INTRODUCTION
The global increase in obesity has become a serious public health concern for both societies and healthcare systems [1]. Globally, 2.5 billion adults are classified as overweight and 890 million as obese, representing more than a twofold increase in adult obesity since 1990 [2]. Obesity is characterized by excessive fat accumulation in adipose tissue as well as in internal organs, such as the liver, heart, pancreatic islets, and skeletal muscles [3]. It is associated with numerous diseases and conditions that are linked to increased mortality, including type 2 diabetes mellitus, cardiovascular disease, metabolic syndrome, chronic kidney disease, hyperlipidemia, hypertension, nonalcoholic fatty liver disease (NAFLD), cancer, obstructive sleep apnea, osteoarthritis, and depression [4]. Obesity can be prevented or managed through several ways, such as diet, exercise, behavioral changes, and medication [5]. However, anti-obesity drugs are known to cause various side effects. For instance, orlistat and sibutramine have been shown to cause certain side effects, including oily stools, increased defecation, fecal urgency, increased blood pressure, dry mouth, constipation, headache, and insomnia [6,7]. Accordingly, the development of plant-based medications for obesity control has generated increased interest due to their minimal side effects. Numerous medicinal plants have been shown to exhibit anti-obesity effects in various animal models. Among these, several species of the Garcinia genus, such as Garcinia cambogia, Garcinia pedunculata, Garcinia indica, and Garcinia atroviridis, have been studied for their anti-obesity properties [8–12].
Garcinia cowa Roxb. ex Choisy (G. cowa), commonly known as Cha muang in Thai, belongs to the family Clusiaceae. In traditional medicine, the fruits and leaves of G. cowa have been used to enhance blood circulation, treat indigestion, and serve as an expectorant [13]. Meanwhile, the bark, root, and latex have been used to reduce fever [13,14]. Previous studies showed that G. cowa extracts have been reported to exhibit antitumor-promoting [15], antioxidant, antimutagenic [16], anti-platelet [17], antibacterial [18], cytotoxic [19], and vasorelaxant [20,21] activities.
A previous study by Pattamadilok et al. [22] demonstrated the anti-hyperlipidemic activity of the ethanolic extract of G. cowa leaves in vitro. The phytochemical analysis of G. cowa leaf extracts has identified polyphenolic glycosides, organic acids, including hydroxycitric acid and its lactone, and flavonoids [23,24]. Hydroxycitric acid is believed to aid in weight management and help combat obesity [12]. However, scientific evidence supporting the anti-obesity effects of G. cowa remains insufficient. Therefore, the present study aims to evaluate the anti-obesity potential of an ethanolic extract of G. cowa leaves in rats fed a high-fat diet (HFD).
MATERIALS AND METHODS
Plant materials and extract preparation
Garcinia cowa leaves were sourced from Chiang Rai Province, Thailand. The plant was identified by Assoc. Prof. Rawiwan Charoensup, and a voucher specimen (MFU-NPR0190) was subsequently deposited at the Natural Products Research Laboratory, School of Science, Mae Fah Luang University, Thailand.
The G. cowa extract used in this study is the same fraction employed in our previous research, with no additional preparation performed [20]. This extract was prepared using the same procedures described in our earlier study. Briefly, 1.70 kg of air-dried G. cowa leaves were ground into a powder and extracted using 95% ethanol. The resulting extract was filtered and evaporated to obtain 211.29 g of G. cowa ethanolic extract (GCE), corresponding to a yield of 12.42%. The extract was kept at a temperature of −20 °C for future use.
LC-QTOF-MS analysis
The qualitative analysis of the chemical composition of GCE was previously analyzed in our earlier study using LC-QTOF-MS in negative ionization mode [20].
Animals
Adult male Wistar rats (6–7 weeks old) were purchased from Nomura Siam International Co., Ltd. (Thailand). They were chosen for these experiments due to their tendency to readily gain weight in response to an HFD [25]. All animals were maintained under controlled conditions at 23°C–25°C, with a 12-hour light/dark cycle. Food and water were provided to the animals ad libitum. They underwent a 1-week acclimatization period before the experiments. The experimental protocol was approved by the Animal Ethical Committee of Mae Fah Luang University (Approval No. AR02/62).
Experimental design
The rats were randomly assigned to four experimental groups, with five rats per group. Sample size calculation was performed using G*Power software (version 3.1.9.7), based on effect size estimates from a previous study [26]. According to the calculation, each group comprised five rats (n = 5). The experimental groups were as follows:
Group I (NC): Normal control rats fed with a standard chow diet and given a vehicle for 8 weeks
Group II (HFD): HFD control rats fed with HFD and given a vehicle for 8 weeks
Group III (HFD + GCE200): Rats fed with HFD and administered GCE 200 mg/kg for 8 weeks
Group IV (HFD + GCE400): Rats fed with HFD and administered GCE 400 mg/kg for 8 weeks
The normal control group (Group I) was fed a commercial standard rodent diet (National Laboratory Animal Center, Mahidol University, Thailand). In contrast, the HFD and experimental groups (Groups II, III, and IV) received HFD containing 45 kcal% fat (product No. D12451, Research Diet Inc., New Brunswick, NJ, USA). Both the normal control and HFD control groups (Groups I and II) received oral gavage vehicle administration, while the treatment groups (Groups III and IV) received GCE at doses of 200 and 400 mg/kg, respectively, administered once daily via oral gavage.
At the end of the experiment (day 56), the rats were anesthetized by intraperitoneal injection of thiopental sodium (40 mg/kg), following overnight fasting. Once deep anesthesia was achieved, blood samples were collected via cardiac puncture for biochemical analysis. Following euthanasia, organs such as the liver, kidneys, and visceral fat, including the greater omentum, retroperitoneal fat, and epididymal fat, were removed. The liver, kidneys, and fat tissues were washed with 0.9% saline solution, blotted dry, and weighed. A schematic representation of the experimental design is shown in Figure 1.
 | Figure 1. Schematic representation of the experimental design. NC: normal diet group; HFD: high-fat diet group; HFD + GCE200: HFD with GCE (200 mg/kg) group; HFD + GCE400: HFD with GCE (400 mg/kg) group. [Click here to view] |
Body weight, food intake, and feed efficiency ratio (FER)
During the experimental period, the rats’ body weight and food intake were recorded weekly. The calculation of FER was performed using the following formula [27]:
FER (%) = [Body weight gain (g/day) / Food intake (g/day)] × 100
Biochemical analysis
Blood samples were collected in tubes without anticoagulants for biochemical analysis, while additional samples were transferred to sodium fluoride tubes for fasting blood glucose analysis. Triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and fasting blood glucose were measured using an automated chemistry analyzer (COBAS INTEGRA® 400 plus analyzer, Roche, Germany) at Mengrai Lab, a medical laboratory in Chiang Rai, Thailand.
The level of very LDL-C (VLDL-C) in serum was estimated using the formula described by Friedewald et al. [28].
VLDL-C = Triglyceride / 5.
Histological analysis
The liver, retroperitoneal, and epididymal fat were removed, washed with 0.9% saline solution, and fixed in 10% formaldehyde. The tissues were embedded in paraffin, sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin (H&E). Six randomly selected images of both retroperitoneal and epididymal adipose tissue were captured from each animal using a Carl Zeiss fluorescence microscope (AxioScope; Rushmore Precision Co., Ltd.) with ZEN 2.3 software. Adipose tissue images were analyzed using ImageJ (Version 1.52q) to assess adipocyte parameters, including number, diameter, perimeter, and area. For measurements of adipocyte number, diameter, perimeter, and area, 10 randomly selected cells per image per animal were analyzed. Adipocyte diameter was determined by measuring the distance between opposite cell membranes in four planes. Lipid droplet accumulation in liver tissue was evaluated using the steatosis grading component of the NAFLD activity score (NAS) [29]. Steatosis was graded based on the proportion of steatosis area in the liver parenchyma as follows: grade 0, <5%; grade 1, 5%–33%; grade 2, 33%–66%; and grade 3, >66%.
Statistical analysis
The results are presented as the mean ± SEM. Statistical analysis was conducted using SPSS software (version 25.0). Group mean differences were analyzed by one-way ANOVA, followed by a least significant difference (LSD) post hoc test. Differences were considered statistically significant at p < 0.05.
RESULTS
Chemical composition of GCE
The phytochemical composition of GCE was previously characterized in our earlier study using LC-QTOF-MS with negative ionization mode. This demonstrated that GCE contains hydroxycitric acid, citric acid, kaempferol, isovitexin, apigenin, scutellarein, myricetin, eriodictyol, luteolin, quercetin, amentoflavone, isomangiferin, and garcimangosone C [20].
Effect of GCE on body weight, food intake, and FER
The HFD group exhibited significantly higher body weight and weight gain than the NC group (p < 0.05). Animals treated with GCE at a dose of 400 mg/kg demonstrated a marked reduction in body weight and cumulative weight gain (p < 0.05) (Fig. 2A, B). A significant decrease in food intake was observed in the HFD group compared to the NC group (p < 0.001); however, GCE treatment did not affect food intake relative to the HFD group (Fig. 2C). Furthermore, FER was markedly elevated in the HFD group (p < 0.001), but this increase was significantly attenuated by GCE at 400 mg/kg (p < 0.01) (Fig. 2D).
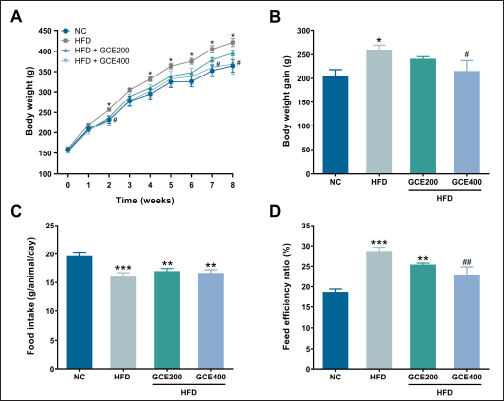 | Figure 2. Effects of GCE on body weight, body weight gain, food intake, and FER. (A) Body weight changes over 8 weeks. (B) Body weight gain. (C) Food intake. (D) FER. NC: normal diet group; HFD: high-fat diet group; HFD + GCE200: HFD with GCE (200 mg/kg) group; HFD + GCE400: HFD with GCE (400 mg/kg) group. Values are expressed as mean ± SEM (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the NC group; #p < 0.05 and ##p < 0.01 compared with the HFD group. [Click here to view] |
Effect of GCE on fat weight
As shown in Figure 3A, the total fat weight, the sum of the weight of greater omentum, epididymal fat, and retroperitoneal fat, was significantly higher in the HFD group than in the NC group (p < 0.01). However, administering GCE at 400 mg/kg significantly reduced total fat weight compared to the HFD group (p < 0.05). There were no significant differences in greater omentum weight among the groups (p > 0.05) (Fig. 3B). Additionally, epididymal and retroperitoneal fat weights were significantly increased in the HFD group compared to those in the NC group (p < 0.01). Although the epididymal fat weight exhibited a decreasing trend in the 400 mg/kg GCE-treated group compared to the HFD group, the reduction did not reach statistical significance (p = 0.098) (Fig. 3C). In contrast, GCE treatment at 400 mg/kg significantly reduced retroperitoneal fat weight compared to the HFD group (p < 0.01) (Fig. 3D).
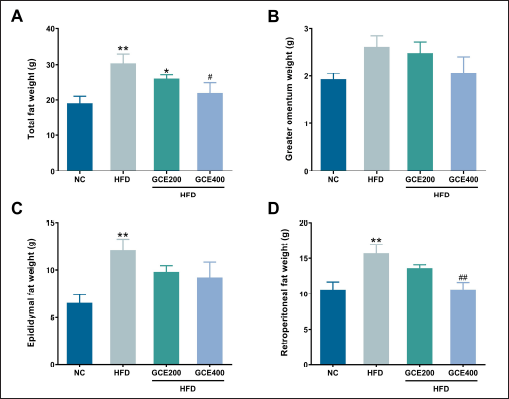 | Figure 3. Effects of GCE on fat weight. (A) Total fat weight. (B) Greater omental fat weight. (C) Epididymal fat weight. (D) Retroperitoneal fat weight. NC: normal diet group; HFD: high-fat diet group; HFD + GCE200: HFD with GCE (200 mg/kg) group; HFD + GCE400: HFD with GCE (400 mg/kg) group. Values are expressed as mean ± SEM (n = 5). *p < 0.05 and **p < 0.01 compared with the NC group; #p < 0.05 and ##p < 0.01 compared with the HFD group. [Click here to view] |
Effect of GCE on adipocytes
Histological sections of epididymal and retroperitoneal adipose tissue from the HFD group showed a lower number per unit area of adipocytes and greater adipocyte diameter, perimeter, and area than the NC group (p < 0.01). Oral supplementation of GCE significantly increased the adipocyte number while reducing their diameter, perimeter, and area in both epididymal and retroperitoneal adipose tissues (p < 0.05) (Fig. 4).
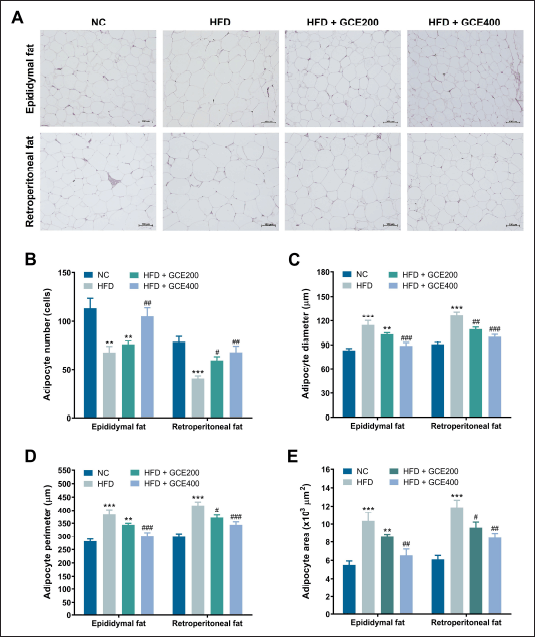 | Figure 4. Effects of GCE on epididymal and retroperitoneal adipose tissue. (A) Representative images of H&E-stained adipose tissue sections. Scale bars: 100 μm. (B) Number of adipocytes. (C) Adipocyte diameter. (D) Adipocyte perimeter. (E) Adipocyte area. NC: normal diet group; HFD: high-fat diet group; HFD + GCE200: HFD with GCE (200 mg/kg) group; HFD + GCE400: HFD with GCE (400 mg/kg) group. Values are expressed as mean ± SEM (n = 5). **p < 0.01 and ***p < 0.001 compared with the NC group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the HFD group. [Click here to view] |
Effect of GCE on organ weight
There were no significant changes in liver and kidney weights among the groups (p > 0.05) (Table 1).
| Table 1. Effect of GCE on organ weight (g). [Click here to view] |
Effect of GCE on histology of the liver
As presented in Figure 5A, consuming an HFD led to noticeably greater fat accumulation in liver tissue compared to the NC group. This was further supported by a significant increase in steatosis scores in the HFD group (p < 0.001). Notably, treatment with GCE at 400 mg/kg significantly reduced steatosis scores compared to the HFD control group (p < 0.01) (Fig. 5B).
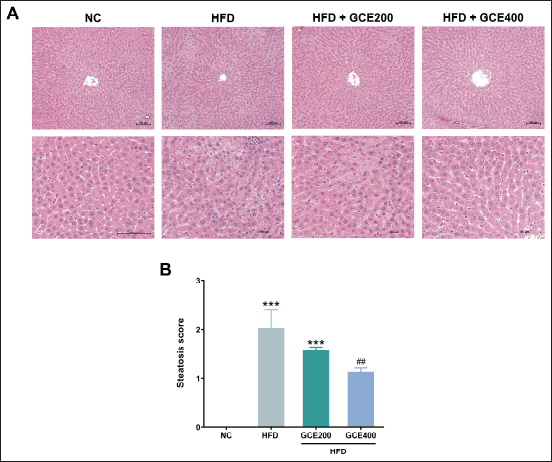 | Figure 5. Effects of GCE on liver tissue. (A) Representative images of H&E-stained liver tissue sections. Scale bars: 100 μm. (B) Steatosis score. NC: normal diet group; HFD: high-fat diet group; HFD + GCE200: HFD with GCE (200 mg/kg) group; HFD + GCE400: HFD with GCE (400 mg/kg) group. Values are expressed as mean ± SEM (n = 5). ***p < 0.001 compared with the NC group; ##p < 0.01 compared with the HFD group. [Click here to view] |
Effect of GCE on biochemical parameters
No significant differences in triglycerides, total cholesterol, HDL-C, LDL-C, VLDL-C, AST, ALT, or blood glucose were observed between the groups (p > 0.05) (Table 2).
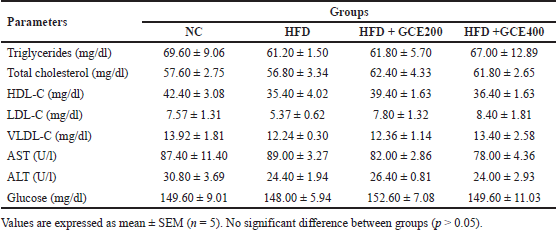 | Table 2. Effect of GCE on biochemical parameters. [Click here to view] |
DISCUSSION
The present study investigated the anti-obesity effects of GCE in HFD-fed Wistar rats. The findings revealed that HFD consumption significantly increased both body weight and weight gain, consistent with previous research demonstrating that HFD contributes to weight gain due to elevated energy intake [30,31]. Notably, treatment with GCE effectively reduced body weight and weight gain compared to untreated HFD-fed rats, indicating that GCE can mitigate HFD-induced weight gain. Importantly, food intake was lower in all HFD-fed rats than those fed the normal diet. The high energy content of HFD may explain the reduced food intake observed in rats, which aligns with previous studies reporting decreased food consumption in HFD-fed animals [32–34]. However, food intake did not differ significantly between the GCE-treated groups and the untreated HFD control group, suggesting that the reduced weight gain in GCE-treated rats was not due to decreased food consumption. Additionally, the study found that the FER was higher in the HFD group, which was significantly decreased following GCE treatment. The findings imply that rats fed an HFD and treated with GCE had a reduced efficiency in converting consumed food into body mass. By lowering FER, GCE may influence energy expenditure, lipid metabolism, and nutrient absorption.
A major characteristic of obesity is the increased accumulation of fat in the body, and in rodents, intra-abdominal adipose tissue expansion occurs due to cellular hypertrophy [32]. Excessive visceral fat is a key indicator of metabolic disorders, including cardiovascular disease, atherosclerosis, dyslipidemia, and hypertension [26]. The present results reveal a significant increase in visceral fat mass in the HFD control group, consistent with previous findings showing a marked rise in fat pad weight in HFD control animals [35,36]. Additionally, HFD consumption led to adipocyte enlargement by increasing their diameter, perimeter, and area while reducing their number per unit area. Treatment with GCE significantly reduced visceral fat weight. The reduction in fat weight may result from a decrease in fat pad mass, attributed either to a decrease in new adipocyte formation from precursor cells or to a reduction in adipocyte size caused by fat storage [37]. We confirm this observation, as administration of GCE increased the number of adipocytes per unit area while significantly reducing their size, suggesting a shift toward smaller adipocytes and potentially reduced fat accumulation. These findings demonstrate the anti-obesity potential of GCE in HFD-fed rats by reducing visceral fat mass and adipocyte size.
Several studies have reported a significant increase in organ weights, such as the liver, kidney, and heart in HFD-fed animals [3,26]. With the progression of obesity, an increase in liver weight occurs as a result of abnormal glycosylation or fibrosis, which are associated with hepatocyte hypertrophy and hepatic steatosis [38]. In obesity, kidney weight also increases, resulting from swelling, inflammation, and necrotic changes in the tissue [33]. However, our findings suggest a trend of increased liver weight in the HFD group, while GCE treatment appeared to reduce it, although these changes were not statistically significant. Meanwhile, kidney weight remained consistent across all groups. These results align with previous findings by Kim et al. [39] who reported that the same HFD used in our study had no significant effect on liver or kidney weight.
Obesity has been found to contribute to hepatic steatosis, marked by excessive hepatic fat accumulation, and is associated with oxidative stress-induced liver cell damage [40]. Research indicates that feeding mice and rats an HFD induces liver steatosis [41,42]. In our study, the HFD control group exhibited significantly greater lipid accumulation in liver tissues compared to the normal group. Administration with GCE diminished this HFD-induced liver lipid accumulation, indicating that GCE may mitigate lipid buildup in liver tissues caused by HFD. However, serum ALT and AST levels, well-known enzymes used as indicators of liver function and important markers for predicting toxicity [43], showed no significant changes. These findings suggest that while the HFD used in this study for 8 weeks induces hepatic steatosis, it does not cause hepatotoxicity.
Recent research has shown that consumption of HFD increases serum levels of total cholesterol, triglycerides, and LDL-C while decreasing HDL-C levels [44,45]. This results in serum lipid imbalances, which are associated with increased risk for several diseases [46]. In contrast, our findings showed no differences in triglycerides, total cholesterol, LDL-C, HDL-C, or VLDL-C levels between the experimental groups. This may be because the percentage of energy from fat in the diet used in the current study was insufficient to cause significant disruptions in lipid metabolism within the 8-week duration. The results of this study align with previous research by Bréger et al. [47] which reported that experimental rats fed an HFD containing 45% energy from fat, similar to the diet used in the present study, for 12 weeks exhibited no changes in plasma triacylglycerols, total cholesterol, LDL-C, or HDL-C levels relative to those on a normal diet. The in vitro findings demonstrated that the ethanolic extract of G. cowa leaves possesses anti-hyperlipidemic activity [22]. However, since no changes in blood lipid levels were observed in the experimental rats fed the HFD in this study, it was not possible to evaluate the lipid-lowering effects of the extract. Further studies should explore the anti-hyperlipidemic effects of G. cowa extract using a diet with a higher fat-energy percentage or an extended study duration.
In a previous study, a qualitative analysis of compounds in the ethanolic extract of G. cowa leaves was conducted using LC-QTOF-MS. The findings revealed that the extract contained various bioactive compounds, including hydroxycitric acid, citric acid, kaempferol, isovitexin, apigenin, scutellarein, myricetin, eriodictyol, luteolin, quercetin, amentoflavone, isomangiferin, and garcimangosone C [20]. Hydroxycitric acid is a widely recognized herbal dietary supplement known for its effectiveness in treating obesity [48]. This compound, derived from citric acid, is found in plant species native to South Asia, including G. cambogia, G. indica, and G. atroviridis [49]. Studies in rats have demonstrated that hydroxycitric acid reduces weight regain and suppresses food intake [50]. In humans, it promotes weight loss without stimulating the central nervous system [51] and helps reduce obesity-related visceral fat accumulation [52]. Additionally, hydroxycitric acid has been shown to decrease de novo lipogenesis during carbohydrate overfeeding in humans, potentially supporting weight maintenance [53]. Research also indicates that hydroxycitric acid reduces body weight regain in rats following substantial weight loss, likely due to its inhibitory effect on lipogenesis [54]. Hydroxycitric acid lowers acetyl-CoA levels, thus suppressing lipogenesis by limiting the availability of precursors required for the synthesis of fatty acids and cholesterol [55].
In addition to hydroxycitric acid, flavonoids such as kaempferol, apigenin, scutellarein, myricetin, eriodictyol, and quercetin have been shown to possess significant anti-obesity effects. A previous study by Torres-Villarreal et al. [56] highlighted the anti-obesity properties of kaempferol, which suppresses adipogenesis and enhances lipolysis in 3T3-L1 adipose cell lines. Furthermore, recent research indicates that kaempferol stimulates the browning of white adipose tissue through the AMPK/SIRT1/PGC-1α pathway, demonstrating its anti-obesity properties [57]. Apigenin has also demonstrated notable anti-obesity effects. In HFD-induced obese mice, it improved dyslipidemia, hepatic steatosis, and insulin resistance [58]. Moreover, apigenin significantly reduced visceral adipose tissue in HFD-fed mice by inhibiting adipogenesis via the signal transducer and activator of transcription 3 and cluster of differentiation 36 signaling axis [59]. Scutellarein has been shown to significantly alleviate HFD-induced obesity, hyperlipidemia, inflammation, and fatty liver [60]. Similarly, myricetin has exhibited anti-obesity effects in several animal studies. Myricetin treatment effectively reduces body weight, serum glucose, triglycerides, cholesterol, and the size of epididymal adipocytes, while also lowering fat mass in HFD-induced obese C57BL/6 mice [61]. Additionally, myricetin inhibits the differentiation of 3T3-L1 preadipocytes and enhances adipocyte lipolysis [62]. Eriodictyol supplementation in HFD-induced obese mice significantly reduced dyslipidemia and adiposity by promoting fecal lipid excretion and suppressing lipogenesis in white adipose tissue. It also alleviated hepatic steatosis by partially inhibiting hepatic lipogenesis and enhancing fatty acid oxidation in the liver [63]. In a previous study, Jung et al. [64] reported that quercetin reduced body weight gain, liver weight, and white adipose tissue weight, as well as lowered serum lipid levels, liver fat accumulation, and lipid droplet size in epididymal adipose tissue. Its anti-obesity effects are likely associated with the regulation of genes related to lipid metabolism. In Western diet-fed mice, chronic quercetin intake alleviated hepatic lipid accumulation, likely by reducing the expression of steatosis-linked genes [65]. Lastly, amentoflavone exhibits anti-obesity properties by inhibiting body weight gain, reducing perirenal adipose tissue weight, and lowering serum triglyceride levels in HFD-fed rats, as well as suppressing 3T3-L1 adipocyte differentiation [66]. Collectively, these phytochemicals, including hydroxycitric acid, apigenin, kaempferol, apigenin, scutellarein, myricetin, eriodictyol, quercetin, and amentoflavone, are likely responsible for the anti-obesity effects of GCE. However, additional research is required to investigate the anti-obesity mechanisms of GCE in greater detail, as well as to isolate and characterize the active constituents responsible for this activity.
The lack of significant changes in lipid profiles or glucose levels represents a limitation of this study and may be due to the study duration or dietary regimen. Future studies with a higher dietary fat content or extended treatment duration are recommended to further validate and strengthen the observed findings from this study.
CONCLUSION
This study demonstrates that GCE effectively reduces body weight, cumulative weight gain, FER, and total fat mass, particularly in retroperitoneal fat, and adipocyte size. Furthermore, GCE significantly mitigates HFD-induced hepatic fat accumulation. The anti-obesity effects of GCE in rats fed an HFD are probably due to its bioactive compounds, such as hydroxycitric acid and flavonoids. These findings indicate that GCE has the potential for development as a therapeutic or nutraceutical agent in obesity prevention and treatment.
ACKNOWLEDGMENT
The authors sincerely thank Prof. Dr. Surat Laphookhieo for providing the extract and Dr. Roger Timothy Callaghan for his assistance with reviewing the English of the manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was supported by the Agricultural Research Development Agency (Public Organization), Thailand (Grant No. CRP6105021500).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals are given in the ‘Materials and Method’ section.
DATA AVAILABILITY
All data are available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Koliaki C, Dalamaga M, Liatis S. Update on the obesity epidemic: after the sudden rise, is the upward trajectory beginning to flatten? Curr Obes Rep. 2023;12(4):514–27. CrossRef
2. World Health Organization. Obesity and overweight. Geneva, Switzerland: WHO [Internet]; 2025 May 7 [cited 2025 May 20]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
3. Kaveripakam SS, Adikay S, Retnasamy G. Anti-obesity efficacy of roots of Stereospermum suaveolens in high fat-induced obese rats. J Young Pharm. 2017;9(2):234–8. CrossRef
4. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne). 2021;12:706978. CrossRef
5. Poston WS, Hyder ML, O’Byrne KK, Foreyt JP. Where do diets, exercise, and behavior modification fit in the treatment of obesity? Endocrine. 2000;13(2):187–92. CrossRef
6. Tak YJ, Lee SY. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Mens Health. 2021;39(2):208–21. CrossRef
7. Yun JW. Possible anti-obesity therapeutics from nature--a review. Phytochemistry. 2010;71(14–15):1625–41. CrossRef
8. Kongchian A, Keawboonlert N, Boonrak T, Lookyee S, Buasri K, Sukongkul N, et al. Anti-hyperlipidemia and anti-obesity properties of Garcinia atroviridis and Camellia sinensis extracts in high-fat diet mice. Walailak J Sci Technol. 2020;17(10):1126–38. CrossRef
9. Lim WF, Nasir SM, Teh LK, James RJ, Izhar MHM, Salleh MZ. The methanolic extract of Garcinia atroviridis (MeGa) reduces body weight and food intake, and improves lipid profiles by altering the lipid metabolism: a rat model. Turk J Biol. 2020;44(6):437–48. CrossRef
10. Majeed M, Majeed S, Nagabhushanam K, Lawrence L, Mundkur L. Garcinia indica extract standardized for 20% Garcinol reduces adipogenesis and high fat diet-induced obesity in mice by alleviating endoplasmic reticulum stress. J Funct Foods. 2020;67(41):103863. CrossRef
11. Sarma R, Kumari S, Elancheran R, Deori M, Devi R. Polyphenol rich extract of Garcinia pedunculata fruit attenuates the hyperlipidemia induced by high fat diet. Front Pharmacol. 2016;7:294. CrossRef
12. Yadav R, Bindra HK, Chaurasia RC. Therapeutic potential of Garcinia cambogia and Emblica officinalis in the management of obesity and lipid profiles in a rat model of diet induced obesity. Int J Basic Clin Pharmacol. 2024;13(5):647–54. CrossRef
13. Panthong K, Hutadilok-Towatana N, Panthong A. Cowaxanthone F, a new tetraoxygenated xanthone, and other anti-inflammatory and antioxidant compounds from Garcinia cowa. Can J Chem. 2009;87(11):1636–40. CrossRef
14. Mahabusarakam W, Chairerk P, Taylor WC. Xanthones from Garcinia cowa Roxb. latex. Phytochemistry. 2005;66(10):1148–53. CrossRef
15. Murakami A, Jiwajinda S, Koshimizu K, Ohigashi H. Screening for in vitro anti-tumor promoting activities of edible plants from Thailand. Cancer Lett. 1995;95(1–2):139–46. CrossRef
16. Negi PS, Jayaprakasha GK, Jena BS. Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit rinds of Garcinia cowa. Int J Food Prop. 2010;13(6):1256–65. CrossRef
17. Jantan I, Jumuddin FA, Saputri FC, Rahman K. Inhibitory effects of the extracts of Garcinia species on human low-density lipoprotein peroxidation and platelet aggregation in relation to their total phenolic contents. J Med Plant Res. 2011;5(13):2699–709.
18. Bulbul-A-Munira, Chowdhury SA. Antibacterial, neuropharmacological and analgesic activities of Garcinia cowa (family: Clusiaceae). Int Conf Bioinform Biomed Eng. 2015;1(2):112–7.
19. Wahyuni FS, Shaari K, Stanslas J, Lajis N, Hamidi D. Cytotoxic compounds from the leaves of Garcinia cowa Roxb. J Appl Pharm Sci. 2015;5(2):6–11. CrossRef
20. Praman S, Teerapattarakan N, Hawiset T. Garcinia cowa leaf ethanolic extract induces vasorelaxation through eNOS/NO/sGC pathway, potassium, and calcium channels in isolated rat thoracic aorta. Pharmacogn J. 2024;16(4):797–804. CrossRef
21. Yorsin S, Sriwiriyajan S, Chongsa W. Vasorelaxing effect of Garcinia cowa leaf extract in rat thoracic aorta and its underlying mechanisms. J Tradit Complement Med. 2023;13(3):219–25. CrossRef
22. Pattamadilok D, Niumsakul S, Limpeanchob N, Ingkaninan K, Wongsinkongman P. Chemical constituents and anti-hyperlipidemia activity of Garcinia cowa leaves. J Thai Tradit Altern Med. 2010;8(2–3):152–9.
23. Jena BS, Jayaprakasha GK, Sakariah KK. Organic acids from leaves, fruits, and rinds of Garcinia cowa. J Agric Food Chem. 2002;50(12):3431–4. CrossRef
24. Shiekh KA, Benjakul S, Sae-Leaw T. Effect of Chamuang (Garcinia cowa Roxb.) leaf extract on inhibition of melanosis and quality changes of Pacific white shrimp during refrigerated storage. Food Chem. 2019;270:554–61. CrossRef
25. Bastías-Pérez M, Serra D, Herrero L. Dietary options for rodents in the study of obesity. Nutrients. 2020;12(11):3234. CrossRef.
26. Arika WM, Kibiti CM, Njagi JM, Ngugi MP. Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats. Heliyon. 2019;5(11):e02800. CrossRef
27. Lee SJ, Choi HN, Kang MJ, Choe E, Auh JH, Kim JI. Chamnamul [Pimpinella brachycarpa (Kom.) Nakai] ameliorates hyperglycemia and improves antioxidant status in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2013;7(6):446–52. CrossRef
28. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
29. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. CrossRef.
30. Bruder-Nascimento T, Ekeledo OJ, Anderson R, Le HB, Belin de Chantemèle EJ. Long term high fat diet treatment: an appropriate approach to study the sex-specificity of the autonomic and cardiovascular responses to obesity in mice. Front Physiol. 2017;8:32. CrossRef
31. Kovacs P, Hajnal A. Short-term high-fat diet consumption increases body weight and body adiposity and alters brain stem taste information processing in rats. Chem Senses. 2022;47:bjac020. CrossRef
32. Kim CM, Yi SJ, Cho IJ, Ku SK. Red-koji fermented red ginseng ameliorates high fat diet-induced metabolic disorders in mice. Nutrients. 2013;5(11):4316–32. CrossRef
33. Kim MR, Kim JW, Park JB, Hong YK, Ku SK, Choi JS. Anti-obesity effects of yellow catfish protein hydrolysate on mice fed a 45% kcal high-fat diet. Int J Mol Med. 2017;40(3):784–800. CrossRef
34. Wu YHS, Chiu CH, Yang DJ, Lin YL, Tseng JK, Chen YC. Inhibitory effects of litchi (Litchi chinensis Sonn.) flower-water extracts on lipase activity and diet-induced obesity. J Funct Foods. 2013;5(2):923–9. CrossRef
35. Cho YR, Lee JA, Kim YY, Kang JS, Lee JH, Ahn EK. Anti-obesity effects of Clausena excavata in high-fat diet-induced obese mice. Biomed Pharmacother. 2018;99:253–60. CrossRef
36. Rahman HA, Sahib NG, Saari N, Abas F, Ismail A, Mumtaz MW, et al. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement Altern Med. 2017;17(1):122. CrossRef
37. Garg A, Singh R. Antiobesity activity of aqueous and ethanol extracts of Aegle marmelos leaves in high fat diet induced obese rats. Int J Pharm Sci Rev Res. 2015;30(1):53–60.
38. Kang SJ, Lee JE, Lee EK, Jung DH, Song CH, Park SJ, et al. Fermentation with Aquilariae Lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients. 2014;6(9):3536–71. CrossRef
39. Kim CH, Lee JM, Jang YS, Jeon JH, Lim SS, Kim SC, et al. Anti-obesity effect of Solidago virgaaurea extract in high-fat diet-fed SD rat. Animal Cells Syst (Seoul). 2016;20(6):335–43. CrossRef
40. Kim BM, Cho BO, Jang SI. Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity. Int J Mol Med. 2019;43(1):603–13. CrossRef
41. Meli R, Mattace Raso G, Irace C, Simeoli R, Di Pascale A, Paciello O, et al. High fat diet induces liver steatosis and early dysregulation of iron metabolism in rats. PLoS One. 2013;8(6):e66570. CrossRef
42. Tsuru H, Osaka M, Hiraoka Y, Yoshida M. HFD-induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci Rep. 2020;10(1):17593. CrossRef
43. El Hilaly J, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91(1):43–50. CrossRef
44. Abbas MA, Boby N, Lee EB, Hong JH, Park SC. Anti-obesity effects of Ecklonia cava extract in high-fat diet-induced obese rats. Antioxidants (Basel). 2022;11(2):310. CrossRef
45. Ai ZL, Zhang X, Ge W, Zhong YB, Wang HY, Zuo ZY, et al. Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism. World J Gastroenterol. 2022;28(43):6131–56. CrossRef
46. Jung YC, Kim HW, Min BK, Cho JY, Son HJ, Lee JY, et al. Inhibitory effect of olive leaf extract on obesity in high-fat diet-induced mice. In Vivo. 2019;33(3):707–15. CrossRef
47. Bréger G, André A, Cotte C, Hammaidi A, Amérand A, Faivre C, et al. Anti-obesity effects evaluation of a blackcurrant leaf standardized hydro-alcoholic extract in Wistar rat subjected to a high-fat diet. Biology (Basel). 2024;13(12):999. CrossRef
48. Onakpoya I, Hung SK, Perry R, Wider B, Ernst E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. J Obes. 2011;2011:509038. CrossRef
49. Roongpisuthipong C, Kantawan R, Roongpisuthipong W. Reduction of adipose tissue and body weight: effect of water soluble calcium hydroxycitrate in Garcinia atroviridis on the short term treatment of obese women in Thailand. Asia Pac J Clin Nutr. 2007;16(1):25–9.
50. Leonhardt M, Langhans W. Hydroxycitrate has long-term effects on feeding behavior, body weight regain and metabolism after body weight loss in male rats. J Nutr. 2002;132(7):1977–82. CrossRef
51. Downs BW, Bagchi M, Subbaraju GV, Shara MA, Preuss HG, Bagchi D. Bioefficacy of a novel calcium-potassium salt of (-)-hydroxycitric acid. Mutat Res. 2005;579(1–2):149–62. CrossRef
52. Hayamizu K, Ishii Y, Kaneko I, Shen M, Okuhara Y, Shigematsu N, et al. Effects of garcinia cambogia (Hydroxycitric acid) on visceral fat accumulation: a double-blind, randomized, placebo-controlled trial. Curr Ther Res Clin Exp. 2003;64(8):551–67. CrossRef
53. Kovacs EM, Westerterp-Plantenga MS. Effects of (-)-hydroxycitrate on net fat synthesis as de novo lipogenesis. Physiol Behav. 2006;88(4–5):371–81. CrossRef
54. Leonhardt M, Hrupka B, Langhans W. Effect of hydroxycitrate on food intake and body weight regain after a period of restrictive feeding in male rats. Physiol Behav. 2001;74(1–2):191–6. CrossRef
55. Chuah LO, Ho WY, Beh BK, Yeap SK. Updates on antiobesity effect of Garcinia Origin (-)-HCA. Evid Based Complement Alternat Med. 2013;2013:751658. CrossRef
56. Torres-Villarreal D, Camacho A, Castro H, Ortiz-Lopez R, de la Garza AL. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J Physiol Biochem. 2019;75(1):83–8. CrossRef
57. Xu C, Zhang X, Wang Y, Wang Y, Zhou Y, Li F, et al. Dietary kaempferol exerts anti-obesity effects by inducing the browing of white adipocytes via the AMPK/SIRT1/PGC-1α signaling pathway. Curr Res Food Sci. 2024;8:100728. CrossRef
58. Jung UJ, Cho YY, Choi MS. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 2016;8(5):305. CrossRef
59. Su T, Huang C, Yang C, Jiang T, Su J, Chen M, et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol Res. 2020;152:104586. CrossRef
60. Lin Y, Ren N, Li S, Chen M, Pu P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed Pharmacother. 2019;117:109042. CrossRef
61. Su HM, Feng LN, Zheng XD, Chen W. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J Zhejiang Univ Sci B. 2016;17(6):437–46. CrossRef
62. Wang Q, Wang ST, Yang X, You PP, Zhang W. Myricetin suppresses differentiation of 3 T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr Res. 2015;35(4):317–27. CrossRef
63. Kwon EY, Choi MS. Dietary eriodictyol alleviates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obese mice. Int J Mol Sci. 2019;20(5):1227. CrossRef
64. Jung CH, Cho I, Ahn J, Jeon TI, Ha TY. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. 2013;27(1):139–43. CrossRef
65. Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res. 2011;55(4):530–40. CrossRef
66. Chen G, Han Y, He W, Liang F. Amentoflavone protects against high fat-induced metabolic dysfunction: possible role of the regulation of adipogenic differentiation. Int J Mol Med. 2016;38(6):1759–67. CrossRef