INTRODUCTION
Stilbenes are phenolic compounds having two aromatic rings with −OH groups and are linked by a double-bonded ethylene bridge (Akinwumi et al., 2018; El Khawand et al., 2018). Found in higher plants, stilbenes exist as monomeric, dimeric, trimeric, oligomeric, and polymeric forms, or as glycosides. Stilbenes possess biological activities, such as anti-diabetic, anti-obesity, cardioprotective, neuroprotective, anti-inflammatory, anti-atherosclerosis, and anti-cancer properties (Akinwumi et al., 2018). Resveratrol and pterostilbene represent two of the monomeric stilbenes with well-studied biological activities and molecular effects (Tsai et al., 2017).
It was reported that the consumption of red wine in France has cardioprotective effects and reduces the risk of cardiovascular diseases (Renaud and de Lorgeril, 1992). The low occurrence of coronary heart diseases among the French people, despite their high-fat diet, is popularly known as the French paradox (Sun et al., 2002). These health benefits of red wine have been attributed to resveratrol (Siemann and Creasy, 1992). Since then, much research was conducted on the cardioprotective and other medicinal properties of resveratrol, a major compound of red grapes and red wine (Catalgol et al., 2012). Pharmacological activities of resveratrol include cardioprotective (Hsieh and Wu, 2018), antioxidant (Cavallini et al., 2016), anti-inflammatory (de Sẚ Coutinho et al., 2018), anti-atherosclerosis (Bonnefont-Rousselot, 2016), anti-aging (Bhullar and Hubbard, 2015; Li et al., 2018), anti-diabetic (Szkudelski and Szkudelska, 2011), anti-osteoporosis (Tou et al., 2015), and anti-obesity (de Ligt et al., 2015; Pan et al., 2018) properties.
Among the many pharmacological activities of resveratrol (Baur and Sinclair, 2006; Berman et al., 2017), its anti-cancer properties are most well-known. Since its first report by Jang et al. (1997), there are many reviews on the subject (Rauf et al., 2018; Varoni et al., 2016). Displaying various molecular mechanisms, resveratrol has shown to be a promising and multi-target agent for cancer prevention and treatment.
In recent years, the neuroprotective effects of resveratrol have also gained much research interest. The overall health benefits of resveratrol toward age-related diseases have become a topic of intense investigations (Timmers et al., 2012). Reviews on the neuroprotective properties of resveratrol are mostly age-related neurodegenerative disorders related to Alzheimer’s disease and Parkinson’s disease (Tellone et al., 2015), brain degeneration (Poulose et al., 2015), and neurological disorders, such as stroke and CNS injury (Lopez et al., 2015). Currently, there is one comprehensive review by Bastianetto et al. (2015) that summarized recent findings on the molecular mechanisms of action and discussed possible roles of resveratrol in the prevention of various neurological disorders.
Pterostilbene was reported by Remsberg et al. (2008) to have anti-cancer, anti-inflammatory, antioxidant, and analgesic effects on rats. These findings generated much research excitement in pterostilbene and subsequently, properties, such as anti-cancer (McCormack and Mc Fadden, 2012; Nutakul et al., 2011), anti-inflammatory (Dvorakova and Landa, 2017), neuroprotective (Poulose et al., 2015; Wang et al., 2016), anti-obesity (Aguirre et al., 2016; Pan et al., 2018), anti-diabetic (Tastekin et al., 2018), antioxidant (McCormack and McFadden, 2013; Rimando et al., 2002), anxiolytic (Al Rahim et al., 2013), and anti-aging (Li et al., 2018) activities have been documented. Recently, the promising therapeutic potential of pterostilbene and its mechanistic insight based on recent preclinical evidence was reviewed by Kosuru et al. (2016).
This comparative overview on the pharmacological properties of resveratrol and pterostilbene is appropriate and timely as the surge in the number of research studies in recent years has generated a wealth of new knowledge. Such useful information will set the platform for scientists to conduct further research on resveratrol, pterostilbene, and other derivatives. References cited in this overview were procured from databases downloaded from Science Direct, Google Scholar, and PubMed.
CHEMISTRY
Resveratrol or trans-3,4′,5-trihydroxystilbene is a monomer stilbene with a molecular formula of C14H12O3 and a molecular weight of 228.25 g/mol. The molecule has two aromatic rings, linked by an ethylene bridge with an ethene double bond (Fig. 1). Ring A has two hydroxyl (−OH) groups at C3 and C5, and ring B has one −OH group at C4′ (Tsai et al., 2017). Resveratrol has a 6−2−6 carbon skeleton with m-hydroquinone and 4′-hydroxystyryl moieties involving rings A and B, respectively (Niesen et al., 2013).
In food products, resveratrol commonly occurs in the trans form rather than in the cis form (Anisimova et al., 2011). When resveratrol is exposed to ultraviolet and visible light, trans to cis isomerization occurs (Silva et al., 2013). The rarer cis-resveratrol is less stable and is not commercially available (Cottart et al., 2010). Red wine is rich in trans-resveratrol, and its moderate consumption has health benefits of lower rates of prostate cancer (Schoonen et al., 2005). Against PC-3 prostate cancer cells, trans-resveratrol was reported to be a more effective anti-cancer agent than cis-resveratrol and dihydro-resveratrol (Anisimova et al., 2011). Earlier, trans-resveratrol has been reported to be 10 times more potent in inducing apoptosis of HL60 leukemia cells as compared to cis-resveratrol (Roberti et al., 2003).
Pterostilbene or trans-3,5-dimethoxy-4′-hydroxystilbene has a molecular formula of C16H16O3, molecular weight of 256.30 g/mol (Kosuru et al., 2016; McCormack and Mc Fadden, 2012; Tsai et al., 2017). Its other names are dimethoxy resveratrol, 3,5′-dimethoxy-4-stilbenol and 4-[2-(3,5-dimethoxyphenyl) ethenyl] phenol. Being a dimethylated analog of resveratrol, pterostilbene is structurally similar to resveratrol by having one hydroxyl group at C4′ of ring B but differs by having two −OCH3 groups at C3 and C5 of ring A (Fig. 2). Like resveratrol, the trans form of pterostilbene is more abundant than the cis form (Kosuru et al., 2016).
BIOSYNTHESIS AND PLANT SOURCES
In plants, the biosynthesis of resveratrol and pterostilbene shares similar substrates and biosynthetic pathway as flavonoids (Jeandet et al., 2010; Poulose et al., 2015). Unlike flavonoids which are produced by most plants, only a few plant species synthesize these stilbenes. The biosynthetic pathway of resveratrol begins with phenylalanine of the shikimate pathway, which undergoes various enzymatic reactions to produce p-coumaroyl-CoA. In the presence of malonyl-CoA and stilbene synthase, trans-resveratrol is produced via an aldol reaction. Trans-resveratrol is then converted to pterostilbene by O-methyl transferase.
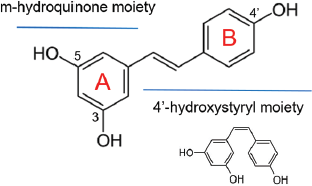 | Figure 1. The molecular structures of trans-resveratrol with cis-resveratrol as inset.
[Click here to view] |
Resveratrol was first isolated from the roots of Veratrum grandiflorum and later from the roots of Polygonum cuspidatum (Nonomura et al., 1963). From P. cuspidatum, an important traditional medicine in China, the content of resveratrol has been reported to be 1.8 mg/g (Zhao et al., 2005). The compound has been isolated from more 70 plant species, including grapes and red wine (Rege et al., 2014). Red grapes and red wine, as shown in Figure 3, are the main dietary sources of resveratrol. Red grapes contain mainly of piceid (1.5−7.3 μg/g), while red wine is rich in resveratrol (1.0−18 μg/ml) (Burns et al., 2002). From the skin of grapes, the content of resveratrol ranged from 2.48 to 6.47 μg/g (Rimando et al., 2004). The concentration of trans-resveratrol in red wine is six times higher than in white wine, which contains high levels of cis-resveratrol (Rege et al., 2014). A possible explanation is that red wine is produced without removing the skin of grapes, whereas white wine is fermented after the removal of the skin.
Pterostilbene was first isolated from Pterocarpus santalinus (sandalwood) in 1940 (Seshadri, 1972), and later identified in Vitis vinifera (grape vine) (Adrian et al., 2000; Langcake et al., 1979), Pterocarpus marsupium (Indian kino) (Manickam et al., 1997; Maurya et al., 1984), Vaccinium berries (Rimando et al., 2004), and Arachis hypogaea (peanut) (Sobolev et al., 2011).
PHARMACOLOGICAL PROPERTIES
Resveratrol and pterostilbene exhibit many similarities in pharmacological properties (Akinwumi et al., 2018; Tsai et al., 2017; Wang and Sang, 2018). They include antioxidant, neuroprotective anti-cancer, cardioprotective, analgesic, anti-atherosclerosis, anti-aging, anti-diabetic, anti-inflammatory, and anti-obesity activities.
Studies have shown that both resveratrol and pterostilbene are able to cross the blood–brain barrier and influence brain activity (Lange and Lee, 2017). The blood–brain barrier is a diffusion barrier essential for the normal functioning of the central nervous system. Located at the capillaries between the blood and cerebral tissue, the blood–brain barrier has endothelial cells with tight junctions that impede the influx of most blood-borne compounds from entering the brain (Ballabh et al., 2004; Kanwar et al., 2012). Small lipophilic molecules, such as oxygen (O2) and carbon dioxide (CO2), can diffuse through plasma membranes while nutrients, such as glucose and amino acids, and larger molecules, including insulin, leptin, and iron transferrin, enter the brain via transporters and receptor-mediated endocytosis, respectively. The blood–brain barrier has been reported to prevent 98% of small molecules and 100% of large molecules from reaching the brain (Kanwar et al., 2012).
A study by Wang et al. (2002) demonstrated that the resveratrol can cross the blood–brain barrier and protects against cerebral ischemic injury in gerbils. The study noted that after injection into these animals, resveratrol rapidly enters the bloodstream as glucuronide conjugate, crosses the blood–brain barrier, and is subsequently enters the brain tissue. Resveratrol was retained in the brain for up to 4 hours.
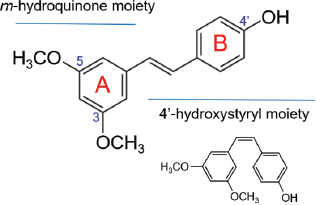 | Figure 2. The molecular structures of trans-pterostilbene with cis-pterostilbene as inset.
[Click here to view] |
Although there are structural and bioactivity similarities between resveratrol and pterostilbene, pharmacological properties of pterostilbene are often stronger than those of resveratrol (Table 1).
The stronger pharmacological properties in pterostilbene than resveratrol have been attributed to the dimethoxy groups at C3 and C5 of ring A (Fig. 2). With these structural characteristics, pterostilbene is more lipophilic, enhancing its membrane permeability, bioavailability, and bioactivity (Kapetanovic et al., 2011; McCormack and Mc Fadden, 2012; Wang and Sang, 2018). Overall, pterostilbene performs better in membrane permeability and metabolic stability than resveratrol. This increases the bioavailability, and enhances the pharmacokinetic profile and pharmacological activities of pterostilbene.
Studies have shown that both resveratrol and pterostilbene are safe for human consumption. In a clinical trial conducted on 40 healthy volunteers, resveratrol was found to be safe after daily doses of 0.5, 1.0, 2.5, and 5.0 g for 29 days, with the exception of 2.5 and 5.0 g doses which caused some gastrointestinal discomfort (Brown et al., 2010). In two studies, 28 daily doses of 50, 150, or 500 mg/kg body weight, and 90 daily dose of 700 mg/kg body weight of Resvida™ (high-purity resveratrol) did not have any adverse effects on rats (Williams et al., 2009). For pterostilbene, a clinical trial (randomized, double-blind, and placebo-controlled) conducted in 80 healthy volunteers for 6–8 weeks, demonstrated that the pterostilbene is generally safe for consumption at doses of up to 250 mg/day (Riche et al., 2013). Biochemical analysis showed that the pterostilbene had no adverse reactions on liver, kidney, and glucose markers.
Kapetanovic et al. (2011) reported that there was greater oral absorption and cellular uptake of pterostilene in rats than resveratrol. One of the studies indicated that the pterostilbene (orally administered) displayed 95% bioavailability, as compared to resveratrol with only 20% bioavailability. In addition, the half-life of resveratrol in the blood was found to be 14 minutes (Asensi et al., 2002), whereas pterostilbene with two –OCH3 groups had a half-life of 105 minutes or seven times longer than resveratrol (Remsberg et al., 2008).
.png) | Table 1. Comparative pharmacological properties of pterostilbene (PS) and resveratrol (RV).
[Click here to view] |
REFERENCES
Adrian M, Jeandet P, Douillet-Breuil AC, Tesson L, Bessis R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J Agric Food Chem, 2000; 48:6103–5. CrossRef
Aguirre L, Milton-Laskibar I, Hijona E, Bujanda L, Rimando AM, Portillo MP. Effects of pterostilbene in brown adipose tissue from obese rats. J Physiol Biochem, 2016; 73:457–64. CrossRef
Akinwumi B, Bordun KA, Anderson H. Biological activities of stilbenoids. Int J Mol Sci, 2018; 19:792. CrossRef
Al Rahim M, Rimando AM, Silistreli K, El-Alfy AT. Anxiolytic action of pterostilbene: involvement of hippocampal ERK phosphorylation. Planta Med, 2013; 79:723–30. CrossRef
Anisimova NY, Kiselevsky MV, Sosnov AV, Sadovnikov SV, Stankov IN, Gakh AA. Trans-, cis- and dihydro-resveratrol: a comparative study. Chem Central J, 2011; 5:88. CrossRef
Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med, 2002; 33:387–98. CrossRef
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview—structure, regulation and clinical implications. Neurobiol Dis, 2004; 16:1–3. CrossRef
Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta—Mol Basis Dis, 2015; 1852:1195–201. CrossRef
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov, 2006; 5:493–506. CrossRef
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol, 2017; 1:35. CrossRef
Bhullar KS, Hubbard BP. Lifespan and health-span extension by resveratrol. Biochim Biophys Acta—Mol Basis Dis, 2015; 1852:1209–18. CrossRef
Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients, 2016; 8:250. CrossRef
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res, 2010; 70:9003–11. CrossRef
Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem, 2002; 50:3337–40. CrossRef
Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol, 2012; 3:141. CrossRef
Cavallini G, Straniero S, Donati A, Bergamini E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J Nutr Health Aging, 2016; 20:540–5. CrossRef
Chakraborty S, Kumar A, Butt NA, Zhang L, Williams R, Rimando AM, Biswas PK, Levenson AS. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: in silico approach to understand biological actions. Mol BioSyst, 2016; 12:1702–9. CrossRef
Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuro-modulator in aging and Alzheimer’s disease. Neurobiol Aging, 2012; 33:2062–71. CrossRef
Chatterjee K, AlSharif D, Mazza C, Syar P, Al Sharif M, Fata JE. Resveratrol and pterostilbene exhibit anticancer properties involving the downregulation of HPV oncoprotein E6 in cervical cancer cells. Nutrients, 2018; 10:243. CrossRef
Choo QY, Yeo SC, Ho PC, Tanaka Y, Lin HS. Pterostilbene surpassed resveratrol for anti-inflammatory application: potency consideration and pharmacokinetics perspective. J Funct Foods, 2014; 11:352–62. CrossRef
Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res, 2010; 54:7–16. CrossRef
de Ligt M, Timmers S, Schrauwen P. Resveratrol and obesity: can resveratrol relieve metabolic disturbances? Biochim Biophys Acta—Mol Basis Dis, 2015; 1852:1137–44. CrossRef
de Sá Coutinho D, Pacheco M, Frozza R, Bernardi A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci, 2018; 19:1812. CrossRef
Dvorakova M, Landa P. Anti-inflammatory activity of natural stilbenoids: a review. Pharmacol Res, 2017; 124:126–45. CrossRef
El Khawand T, Courtois A, Valls J, Richard T, Krisa S. A review of dietary stilbenes: sources and bioavailability. Phytochem Rev, 2018; 17:1007–29. CrossRef
Langcake P, Cornford CA, Pryce RJ. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry, 1979; 18:1025–7. CrossRef
Hsieh TC, Wu JM. 2018. Unraveling and trailblazing cardioprotection by resveratrol. In: Wu JM, Hsieh TC, editors. Resveratrol: State-of-the-Art Science and Health Applications. World Publishing, Singapore, pp. 1–28, 2018. CrossRef
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 1997; 275:218–20. CrossRef
Jeandet P, Delaunois B, Conreux A, Donnez D, Nuzzo V, Cordelier S, Clément C, Courot E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors, 2010; 36:331−41. CrossRef
Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem, 2002; 50:2731–41. CrossRef
Kanwar JR, Sriramoju B, Kanwar RK. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int J Nanomed, 2012; 7:3259–78. CrossRef
Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol, 2011; 68:593–601. CrossRef
Kosuru R, Rai U, Prakash S, Singh A, Singh S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur J Pharmacol, 2016; 789:229–43. CrossRef
Lange KW, Li S. Resveratrol, pterostilbene, and dementia. BioFactors, 2018; 44:83–90. CrossRef
Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors, 2018; 44:69–82. CrossRef
Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int, 2015; 89:75–82. CrossRef
Manickam M, Ramanathan M, Jahromi MA, Chansouria JP, Ray AB. Anti-hyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod, 1997; 60:609–10. CrossRef
Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exper Ther, 2002; 302:369–73. CrossRef
Maurya R, Ray AB, Duah FK, Slatkin DJ, Schiff PL. Constituents of Pterocarpus marsupium. J Nat Prod. 1984; 47:179–81. CrossRef
McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res, 2012; 173:53–61. CrossRef
McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev, 2013; Article ID 575482, 15 p. CrossRef
Meng XL, Yang JY, Chen GL, Wang LH, Zhang LJ, Wang S, Li J, Wu CF. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem Biol Interact, 2008; 174:51–9. CrossRef
Messina F, Guglielmini G, Curini M, Orsini S, Gresele P, Marcotullio MC. Effect of substituted stilbenes on platelet function. Fitoterapia, 2015; 105:228–33. CrossRef
Mikstacka R, Rimando AM, Ignatowicz E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum Nutr, 2010; 65:57–63. CrossRef
Niesen DB, Hessler C, Seeram NP. Beyond resveratrol: a review of natural stilbenoids identified from 2009–2013. J Berry Res, 2013; 3:181–96.
Nonomura S, Kanagawa H, Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-jo-kon (Polygonum cuspidatum Sieb. et Zucc.). Yakugaku Zasshi, 1963; 83:988–90. CrossRef
Nutakul W, Sobers HS, Qiu P, Dong P, Decker EA, McClements DJ, Xiao H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: a side-by-side comparison. J Agric Food Chem, 2011; 59:10964–70. CrossRef
Pan MH, Wu JC, Ho CT, Lai CS. Anti-obesity molecular mechanisms of action: resveratrol and pterostilbene. BioFactors, 2018; 44:50–60. CrossRef
Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS, Suh N. Anti-inflammatory action of pterostlbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res, 2009; 2:650−7. CrossRef
Poulose SM, Thangthaeng N, Miller MG, Shukitt-Hale B. Effects of pterostilbene and resveratrol on brain and behavior. Neurochem Int, 2015; 89:227−33. CrossRef
Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr, 2018; 58:1428−47. CrossRef
Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci, 2014; 6:218. CrossRef
Remsberg CM, Yáñez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, anti-inflammatory, antioxidant and analgesic activity. Phytother Res, 2008; 22:169−79. CrossRef
Renaud SD, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet, 1992; 339:1523–6. CrossRef
Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, Griswold M. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol, 2013; Article ID 463595: 5 p. CrossRef
Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem, 2002; 50:3453–7. CrossRef
Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α-isoform, lowers plasma lipoproteins and cholesterol in hyper-cholesterolemic hamsters. J Agric Food Chem, 2005; 53:3403–7. CrossRef
Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J Agric Food Chem, 2004; 52:4713–9. CrossRef
Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M. Synthesis and biological evaluation of resveratrol and analogs as apoptosis-inducing agents. J Med Chem, 2003; 46:3546–54. CrossRef
Rossi M, Caruso F, Antonioletti R, Viglianti A, Traversi G, Leone S, Basso E, Cozzi R. Scavenging of hydroxyl radical by resveratrol and related natural stilbenes after hydrogen peroxide attack on DNA. Chem Biol Interact, 2013; 20:175–85. CrossRef
Schoonen WM, Salinas CA, Kiemeney LAL, Stanford JL. Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer, 2005; 113:133–40. CrossRef
Seshadri TR. Polyphenols of Pterocarpus and Dalbergia woods. Phytochemistry, 1972; 11:881–98. CrossRef
Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Eno Vitic, 1992; 43:49–52.
Silva CG, Monteiro J, Marques RR, Silva AM, Martínez C, Canle M, Faria JL. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem Photobiol Sci, 2013; 12:638–44. CrossRef
Sobolev VS, Khan SI, Tabanca N, Wedge DE, Manly SP, Cutler SJ, Coy MR, Becnel JJ, Neff SA, Gloer JB. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J Agric Food Chem, 2011; 59:1673–82. CrossRef
Sun AY, Simonyi A, Sun GY. The “French paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med, 2002; 32:314−8. CrossRef
Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci, 2011; 1215:34–9. CrossRef
Tastekin B, Pelit A, Polat S, Tuli A, Sencar L, Alparslan MM, Daglioglu YK. Therapeutic potential of pterostilbene and resveratrol on biomechanic, biochemical, and histological parameters in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med, 2018; Article ID 9012352: 10 p. CrossRef
Tellone E, Galtieri A, Russo A, Giardina B, Ficarra S. Resveratrol: a focus on several neurodegenerative diseases. Oxidat Med Cell Longevity, 2015; Article ID 392169:14 p. CrossRef
Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging, 2012; 4:146–58. CrossRef
Tou JC. Evaluating resveratrol as a therapeutic bone agent: preclinical evidence from rat models of osteoporosis. Ann N Y Acad Sci, 2015; 1348:75−85. CrossRef
Tsai HY, Ho CT, Chen YK. Biological actions and molecular effects of resveratrol, pterostilbene and 3’-hydroxypterostilbene. J Food Drug Anal, 2017; 25:134−47. CrossRef
Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr, 2016; 3:8. CrossRef
Wang B, Liu H, Yue L, Li X, Zhao L, Yang X, Wang X, Yang Y, Qu Y. Neuroprotective effects of pterostilbene against oxidative stress injury: involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res, 2016; 1643:70−9. CrossRef
Wang P, Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors, 2018; 44:16–25. CrossRef
Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res, 2002; 958:439–47. CrossRef
Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol, 2009; 47:2170–82. CrossRef
Zhao RZ, Liu S, Zhou LL. Rapid quantitative HPTLC analysis, on one plate, of emodin, resveratrol and polydatin in the Chinese herb Polygonum cuspidatum. Chromatographia, 2005; 61:311–4. CrossRef



.png)