INTRODUCTION
Heart failure (HF) continues to be a growing concern in global public health and remains one of the primary causes of hospitalization. It is estimated that one in five individuals will experience HF at some point in their lives, with the condition currently affecting approximately 63 million people worldwide [1]. Despite significant advancements in the management of HF, mortality rates remain considerable—particularly in the Indian subcontinent, where the INTER-CHF study reported a mortality rate of 23% [2]. Diabetes mellitus (DM) is recognized as both an independent risk factor and a prognostic marker for HF. Myocardial injury, both atherosclerotic and non-atherosclerotic, often begins silently in the early stages of diabetes—frequently preceding any clinical signs of HF. Acute myocardial infarction (MI) represents the most frequent underlying cause of HF and is also a strong predictor of mortality in these patients [3]. Reports suggest that the incidence of HF among individuals hospitalized for MI ranges between 14% and 36%, depending on the study [4]. Standard treatment options for managing HF following MI typically include beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), aldosterone antagonists, early revascularization, and mechanical circulatory support. More recently, angiotensin receptor neprilysin inhibitors (ARNIs) have been evaluated in this setting, as demonstrated by the PARADISE-MI trial. However, ARNIs did not show superiority over ramipril in reducing key outcomes such as cardiovascular (CV) death, first hospitalization for HF, or outpatient HF [5]. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, initially approved for the treatment of type 2 DM (T2DM), have recently been studied in large-scale, placebo-controlled trials assessing their CV safety and effectiveness in patients with T2DM [6–8]. These agents have consistently demonstrated a beneficial impact by reducing HF risk and prolonging hospitalization-free survival. Nevertheless, the precise mechanisms driving these effects remain poorly understood, and there is limited research evaluating the role of SGLT2 inhibitors in cardiac remodeling following MI. The primary objective of this study was to assess the impact of SGLT2 inhibitors on left ventricular (LV) function and volume in diabetic patients following acute MI after 3–6 months of optimized medical therapy. By examining these parameters, the study aims to indirectly explore the potential role of SGLT2 inhibitors in promoting favorable LV remodeling in this patient population. If beneficial, SGLT2 inhibitors could represent a promising adjunctive therapy in diabetic patients with acute MI and impaired LV function—an area not previously investigated.
METHODS
Participant recruitment
This research was designed as a single-center, prospective, randomized case–control study and was conducted over a span of 24 months in the Department of Cardiology at a tertiary care hospital in southern India. The study received ethical clearance from the Institutional Ethics Committee (IEC No. 427/2020). A total of 90 participants were enrolled based on predefined eligibility criteria. The sample size was calculated based on the comparison of two independent means using the formula: n = [(Zα/2 + Zβ)2σ2]/d2. Assuming a two-sided alpha level of 0.05 (Zα/2 = 1.96), a power of 80% (Zβ = 0.84), an SD of 10 (based on data from the study by Soga et al. [9]), and a minimum detectable difference of seven units in LV mass index (LVMI), the required sample size was calculated to be approximately 25 patients per group. To account for 15% of dropouts, 30 patients were recruited in each group. The final sample size was determined based on a 2:1 allocation ratio, with 60 patients in the case group and 30 in the control group, to ensure adequate power for detecting differences in the primary outcome while accounting for feasibility and expected dropout rates.
Inclusion criteria consisted of patients with extensive MI and an LV ejection fraction (LVEF) of less than 50%, along with either preexisting or newly diagnosed T2DM, as defined by the American Diabetes Association guidelines [10]. Eligible patients were also required to have an estimated glomerular filtration rate (eGFR) of at least 30 ml/min/1.73 m² and to be receiving optimal post-MI medical therapy, which included dual antiplatelet agents (aspirin and ticagrelor), statins, beta-blockers, ARBs or ACEIs, aldosterone antagonists, and/or diuretics, where indicated or tolerated. Exclusion criteria encompassed the presence of any of the following: severe infections, significant hepatic or renal dysfunction (defined as eGFR < 30 ml/min/1.73 m²), alcohol or substance abuse, pregnancy or lactation, potential or planned pregnancy during the study duration, dehydration, active urinary tract or genital infections, known hypersensitivity to any of the study medications, a history of diabetic ketoacidosis, diabetic coma or pre-coma, prior or current pancreatitis, and uncontrolled tachyarrhythmias.
Procedure
Patients who fulfilled the inclusion criteria were enrolled at the time of admission for acute MI after obtaining informed consent. The baseline characteristics of the patients included age, sex, body mass index (BMI) and body surface area (BSA), comorbidities, type of MI at presentation, fasting and random sugars, glycated hemoglobin (HbA1c), duration of diabetes and diabetes medication, and other cardiac medications initiated as part of standard therapy. Echocardiography parameters were recorded at the index admission for MI. Standard echocardiographic measurements were obtained in accordance with the current guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging [11,12].Click or tap here to enter text.
Echocardiographic evaluation
At baseline, the following parameters were assessed by echocardiogram:
- LV mass and LVMI
- Relative wall thickness (RWT)
- LVEF
- Area length ejection fraction (ALEF)
- LV end-diastolic volume (LVEDV) and indexed LV end diastolic volume (LVEDVI)
- LV end-systolic volume (LVESV) and indexed LV end systolic volume (LVESVI)
- LV end-systolic dimension and LV end-diastolic dimension
- LV interventricular septal and posterior wall thickness
- Left atrial (LA) anteroposterior (AP) dimension
- E/E’: the ratio between early mitral inflow velocity and mitral annular early diastolic velocity
- LA volume (four-chamber and two-chamber views) and LA volume index.
LV mass calculation
LV mass was calculated by the following formula:
LV mass (g) = 0.8{1.04[([LVEDD + IVSd + PWd]3 – LVEDD3)]} + 0.6
LVEDD = LV end-diastolic dimension (mm)
IVSd = interventricular septal thickness at end-diastole (mm)
PWd = posterior wall thickness at end-diastole (mm)
1.04 = Specific gravity of the myocardium (g/cm3)
RWT = 2 × PWd/LVEDD
Measurements of the IVSd, LVEDD, and PWd were performed in accordance with the guidelines set forth by the American Society of Echocardiography. LV mass was indexed to BSA to obtain the LVMI. Linear internal dimensions of the left ventricle were obtained from the parasternal long-axis view, with measurements taken perpendicular to the LV long axis at the level of the mitral valve leaflet tips. Volumetric assessments were conducted by tracing the endocardial border in both the apical four- and two-chamber views. LVEF was derived using M-mode measurements to estimate the end-diastolic volume and end-systolic volume, while the apical longitudinal ejection fraction (ALEF) was calculated using Simpson’s biplane method (Fig. 1). Transmitral flow velocities were recorded from the apical four-chamber window during quiet expiration, with the pulsed Doppler sample positioned at the tips of the mitral leaflets. Peak early (E) and late (A) diastolic velocities, along with the E/A ratio, were measured (Fig. 2). Pulsed-wave tissue Doppler imaging was performed in the apical four-chamber view to obtain the e’ velocities at the lateral and septal basal segments, from which an average e’ velocity was calculated (Fig. 3). The mitral E/e’ ratio was subsequently derived from these values. LA volume was assessed using frames frozen just prior to mitral valve opening from both apical four- and two-chamber views.
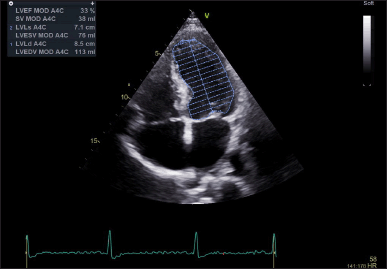 | Figure 1. Left ventricular ejection fraction assessment using the volumetric method—Simpson’s method. EF = ejection fraction; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume. [Click here to view] |
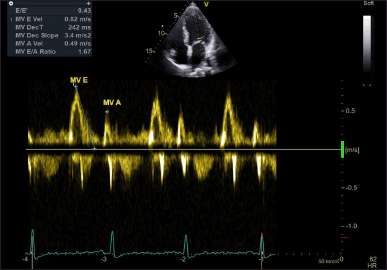 | Figure 2. Mitral valve Doppler flow pattern. MV A = late diastolic velocity during atrial contraction; MV E = early diastolic velocity. [Click here to view] |
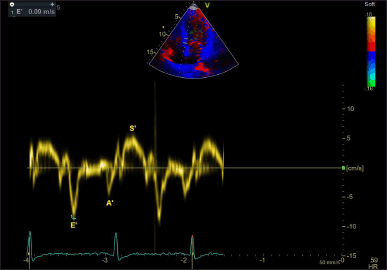 | Figure 3. Tissue annular velocity tracing drawn at the medial mitral annulus. A’ = late diastolic tissue annular velocity; E’ = early diastolic tissue annular velocity; S’ = systolic tissue annular velocity. [Click here to view] |
Following randomization, all patients received optimal medical therapy, which included dual antiplatelet agents, statins, beta-blockers, ACEIs, or ARBs for those intolerant to ACEIs, as well as diuretics, aldosterone antagonists, and vasodilators where indicated. The antiplatelet regimen consisted of 75 mg aspirin and 180 mg ticagrelor daily; patients on anticoagulation with non-vitamin K oral anticoagulants were instead prescribed clopidogrel in place of ticagrelor. In addition to the standard antidiabetic regimen, patients assigned to the intervention group were started on 10 mg dapagliflozin. The diabetic medication for patients in the control group was adjusted as needed. Follow-up echocardiographic evaluation was planned for a median period of 3–6 months post-MI; however, the follow-up duration varied due to interruptions caused by the COVID-19 pandemic. Repeat echocardiography was performed as part of routine clinical care at follow-up visits.
The primary endpoints of the study included changes in LV mass and LVMI from baseline to follow-up. Secondary endpoints included changes in RWT, LVEF, ALEF, LVEDV and LVEDVI, LVESV and LVESVI, LV end-systolic and end-diastolic dimensions, interventricular septal and posterior wall thickness, LA AP dimension, E/e’ ratio, LA volumes from the four- and two-chamber views, and LA volume index. All data were analyzed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY).
Statistical analysis
The data were compiled using descriptive statistics. Continuous data were given as means with SD, while categorical variables were displayed as frequencies and percentages. The independent (unpaired) t-test was used to compare means between two unrelated groups, while the paired sample t-test was used to compare paired groups. To evaluate relationships between categorical data, the chi-square test was applied. The Fisher’s exact test was employed as a backup in cases when the predicted frequency in a 2 × 2 contingency table was less than 5. Repeated measures of ANOVA were used for comparing mean differences between two independent groups evaluated at two time points. For all analyses, a p-value of less than 0.05 was deemed statistically significant.
RESULTS
The study enrolled 90 patients who presented with acute MI with diabetes and LV systolic dysfunction (EF less than 50%). Sixty patients were randomized to the case group and 30 to the control group in a 2:1 manner according to the treating physician’s discretion. Five patients in the case group were lost to follow-up, four died before follow-up, and one patient discontinued SGLT2 therapy due to renal failure. In the control group, five patients were lost to follow-up, and three patients died before follow-up. A total of 72 patients were enrolled and completed the study protocol; 50 in the case group and 22 in the control group. Table 1 shows the baseline characteristics of the two groups (Fig. 4).
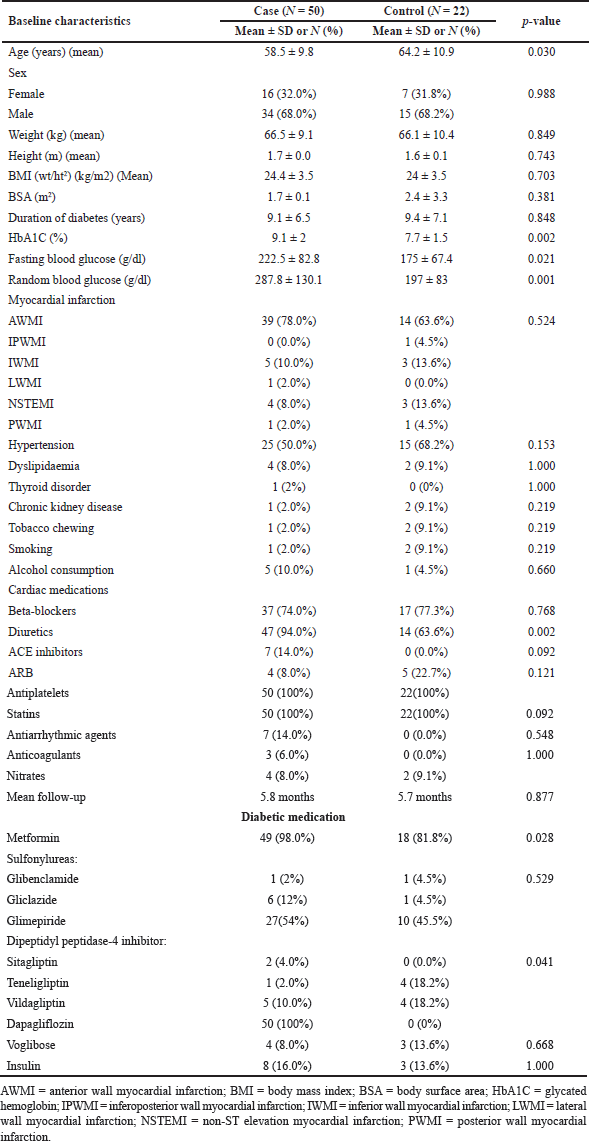 | Table 1. Baseline characteristics among cases and controls. [Click here to view] |
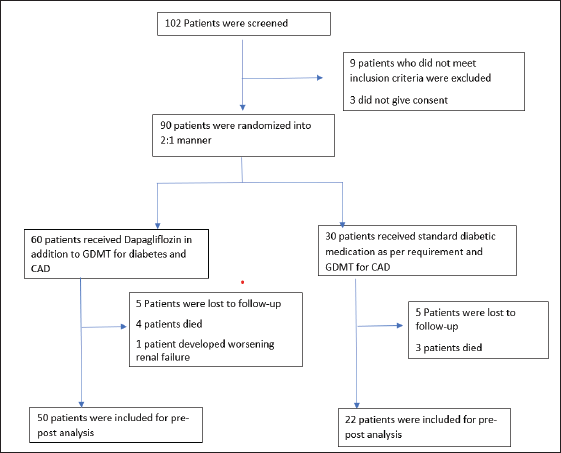 | Figure 4. CONSORT flow diagram of participant recruitment. [Click here to view] |
Demographic and clinical findings
The mean age in the control group was 64.2 ± 10.9 years, significantly greater than that in the control group (58.5 ± 9.8 years; p = 0.030). The number of males and females was equally balanced in both cases [males: 34(68%), females: 16(32%)] and controls [males: 15(68.2%), females: 7(31.8%)]. The height and weight in the case group [1.7 ± 0.0 m, 66.5 ± 9.1 kg] and in the control group [1.6 ± 0.1 m, 66.1 ± 10.4 kg] were similar. The mean HbA1c levels, fasting blood sugar levels, and random blood sugar levels were greater in the −9.1% (SD ± 2), 222.5 mg/dl (SD ± 82.8), and 287.8 mg/dl (SD ± 130.1) groups than in the control group [−7.7% (SD ± 1.5), 175 mg/dl (SD ± 67.4) and 197 mg/dl (SD ± 83)] (p values of 0.002, 0.021, and 0.001, respectively). Greater metformin use was observed among the patients, whereas greater DPP-4 inhibitor use was observed among the controls. The majority of patients in both the case and control groups suffered from anterior wall MI (AWMI) (8% vs. 63.6%). Among the other comorbidities, hypertension was most prevalent (case - 50% vs. control - 68.2%), followed by dyslipidemia (case - 8% vs. control - 9.1%), although the difference was not statistically significant. Patients in both the case and control groups were started on appropriate cardiac medication. The use of diuretics was greater among patients in the treatment group than among those in the control group (94% vs. 63.6%). Equal proportions of patients were taking beta-blockers (74% vs. 77.3%) and ACEIs/ARBs (22% vs. 22.7%). The median follow-up in the case group was 5 months, and that in the control group was 5.5 months (p = 0.877) (Table 1).
Conventional echocardiographic findings
Table 2 shows the echocardiographic parameters at baseline and follow-up. Intragroup differences from baseline to follow-up were also compared. The mean LVEF among cases was 42.6% (SD ± 5.2), and it was 45.1% (SD ± 6.0) among the controls. At the same time, the ALEF was 41.6% (SD ± 4.8) among the patients and 44.1% (SD ± 5.1) among the controls. The baseline ALEF was greater in the control group (p = 0.044). The mean LV mass at baseline between the case and control groups (222 ± 48.4 vs. 227 ± 63.3 g). Similarly, the LVMI was equal between the two groups (128 ± 27.6 vs. 127.8 ± 30.4 g/m2). Other parameters, including LV RWT, LV septal and posterior wall thickness, LV end-systolic dimension and LV end-diastolic dimension, LVEDV and LVEDVI, and LVESV and LVESVI, and E/e’, were similar between the two groups at baseline. The LA volume and the LA volume index were higher in the control group (p = 0.020 and 0.021, respectively). At follow-up, the LV mass and LVMI were significantly lower in the patients (190.8 and 110.9 g/m2, respectively) than in the controls (220.8 and 127.1 g/m2, respectively) (p = 0.045 and 0.05, respectively). The LV RWT was also significantly lower in the patient group than in the control group (p = 0.038). The LV septal and posterior wall thickness were significantly lower among cases compared to the control group (p = 0.009 and 0.045, respectively). The EF and ALEF were higher in the control group (51.1% and 51.1%, respectively) than in the case group (46.3% and 45%, respectively) (p = 0.025 and 0.002, respectively). The LVEDV and LVESV were comparable between the two groups; however, the indexed LVEDV and LVESV were lower in the control group than in the cases (p = 0.042 and 0.046, respectively). E/E’, LA volumes (four chambers, two chambers), LA volume index, and LV end-diastolic and systolic dimensions did not show significant changes at follow-up.
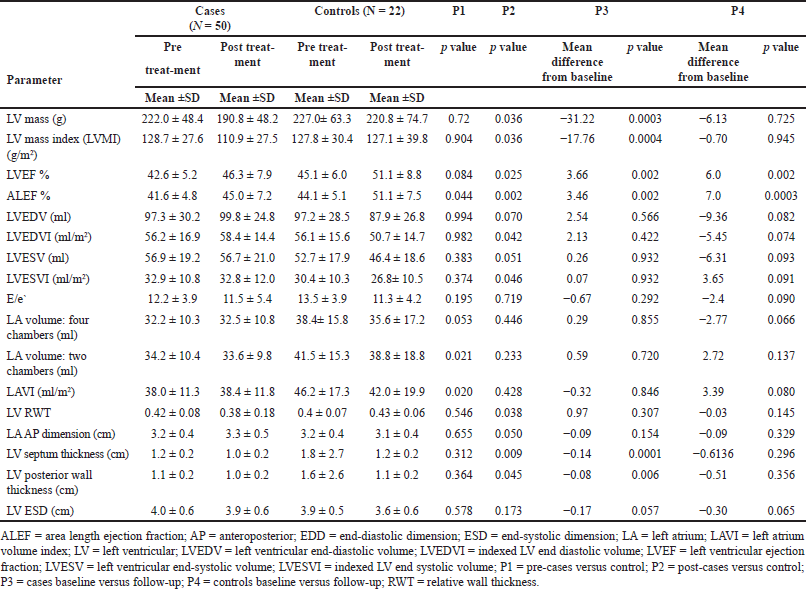 | Table 2. Comparison of echocardiographic parameters among cases versus controls. [Click here to view] |
Changes in LV mass
The mean LV mass among cases showed a significant decrease from pre-treatment values of 222–190.8 g (−31.22 ± 56.19 g) (p = 0.0003). Similar changes were reflected in the LVMI, which decreased from 128.67 g/m2 at baseline to 110.9 g/m2 at follow-up (−17.76 ± 32.94 g/m2) (p = 0.0004). The LVEF increased from the baseline mean of 42.64% to 46.3% (3.66 ± 7.74%) (p = 0.002). The ALEF increased from 41.56% to 45.02% (3.46% ± 7.54%) (p = 0.002). The LV septal thickness significantly reduced by −0.14 (p = 0.0001) from the baseline value. Similarly, a mean reduction in posterior wall thickness of −0.08 (p = 0.0006) from the baseline value was noted. Other parameters, including LVEDV and LVESV, the indexed LVEDV and LVESV, the end-systolic dimension, the end-diastolic dimension, the LA (four chambers and two chambers), the LA volume index, and the LA AP dimension, did not show significant changes from baseline to follow-up. The mean LV mass in the control group decreased from pretreatment values of 227–220.8 g at follow-up, but the difference was not statistically significant (−6.13 g; p = 0.72). Similar changes were detected in the LVMI, which decreased from 127.8 g/m2 at baseline to 127.1 g/m2 at follow-up (−0.70 g/m2; p = 0.945). The EF improved from 45.1% to 51.1% at baseline (6.00%; p = 0.002), and the ALEF improved from 44.1% at baseline to 51.1% at follow-up (7%; p = 0.0003). The E/E’ in the control group did not significantly change from baseline (−2.4; p = 0.092). LVEDV and LVESV, the indexed LVEDV and LVESV, the end-systolic dimension, the end-diastolic dimension, the LA (four chambers and two chambers), the LA volume index, and the LA AP dimension did not significantly change from baseline to follow-up.
DISCUSSION
SGLT2 inhibitors as class I recommendations
The role of SGLT2 inhibitors in CV disease has been well established. The 2022 American Heart Association guidelines classify SGLT2 inhibitors as class I recommendations for HF with reduced ejection fraction (HFrEF) and class IIa for HF with mid-range ejection fraction, both to reduce the risk of CV death and hospitalization, regardless of diabetes status [13]. Similarly, the 2021 European Society of Cardiology guidelines recommend SGLT2 inhibitors for preventing CV events in type 2 diabetes patients and reducing HF hospitalizations in those with HFrEF and type 2 diabetes [14]. Evidence from meta-analyses of major trials also supports benefits for HF with preserved ejection fraction (HFpEF) in reducing CV death and hospitalization [15]. Given these beneficial effects, there is a rationale for using SGLT2 inhibitors in treating LV dysfunction after acute MI. Animal studies have shown that SGLT2 inhibitors positively affect cardiac remodeling during the early post-MI period by reducing inflammation and oxidative stress, enhancing cardiac myocyte metabolism, improving vascular function, and dampening sympathetic activation [16]. Our study specifically examined the effects of SGLT2 inhibitors in patients with acute MI and LV dysfunction [ejection fraction (EF) < 50%].
Baseline characteristics of patients
Fifty diabetic patients with acute MI and LV dysfunction were enrolled in the case group, and 22 controls were included. All participants underwent revascularization through primary percutaneous coronary intervention (PCI), with no residual significant coronary artery disease. The baseline characteristics of both groups were largely similar, except for differences in age, as the control group had an older average age. Other factors, such as gender, BMI, and hypertension, were equally distributed between the groups. The average duration of diabetes was also similar in both groups, and there was no significant difference in the follow-up period. Previous studies, such as one by Soga et al. [9] focused on patients with diabetes and HF. Their study excluded patients older than 75, those with uncontrolled diabetes, and those with extreme HbA1c levels. Another study by Matsutani et al. [17] excluded those with acute MI or HF, unlike our study, which included both controlled and insulin-dependent diabetic patients, and those above 75 years of age. Additionally, our study included a comparison group of patients receiving guideline-directed medical therapy (GDMT) but not dapagliflozin, which sets our research apart from earlier studies. Most of the patients in both the case and control groups had AWMI as the cause of LV dysfunction, with other types of MI being similarly distributed between the groups. Both groups received GDMT, including beta-blockers, ACE inhibitors (or ARBs for those intolerant to ACE inhibitors), diuretics, aldosterone antagonists, and dual antiplatelet therapy with aspirin and ticagrelor. However, a greater proportion of the case group was on metformin, while the control group had more patients on DPP-4 inhibitors. A meta-analysis of the effects of oral hypoglycemic agents on LV function showed that GLP-1 agonists most effectively improved LVEF, while other drugs, including metformin and DPP-4 inhibitors, showed no significant effect on LVEF [18]. Importantly, insulin-dependent diabetes was equally distributed between the groups in our study, which previous studies had excluded due to insulin’s potential impact on LV mass.
LV mass and volume
Our study found no significant baseline differences in LV mass between the groups, but the control group had higher LVEF and LA volumes. At follow-up, patients on dapagliflozin (the case group) showed a significant reduction in LV mass and LVMI compared to the control group. Specifically, LV mass decreased by 31.2 g (14%) from baseline in the case group, a larger reduction than seen in previous studies, such as the DAPA-LVH trial and the EMPAHEART-Cardiolink-6 trial [19,20]. Although no significant changes in LVEDV or LVESV were observed, which aligns with findings from other studies where EF was preserved, the reduction in LV mass was an indicator of positive LV remodeling. This reduction could not be solely attributed to dapagliflozin since PCI and GDMT, including ACE inhibitors and beta-blockers, also contribute to LV remodeling. The interventricular septal thickness and posterior wall thickness were lower in the case group compared to the control group, along with a significant reduction in RWT. These changes point to beneficial LV remodeling. However, while the case group had a smaller increase in LVEF (3.66%) compared to the control group (6%), this could be due to the control group’s higher baseline EF and the timing of revascularization. Interestingly, studies such as the EMPAHEART-Cardiolink-6 trial and DAPA-LVH trial also reported positive changes in LV mass and dimensions with SGLT2 inhibitors [19,20], although our study found a greater reduction in LV mass. The exact mechanisms behind these differences remain unclear and warrant further investigation.
LV diastolic function
The E/e’ ratio, an indicator of diastolic dysfunction, did not show significant changes from baseline to follow-up in either group. Other studies, such as those by Soga et al. [9] found that dapagliflozin significantly reduced E/e’, while studies by Matsutani et al. [17] and Sakai et al. [21] found improvements in diastolic function without changes in LV mass. These studies, however, involved patients who were more stable, having received long-term GDMT, which might explain the more pronounced effects on diastolic function observed in those studies compared to our research [22–24]. Yet, it is important to note that these echocardiographic studies were performed on patients with stable HFpEF and HFrEF who previously received GDMT with ACE inhibitors/ARBs or beta-blockers, and fewer patients were on diuretics. SGLT2 inhibitors were used as an add-on therapy. ARB/ARNI//ACE inhibitors have been shown to improve diastolic function in echocardiographic studies [25,26]. SGLT2 inhibitors exert pleiotropic effects beyond glycemic control, influencing myocardial energetics, reducing cardiac workload, and promoting reverse cardiac remodeling [27].
Limitations
Limitations of our study include the small sample size, which could lead to potential biases, and the short follow-up period (mean of 5.8 months). Additionally, our study was conducted at a single center, which introduces selection bias, and the impact of timing and outcomes of revascularization after MI was not considered. The differences in baseline characteristics, such as age and medication use, further complicate the analysis, although GDMT was matched across groups. Larger-scale studies with longer follow-up and more consistent data on the timing of revascularization and biochemical markers such as NT-proBNP and troponin are necessary to fully understand the effects of SGLT2 inhibitors on LV remodeling and clinical outcomes in patients with acute MI and LV dysfunction.
CONCLUSION
In patients with acute MI and LV dysfunction, SGLT2 inhibitors have improved LV remodeling by reducing LV mass and LVMI with concomitant reductions in the interventricular septum, posterior wall, and RWT. The diastolic parameters did not significantly differ between pre- and post-treatment values in either cohort. Larger studies with longer follow-up periods are required to determine the cumulative effect of SGLT2 inhibitors on diastolic function. This is the first study in humans to investigate the effect of SGLT inhibitors on acute MI with LV dysfunction.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals are given in the ‘Methods’ section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
CONSENT FORM
Consent was taken from the patient before collecting the information and manuscript preparation.
PRIOR PUBLICATION OR UNDER CONSIDERATION
This study is not under consideration for publication anywhere or published before.
REFERENCES
1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. https://doi.org/10.1016/S0140-6736(17)32154-2. Erratum in: Lancet. 2017;390(10106):e38. doi: CrossRef
2. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017;5(7):e665–72. CrossRef
3. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163(19):2345–53. CrossRef
4. Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS, Roger VL. Heart failure after myocardial infarction: a review. Am J Med. 2002;113(4):324–30. CrossRef
5. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, et al. Angiotensin receptor–neprilysin inhibition in acute myocardial infarction. N Engl J Med. 2021;385(20):1845–55. CrossRef
6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. CrossRef
7. Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137(4):323–34. CrossRef
8. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347–57. CrossRef
9. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure UMIN000019789 UMIN. Cardiovasc Diabetol. 2018;17(1):1–8. CrossRef
10. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2019;43(Supplement_1):S14–31. CrossRef
11. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):1321–60. CrossRef
12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–71. CrossRef
13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. CrossRef
14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contributio. Eur Heart J. 2021;42(36):3599–726. CrossRef
15. Tsampasian V, Elghazaly H, Chattopadhyay R, Ali O, Corballis N, Chousou PA, et al. Sodium glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(6):e227–9. CrossRef
16. Lahnwong C, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19(1):91. CrossRef
17. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. CrossRef
18. Zhang DP, Xu L, Wang LF, Wang HJ, Jiang F. Effects of antidiabetic drugs on left ventricular function/dysfunction: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2020;19(1):10. CrossRef
19. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–702. CrossRef
20. Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41(36):3421–32. CrossRef
21. Sakai T, Miura S. Effects of sodium-glucose cotransporter 2 inhibitor on vascular endothelial and diastolic function in heart failure with preserved ejection fraction - novel prospective cohort study. Circ Rep. 2019;1(7):286–95. CrossRef
22. van Loon RB, Veen G, Kamp O, Baur LHB, van Rossum AC. Left ventricular remodeling after acute myocardial infarction: the influence of viability and revascularization - an echocardiographic substudy of the VIAMI-trial. Trials. 2014;15(1):329. CrossRef
23. Khattar RS. Effect of ACE-inhibitors and beta-blockers on left ventricular remodeling in chronic heart failure. Minerva Cardioangiol. 2003;51(2):143–54.
24. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25. CrossRef
25. Pericas P, Mas-Lladó C, Ramis-Barceló MF, Valadrón I, Noris Mora M, Pasamar Márquez L, et al. Impact of sacubitril–valsartan treatment on diastolic function in patients with heart failure and reduced ejection fraction. High Blood Press Cardiovasc Prev. 2021;28(2):167–75. CrossRef
26. Willenheimer R, Rydberg E, Öberg L, Juul-Möller S, Erhardt L. ACE inhibition with ramipril improves left ventricular function at rest and post exercise in patients with stable ischaemic heart disease and preserved left ventricular systolic function. Eur Heart J. 1999;20(22):1647–56. CrossRef
27. Piccirillo F, Lanciotti M, Nusca A, Frau L, Spanò A, Liporace P, et al. Sodium-glucose transporter-2 inhibitors (SGLT2i) and myocardial ischemia: another compelling reason to consider these agents regardless of diabetes. Int J Mol Sci. 2025;26(5):2103. CrossRef