INTRODUCTION
Drug interactions appear when the effect of a drug is altered by co-administration with another drug, food, or herbal product [1]. DDIs, which alter the efficacy or safety of drugs, are categorized as pharmaceutical, pharmacokinetic, and pharmacodynamic interactions. Pharmaceutical interactions involve physicochemical changes during drug administration, affecting absorption, distribution, metabolism, and excretion [2,3]. Pharmacokinetic interactions occur when drug-metabolizing enzymes are inhibited or induced, while pharmacodynamic interactions change pharmacological responses through concurrent drugs [4]. Elderly patients who often take multiple medications are at high risk of experiencing specific food–drug interactions [5,6]. The interaction between drugs and herbal products is a safety concern, and these interactions are especially important for drugs with narrow therapeutic indices [7]. Although the current literature emphasizes the need to monitor these interactions in polypharmacy [4], further research targeting specific populations or lesser explored substances like cannabis is crucial [8]. The prevalence of drug interactions with DOACs varies across studies and populations, particularly in clinical practice. A retrospective study of hospitalized patients over the age of 65 identified drug interactions in 37% of cases, with pharmacodynamic interactions accounting for 29% and pharmacokinetic interactions for 16% [9]. In addition, another study in hospitalized older adults reported a prevalence of pharmacokinetic interactions with DOACs of 16.9% at admission and 20.7% at discharge. In comparison, pharmacodynamic interactions were observed in 20.7% and 20.2% of cases, respectively [10]. Similarly, in patients with atrial fibrillation, 30.4% were co-prescribed potentially interacting medications, most commonly enzyme inhibitors. However, these interactions were not associated with an increased risk of embolic or hemorrhagic adverse events [11]. Furthermore, a retrospective cohort study of patients with cancer-associated thrombosis (CAT) receiving anticoagulants found that 41.6% experienced drug–drug interactions (DDIs), with a significantly higher prevalence among those using DOACs (50.9%) compared to low molecular weight heparins (19.3%) [12]. These findings reveal the high prevalence of DDIs with DOACs in polymedicated and elderly patients, as well as those with cancer, highlighting the importance of thorough treatment monitoring. This evidence reinforces the need for a bibliometric analysis to map research trends and identify gaps in knowledge within this field.
Since the Food and Drug Administration (FDA) approved dabigatran in 2010, DOACs have become essential for managing cardiovascular conditions such as atrial fibrillation and venous thromboembolism (VTE) [13–15]. While DOACs have fewer interactions than vitamin K antagonists, certain drugs can interfere with their metabolism, mainly through cytochrome P450 enzymes or P-glycoprotein transport, impacting their therapeutic effects [1]. This issue is particularly relevant for CAT patients, who often have complex drug regimens [16–18]. Studies indicate that enzyme-inducing antiseizure medications (ASMs) reduce DOAC levels, impacting dosage needs; for instance, rivaroxaban shows significant pharmacokinetic changes with these ASMs [19]. In addition, drugs such as carbamazepine, phenytoin, rifampicin, and enzalutamide, which have class X interactions with DOACs, are linked to an approximately threefold increase in mortality rates [20].
Various DDI databases, such as drug interactions facts, Stockley, DailyMed, WebMD, National Drug File, DrugBank, Lexicomp, and TWOSIDES, are available for researchers, clinicians, and patients. However, these databases face challenges in staying updated, leaving critical DDI information embedded in biomedical literature [21]. A database often requires a thorough evaluation through in-depth bibliometric analysis or comprehensive literature reviews [22].
Bibliometric research provides a structured overview of current literature, identifies trends, and highlights gaps, establishing a roadmap for future research. Network metrics enhance bibliometric analysis by offering insights into the relative importance of research constituents (e.g., authors, institutions, and countries), beyond publication and citation counts. Metrics such as betweenness, degree, and closeness centrality are widely used to contextualize research impact and enrich discussions in bibliometric studies, making them a valuable tool for comprehensive assessments [23]. Betweenness centrality measures a node’s ability to act as a bridge for information exchange between otherwise disconnected groups, where each node represents a research entity. In contrast, degree centrality indicates the number of direct connections a research entity has within the network, while closeness centrality reflects how efficiently a node can transmit information by maintaining proximity to other nodes in the network [23]. Previous bibliometric studies on DOACs include “Research Trends in Anticoagulation Therapy over the Last 25 Years” by Mian et al. [24]. The objective of this research is to address the gap in the literature related to the prevalence and clinical impact of DDIs with DOACs. Despite numerous studies on this topic, comprehensive analyses that map the evolution of research and identify hotspots and emerging trends are still lacking. This research provides key insights to guide future investigations, improving our understanding of interactions with DOACs. Optimizing DOAC clinical benefits by identifying and managing potential drug interactions remains a priority in both clinical practice and public health.
MATERIALS AND METHODS
The current study was a retrospective descriptive bibliometric analysis of drug interactions with DOACs extracted from research published in Scopus article abstracts. This approach of performance analysis and science mapping allowed for identifying the highlights and emerging trends within this research field.
Data source
We used the Scopus database as our data source. Elsevier launched Scopus in 2004. Over the years, it has earned recognition as a comprehensive bibliographic data source and is very reliable [22,25]. Scopus was selected as the primary database due to its extensive coverage of high-impact journals and structured metadata, which facilitate bibliometric analysis. Scopus indexes a broader range of journals across multiple disciplines, including health and biomedical sciences, and also includes content from many specialized databases, such as Embase and Medline [22,26,27]. In addition, its citation tracking capabilities and structured metadata enable robust co-authorship and keyword co-occurrence analyses [28].
Data retrieval strategy
Preliminary search
A preliminary search was realized in the Scopus and Dimensions AI databases using the keywords “drug interaction” AND “direct oral anticoagulant,” resulting in 523 and 489 documents, respectively. Publications on drug interactions of DOACs were observed starting from 2010 in Scopus and 2011 in Dimensions, with a trend of increasing the number of publications annually in both databases. Because the FDA approved dabigatran in October 2010, it was decided to begin the search for drug interactions of DOACs from 2011 to 2023 to cover full years.
Data retrieval
In this study, all publications were retrieved and downloaded from the Scopus database on August 04, 2024. To ensure consistency, all data extraction procedures were conducted on the same day to prevent any fluctuations in citation counts. The search equation employed was as follows: TITLE-ABS-KEY [(“drug interact*” OR co-medication OR “concomitant use” OR concurrent OR co-administ* OR “pharmac* interaction” OR “cytochrome P450 inhibitor” OR “CYP3A4 inhibit*” OR “cytochrome P450 inducer” OR “P-glycoprotein inhibit*” OR “P-gp inhibit*” OR “P-glycoprotein induc*” OR “P-gp inducer”) AND (“direct oral anticoagulant” OR “non-vitamin K oral anticoagulant” OR “direct-acting oral anticoagulant” OR “novel oral anticoagulant” OR dabigatran OR “direct thrombin inhibitor” OR rivaroxaban OR apixaban OR edoxaban OR “direct factor Xa inhibitor”)] AND PUBYEAR > 2010 AND PUBYEAR < 2024 AND [ LIMIT-TO ( DOCTYPE, “ar”)]. To obtain as many relevant sources as possible, thesaurus and wildcard characters (*) were used to represent one or more other characters and allow for variable endings of keywords. For example, interact* also includes the plural of interaction, interactions, or the variant word interacting. The literature publication period was 2011–2023, without any restrictions on language. The publication type was limited to articles.
Inclusion and exclusion criteria
Figure 1 presents the specific inclusion and exclusion criteria. The flowchart illustrates the publication search and selection process employed by Yao et al. [29].
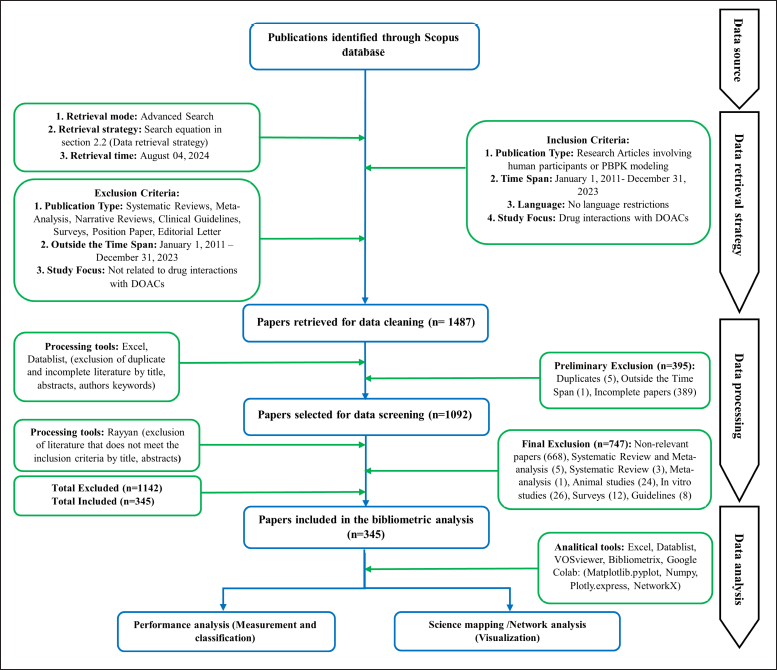 | Figure 1. Flowchart of the publication search and selection process. [Click here to view] |
Data processing
Validation of retrieved data
A total of 1487 articles were retrieved from Scopus. The titles and abstracts of these articles were downloaded as a CSV file. To validate the search strategy and ensure the absence of false-positive results, we reviewed the top 20 most cited documents [30]. The CSV file was imported into the Datablist tool [31], where a filter of the most representative keywords was applied to the title, abstract, and author keywords fields to verify the accuracy of the data retrieval strategy. After filtering, 19 out of 20 of the most cited articles were retained, indicating a 95% precision in the search strategy. Moreover, the number of publications of the top authors was positively correlated with the number of total publications related in Scopus of these authors, suggesting the absence of false-negative results. These two methods have been applied in previously published bibliometric analyzes [30]. This validation confirms the reliability of the retrieved data and helps minimize the inclusion of literature not related to the research topic.
Data cleaning and screening
The dataset was manually reviewed using the same tool to exclude duplicate and incomplete entries [31], resulting in a total of 1092 articles. These articles were then exported to a CSV file and imported into the Rayyan tool [32], where they were manually screened by two pharmacist reviewers (I.H.T. and N.G.S.) to exclude literature not related to the research topic. ChatGPT [33] was used as an assistive tool to address reviewer disagreements and provide alternative perspectives. However, the same two reviewers manually validated all artificial intelligence (AI) tool-generated suggestions to ensure consistency, scientific accuracy, and relevance to the study before inclusion in the final dataset. This approach aligns with previous studies that have explored the potential of AI tools, including ChatGPT, in assisting literature screening [34–36].
Final paper selection and data export for bibliometric analysis
After removing 747 articles not related to the inclusion criteria, 345 articles were included for the data analysis. Table 1 summarizes the relevant information from these papers. All 345 retrieved articles were downloaded as a CSV file to Microsoft Excel 365. The dataset contained complete information on each publication for bibliometric analysis, including literature title, abstract, author list, journal name, keywords, publication year, countries/regions, affiliations, reference list, and citations.
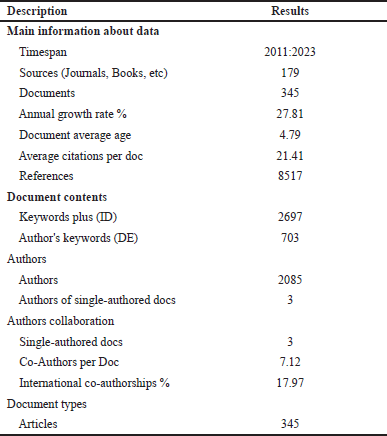 | Table 1. Main information. [Click here to view] |
Keyword and institutions standardization
For analysis in VOSviewer [version 1.6.20 © 2009-2023 Nees Jan van Eck and Ludo Waltman], 449 keywords with similar meanings but different formats were standardized. For example, “drug-interaction” was changed to “drug interaction,” “DDI” to “drug–drug interaction,” “anticoagulant agent” to “anticoagulant,” and “DOAC” to “direct oral anticoagulant.” In addition, for institutional analysis, 722 institutions belonging to the same core group were standardized [37].
Data analysis
Performance analysis of publication and citation data
Microsoft Excel 365, Datablist, Google Colab (Python libraries: Matplotlib.pyplot, Numpy, Plotly.express) [38], and the R package bibliometrix [Comprehensive Science Mapping Analysis, version 4.1.4 ©] were employed to conduct essential statistical analyses of the annual publication and citation counts [29,31,38].
Science mapping and network analysis
The evaluation was carried out on data from countries/regions, institutions, authors, journals, documents, and keywords. The analysis also involved collaborative assessments among countries, organizations, and authors, bibliographic coupling analysis of organizations and authors, and analysis of keyword co-occurrence using VOSviewer [version 1.6.20 © 2009-2023 Nees Jan van Eck and Ludo Waltman]. In this study, optimization of VOSviewer hyperparameters involved adjusting the Normalization (Method), Layout (Attraction and Repulsion), Clustering (Resolution and Minimum Cluster Size), and Rotate/Flip (Degrees to Rotate) values for each analysis to achieve an optimal network layout in which strongly related nodes were positioned together forming clusters and weakly related nodes were positioned far apart. This configuration optimized the spatial distribution of network nodes, reducing overlap and congestion and allowing for a clearer visualization of cooperative relationships [29,39]. The network metrics (betweenness centrality, degree centrality, and closeness centrality) were calculated using VOSviewer [version 1.6.20 © 2009-2023 Nees Jan van Eck and Ludo Waltman], and Google Colab (Python libraries: Networkx, Matplotlib.pyplot) [23].
RESULTS AND DISCUSSION
Publication and citation trends
Figure 2 illustrates the total number of publications and citations related to research on DOAC drug interactions from 2011 to 2023. In the first 3 years, both publications and citations showed a continuous increase. Since 2018, the number of published papers has grown steadily, reaching a peak of 57 in 2023. The decrease in the number of citations over the last 3 years is due to the limited time available for these publications to accumulate citations, given their recent publication, an effect documented in other bibliometric analyses [29]. Table 2 presents the annual number of publications and citation frequency. TP denotes total publications, while TC represents total citations. Four citation thresholds were established to assess the impact of publications. These thresholds categorize papers based on citation counts of ≥100, ≥10, ≥1, and 0. This classification provides insight into the citation distribution and influence of the published studies.
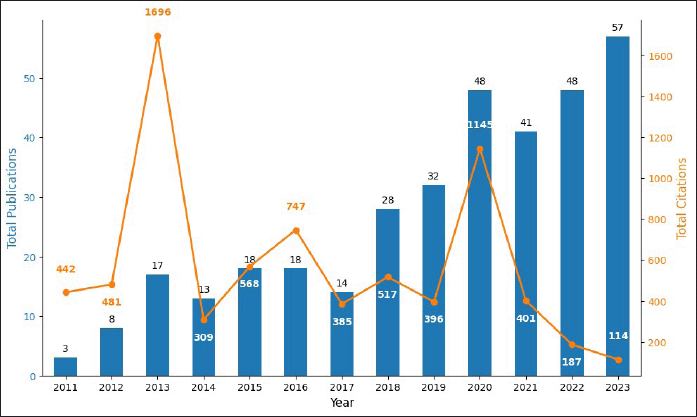 | Figure 2. Number of publications and citations from 2011 to 2023. The graph, based on data from Scopus, was created using Matplotlib.pyplot and Numpy Python libraries in Google Colab. [Click here to view] |
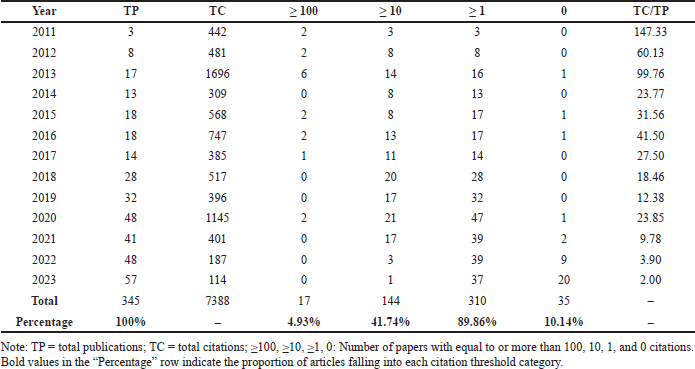 | Table 2. Total publications, total citations, and average citations per year (2011 to 2023). [Click here to view] |
Additional analyses were conducted to enhance the evaluation of research impact beyond citation-based metrics. A visualization map of international collaboration (Section 3.2) and a keyword co-occurrence analysis (Section 3.7) provide a broader perspective on knowledge evolution in research drug interactions with DOACs. Further contextualization of the contributions and impact of publications was achieved through analyses of institutions (Section 3.3), journals (Section 3.4), authors (Section 3.5), and documents (Section 3.6) provide further context for understanding the contributions and impact of publications in this field. These approaches provide valuable insights into the quality and relevance of the research while accounting for variations in citation practices across different disciplines.
Country/region analysis
Geographic distribution of publications by country/region
The global distribution of DOAC research, mapped across 345 papers from 45 countries (Fig. 3A), highlights North America, Europe, and East Asia as primary contributors, likely due to more robust research funding, frequent clinical trials, and widespread clinical adoption of DOACs. In contrast, regions such as South America, Central America, and Africa show limited research activity, potentially reflecting resource limitations or lower use of these medications in clinical practice.
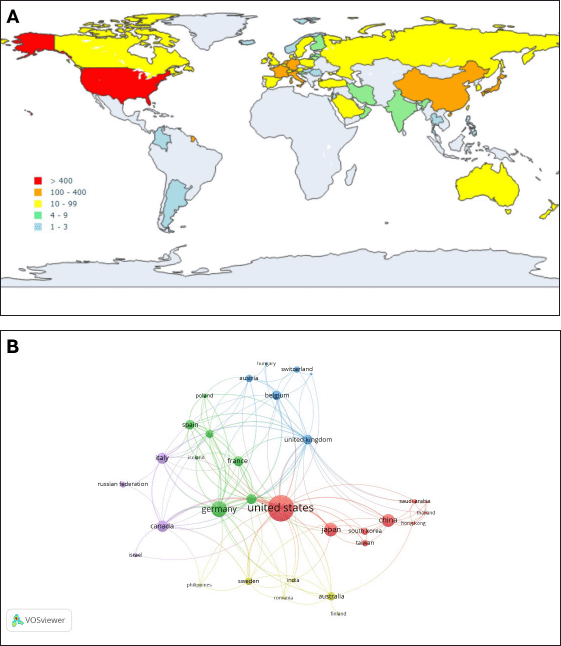 | Figure 3. (A) Geographic distribution of the total number of publications (counted per author) in different countries and regions. The map chart, based on data from Scopus, was generated using Plotly.express Python libraries in Google Colab. (B) Visualization of country cooperation. The network visualization map, based on data from Scopus, was created using VOSviewer to visualize co-authorship and inter-country collaborations. The analysis settings were as follows: Normalization (Method: Association strength), Layout (Attraction: 2, Repulsion: 0), Clustering (Resolution: 1, Minimum cluster size: 4), and Rotate/flip (Degrees to rotate: 90). Each node represents a country, and its size shows its contribution to the network. The edges indicate cooperative links, and their thickness represents the strength of the collaboration. [Click here to view] |
Visualization of country collaborations
The network visualization of country collaboration (Fig. 3B) highlights the United States as the most influential and central country, displaying the largest node size and the highest number of connections. This suggests its dominant role in global research collaborations on drug interactions with DOACs. The United Kingdom, Germany, France, and China also exhibit strong connectivity, acting as key hubs that facilitate international cooperation.
Countries are categorized into clusters of distinct colors. Germany, France, the Netherlands, and Spain create a highly interconnected cluster that showcases their strong scientific collaboration. Similarly, China, Japan, South Korea, and Taiwan are closely linked and reflect a regional research network. Canada shows strong connections with the United States and Germany, reinforcing its global research presence.
The visualization effectively captures the global distribution of research efforts. North America and Europe serve as primary research hubs, while Asia and other regions maintain significant but more regionally focused collaborations.
Table 3 presents the top 10 countries contributing to research on drug interactions with DOACs, highlighting TP, TC, citation impact (TC/TP), total link strength (TLS), and the publication year start (PY_start). The United States leads with the highest number of publications (116, 33.62%), citations (4162), and collaboration strength (TLS = 90), demonstrating its dominant role in the field. Germany follows with 44 publications (12.75%), the highest citation impact (TC/TP = 53.23), and strong research connectivity (TLS = 65). Canada exhibits notable citation impact (TC/TP = 46.45) despite a lower publication count (22, 6.38%). Japan (30, 8.70%) and China (28, 8.12%) also contribute significantly but with comparatively lower citation impact. The United Kingdom (TLS = 43) and the Netherlands (TLS = 34) play crucial roles in international research networks. Most leading countries initiated research in 2011, except for France (2013), the Netherlands (2014), the United Kingdom (2015), and Italy (2016).
These results highlight the United States, Germany, and Canada as key contributors, with Germany and Canada excelling in citation impact. At the same time, the United Kingdom and the Netherlands reinforce global collaboration.
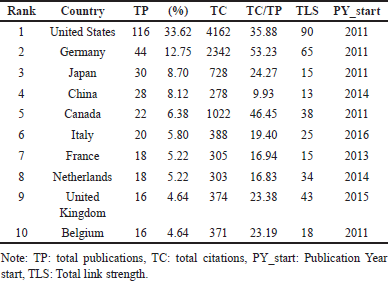 | Table 3. Top 10 countries and regions with the highest production. [Click here to view] |
Network metrics of country collaborations
Figure 4A illustrates that the United Kingdom possesses the highest betweenness centrality (0.0918), (Fig. 6) positioning it as a crucial intermediary in global interactions—the United States (0.0895) closely trails, (Fig. 6A) reinforcing its strategic influence. France (0.0534), Australia (0.0419), and China (0.0356) also serve essential bridging roles, facilitating connections between various regions and improving network efficiency. Figure 4B illustrates that the United States has the highest degree of centrality (24), indicating its extensive (Fig. 6B) direct connections. The United Kingdom closely follows with a score of 21, emphasizing its strong global ties. Similarly, Germany (17), the Netherlands (16), and Canada (12) rank among the top locations for degree centrality, demonstrating their active involvement in international collaborations. In terms of accessibility within the network, Figure 4C shows that the United States (0.5748) ranks highest in closeness (Fig. 6C) centrality, demonstrating its ability to reach other entities quickly. The United Kingdom (0.5327) follows, reinforcing its influence. Germany (0.4854), the Netherlands (0.4748), Canada, and Italy (0.4283) maintain strong positions, ensuring efficient communication and resource flow.
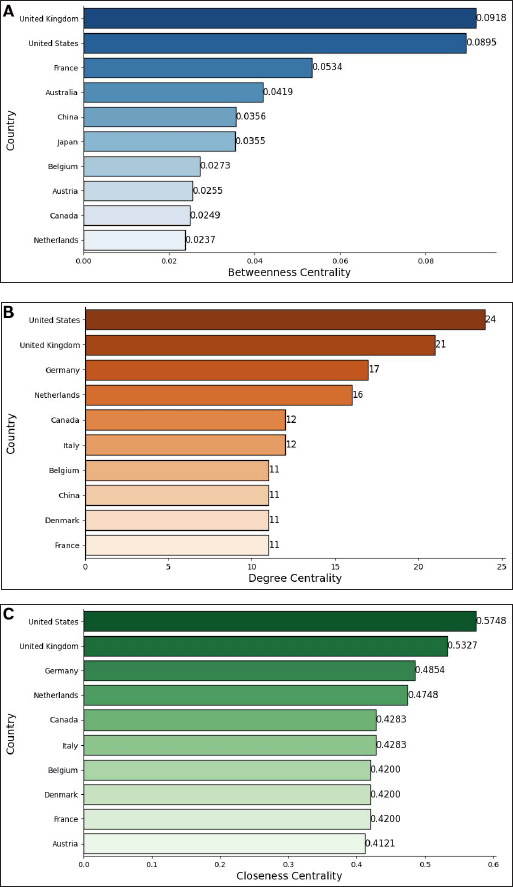 | Figure 4. Network metrics of country collaborations: (A) Betweenness centrality. (B) Degree centrality. (C) Closeness centrality. The graphs, based on data from Scopus, were created using Networkx and Matplotlib.pyplot Python libraries in Google Colab. [Click here to view] |
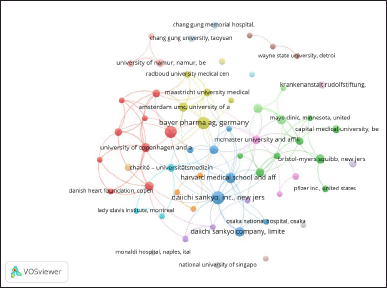 | Figure 5. Network visualization map of institutions Co-authorship analysis. The network visualization map, based on data from Scopus, was created using VOSviewer to visualize co-authorship among institutions. The analysis settings were as follows: Normalization (Method: Association strength), Layout (Attraction: 4, Repulsion: -3), Clustering (Resolution: 1, Minimum cluster size: 3), and Rotate/flip (Degrees to rotate: 90). Each node represents an institution, and its size corresponds to that institution’s contribution to the network. The edges indicate collaborative relationships between institutions, and their thickness reflects the strength of the collaboration. [Click here to view] |
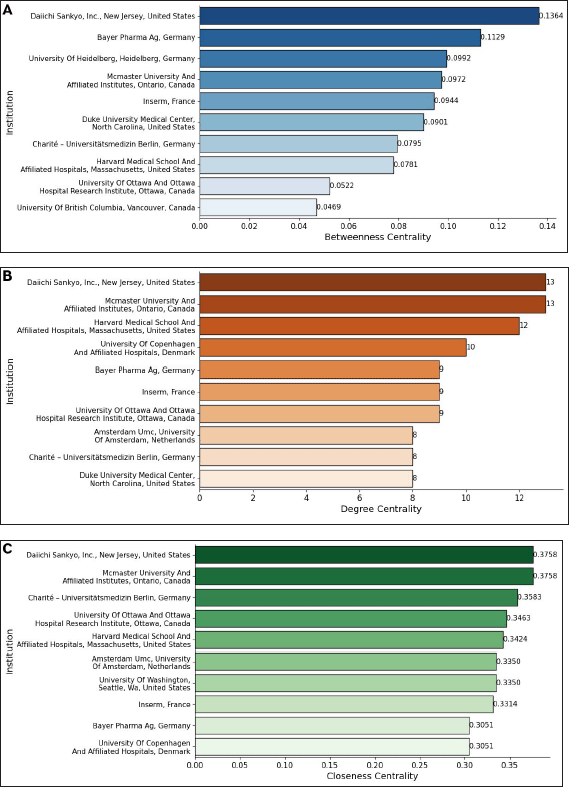 | Figure 6. Network metrics of institutions co-authorship: (A) Betweenness centrality. (B) Degree centrality. (C) Closeness centrality. The graphs, based on data from Scopus, were created using Networkx and Matplotlib.pyplot Python libraries in Google Colab. [Click here to view] |
The United States and the United Kingdom emerged as dominant global actors in DOAC research, leading across all centrality measures and reinforcing their pivotal role in international collaborations. The United States shows the highest research output, with 116 articles and 4162 citations, reflecting its strong influence on drug interaction with DOAC studies. The United Kingdom, Germany, and the Netherlands also maintain significant positions, sustaining network connectivity and knowledge dissemination. The betweenness centrality of France, Australia, and China suggests that these countries serve as key intermediaries, bridging different research hubs. Meanwhile, Canada and Italy ensure stable accessibility, contributing to efficient global exchanges. These findings align with the focus of research efforts in North America and Europe, where high DOAC usage and significant funding for cardiovascular research propel scientific advancements. The structural hubs identified in this analysis underscore the interconnected nature of global DOAC research, influencing international collaboration and the flow of knowledge.
Institutional analysis
Institutional co-authorship network analysis
The institutional co-authorship analysis identified 61 institutions researching drug interactions with DOACs, of which 44 formed 11 international and interdisciplinary collaborative clusters (Fig. 5). Cluster 1 (red) is led by Inserm (France), with 12 publications and 232 citations, and includes European institutions such as the University of Copenhagen and Aalborg University (Denmark), establishing a strong academic network. Cluster 2 (green) is distinguished by the involvement of Boehringer Ingelheim International GmbH (Germany), with eight publications and 1115 citations, along with key pharmaceutical industry players such as Boehringer Ingelheim Pharmaceuticals, Inc. (USA), Bristol–Myers Squibb, and Pfizer Inc. (USA), suggesting a focus on translational and clinical research. Cluster 3 (blue) is dominated by Daiichi Sankyo, Inc. (USA), with 14 publications and 843 citations, and Harvard Medical School (USA), with nine publications and 679 citations. This group includes institutions such as Daiichi Sankyo Company, Limited (Japan), and the University of Ottawa and Ottawa Hospital Research Institute (Canada), reflecting strong North American–Asian collaboration. Finally, Cluster 4 (yellow) is centered around Bayer Pharma AG (Germany), with 13 publications and 711 citations. It is closely linked to Amsterdam UMC and Maastricht University Medical Center (Netherlands), highlighting a European research focus on DOAC pharmacodynamics and pharmacokinetics.
According to Table 4, Boehringer Ingelheim Pharmaceuticals, Inc. (United States) has the highest TC/TP ratio (150.57), followed closely by Boehringer Ingelheim International GmbH (Germany) (139.38) and McMaster University and Affiliated Institutes (Canada) (136.33), indicating their strong citation impact per publication. Bristol–Myers Squibb (United States) (87) and Harvard Medical School and Affiliated Hospitals (United States) (75.44) also have a significant influence in the field. In terms of TLS, Daiichi Sankyo, Inc. (United States) leads with 24, followed by the University of Copenhagen and Affiliated Hospitals (Denmark) (21), McMaster University and Affiliated Institutes (Canada) (20), and Harvard Medical School and Affiliated Hospitals (United States) (17), highlighting their extensive collaborative networks. These results align with the institutional co-authorship analysis in Figure 5, in which Daiichi Sankyo, Harvard Medical School, and Boehringer Ingelheim were also identified as key players.
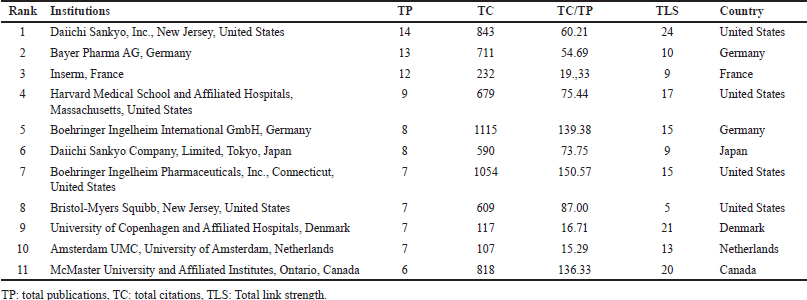 | Table 4. Major research institutions and number of publications. [Click here to view] |
Institutional bibliographic coupling network analysis
Supplementary Figure S1 presents the results of the institutional bibliographic coupling analysis, emphasizing the structural relationships among research institutions. The network consists of 42 institutions grouped into six clusters, with 587 links and a TLS of 4264. Among the most influential institutions, Harvard Medical School and Affiliated Hospitals (United States) show the highest TLS (1724), followed by Daiichi Sankyo, Inc. (United States) with 1505, University of Copenhagen and Affiliated Hospitals (Denmark) with 1456, Utrecht University and University Medical Center Utrecht (Netherlands) with 1365, and McMaster University and Affiliated Institutes (Canada) with 1272. These metrics indicate a well-connected network where key institutions display strong bibliographic coupling, reflecting shared research interests and collaborative efforts.
Network metrics of institutions collaborations
The analysis of institutional centrality in the research network reveals significant variations across betweenness, degree, and closeness centrality metrics (Fig. 6). Betweenness centrality (Fig. 6A), which measures an institution’s role as an intermediary in the network, is notably high for Daiichi Sankyo, Inc. (United States) with 0.1363, Bayer Pharma AG (Germany) with 0.1129, and the University of Heidelberg (Germany) with 0.0992. Their ranks indicate their strategic position in linking other institutions. Degree centrality (Fig. 6B), reflecting the number of direct collaborations, ranks Daiichi Sankyo, Inc. (United States) with 13, McMaster University and Affiliated Institutes (Canada) with 13, and Harvard Medical School and Affiliated Hospitals (United States) with 12, among the most connected. Their positions suggest that these institutions are highly engaged in direct research collaborations. Closeness centrality (Fig. 6C), which assesses how quickly an institution can reach others in the network, is highest for Daiichi Sankyo, Inc. (United States) with 0.3758, McMaster University and Affiliated Institutes (Canada) with 0.3758, and Charité—Universitätsmedizin Berlin (Germany) with 0.3583. Their positions indicate efficient access to the entire network, reinforcing their influence.
The relationship between pharmaceutical companies and universities shows a collaborative framework that conjugates scientific innovation with clinical practice application. These partnerships will likely contribute to a strong knowledge of DOAC safety and efficacy, as well as drug interactions.
Journal analysis
The journal analysis indicates that research on drug interactions with DOAC has been published in several prominent journals. As shown in Table 5, the British Journal of Clinical Pharmacology leads in publication volume, with 25 articles and an h-index of 14, demonstrating its significant influence in the field. This journal has accumulated 1,391 citations, achieving a TC/TP ratio of 55.64, emphasizing its strong impact on clinical pharmacology research. The Journal of Thrombosis and Thrombolysis follows with 17 publications, an h-index of 9, and a total of 281 citations, resulting in a TC/TP ratio of 16.53. Similarly, the European Journal of Clinical Pharmacology has 10 publications, an h-index of 7, and 173 total citations, with a TC/TP ratio of 17.30. These journals exhibit a notable presence in DOAC research, contributing relevant studies to the field. The Journal of Clinical Pharmacology and the Journal of Thrombosis Research share the same h-index (6) and publication count (8) but differ significantly in total citations: 288 and 105, respectively. Their TC/TP ratios are 36 and 13.3, respectively. Notably, the Journal of Thrombosis and Haemostasis, despite ranking seventh in publication count (7 articles), demonstrates the highest TC/TP ratio at 137.43, an h-index of 7, and has accumulated 962 citations. This underscores the journal’s influence and the substantial impact of select studies published in it. The CiteScore metric also highlights key differences in journal impact. The Journal of Thrombosis and Haemostasis stands out with the highest CiteScore (138), followed by the Journal of Thrombosis and Thrombolysis (18.20) and Thrombosis and Haemostasis (16.50).
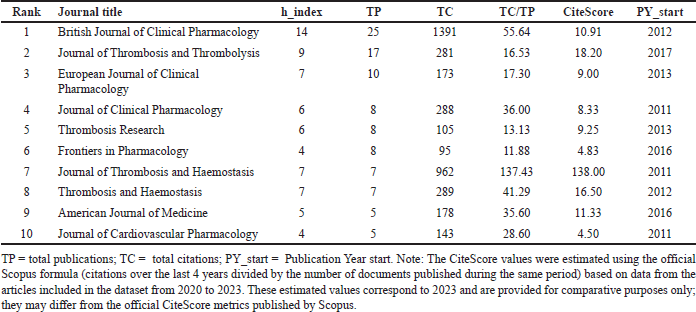 | Table 5. Top 10 journals in terms of publications. [Click here to view] |
These results indicate that while some journals excel in publication volume, others exhibit a disproportionately high citation impact. This suggests that fewer yet highly influential studies play a significant role in the field. The British Journal of Clinical Pharmacology consistently reflects its ongoing contribution to DOAC research, whereas the Journal of Thrombosis and Haemostasis demonstrates a more selective but profoundly impactful approach.
Author analysis
Author co-authorship network analysis
Among the 2085 authors who have contributed to research on DOAC interactions, 55 key authors meet Price’s Law (at least three publications), forming 11 collaboration clusters (Fig. 7). The visualization in VOSviewer reveals the collaborative structure among researchers in the field of DOAC interactions. Several co-authorship clusters stand out, including Cluster 1 (red): Primarily made up of authors from Comenius University in Slovakia, this group focuses on the interactions between DOACs and proton pump inhibitors as well as statins in patients with atrial fibrillation. The group consists of eight authors; key authors here include Bolek Tomáš, Galajda Pedro, and Kubisz Peter [41–44]. Cluster 2 (green): This represents researchers from Ghent University, Belgium, who focus on managing DOAC pharmacological interactions in outpatients with atrial fibrillation. Key authors here include Koen Boussery, Andreas Capiau, and Els Mehuys, who maintain strong interconnections with multiple researchers [45–48]. Cluster 3 (blue): Includes researchers from Bristol–Myers Squibb, a pharmaceutical company with a strong presence in pharmacokinetic and pharmacodynamic studies of apixaban. Notable authors include Wang Jessie, Song Yan, and Lacreta Frank, who hold multiple connections within the network [49–54].
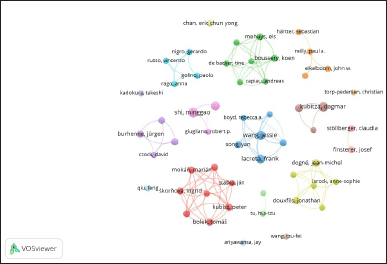 | Figure 7. Network visualization map of authors Co-authorship analysis. The network visualization map, based on data from Scopus, was created using VOSviewer to visualize co-authorship among authors. The analysis settings were as follows: Normalization (Method: Association strength), Layout (Attraction: 4, Repulsion: -2), Clustering (Resolution: 1, Minimum cluster size: 3), and Rotate/flip (Degrees to rotate: 90). Each node corresponds to an author, with the node size reflecting the number of publications or contributions. The edges between nodes indicate co-authorship, with their thickness representing the strength of the collaboration. [Click here to view] |
Table 6 provides an overview of the most productive authors in the field of drug interactions with DOAC based on their TP, TC, TC/TP ratio, TLS in the co-authorship network, and PY_start. Kubitza Dagmar (Germany) leads the ranking with seven publications, 620 citations, and a TC/TP ratio of 88.57. Her research, which began in 2012, has been pivotal in understanding the pharmacokinetics and DDIs of DOACs. Shi Minggao (United States) has also contributed seven publications, with 522 citations and a TC/TP ratio of 74.57 since 2011. His research has focused on DOAC metabolism and its clinical applications. Lacreta Frank and Wang Jessie (United States) each have six publications with 597 citations, resulting in a TC/TP ratio of 99.50. Their work, which began in 2012, has significantly influenced the clinical development of apixaban, as evidenced by their strong collaboration links (TLS = 14) within the network. Mendell Jeanne (United States) follows closely, with six publications, 519 citations, and a TC/TP ratio of 86.50. Since 2011, her studies have emphasized drug interactions with edoxaban.
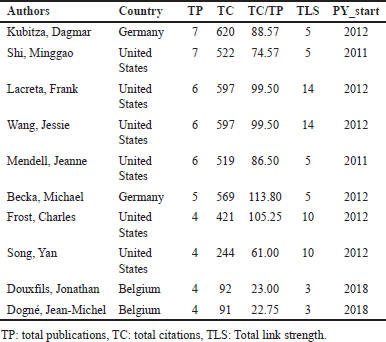 | Table 6. Top 10 most productive authors. [Click here to view] |
This underscores the prominence of North American and European researchers in advancing DOAC interaction studies.
Author bibliographic coupling network analysis
Supplementary Figure S2 presents the network visualization map of the authors’ bibliographic coupling analysis. This analysis highlights the interconnections between authors based on shared references, emphasizing the structure and collaboration patterns within the field of research on drug interactions with DOACs. The author bibliographic coupling network features 55 authors interconnected by 1300 links, with a total link strength of 18860, highlighting the extensive intellectual relationships within research on drug interactions with DOACs. The network is organized into five distinct clusters, each representing research communities with closely related thematic focuses. The high number of links and strong coupling strength indicate a well-established field with frequent cross-referencing among researchers, reflecting shared research interests and collaborative efforts. This structure underscores the significant interdisciplinary engagement and the evolution of scientific knowledge in the study of DOAC interactions, fostering advancements in pharmacokinetics, clinical applications, and safety assessments.
Network metrics of authors’ collaborations
The analysis of author collaboration networks provides key insights into the structural characteristics of knowledge dissemination in the field. Figure 8 presents three centrality measures: betweenness, degree, and closeness centrality, highlighting the most influential authors within the network. The top three authors with the highest betweenness centrality (Fig. 8A) were Grimonprez Maxim (0.0015), Larousse Lies (0.0015), and Reilly Paul A. (0.0014), indicating their crucial role in connecting different research groups. Other authors, such as Boyd Rebecca A., Byon Wonkyung, Frost Charles, and Giugliano Robert P., had moderate values (0.0007), suggesting a less central but still significant position. Meanwhile, De Backer Tine and Van Tongelen Inge exhibited the lowest betweenness centrality (0.0003), indicating a more peripheral role. These results suggest that a few key authors act as main connectors, while most researchers play a less central role in the network structure. The highest degree of centrality (7) was observed for Bolek Tomáš, Galajda Peter, Kubisz Peter, Moká?, Marián, Samoš Matej, Stan?iaková Lucia, Staško Ján, and Škor?ová Ingrid, indicating their extensive collaboration with multiple researchers. In contrast, Boussery, Koen, and Capiau, Andreas, had a slightly lower degree of centrality (6), suggesting that they maintain a high but slightly fewer number of direct co-authorship connections. These results highlight the presence of a core group of highly connected researchers, fostering strong collaborative interactions within the network. The highest closeness centrality value (0.1296) was shared by Bolek Tomáš, Galajda Peter, Kubisz Peter, Moká? Marián, Samoš Matej, Stan?iaková Lucia, Staško Ján, and Škor?ová Ingrid, suggesting that they are centrally positioned within the network and can quickly connect with other authors. In contrast, Boussery Koen and Capiau Andreas had a slightly lower closeness centrality (0.1111), meaning they are still well-connected but may require more intermediaries to reach other researchers. These findings emphasize the central role of the top-ranking authors in facilitating collaboration and knowledge exchange.
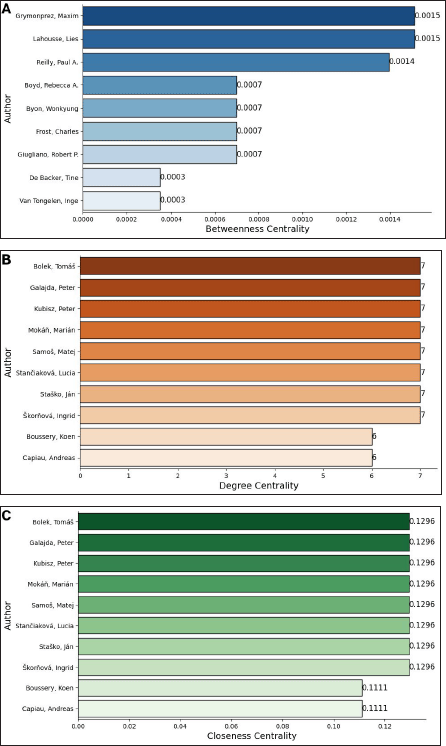 | Figure 8. Network metrics of authors co-authorship: (A) Betweenness centrality. (B) Degree centrality. (C) Closeness centrality. The graphs, based on data from Scopus, were created using Networkx and Matplotlib.pyplot Python libraries in Google Colab [Click here to view] |
Documents analysis
Citation analysis of key influential research on drug interactions with DOACs
Table 7 highlights the top 10 most highly cited papers on DOAC interactions, reflecting their significant role in advancing clinical understanding and safe use of DOACs [55–64]. The study by Dans et al. [55], published in Circulation, has the highest citation count (427). This pivotal work investigates the concomitant use of antiplatelet therapy with dabigatran or warfarin in patients undergoing long-term anticoagulation therapy (RE-LY trial). It presents essential insights into the safety and efficacy of combination therapy. This work underscores the complexities of managing DOAC interactions with commonly prescribed medications [55]. McBane et al. [56] (Journal of Thrombosis and Haemostasis, 390 citations) compared apixaban and dalteparin in CAT. Among 300 patients, 74% were receiving concurrent chemotherapy. Apixaban showed lower VTE recurrence (0.7% vs. 6.3%, p = 0.0281) without increasing major bleeding (0% vs. 1.4%) [56]. Mueck et al. [57], in the Journal of Clinical Pharmacology (322 citations), evaluates the co-administration of rivaroxaban with drugs that share its elimination pathways, analyzing pharmacokinetic effects in healthy individuals [57]. Frost et al. [51], in the British Journal of Clinical Pharmacology (301 citations), focuses on apixaban’s single-dose safety, pharmacokinetics, pharmacodynamics, and food interactions, providing foundational pharmacological data for clinical decision-making [51]. Liesenfeld et al. [58] (Journal of Thrombosis and Haemostasis, 287 citations) investigated the population pharmacokinetics of dabigatran etexilate in nonvalvular atrial fibrillation, offering deeper insights into its metabolism and elimination. The study revealed that co-administration with PPIs, amiodarone, and verapamil significantly impacted its bioavailability [58].
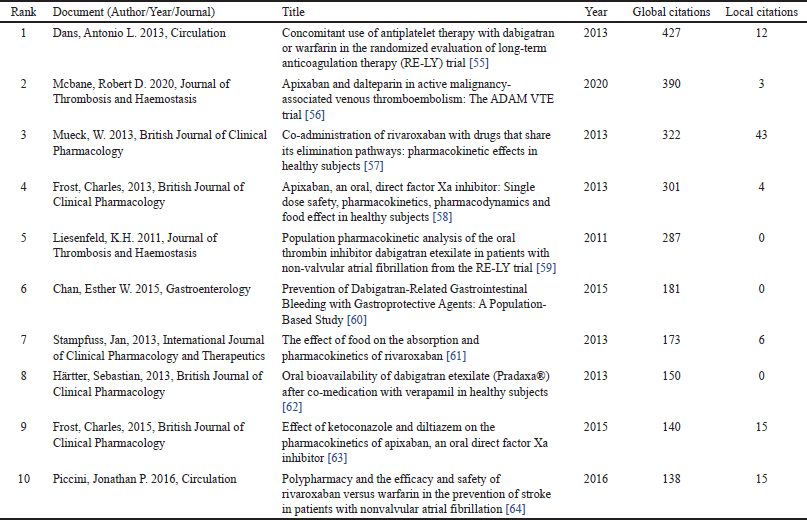 | Table 7. Top 10 highly cited papers. [Click here to view] |
Relevant insights into DOAC pharmacokinetics, pharmacodynamics, and interactions with other drugs are highlighted, emphasizing their significance in researching complex clinical interactions. The high citation counts of these papers underscore their role in shaping clinical guidelines and informing safe DOAC use in complex scenarios, such as CAT and polypharmacy [55–61,53,62].
Drug interactions of DOACs in the context of COVID-19
Recent research trends have highlighted specific areas of critical clinical concern regarding DOAC interactions. The five most-cited articles analyzing DOAC interactions within the context of COVID-19 were identified (Supplementary Table S1), reflecting key pharmacokinetic and clinical considerations during the pandemic [63–67].
The most highly cited study, by Testa et al. [63] in the Journal of Thrombosis and Haemostasis (129 citations), explores the impact of antiviral therapies on DOAC plasma levels in severe COVID-19 cases. The findings underscore a notable increase in anticoagulant concentrations, likely due to interactions affecting DOAC metabolism. This raises concerns about elevated bleeding risk in these patients. This study played a pivotal role in guiding anticoagulation management during the early phases of the pandemic [63]. The study by Fröhlich et al. [64] in Clinical Research in Cardiology (43 citations) investigates the impact of concomitant long-term medication, with a focus on ACE inhibitors and oral anticoagulation, on clinical outcomes in patients hospitalized with COVID-19. Potere et al. [65], in the Journal of Thrombosis and Thrombolysis (9 citations), investigate the effect of corticosteroids on DOAC plasma levels in COVID-19 patients. Given the widespread use of dexamethasone in severe cases, this study highlights the pharmacokinetic interactions that may alter DOAC efficacy and safety, emphasizing the need for dose adjustments or alternative anticoagulation strategies [65].
Collectively, these studies highlight the complexity of DOAC management in COVID-19 patients, particularly concerning antiviral agents, corticosteroids, and inflammatory-mediated pharmacokinetic changes. The emerging evidence underscores the necessity of individualized anticoagulation strategies that balance thrombotic protection with bleeding risk in this high-risk population [63–67].
PBPK modeling in DOAC drug interactions research
Another critical area is PBPK modeling for evaluating DDIs with DOACs, which has gained traction as a precision tool in recent years. This approach integrates physiological, biochemical, and drug-specific parameters to predict complex interactions in various patient populations. The five most-cited studies on PBPK modeling were identified (Supplementary Table S2), reflecting the increasing role of computational pharmacokinetics in optimizing anticoagulant therapy [68–72]. The most highly cited study, by Zhao and Hu. [70] in the British Journal of Pharmacology (34 citations), explores P-glycoprotein-mediated DDIs involving dabigatran etexilate. The authors validate in vitro and in vivo PBPK models to accurately predict transport-mediated interactions, demonstrating how computational modeling can enhance the risk assessment of DOAC co-administration with P-glycoprotein inhibitors or inducers [68]. The study by Xu et al. [69] in the European Journal of Clinical Pharmacology (25 citations) expands the PBPK application to both drug–drug and drug–disease interactions with rivaroxaban. Their findings highlight the impact of hepatic and renal impairment on rivaroxaban pharmacokinetics, stressing the importance of PBPK modeling in tailoring anticoagulation therapy for patients with comorbid conditions [69]. Ismail et al. [70], in the Journal of Clinical Pharmacology (22 citations), use minimal PBPK modeling to explore the interaction of rivaroxaban with verapamil in healthy and really impaired individuals. Their study provides valuable insights into dose adjustments required to mitigate the risk of bleeding complications in patients with altered drug clearance [70].
Together, these studies illustrate the growing role of PBPK modeling in predicting DOAC pharmacokinetics and interactions, particularly in patients with renal/hepatic dysfunction or concomitant medications affecting drug transport and metabolism. The increasing reliance on computational modeling underscores its potential in enhancing clinical decision-making, minimizing risks, and optimizing anticoagulation therapy [68–72].
Drug interactions of DOACs in oncology
The five most-cited studies on DOAC interactions in oncology highlight the challenges of managing anticoagulation in cancer patients, where DDIs with anticancer therapies can significantly alter anticoagulant efficacy and safety (Supplementary Table S3). These studies provide crucial insights into pharmacokinetic and pharmacodynamic interactions, bleeding risks, and clinical outcomes in this complex patient population. [56,57,73–75]. The most highly cited study, by McBane et al. [56] in the Journal of Thrombosis and Haemostasis (390 citations), focuses on the ADAM VTE trial, which evaluated apixaban versus dalteparin in patients with malignancy-associated VTE (see section 3.6.1).
A pharmacokinetic perspective is provided by Mueck et al. [57] in the British Journal of Clinical Pharmacology (322 citations). This study investigates the co-administration of rivaroxaban with drugs that share its metabolic and elimination pathways (see section 3.6.1). The study by Verso et al. [73] in the European Journal of Cancer (43 citations) evaluates the effects of concomitant administration of anticancer agents with apixaban or dalteparin on recurrence and bleeding risks in patients with cancer-associated VTE. Their results provide clinically relevant insights into optimizing DOAC use in patients receiving chemotherapy or immunotherapy, where bleeding risk is a critical concern [73].
Collectively, these studies reinforce the importance of individualizing DOAC therapy in oncology, accounting for drug interactions, genetic variability, and disease-specific factors to enhance safety and efficacy in anticoagulation management [56,57,73–75].
Regional perspectives on drug interactions with DOACs
In the local context, a 2016 study published in the Revista Colombiana de Cardiología by Machado-Alba et al. [76] offers important insights into DOAC prescription patterns and their economic impact in Colombia. The study found that 77.7% of patients were taking concurrent medications, leading to potential interaction risks with DOACs, particularly with antiarrhythmics, antihypertensives, lipid-lowering agents, and anti-ulcer drugs. A high-risk cardiovascular group (7.6%) was identified because of the concomitant use of these specific drug classes. The study also emphasized an increased bleeding risk when rivaroxaban or dabigatran was taken with P-glycoprotein inhibitors such as verapamil and amiodarone, highlighting the need for careful management [76].
Case reports on drug–herb interactions with DOACs
A 2019 case report by Maadarani et al. [77] in the European Journal of Case Reports in Internal Medicine underscores the severe risks associated with drug–herb interactions involving DOACs. This report describes an 80-year-old patient with non-valvular atrial fibrillation on dabigatran who experienced fatal gastrointestinal bleeding after consuming a mixture of ginger and cinnamon. The herbs likely enhanced the anticoagulant effects of dabigatran, leading to uncontrollable bleeding despite intensive resuscitation measures. This case highlights the critical need for patient awareness and medical guidance regarding drug–herb interactions with DOACs, as such combinations can pose significant, sometimes fatal, risks [77].
Keyword analysis
Overview of keyword selection and primary themes
The keyword co-occurrence analysis in this study identified 428 relevant terms. Of the initial set of 471 keywords meeting the minimum threshold of five occurrences, 43 were excluded because they were irrelevant or overly general. This refined selection provides a focused representation of key research themes in DOAC interactions. Table 8 presents the top 30 most frequently occurring keywords, revealing critical areas of interest. The most common terms include “Anticoagulant” (n = 244), “Rivaroxaban” (n = 193), “Atrial fibrillation” (n = 180), “Major clinical study” (n = 178), and “Drug interaction” (n = 161). These keywords highlight the central role of DOACs in contemporary research, with particular emphasis on their clinical use, safety, and interaction profiles. A deeper look at Degree Centrality (DC) and TLS further elucidates keyword relevance. “Anticoagulant” (DC = 427, TLS = 6295) and “Rivaroxaban” (DC = 425, TLS = 4954) exhibit the highest degree of centrality, highlighting their critical role within the network. Other terms, such as “Drug interaction” (DC = 419, TLS = 4264) and “Atrial fibrillation” (DC = 413, TLS = 4668), also display significant connectivity, reflecting their integration across various research fields. The pharmacokinetic and pharmacodynamic aspects of DOAC interactions are evident in terms such as “Pharmacokinetics” (n = 63, DC = 329, TLS = 1576), “Drug-blood level” (n = 69, DC = 352, TLS = 1800), and “Factor Xa inhibitor” (n = 75, DC = 372, TLS = 2017). These keywords emphasize the molecular mechanisms underlying drug interactions, particularly those involving metabolic pathways and blood concentration levels. Finally, “Amiodarone” (n = 63, DC = 355, TLS = 1863) and “Drug–drug interaction” (n = 63, DC = 348, TLS = 1418) highlight frequent co-prescription scenarios and their implications for DOAC metabolism and effectiveness. The inclusion of “Venous thromboembolism” (n = 61, DC = 355, TLS = 1635) and “Gastrointestinal hemorrhage” (n = 56, DC = 319, TLS = 1613) suggests a growing focus on DOAC use beyond atrial fibrillation, particularly in conditions requiring long-term anticoagulation. The degree of centrality and connectivity of these keywords further reinforce their scientific relevance, guiding future research directions in the field.
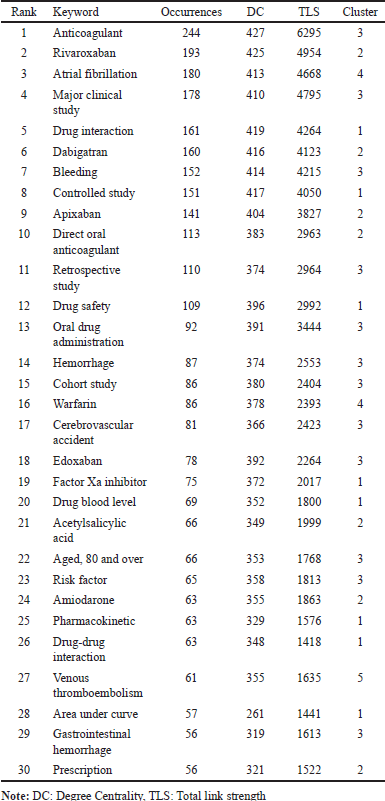 | Table 8. Top 30 keywords most frequent occurrences. [Click here to view] |
Clustered thematic keyword co-occurrence analysis
The clustered co-occurrence map (Fig. 9A) organizes keywords into five distinct thematic clusters, each representing a key research focus within DOAC-related drug interactions. Cluster 1 (red): DDIs and Pharmacokinetic Parameters of DOACs; this cluster, centered around “Drug interaction” (n = 161), emphasizes the pharmacokinetic mechanisms underlying DOAC interactions. Key terms such as “Drug safety” (n = 109), “Drug blood level” (n = 69), “CYP3A4” (n = 41), and “P-glycoprotein” (n = 27) indicate a strong emphasis on metabolic pathways and transporter-mediated interactions. The presence of “Area under curve” (n = 57), “Maximum plasma concentration” (n = 36), “Metabolism” (n = 34), and “Drug elimination” (n = 13) suggests ongoing research into how DOAC plasma levels are affected by inhibitors and inducers of CYP3A4 and P-glycoprotein. These findings highlight the significance of therapeutic drug monitoring in optimizing anticoagulation therapy and reducing adverse events. Cluster 2 (green): DDIs of DOACs: Impact of CYP3A4 and P-glycoprotein Inhibitors and Inducers; centered around “Rivaroxaban” (n = 193), this cluster analyzes enzyme-mediated interactions. The notable co-occurrence of “Amiodarone” (n = 63), “Verapamil” (n = 40), “Diltiazem” (n = 31), “Carbamazepine” (n = 21), and “Fluconazole” (n = 21) underscores clinically significant DDIs, especially those influencing DOAC clearance. Cluster 3 (blue): Risk of Bleeding, Comorbidities, and DDIs of DOACs with Concurrent Medications; this is the largest and most interconnected cluster, dominated by “Anticoagulant” (n = 244) and “Major clinical study” (n = 178), reflecting broad research on DOAC pharmacodynamics. The strong connectivity of “Bleeding” (n = 152), “Warfarin” (n = 86), and “Hemorrhage” (n = 87) underscores comparative effectiveness studies and the safety profile of DOACs versus vitamin K antagonists. The presence of “Hypertension” (n = 65), “Diabetes mellitus” (n = 49), and “Chronic kidney disease” (n = 47) suggests that comorbidities significantly influence DOAC interactions. Cluster 4 (yellow): Clinical Monitoring and DDIs of DOACs in Patients with Comorbidities and Concurrent Medications; with “Atrial fibrillation” (n = 180) as the leading keyword, this cluster highlights the clinical application of DOACs. The presence of “Cohort study” (n = 86), “Randomized controlled trial” (n = 45), and “Case report” (n = 48) indicates that evidence-based medicine plays a pivotal role in DOAC research. Cluster 5 (purple): DDIs in Oncology Patients on DOAC Therapy; this cluster, centered on “Venous thromboembolism” (61 occurrences), highlights the unique challenges of DOAC use in oncology.
The five thematic clusters identified in Figure 9A illustrate a multidimensional research landscape for DOAC drug interactions. While pharmacokinetics (Clusters 1 and 2) and clinical safety (Clusters 3 and 4) remain predominant themes, emerging domains such as oncology-related DDIs (Cluster 5) emphasize new frontiers in anticoagulation research. The high centrality of “Anticoagulant,” “Rivaroxaban,” and “Drug interaction” highlights their crucial role in shaping future investigations. These findings offer valuable insights for optimizing DOAC therapy across diverse clinical situations, especially in high-risk and polypharmacy populations.
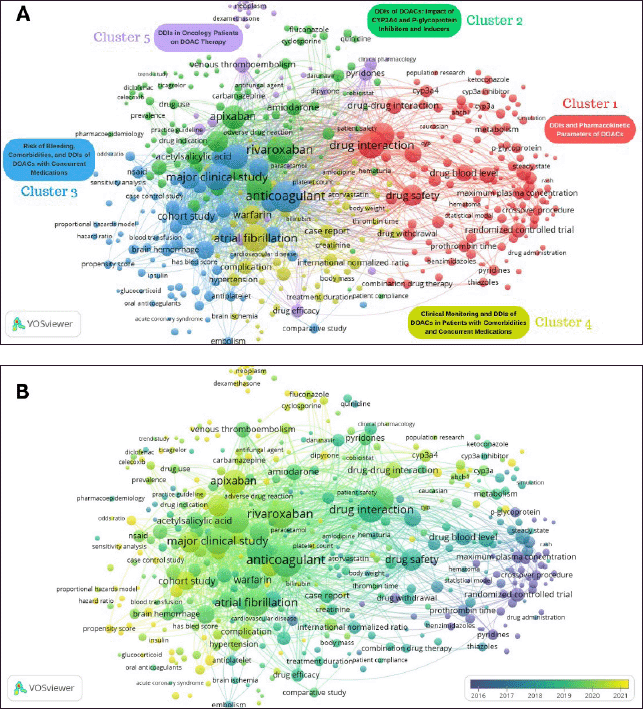 | Figure 9. Keywords analysis for research of drug interactions of DOACs: (A) Clustering co-occurrence map of keywords. The network visualization map, based on data from Scopus, was created using VOSviewer to visualize keyword co-occurrence and additional cluster labels were added for better interpretation. (B) Distribution of keywords based on the average time of appearance. The network visualization map, based on data from Scopus, was created using VOSviewer to illustrate the temporal distribution of keywords across the publications. In both Figure A and B, the analysis settings were as follows: Normalization (Method: Association strength), Layout (Attraction: 1, Repulsion: 0), Clustering (Resolution: 1, Minimum cluster size: 35), and Rotate/flip (Degrees to rotate: 90). Each node represents keywords, with node size indicating the frequency of occurrence in the dataset. The edges connect keywords that co-occur in the same publications, with line thickness reflecting the strength of their association. [Click here to view] |
Evolution of research trends through keyword analysis
Figure 9 B depicts the evolution of research interests, illustrating how drug interaction studies related to DOACs have evolved. The distribution of keywords based on their average time of appearance highlights a progressive shift towards pharmacokinetic modeling, clinical safety, and DDIs with newly introduced therapeutic agents. Keywords such as “ABC transporter subfamily B,” “Clinical outcomes,” and “PBPK model” reflect a growing emphasis on pharmacokinetic precision and computational modeling in recent studies. These trends indicate a shift toward mechanistic understanding and predictive modeling of DOAC interactions, especially aimed at enhancing clinical decision-making and personalized medicine strategies. The co-occurrence of keywords related to anticancer therapies suggests a rising concern regarding DOAC interactions in oncology patients. Specific anticancer agents, including imatinib, doxorubicin, and bicalutamide, have gained research attention due to their potential modulation of CYP3A4 and P-glycoprotein pathways, which significantly affect DOAC metabolism and bioavailability. Furthermore, antifungal agents such as ketoconazole and fluconazole emerge as common themes, reinforcing their established role as strong CYP3A4 inhibitors that may elevate DOAC plasma concentrations and the risk of bleeding. This is in line with the increasing evidence that calls for careful monitoring of DOAC therapy in patients who require long-term antifungal prophylaxis. Moreover, macrolide antibiotics such as azithromycin and clarithromycin emerge as key contributors to pharmacokinetic DDIs, particularly relevant in the context of COVID-19 treatments, where co-administration with DOACs required careful risk-benefit assessments due to their effects on CYP3A4 and P-glycoprotein activity. The presence of keywords related to chronic kidney disease and renal impairment highlights the ongoing challenge of optimizing DOAC use in patients with multiple comorbidities. Given that renal clearance plays a crucial role in DOAC elimination, dose adjustments, and therapeutic monitoring remain key areas of research to prevent thromboembolic and bleeding complications in high-risk populations. In addition, corticosteroids such as dexamethasone and prednisone, as well as enzyme inducers such as phenobarbital and carbamazepine, are attracting attention for their ability to modify DOAC pharmacokinetics through CYP3A4 induction, which may diminish anticoagulant efficacy and raise thrombotic risk.
Overall, Figure 9B illustrates a progressive evolution in DOAC drug interaction research. It transitions from general safety concerns to highly specialized pharmacokinetic and clinical outcome-driven studies, reflecting the growing complexity of anticoagulation management in diverse patient populations.
Keyword density visualization
Supplementary Figure S3 presents a density visualization of keyword analysis for research on drug interactions of DOACs. This representation intuitively explains the prominence, concentration, and interconnectedness of keywords, highlighting dominant research themes and emerging topics within the field of DOAC drug interactions. The densest clusters of keywords revolve around rivaroxaban, apixaban, warfarin, anticoagulant, atrial fibrillation, and drug interaction, indicating that these topics form the core focus of DOAC research. The strong concentration of major clinical studies, cohort studies, and drug safety reinforces the emphasis on real-world evidence, clinical efficacy, and safety profiles of DOACs in various patient populations. A significant cluster is observed around CYP3A4, p-glycoprotein, and DDI, underscoring the pharmacokinetic mechanisms driving DOAC metabolism and clearance. Metabolism-related keywords such as CYP3A inhibitor, maximum plasma concentration, and drug blood level suggest an increasing focus on DOAC plasma level monitoring and individualized dosing strategies.
The density visualization in Supplementary Figure S3 highlights the interconnected nature of DOAC research, emphasizing drug–drug interactions, clinical safety, and metabolic pathways.
FUTURE PERSPECTIVES
The observed trends suggest that future research will likely continue focusing on personalized DOAC therapy using PBPK models and real-world clinical data, refining anticoagulant strategies for patients receiving cardiovascular, antifungal, antiviral, and oncologic treatments, addressing complex DDIs involving CYP3A4/P-glycoprotein inhibitors and inducers, and developing new risk stratification tools to optimize DOAC use in renal impairment.
LIMITATIONS
To the best of our knowledge, this is the first bibliometric analysis to extensively map and analyze research on drug interactions involving DOACs using the Scopus database. Unlike traditional narrative reviews, this study employed multiple bibliometric tools for performance analysis, science mapping, and network analysis (visualization), which enhanced the clarity, impact, and objectivity of the findings. Our study provides valuable insights into the trends, key research areas, and evolving focus of DOAC interaction research from 2011 to 2023, thereby offering a comprehensive view of the development of this field. However, some limitations must be acknowledged. First, this analysis was confined to the Scopus database, which, while extensive, may overlook relevant studies indexed in other databases, such as Web of Science and PubMed. Consequently, certain influential publications might have been omitted, potentially affecting the comprehensiveness of our findings.
In addition, a major limitation of bibliometric analyses is the absence of a formal quality assessment for the studies included. Although this method facilitates a broad and objective mapping of research trends, it does not assess the methodological rigor, risk of bias, or overall quality of the selected individual studies.
Future research could build on this work by incorporating multiple databases to create a more diverse and comprehensive dataset. This would deepen our understanding of drug interactions with DOACs and reveal additional insights into regional research trends. Furthermore, to enhance the validity of findings, bibliometric analyses should be complemented by systematic reviews that integrate established quality assessment frameworks. This combined approach would strengthen the interpretation of data on drug interactions with DOACs, ensuring that clinical and pharmacological conclusions are grounded not only in citation impact but also in methodological rigor and evidence quality.
CONCLUSION
This bibliometric analysis provides a comprehensive overview of landscape research on drug interactions involving DOACs. It reveals a significant increase in publications and key findings for future research and application in clinical practice and public health. Developed countries and institutions across robust international collaborations have contributed to the development and evolution of the field, highlighting the critical role of partnerships in expanding knowledge on the safety and efficacy of DOACs for the management of drug interactions. By mapping trends and focal points in the research, this analysis offers valuable insights to guide future investigations and support improvements in clinical outcomes for patients receiving DOACs. Emerging research directions, such as the application of PBPK modeling and exploration of interactions in oncology and COVID-19 treatment, underscore the complex challenges of managing DDIs in patients with polypharmacy.
ACKNOWLEDGMENTS
The authors of this study acknowledge Ing. Oscar Oviedo for providing technical advice concerning the data retrieval strategy and processing.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FINANCIAL SUPPORT
There is no funding to report.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects; thus, it does not require formal approval from the Ethics Committee.
DATA AVAILABILITY
All the data are available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. SPS - Specialist Pharmacy Service. Understanding drug interactions [Internet]. London, UK: NHS Specialist Pharmacy Service; 2024 [cited 2024 Jul 20]. Available from: https://www.sps.nhs.uk/articles/understanding-drug-interactions/
2. Neves LMB, Castro Silva LD, De Melo MTB, Silva Nobre YV, Paulino ET, Nogueira Ribeiro ÊA, et al. Drug interactions pharmacology: a narrative review. Am J Pharmacol Toxicol. 2022;17(1):27–36. CrossRef
3. Corrie K, Hardman JG. Mechanisms of drug interactions: pharmacodynamics and pharmacokinetics. Anaesth Intensive Care Med. 2020;21(5):219–22. CrossRef
4. Jeong E, Malin B, Nelson SD, Su Y, Li L, Chen Y. Revealing the dynamic landscape of drug-drug interactions through network analysis. Front Pharmacol. 2023;14:1211491. CrossRef
5. Jarosz M, Wolanicka K. Relations between occurrence of the risk of food-drug interactions and patients’ socio-demographic characteristics and selected nutrition habits. Pol J Food Nutr Sci. 2011;61(3):211–8. CrossRef
6. Degefu N, Getachew M, Amare F. Knowledge of drug–food interactions among healthcare professionals working in Public Hospitals in Ethiopia. J Multidiscip Healthc. 2022;Volume 15:2635–45. CrossRef
7. Dülger G. Herbal drugs and drug interactions. MARMARA Pharm J. 2012;1(16):9–22. CrossRef
8. Lopera V, Rodríguez A, Amariles P. Clinical relevance of drug interactions with Cannabis: a systematic review. J Clin Med. 2022;11(5):1154. CrossRef
9. Forbes HL, Polasek TM. Potential drug–drug interactions with direct oral anticoagulants in elderly hospitalized patients. Ther Adv Drug Saf. 2017;8(10):319–28. CrossRef
10. Decaix T, Kemache K, Gay P, Ketz F, Laprévote O, Pautas É. Pharmacokinetics and pharmacodynamics of drug?drug interactions in hospitalized older adults treated with direct oral anticoagulants. Aging Clin Exp Res. 2024;36(1):113. CrossRef
11. Sanborn D, Sugrue A, Amin M, Mehta R, Farwati M, Deshmukh AJ, et al. Outcomes of direct oral anticoagulants co-prescribed with common interacting medications. Am J Cardiol. 2022;162:80–5. CrossRef
12. Wang TF, Hill M, Mallick R, Chaudry H, Unachukwu U, Delluc A, et al. The prevalence of relevant drug-drug interactions and associated clinical outcomes in patients with cancer-associated thrombosis on concurrent anticoagulation and anticancer or supportive care therapies. Thromb Res. 2023;231:128–34. CrossRef
13. Houben A, Bonhomme V, Senard M. Clinical use of direct oral anticoagulants and reversal: consideration for vascular surgeons. J Vasc Dis. 2023;2(2):230–5. CrossRef
14. Khairani CD, Bejjani A, Assi A, Porio N, Talasaz AH, Piazza G, et al. Direct oral anticoagulants for treatment of venous thrombosis: illustrated review of appropriate use. Res Pract Thromb Haemost. 2024;8(4):102424. CrossRef
15. Darche FF, Fabricius LC, Helmschrott M, Rahm AK, Ehlermann P, Bruckner T, et al. Oral anticoagulants after heart transplantation—comparison between vitamin K antagonists and direct oral anticoagulants. J Clin Med. 2023;12(13):4334. CrossRef
16. Hellfritzsch M, Henriksen JN, Holt MI, Grove EL. Drug–drug interactions in the treatment of cancer-associated venous thromboembolism with direct oral anticoagulants. Semin Thromb Hemost. 2024;50(03):489–98. CrossRef
17. Van Der Linden L, Vanassche T, Van Cutsem E, Van Aelst L, Verhamme P. Pharmacokinetic drug–drug interactions with direct anticoagulants in the management of cancer-associated thrombosis. Br J Clin Pharmacol. 2023;89(8):2369–76. CrossRef
18. Tsoukalas N, Brito-Dellan N, Font C, Butler T, Rojas-Hernandez CM, Butler T, et al. Complexity and clinical significance of drug–drug interactions (DDIs) in oncology: challenging issues in the care of patients regarding cancer-associated thrombosis (CAT). Support Care Cancer. 2022;30(10):8559–73. CrossRef
19. Ranzato F, Roberti R, Deluca C, Carta M, Peretti A, Polo D, et al. Pilot study on the probability of drug-drug interactions among direct oral anticoagulants (DOACs) and antiseizure medications (ASMs): a clinical perspective. Neurol Sci. 2024;45(1):277–88. CrossRef
20. Ersoy ?, Ersoy P. Impact of drug interactions with direct oral anticoagulants on mortality in elderly with atrial fibrillation during the COVID-19 pandemic. Med Clín. 2023;160(2):71–7. CrossRef
21. Dou M, Tang J, Tiwari P, Ding Y, Guo F. Drug–drug interaction relation extraction based on deep learning: a review. ACM Comput Surv. 2024;56(6):1–33. CrossRef
22. Pranckut? R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications. 2021;9(1):12. CrossRef
23. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. 2021;133:285–96. CrossRef
24. Mian MK, Sreedharan S, Limaye NS, Hogan C, Darvall JN. Research trends in anticoagulation therapy over the last 25 years. Semin Thromb Hemost. 2020;46(08):919–31. CrossRef
25. Öztürk O, Kocaman R, Kanbach DK. How to design bibliometric research: an overview and a framework proposal. Rev Manag Sci. 2024;18(11):3333–61. CrossRef
26. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22(2):338–42. CrossRef
27. Mongeon P, Paul-Hus A. The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics. 2016;106(1):213–28. CrossRef
28. Martín-Martín A, Orduna-Malea E, Thelwall M, Delgado López-Cózar E. Google Scholar, Web of Science, and Scopus: a systematic comparison of citations in 252 subject categories. J Informetr. 2018;12(4):1160–77. CrossRef
29. Yao R, Shen Z, Xu X, Ling G, Xiang R, Song T, et al. Knowledge mapping of graph neural networks for drug discovery: a bibliometric and visualized analysis. Front Pharmacol. 2024;15:1393415. CrossRef
30. Al-Taani GM, Al-Azzam SI, Alzoubi KH, Sweileh WM, Muflih S. Polypharmacy in the elderly: a bibliometric and visualization analysis. Electron J Gen Med. 2024;21(1):em555. CrossRef
31. Datablist App [Internet]. Datablist.com. [cited 2024 Aug 6]. Available from https://app.datablist.com/home
32. Shanaa A. Rayyan – intelligent systematic review [Internet]. Doha, QA: Rayyan Systems; 2021 [cited 2024 Aug 6]. Available from: https://www.rayyan.ai/
33. OpenAI. ChatGPT [Internet]. 2023 [cited 2024 Sep 1]. Available from: https://openai.com
34. Syriani E, David I, Kumar G. Screening articles for systematic reviews with ChatGPT. J Comput Lang. 2024;80:101287. CrossRef
35. Issaiy M, Ghanaati H, Kolahi S, Shakiba M, Jalali AH, Zarei D, et al. Methodological insights into ChatGPT’s screening performance in systematic reviews. BMC Med Res Methodol. 2024;24(1):78. CrossRef
36. Spillias S, Tuohy P, Andreotta M, Annand-Jones R, Boschetti F, Cvitanovic C, et al. Human-AI collaboration to identify literature for evidence synthesis. Cell Rep Sustain. 2024;1(7):100132. CrossRef
37. Wei L, Zhang B, Wang L, Liu A. Mapping the evolution of acne research based on 100 top-cited articles: a bibliometric analysis of trends and hotspots from 2014 to 2023. Medicine (Baltimore). 2024;103(21):e37657. CrossRef
38. Colab.Google [Internet]. colab.google. [cited 2024 Sep 8]. Available from https://colab.google/
39. Gálvez C. Evolution of the field of social media research through science Maps (2008-2017). Commun Soc. 2019;32(2):61–76. CrossRef
40. Us PW. CiteScore hub [Internet]. www.elsevier.com. [cited 2024 Sep 8]. Available from: https://www.elsevier.com/promotions/citescore-hub-explore-journal-rankings-track-trends-compare-performance-predict-directions
41. Bolek T, Samoš M, Stan?iaková L, Ivanková J, Škor?ová I, Staško J, et al. The impact of proton pump inhibition on dabigatran levels in patients with atrial fibrillation. Am J Ther. 2019;26(3):e308–13. CrossRef
42. Bolek T, Samoš M, Škor?ová I, Stan?iaková L, Staško J, Korpallová B, et al. Does proton pump inhibition change the on-treatment anti-Xa activity in xabans-treated patients with atrial fibrillation? a pilot study. J Thromb Thrombolysis. 2019;47(1):140–5. CrossRef
43. Schnierer M, Samoš M, Bolek T, Škor?ová I, Nosáková L, Bánov?in P, et al. The effect of proton pump inhibitor withdrawal on dabigatran etexilate plasma levels in patients with atrial fibrillation: a washout study. J Cardiovasc Pharmacol. 2020;75(4):333–5. CrossRef
44. Škor?ová I, Samoš M, Bolek T, Stan?iaková L, Vádelová ?, Galajda P, et al. Does atorvastatin therapy change the anti-Xa activity in xabans-treated patients with atrial fibrillation? Pharmacol Res Perspect. 2021;9(3):e00730. CrossRef
45. Grymonprez M, Carnoy L, Capiau A, Boussery K, Mehuys E, De Backer TL, et al. Impact of P-glycoprotein and CYP3A4-interacting drugs on clinical outcomes in patients with atrial fibrillation using non-vitamin K antagonist oral anticoagulants: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. 2023;9(8):722–30. CrossRef
46. Capiau A, Mehuys E, De Bolle L, Van Tongelen I, De Backer T, Boussery K. Drug–drug interactions with direct oral anticoagulants: development of a consensus list for ambulatory care. Int J Clin Pharm. 2023;45(2):364–74. CrossRef
47. Mehuys E, De Backer T, De Keyser F, Christiaens T, Van Hees T, Demarche S, et al. Prevalence and management of drug interactions between nonsteroidal anti-inflammatory drugs and antithrombotics in ambulatory care. Br J Clin Pharmacol. 2022;88(8):3896–902. CrossRef
48. Capiau A, De Backer T, Grymonprez M, Lahousse L, Van Tongelen I, Mehuys E, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation according to the drug labelling and the EHRA Practical Guide. Int J Cardiol. 2021;328:97–103. CrossRef
49. Barrett Y, Wang J, Song Y, Pursley J, Wastall P, Wright R, et al. A randomised assessment of the pharmacokinetic, pharmacodynamic and safety interaction between apixaban and enoxaparin in healthy subjects. Thromb Haemost. 2012;107(05):916–24. CrossRef
50. Upreti VV, Song Y, Wang J, Byon W, Boyd RA, Pursley JM, et al. Effect of famotidine on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Clin Pharmacol Adv Appl. 2013;5:59–66. CrossRef
51. Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, et al. Apixaban, an oral, direct factor X a inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476–87. CrossRef
52. Frost C, Shenker A, Gandhi MD, Pursley J, Barrett YC, Wang J, et al. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. Br J Clin Pharmacol. 2014;78(4):877–85. CrossRef
53. Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor X a inhibitor. Br J Clin Pharmacol. 2015;79(5):838–46. CrossRef
54. Frost C, Song Y, Yu Z, Wang J, Lee L, Schuster A, et al. The effect of apixaban on the pharmacokinetics of digoxin and atenolol in healthy subjects. Clin Pharmacol Adv Appl. 2017;Volume 9:19–28. CrossRef
55. Dans AL, Connolly SJ, Wallentin L, Yang S, Nakamya J, Brueckmann M, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation. 2013;127(5):634–40. CrossRef
56. McBane RD, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–21. CrossRef
57. Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–66. CrossRef
58. Liesenfeld K H., Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9(11):2168–75. CrossRef
59. Chan EW, Lau WCY, Leung WK, Mok MTC, He Y, Tong TSM, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586–95. CrossRef
60. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(07):549–61. CrossRef
61. Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate ( P radaxa® ) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75(4):1053–62. CrossRef
62. Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016;133(4):352–60. CrossRef
63. Testa S, Prandoni P, Paoletti O, Morandini R, Tala M, Dellanoce C, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320–3. CrossRef
64. Fröhlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, et al. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021;110(7):1041–50. CrossRef
65. Potere N, Candeloro M, Porreca E, Marinari S, Federici C, Auciello R, et al. Direct oral anticoagulant plasma levels in hospitalized COVID-19 patients treated with dexamethasone. J Thromb Thrombolysis. 2022;53(2):346–51. CrossRef
66. Launay M, Demartin AL, Ragey SP, Mismetti P, Botelho-Nevers E, Delavenne X. Severe inflammation, acute kidney injury, and drug–drug interaction: triple penalty for prolonged elimination of apixaban in patients with coronavirus disease 2019: a grand round. Ther Drug Monit. 2021;43(4):455–8. CrossRef
67. Kravchenko OV, Boyce RD, Gomez-Lumbreras A, Kocis PT, Villa Zapata L, Tan M, et al. Drug–drug interaction between dexamethasone and direct-acting oral anticoagulants: a nested case–control study in the National COVID Cohort Collaborative (N3C). BMJ Open. 2022;12(12):e066846. CrossRef
68. Zhao Y, Hu Z. Physiologically based pharmacokinetic modelling and in vivo [I]/ Ki accurately predict P -glycoprotein-mediated drug-drug interactions with dabigatran etexilate. Br J Pharmacol. 2014;171(4):1043–53. CrossRef
69. Xu R, Ge W, Jiang Q. Application of physiologically based pharmacokinetic modeling to the prediction of drug-drug and drug-disease interactions for rivaroxaban. Eur J Clin Pharmacol. 2018;74(6):755–65. CrossRef
70. Ismail M, Lee VH, Chow CR, Rubino CM. Minimal physiologically based pharmacokinetic and drug-drug-disease interaction model of rivaroxaban and verapamil in healthy and renally impaired subjects. J Clin Pharmacol. 2018;58(4):541–8. CrossRef
71. Kushwah V, Arora S, Tamás Katona M, Modhave D, Fröhlich E, Paudel A. On absorption modeling and food effect prediction of rivaroxaban, a BCS II drug orally administered as an immediate-release tablet. Pharmaceutics. 2021;13(2):283. CrossRef
72. Willmann S, Coboeken K, Kapsa S, Thelen K, Mundhenke M, Fischer K, et al. Applications of physiologically based pharmacokinetic modeling of rivaroxaban—renal and hepatic impairment and drug-drug interaction potential. J Clin Pharmacol. 2021;61(5):656–65. CrossRef
73. Verso M, Munoz A, Bauersachs R, Huisman MV, Mandalà M, Vescovo G, et al. Effects of concomitant administration of anticancer agents and apixaban or dalteparin on recurrence and bleeding in patients with cancer-associated venous thromboembolism. Eur J Cancer. 2021;148:371–81. CrossRef
74. Gulilat M, Keller D, Linton B, Pananos AD, Lizotte D, Dresser GK, et al. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis. 2020;49(2):294–303. CrossRef
75. Wang T, Baumann Kreuziger L, Leader A, Spectre G, Lim MY, Gahagan A, et al. Characteristics and outcomes of patients on concurrent direct oral anticoagulants and targeted anticancer therapies—TacDOAC registry: communication from the ISTH SSC subcommittee on hemostasis and malignancy. J Thromb Haemost. 2021;19(8):2068–81. CrossRef
76. Machado-Alba JE, García-Betancur S, Villegas-Cardona F, Medina-Morales DA. Patrones de prescripción de los nuevos anticoagulantes orales y sus costos económicos en Colombia. Rev Colomb Cardiol. 2016;23(4):277–85. CrossRef
77. Maadarani O, Bitar Z, Mohsen M. Adding herbal products to direct-acting oral anticoagulants can be fatal. Eur J Case Rep Intern Med. 2019;6(8):1. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4611_pdf.pdf]