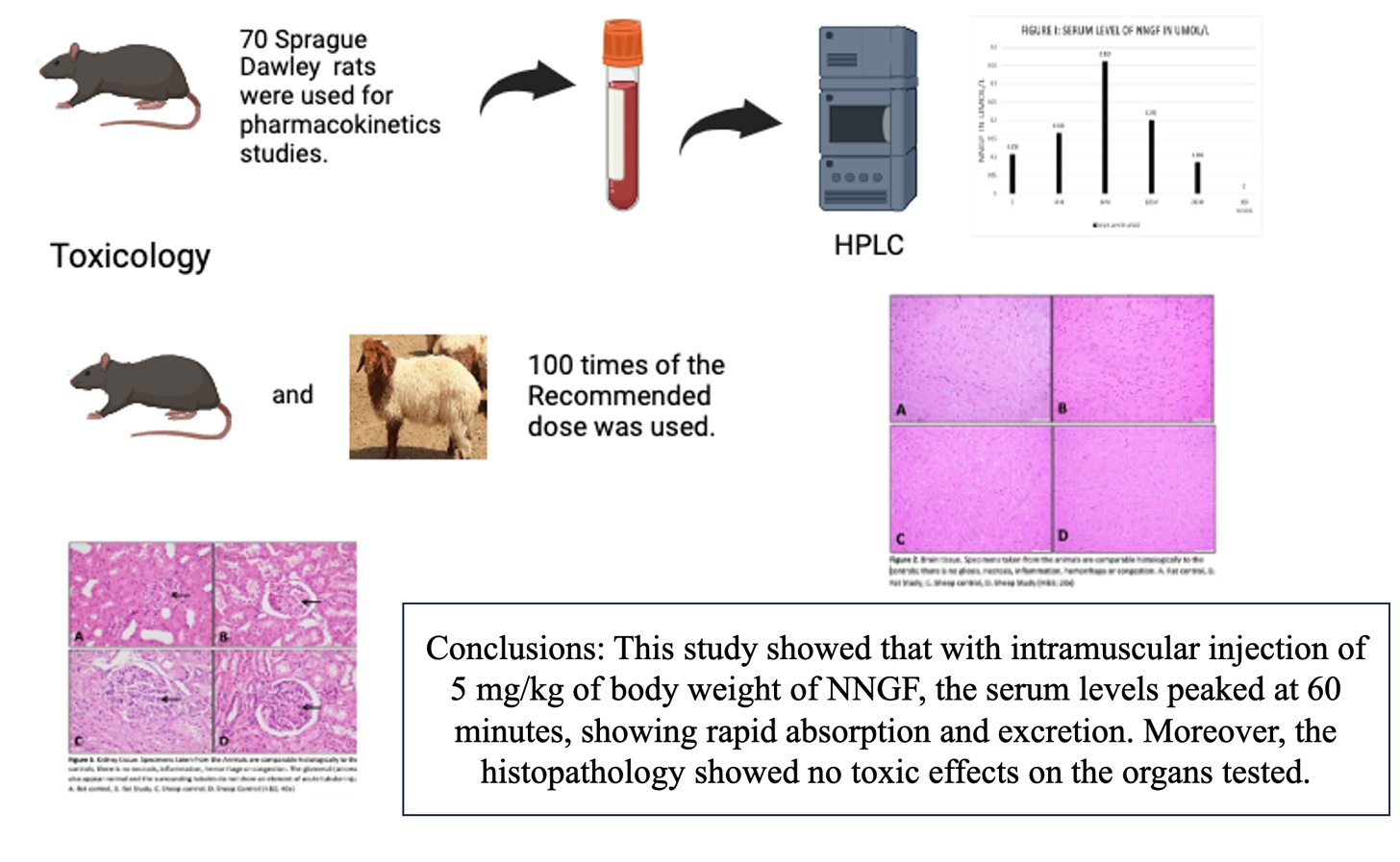
Pharmacokinetics and toxicology studies of new neuronal growth factor
Mir Sadat-Ali, Dakheel A Al-Dakheel, Khulood S. Al Ghamdi, Shahanas Chathoth, Fadel A. Al-Omar, Hassan A. Al Saad, Ayesha Ahmed
DOI: 10.7324/JAPS.2024.112811Pages: 120-124
Antimicrobial activity and time-kill kinetics of Boesenbergia rotunda essential oil and geraniol alcohol against oral bacterial pathogens
Donruedee Sanguansermsri, Phanchana Sanguansermsri, Kittisak Buaban, Rungtip Kawaree, Nalin Wongkattiya
DOI: 10.7324/JAPS.2024.154568Pages: 215-221
_.jpg)
Development of an LC-MS/MS technique and its validation for the determination of infigratinib in human K2EDTA plasma; Pharmacokinetics in healthy rabbits
Kunala Anusha, Gummadi Sowjanya
DOI: 10.7324/JAPS.2024.171011Pages: 148-155
_.webp)
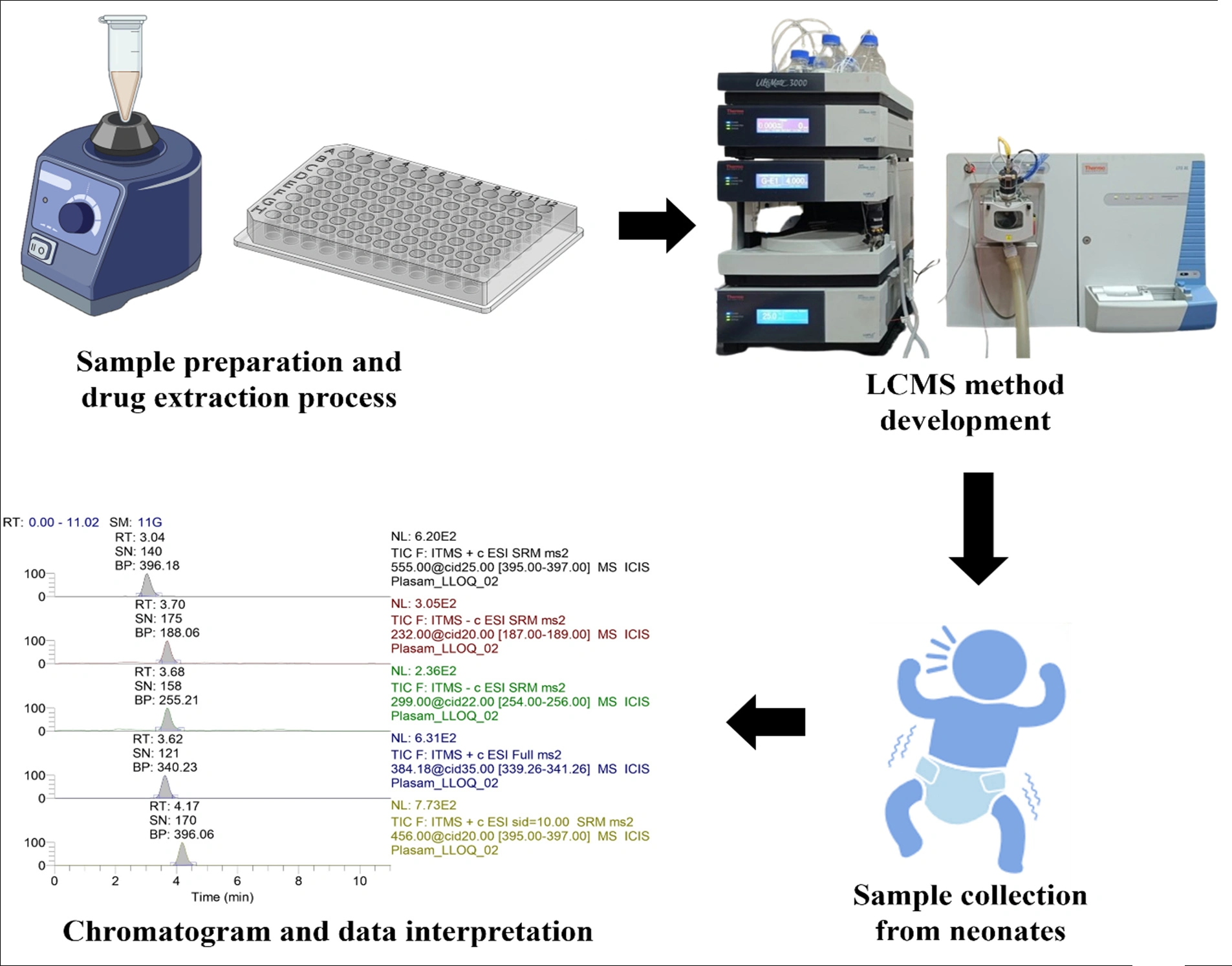
A comparative evaluation of the plasma and DBS-based LC-MS/MS methods for the simultaneous analysis of nine antibiotics for application to pharmacokinetic evaluations and precision dosing in neonates
Bhim Bahadur Chaudhari, Leslie E. Lewis, M. Surulivel Rajan, Ashutosh Gupta, Moumita Saha, Shivani Kunkalienkar, Sudheer Moorkoth
DOI: 10.7324/JAPS.2025.214611Pages: 129-142
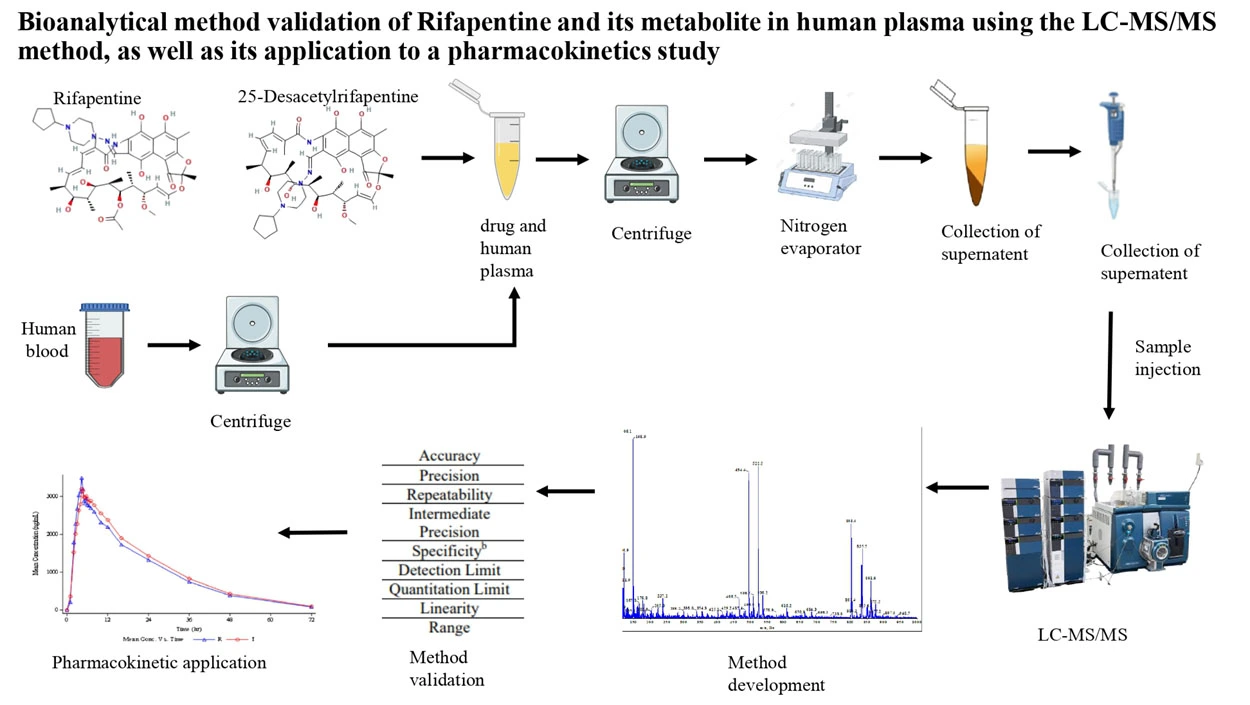
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201
Development and validation of an LC-MS/MS method for pharmacokinetic assessment of tucatinib in rat plasma
Bandaru Venkata Ramarao, Anand Solomon Kamalakaran
DOI: 10.7324/JAPS.2025.202389Pages: 225-233
_.jpg)
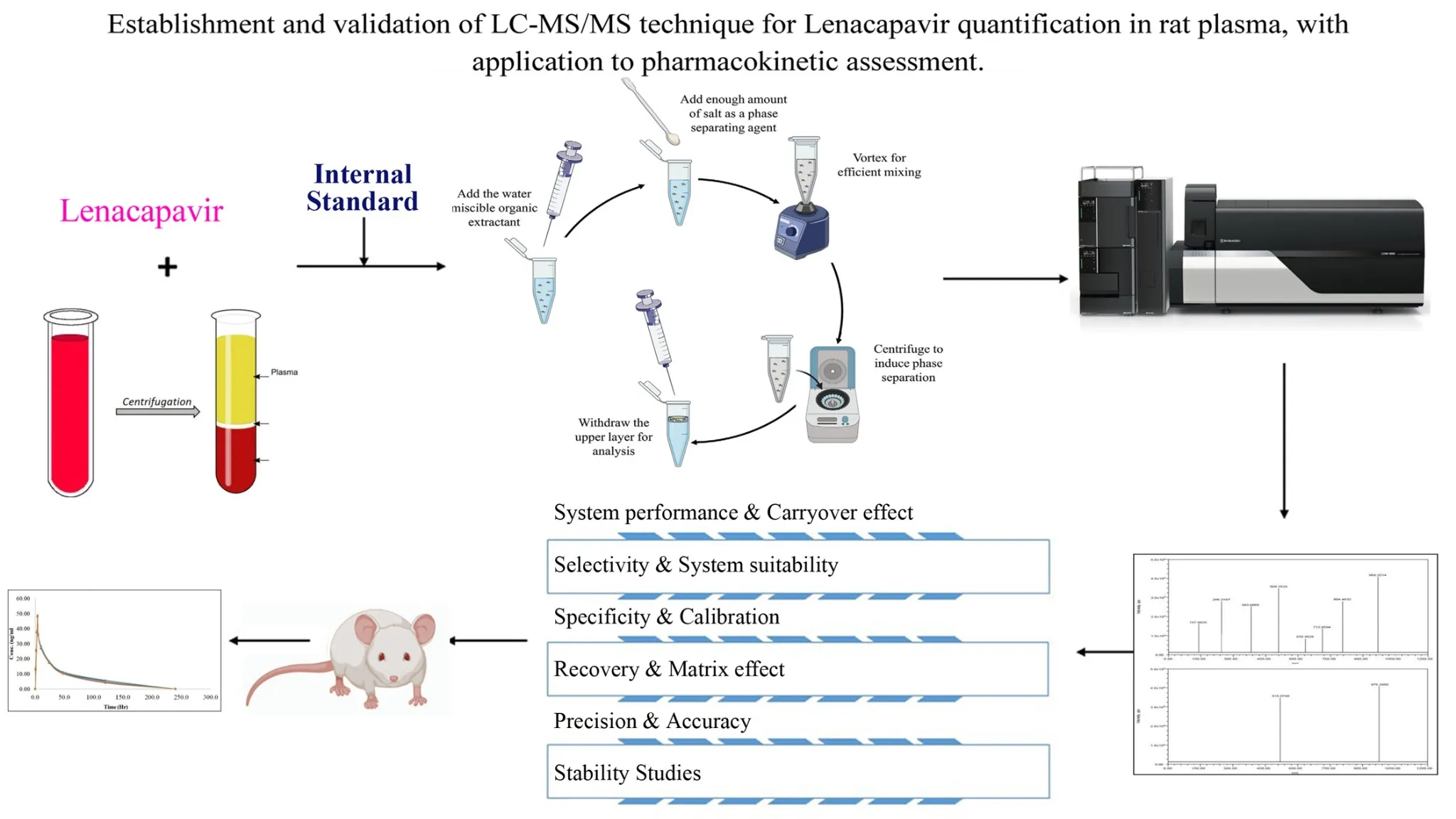
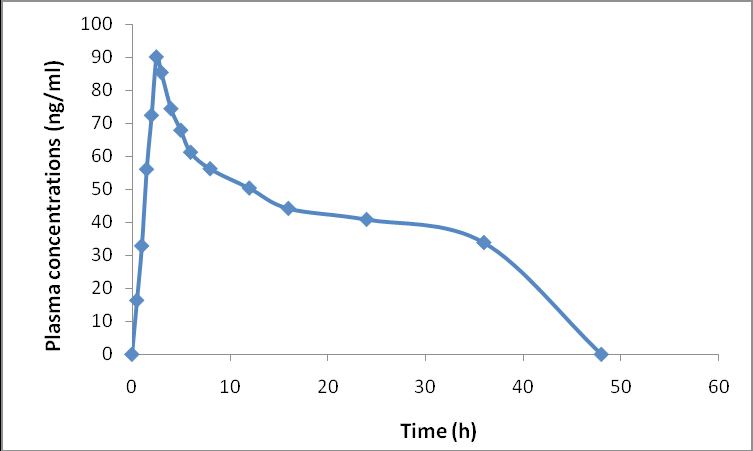
Establishment and validation of LC-MS/MS technique for Lenacapavir quantification in rat plasma, with application to pharmacokinetic assessment
Edward Raju Gope, Srikanth Pottendla, Suneetha Yaparthi
DOI: 10.7324/JAPS.2025.229006Pages: 112-120