INTRODUCTION
Long-term alcohol drinking is now accepted as a serious worldwide medical, economic, and social problem [1]. Excessive alcohol exposure can alter persistence in brain structure and functionality which results in memory decline [2]. Scientific evidence reports that the neurotoxicity of chronic alcohol consumption affects on the cholinergic neuron’s degeneration in the hippocampus, basal forebrain, and cortex [3],[4].
Acetylcholine (ACh) is a main neurotransmitter secreted by cholinergic neurons and it plays a crucial role in cognitive performance. Increasing the levels of acetylcholinesterase (AChE) enzyme activity led to learning and memory impairment [5]. Chronic ethanol intake results in an increase in AChE activity and reduces ACh release in the hippocampus [6]. Therefore, acetylcholinesterase inhibitors can slow the progression of memory decline in cognitive disorders via the enhancement of cholinergic synapses which improve cognition processes [7].
From the available scientific studies, it is evident that chronic alcohol consumption leads to the enhancement of oxidative stress results in neuronal damage [8],[9]. Ethanol metabolism induces oxidative stress by increasing free radical formation while decreasing the level of antioxidant enzyme activities in the brain [10].
Currently, medicines to treat memory deficit and protect brain oxidative damage in alcoholism are less available. Thus, natural antioxidant therapeutic agents have gained increasing attention worldwide [11].
The seed of fruit is the waste product from the household kitchen and fruit industrial processing is an important contributor to generating millions of tons of waste every day [12]. However, several published studies have demonstrated that fruit seed waste is rich in phytoactive substances with functional ingredients, particularly phenolic compounds, with potent antioxidant properties [13,14]. Furthermore, additional supports are found in a recent study that reported many fruit seeds such as Washingtonia ?lifera palm fruit, chia, and grape exhibit AChE activity [15–17]. Therefore, fruit seeds with antioxidant and AChE inhibitory activities may be in treating, protecting, or recovering cognitive deficits and memory decline in alcoholism.
Lychee (Litchi chinensis Sonn.) is a subtropical fruit tree member of the Sapindaceae family, which originated in China; it is widely located in tropical zones including the northern part of Thailand such as Chiang Mai, Chiang Rai, and Phayao [18]. Research findings reveal that L. chinensis seed contains various phytochemical constituents such as catechin, procyanidin A1, procyanidin A2, saponin, quercetin, fatty acid, organic acids, amino acids, volatile oils, and flavonoids [19,20]. In traditional Chinese medicine practices, L. chinensis seed was always used for relieving neuralgic pain, polydipsia, hernia, orchitis, ulcers, fever, and cough [21]. In addition, it is claimed to have multiple pharmacological properties such as anti-hyperglycemia, anti-hyperlipidemia, antivirus, antitumor, and antioxidation effects [22–27]. Moreover, it has been reported to ameliorate d-galactose-induced learning and memory deficits in mice [28].
Based on the antioxidant, neuroprotection activities and the various health promotion benefits of L. chinensis seed mentioned earlier; the present study set out to examine the effect of L. chinensis seed extract in the alleviation of ethanol-induced oxidative brain damage and memory impairment via its antioxidant and AChE inhibitory activities in rats.
MATERIALS AND METHODS
The LC seed extract preparation
The crude L. chinensis seed extract was prepared from the fresh ripe of L. chinensis fruits collected from Mae Jai District of Phayao province, Thailand, at the end of February 2024. The seeds of L. chinensis fruits were removed and washed completely in running tap water. Let L. chinensis seeds dry in a laboratory hot air oven at 60°C for 72 hours. The seeds dried samples were crushed with a grinder. Dried LC seeds powder with distilled water (1:5) was refluxed for 3 hours at 80°C–100°C, residues and distilled water (1:5) at 80°C–100°C for 2 hours to achieve an initial extract. The extracting solvent was filtered using filter paper after cooling to room temperature and concentrated with a vacuum rotary evaporator at a temperature of 40°C and freeze-dried using a lyophilizer (Labconco, MO, USA). The yield of seed extraction was determined as in the following formula: (weight of dried LC seed extract obtained / initial weight of dried LC seed sample) × 100%. Finally, a total of the extract was preserved at −20°C for further study in the experimental animals.
Measurement of total phenolic contents (TPCs) of L. chinensis seed extract
The TPC of the LC seed extract sample was measured according to the Folin-Ciocalteu method [29] with minor adaptations. In this case, gallic acid served as the standard, and the calibration curve was prepared at different concentrations (0.02–0.1 mg/ml). A 0.1 ml diluted seed extract solution was mixed with 2.5 ml 10% fresh Folin–Ciocalteu’s solution and incubated for 5 minutes at normal temperature. Add 0.75 ml 6% sodium carbonate, then incubate and let it rest in the dark room for 30 minutes. Detect the absorbance of the standard and seed extract sample at a wavelength of 765 nm and present as milligrams of gallic acids equivalents per gram based on dry weight (mg GAE/g DW).
Determination of the antioxidant activity of L. chinensis seed extract in vitro assays
DPPH (1,1-diphenyl-2-picrylhydrazyl) assay
This method aims to determine the capability of the extract to scavenge or reduce DPPH radicals using a spectrophotometer. The DPPH assay was measured according to Akowuah et al [30] with some minor adjustments. An aliquot of 0.1 ml of each seed extract solution was added and mixed in 0.9 ml of DPPH solution in methanol (0.1 mM) and incubated for 15 minutes in the dark room. Then, the absorbance was denoted at 517 nm using a spectrophotometer. In this case, ascorbic acid served as a standard. The capability of the extract to neutralize DPPH radicals was reported as IC50 DPPH values. IC50 indicates the concentration of extract required for inhibition of 50% of DPPH free radicals. The percentage of seed extract inhibition (I%) was determined by the formula:
FRAP (Ferric reducing antioxidant power) assay
The measurement of FRAP ability of the L. chinensis seed extract was performed using the previous method [31]. Freshly working FRAP solution was prepared, consisting of acetate buffer (300 mM, pH 5.6), 2,4, 6-Tripyridyl-s-triazine (TPTZ; 10 mM) in HCl (40 mM) and FeCl3 (20 mM). Mix 0.3 ml L. chinensis seed extract with 0.3 ml distilled water and 3 ml of the working FRAP solution by a vortex. The contents were incubated for 30 minutes before determining the absorbance at a wavelength of 595 nm. Trolox served as a standard solution to determine the antioxidant capability. The capability of the seed extract to scavenge free radicals was shown as mM Trolox equivalents per gram based on dry weight (TE/g DW).
Experimental animal studies
Forty male Wistar rats (8 weeks, weighing 220–250 g) were procured from Nomura Siam International Co, Ltd., based in Bangkok, Thailand. They were housed in polypropylene cages (size 10.5 ×19 × 18 inches, 2 rats/cage) at room temperature (25.0°C ± 2°C), relative humidity (55% ?± ?5%), with artificial illumination from 6.00 am to 6.00 pm., and led free access to a standard pellet diet and RO water ad libitum. After acclimatizing for 1 week, all animals were randomly separated into five groups (n = 8) as follows: Control group (alcohol-free group), ethanol (EtOH) group, Don + EtOH (donepezil; positive control group), L. chinensis seed extract 300 + EtOH, and L. chinensis seed extract 600 + EtOH. All rats in the control group were gavaged with reverse osmosis water, and the rats in the positive control or treatment groups were gavaged with donepezil at doses of 3 mg/kg BW or L. chinensis seed extract at doses of 300 and 600 mg/kg BW, respectively. All various substances were given to the experimental rodents once daily at the same time for 42 days.
An experimental model of alcohol-induced cognitive decline
The rodents of all groups except the control group received 5 ml of 25% EtOH (9.875 mg/kg BW) divided into two equal doses per day, 2.5 ml once in the morning and once in the afternoon [32] via a gastric tube for 42 days. Alcohol dependency was assessed the aggressive behaviors through the observation and tremor symptom using the stationary dowel test [33]. The rodent behavioral tests used in this experiment to evaluate the potency of L. chinensis seed extract on cognitive function in rats exposed to chronic alcohol consumption, comprised of two memory procedures performed in the following order: object recognition (ORT) and Morris water maze test (MWM). All behavioral tests were performed every 14 days of intervention and recorded by an unbiased observer.
Object recognition test
The ORT is a common behavioral task to assess non-spatial working memory states in rodents. In this study, the ORT was performed as described previously [34]. The tool consisted of a gray plastic box with the following dimensions: 54 cm × 90 cm × 50 cm. This test comprised of 2 sections such as a training and a testing section. In the training section, two identical similar objects (object A and object A) were located equidistant from each other. The rat was placed and led to explore the objects freely for 5 minutes and then taken back to its cage. The apparatus was wiped clean with a 90% alcohol solution between each trial to eliminate odor cues.
Twenty-four hours after the training section, the testing section was conducted. The rodents were reintroduced to the same process. However, one object was substituted by a new object (object A and object B). Each rat was led to survey the objects freely for 5 minutes. The collective time taken to survey objects was noted by a stopwatch. Object exploration was considered when the rat licked, touched the objects with its paws, or sniffed or its head was oriented towards the object at a distance ≤2 cm. The following considerations were noted: the cumulative time (in seconds) spent exploring the familiar object (Ta) and the cumulative time (in seconds) spent exploring the new object (Tb). Finally, the recognition index (RI) of each rodent was determined with the formula: RI = Tb/(Ta + Tb) [35].
Morris water maze test
The effect of L. chinensis seed extract on spatial learning and memory was further determined using the MWM. This test used was a minor modification of that described in our previous study [36]. A circular plastic pool (height 45 cm and diameter 150 cm) was divided into four quadrants located in a room with environmental cues visible from the inside of the pool. The pool was filled with cloudy water (powder solution) at room temperature (25°C). A circular platform (12.5 cm diameter) was placed at the center in one of the four quadrants and was submerged 2 cm under the opaque water surface. All animals were continuously trained to escape by swimming to the hidden platform for three trials per day (90 seconds/trial). The escape latency or the acquisition time (the time taken to climb onto the hidden platform) for each rat was noted and the cut-off acquisition time is 90 seconds. The retrieval memory was determined on the next day, in this case, the platform was removed from the pool, and each rat was released and led to swim to find the platform for 90 seconds. The time taken in the target quadrant where the platform was previously stationed was denoted as retention time.
Terminal procedures for brain biochemical analysis and oxidative markers
At the end of the experiment, all rodents were deeply anesthetized with a high dose of thiobutabarbital sodium (100 mg/kg, i.p) and transcranial perfused with cold normal saline. After perfusion, the whole brain of each rat was quickly removed and divided into left and right hemispheres. The right hippocampus and cortex of each rat were separated for AChE activity determination whereas the left hippocampus and cortex were used to evaluate the oxidative stress markers including superoxide dismutase (SOD), reduced glutathione (GSH), and malondialdehyde (MDA) levels.
Brain tissue homogenization
Each rat brain tissue (cortex and hippocampus) was weighted and homogenized in 0.1 M of ice-cold phosphate buffer saline (10% w/v, pH 7.4), and centrifuged for 60 minutes at 13,000 rpm at 4°C. The supernatant was collected and kept at −80°C for the determination of the oxidative markers and AChE activity.
Determination of SOD content in rats’ brain
The SOD activities in the cortex and hippocampus tissue were evaluated using a SOD commercial assay kit (Code No. 19160, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions. Results of SOD activity were calculated and expressed in percentage inhibition of WST-1 formazan formation.
Estimation of reduced GSH content in rats’ brain
The GSH concentration was evaluated according to the method previously described by Moron et al. [37] with certain adaptations. Each hippocampus/cortex homogenate supernatant was combined and mixed with 0.1 ml of trichloroacetic acid (TCA, 10%) and centrifuged at 5,000 g for 10 minutes. The upper layer of the supernatant was kept and added to phosphate buffer (0.2 M, pH 8) and 5,5-dinitrobis-2-nitrobenzoic acid (DTNB, 0.04%). GSH content was read at 412 nm and expressed as μmol/g tissue.
Evaluation of MDA content in rats’ brain
MDA, a major marker of lipid peroxidation, was evaluated as described previously [38] with minor changes. Mix 0.1 ml samples of hippocampus/cortex homogenate with the reagent solution consisting of 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid solution (pH 3.5), and 1.5 ml of 0.8% thiobarbituric acid. Heat a mixture solution at 95°C for 60 minutes. After cooling the contents under an ice-box package, the tubes were centrifuged at about 10,000 rpm for 5 minutes. The organic layer was aspirated and measured at 540 nm. In this case, 1,1,3,3-Tetramethoxypropane was served as an external standard. The MDA concentration was assessed using the standard curve and reported as nmol/mg protein.
Assessment of AChE activity in rats’ brain
The AChE enzyme activity in both cortex and hippocampus tissue was estimated spectrophotometrically according to the Ellman assay [39] with slight modification. Cortex or hippocampus homogenate (20%), phosphate buffer (0.1 M, pH 7.4), and 5,5′-dithio-bis-nitrobenzoic acid (DTNB, 1 mM) were mixed. The absorbance was denoted at 405 nm immediately after adding acetylthiocholine iodide (ATCI, 1 mM). Read the absorbance changing every 30-second intervals for 3 minutes. The AChE activity was expressed as μmol/minute/g tissue.
Statistical analysis
All results were presented as mean value ± standard error of the mean (SEM). Data analysis for the phytochemical content and antioxidant capacity data was performed using GraphPad Prism (version 9.0) and investigated by a paired t-test. Data analysis for the memory performance and neurochemical values was assessed through one-way analysis of variance followed by Dunnett’s test for multiple comparisons in GraphPad Prism (version 9.0). Statistical significance was accepted at p-value < 0.05.
RESULTS
The percentage yield, TPCs, and antioxidant activity of L. chinensis seed extract
In the first part of this study, the yield of the aqueous extract of L. chinensis seed was 28.06%. The TPCs (GAE acid equivalents, mg/g DW) of the extract was found to be 319.75 ± 5.11 whereas the IC50 value of DPPH radical scavenging activity was 4.03 ± 1.03 mg/ml. In addition, the extract also showed the FRAP value of 1.93 ± 0.05 mM TE/g DW. All results are presented in Table 1.
 | Table 1. TPCs and antioxidant capacities of the extract of L. chinensis seed. [Click here to view] |
Effects of LC seed extract on cognitive performance in rat model of alcoholism
The effect of L. chinensis extract on non-spatial working memory performance is shown in Table 2. At the beginning of the study, there were no significant differences in either the total exploration time or RI value among the groups. However, compared with the control group, the EtOH group showed a significant decrease (p < 0.05) in both parameters in the ORT test after 21 days of treatment. The pattern of cognitive decline continued throughout the study period. Interestingly, after 21 days, treatment with L. chinensis seed extract at doses of 300 and 600 mg significantly increased (p < 0.05) the total exploration time or RI value compared with the EtOH group; this difference also continued for the remainder of the study. There was a similar alteration in both parameters in rats treated with donepezil (p < 0.05), serving as a positive control.
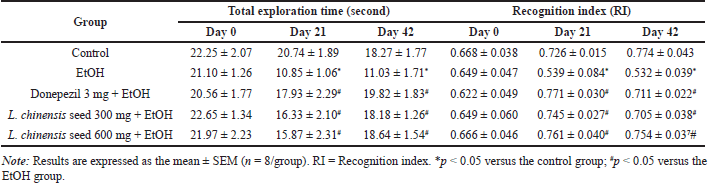 | Table 2. Effect of L. chinensis seed extract on the non-spatial memory of rats exposed to alcohol. [Click here to view] |
This study also assessed the effect of L. chinensis extract on spatial working memory in the MWT. Binge alcohol exposure significantly increased the escape latency time (p < 0.01) as compared to the control group. However, on day 21st of the experiment period, treatment with donepezil and seed extract at 600 mg significantly decreased (p < 0.01 and p < 0.05, respectively) the escape latency time when compared to the EtOH group. In addition, we found that both donepezil and the high dose of seed extract could significantly reduce (p < 0.001 and p < 0.01, respectively) the escape latency time of rats until the end of the study. Meanwhile, treatment with seed extract at 300 mg showed a significantly shorter (p < 0.05) escape latency compared with the EtOH group on day 42nd of the experiment protocol (Fig. 1A). Considering the retention trial, chronic alcohol exposure significantly showed a significantly shorter (p < 0.001) retention time compared with a control group. However, compared with the EtOH group, the rats treated with donepezil and the seed extract at 600 mg exhibited a significant increase (p < 0.001 all) in the retention time from the day 21st of the study protocol until the end of the experiment. Again, the rats treated with seed extract at 300 mg showed a significantly longer (p < 0.01) retention time compared with the EtOH group on day 42nd of the study period (Fig. 1B).
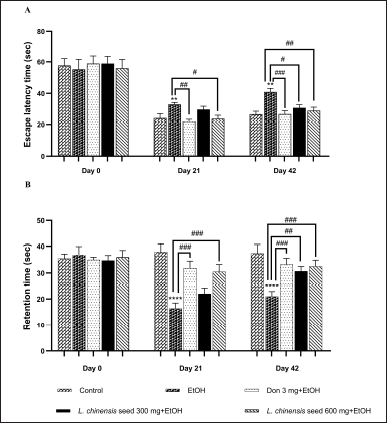 | Figure 1. Effect of L. chinensis seed extract on the spatial memory of rats exposed to alcohol: (A) Escape latency time and (B) Retention time. Values represent the mean ± SEM (n = 8/group). **p < 0.01, ****p < 0.001 versus the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus the EtOH group. [Click here to view] |
Notably, there were no significant differences seen in cognitive performance between both doses of the extract of seed in this experiment.
Effects of L. chinensis seed extract on brain antioxidant enzyme activities and lipid peroxidation
The efficacy of L. chinensis LC seed extract on the activities of antioxidant enzyme and lipid peroxidation in both the hippocampus and the cortex of chronic alcoholic rats were shown in Figures 2A and B. Chronic alcohol administration showed a significantly reduce (p < 0.01 all) the enzyme activity of SOD in both areas of rat’s brain when compared to the control group. However, oral administration of seed extract at 300 and 600 mg significantly attenuated SOD activity in both the hippocampus (p < 0.05) and the cortex (p < 0.01) compared to the EtOH-treated group. There was a similar alteration in SOD activity in both brain regions of rats treated with donepezil (p < 0.05 and p < 0.01, respectively). In addition, our result showed that prolonged alcohol exposure led to a significant reduction in GSH activity in both the hippocampus (p < 0.01) and the cortex (p < 0.001) compared to the control group. However, treatment with both doses of L. chinensis extract significantly restored (p < 0.01 all) the activity of GSH in both brain regions of rats compared to the EtOH group. Similarly, the GSH activity was elevated in both the hippocampus and the cortex of rats treated with donepezil (p < 0.01 and p < 0.05, respectively).
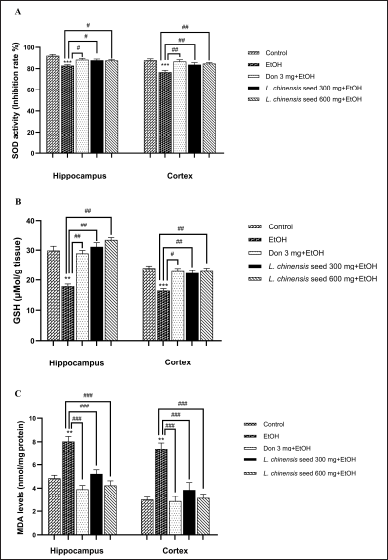 | Figure 2. Effect of L. chinensis seed extract on the brain antioxidant enzymes activities and lipid peroxidation of rats exposed to alcohol: (A) Superoxide dismutase (SOD) activity; (B) Glutathione (GSH) activity, and (C) Malondialdehyde (MDA). Values represent the mean ± SEM (n = 8/group). **p < 0.01, ***p < 0.001 versus the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus the EtOH group. [Click here to view] |
Furthermore, we found that MDA content in both the hippocampus and the cortex of rats exposed to alcohol was significantly higher (p < 0.01 all) than those in the control group. Again, treatment with donepezil, and seed extract at 300 and 600 mg resulted in a significant reduction (p < 0.01 all) in MDA content in both regions of the rat brain (Fig. 2C).
Effects of L. chinensis seed extract on brain AChE activity
As depicted in Figure 3, chronic alcohol exposure allowed a significant increase (p < 0.01 all) AChE activity in both the hippocampus and the cortex compared with the control group. Surprisingly, both doses of the L. chinensis seed extract and donepezil ameliorated this increase compared to the EtOH group (p < 0.01 all).
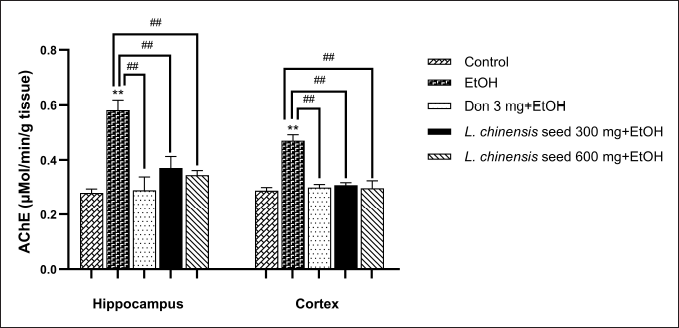 | Figure 3. Effect of L. chinensis seed extract on brain AChE activity of rats exposed to alcohol. Values represent the mean ± SEM (n = 8/ group). **p < 0.01 versus the control group; ##p < 0.01 versus the EtOH group. [Click here to view] |
DISCUSSION
A myriad of research has demonstrated that fruit seed waste is an abundant source of phytoactive compounds with strong antioxidant activity that may help in protecting against several diseases and delay memory decline in neurodegenerative disorders [40]. Additionally, with the rising public concerns about fruit waste production. Our study was conducted on Litchi chinensis Sonn. or L. chinensis seed extract examined its potential antioxidant and anticholinesterase activities in a rat model of alcoholism. Several screening methods were used such as the animal behavior tests together with analyzing the AChE levels and oxidative stress markers in brain tissue homogenates. The results of this study confirmed that L. chinensis seed extract supplementation reverses learning and memory impairment following chronic ethanol exposure by restoration of cholinergic function and mitigation of oxidative stress.
Chronic alcohol drinking can cause damage to virtually every organ, especially the brain results in cognitive decline [41]. The hippocampus is a critical region for cognitive formation, particularly for spatial working memory [42]. Meanwhile, the cortex, particularly the frontal cortex, plays a role in both spatial and non-spatial working memory [43]. Therefore, both spatial and non-spatial working memory impairment have been linked to excessive alcohol drinking [44].
In a recent study, the MWM procedure was performed to investigate the spatial learning ability and reference memories that are linked with hippocampal function. Meanwhile, ORT is a behavioral task to assess non-spatial working memory. In agreement with previous studies [45], our results showed that chronic alcohol consumption leads to worse memory performances in both animal behavior tests, denoted by a decrease in the retention latency but an increase in the escape latency period in MWT, and a reduction in the RI value in the ORT.
Several studies have reported that chronic alcohol administration is associated with the accumulation of ROS leads to dynamic cellular molecular damage such as lipid peroxidation, DNA damage, protein denaturation, and eventually neurodegeneration [11]. Moreover, excessive alcohol drinking results in the reduction of the endogenous antioxidant capacity [46]. Similarly to our results, the rats exposed to chronic alcohol administration showed a gradual loss of memory accompanied by reduced antioxidant enzyme activities and increased brain MDA concentrations in the rats’ brains.
Previous research addressed the relationship between the cholinergic system and the effects of prolonged ethanol exposure by focusing on AChE activity. These studies revealed that ethanol consumption led to the AChE activity enhancement in various regions of the brain [47,48]. Consistent with our findings in this study, the results showed that chronic alcohol consumption induced elevation in AChE activity in both the hippocampus and cortex of rat brain. Moreover, on general appearances observation, rats exposed to chronic alcohol had increased sensitivity to environmental stimuli, rest tremors, startle, increased hair ruffling and puffing, irritability, and hypervigilance like that reported in the previous studies [49]. Therefore, these results suggest that the model in this study to induce alcohol dependence is suitable for evaluating the effects of L. chinensis seed extract against memory deficit in alcoholic rats.
Various studies have proved that the mechanism of action of antioxidant compounds is linked to the modulation of several cell signaling pathways associated with learning and memory processes. Polyphenols and flavonoids help recover cognitive function by improving synaptic plasticity, inducing expression of genes encoding antioxidant enzymes, and up-regulation of neurotrophic factors by increasing cerebral blood circulation [50,51].
Epidemiological studies reveal that the pharmacological effects and antioxidant ability of a wide variety of fruit seeds could help protect and treat several diseases including the complications of chronic alcohol drinking [52,53]. However, up to date, no scientific reports on the cognitive enhancing effects of L. chinensis seed extract attenuate alcohol-induced memory decline in rats. Herein, both doses of seed extract possess potent antioxidants that help ameliorate the worse memory performance in animal models of alcoholism. Either low or high doses of the seed extract exhibited improved cognitive ability in rats exposed to chronic alcohol consumption, as evidenced by an increase in the retention latency and a reduction in the escape latency period in the MWT. In addition, both doses of the extract showed an increase in the RI value in the ORT. Results demonstrated that the extract of L. chinensis seed exerts a cognitive booster effect by restoring the endogenous antioxidant enzyme activities (SOD and GSH) as well as reduced brain MDA contents in chronic alcohol-exposed rats.
As is well known, there is a close correlation between TPC in natural phenolic compounds and antioxidant abilities [54]. Notably, the antioxidant capacity of L. chinensis seed extract in this study, together with the high TPC concentrations (Table 1). Our data are also consistent with prior research by Potisate and Pintha [55] that reveals the TPC of L. chinensis seed extract was 82.43 ± 12.93 mg GAE/g DW and IC50 value of DPPH radical scavenging activity was 1.66 ± 5.52 mg/ml. However, the differences between our results and the study as mentioned earlier, are probably due to variations in the species of lychee, climate zone, soil type, especially the methods of extraction.
A myriad of reports has revealed that the high phenolic content present in some fruit seeds could also possess AChE inhibitory activity [56,57]. Our findings agree with these previous studies as mentioned, herein, both doses of L. chinensis seed extract display significant anticholinesterase activities by decreasing the enzyme AChE in both cortex and hippocampus of ethanol-treated rats. As stated, the AChE enzyme is important in the breakdown of ACh which causes an increase in the ACh contents that are associated with improved learning and memory functions. Therefore, the AChE inhibitory activity shown by the L. chinensis seed extract in this study suggests that the extract is a potential source of active compounds which in turn stimulate an increase in the levels of ACh in the brain of ethanol-treated rats.
Notably, either donepezil or the seed extract could restore memory deficit induced by chronic ethanol consumption. Donepezil is a drug that increases the endogenous ACh concentration by inhibiting the AChE enzyme [58]. However, the L. chinensis seed sample that we used in this experiment is a crude extract containing various phytobiological active compounds with a wide spectrum of pharmacological activities. Therefore, the cognitive enhancing effect of the extract may occur via the additive and/or the synergistic interactions among different biologically active compounds.
Taken together, by a combination of animal behavioral tests and neurobiochemical analysis, this study demonstrates that oral supplementation with the extract of L. chinensis seed ameliorates cognitive impairment following long-term alcohol consumption in rats by modulating the AChE activity and increasing the brain antioxidant status, along with reducing the lipid peroxidation in both hippocampus and cortex of ethanol-treated rats. However, the current study has potential limitations that should be acknowledged. First, there are no previous studies and existing literature in research reports on the cognitive enhancing effects of L. chinensis seed extract in a rat model of alcoholism. Second, this article lacks clarity in presenting quantitative analysis. Third, histopathological evaluation to confirm the cognitive enhancing effect of the seed extract in this experiment has not been conducted. Finally, the correlations with the AChE inhibitory effect and their phenolic constituents of the seed extract have not been elucidated. However, to the best of our knowledge, the deterioration of memory functions is a very complex gradual process and comprehends a wide variety of molecular alterations, but oxidative stress damage and cholinergic dysfunction at least in part participate in this process. Therefore, the current study provides a preliminary step in validating the potency of lychee seed waste utilization by transforming them into value-added products, as a brain booster agent for alcohol-related memory impairment. Further scientific validation to identify the precise mechanisms and the active compounds of the extract is still required prior to moving to clinical trials.
CONCLUSION
This study illustrates a valuable scientific basis for the efficacy of lychee seed extract to combat learning and memory deficits in a rat model of alcoholism. The possible mechanisms by which the extract restores cognitive function may occur via its impact on cholinergic function and the antioxidant system. Thus, the extract of lychee seed, a fruit waste product, had high potential to be developed as novel nutraceutical products and inexpensive adjuvant treatment to boost memory and cognitive abilities in alcoholism.
ACKNOWLEDGMENTS
This research was supported by the University of Phayao and Thailand Science Research and Innovation Fund (Fundamental Fund 2024).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The animal studies protocol and all procedures (code number: 1-030-66) were ratified by the Animal Ethics Committee of the Institutional Animal Care and Use, University of Phayao and performed following the guidelines of the “Principle of laboratory animal care” (NIH publication 85–23, 1985).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
All authors declare that we have not used artificial intelligence (AI)-tools for writing and editing this manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors, and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a social-ecological framework. Alcohol Res. 2016;38(1):35–45.
2. Ma J, Xiong F, Li Z, Dong G, Sun X, Yin W, et al. The effect of chronic alcohol exposure on spatial memory and BDNF-TrkB- PLCγ1 signaling in the hippocampus of male and female mice. Heliyon. 2023;9(6):e16660. CrossRef
3. Macht VA, Vetreno RP, Crews FT. Cholinergic and neuroimmune signaling interact to impact adult hippocampal neurogenesis and alcohol pathology across development. Front Pharmacol. 2022;13:849997. CrossRef
4. Crews FT, Fisher R, Deason C, Vetreno RP. Loss of basal forebrain cholinergic neurons following adolescent binge ethanol exposure: recovery with the cholinesterase inhibitor galantamine. Front Behav Neurosci. 2021;15:652494. CrossRef
5. Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110(4):627–39. CrossRef
6. Cadete-Leite A, Andrade JP, Sousa N, Ma W, Ribeiro-da-Silva A. Effects of chronic alcohol consumption on the cholinergic innervation of the rat hippocampal formation as revealed by choline acetyltransferase immunocytochemistry. Neuroscience. 1995;64(2):357–74. CrossRef
7. Moreta MP, Burgos-Alonso N, Torrecilla M, Marco-Contelles J, Bruzos-Cidón C. Efficacy of acetylcholinesterase inhibitors on cognitive function in Alzheimer’s disease. Review of reviews. Biomedicines. 2021;9(11):1689. CrossRef
8. Cortez I, Brocardo PS, Leasure JL. Changes in affective behavior and oxidative stress after binge alcohol in male and female rats. Brain Sci. 2021;11(9):1250. CrossRef
9. Hernández JA, López-Sánchez RC, Rendón-Ramírez A. Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxid Med Cell Longev. 2016;2016:1543809. CrossRef
10. Augustyniak A, Michalak K, Skrzydlewska E. The action of oxidative stress induced by ethanol on the central nervous system (CNS). Postepy Hig Med Dosw. 2005;59:464–71.
11. Tsermpini EE, Plemenitaš Ilješ A, Dolžan V. Alcohol-induced oxidative stress and the role of antioxidants in alcohol use disorder: a systematic review. Antioxidants (Basel). 2022;11(7):1374. CrossRef
12. Pacheco MT, Moreno FJ, Villamiel M. Chemical and physicochemical characterization of orange by-products derived from industry. J Sci Food Agric. 2019;99(2):868. CrossRef
13. Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10(2):221. CrossRef
14. Kittipongpittaya K, Puangploy P, Kullamethee P, Fakkheow P, Kareevate P, Philkliang B. Antioxidant activities of extract from Makmao seed waste. Asia Pac J Sci Technol. 2021;26(2):26.
15. Floris S, Fais A, Rosa A, Piras A, Marzouki H, Medda R, et al. Phytochemical composition and the cholinesterase and xanthine oxidase inhibitory properties of seed extracts from the Washingtonia filifera palm fruit. RSC Adv. 2019;9:21278–87. CrossRef
16. Kobus-Cisowska J, Szymanowska D, Maciejewska P, Kmiecik D, Gramza-Micha?owska, A, Kulczy?ski,B, et al. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron J Biotechnol. 2018;37:1–10. CrossRef
17. Saadh MJ. Potential protective effects of red grape seed extract in a rat model of malathion-induced neurotoxicity. Vet World. 2023;16(2):380–5. CrossRef
18. Subhadrabandhu S, Yapwattanaphun C. Lychee and longan production in Thailand. Acta Horticulturae. 2001;558:49–57. CrossRef
19. Zhang Y, Jin D, An X, Duan L, Duan Y, Lian F. Lychee seed as a potential hypoglycemic agent, and exploration of its underlying mechanisms. Front Pharmacol. 2021;12:737803. CrossRef
20. Xiao LY, Pan ZJ, Rao WN. The research of protective effect of Litchi seed of experimental liver injury in mice. Chin J Trad Chin Med Pharm. 2005;20:42–4.
21. Sun W, Shahrajabian MH, Shen H, Cheng Q. Lychee (Litchi chinensis Sonn.), the king of fruits, with both traditional and modern pharmacological health benefits. Phcog Commn. 2021;11:22–5. CrossRef
22. Deng ZJ, Guo JW, Pan JQ. Pharmacologic and pharmacodynamic effects of effective element of Litchi and Litchi seed. Pharm Today. 2009;5:7–9.
23. Xiao ZJ, Guo JW, Xu F. Effect of litchi saponin and litchi flavones on insulin resistance in HepG2 cells. J Pharm Pract. 2015;4:316–8.
24. Zhang YM, Yuan H, Tian JX, Shen L, Yong YH. Effects of saponin of litchi seed on gluconeogenesis and metabolism of blood lipid in mice. J Hangzhou Teach Coll. 2005;6:435–6.
25. Zhang J, Zhang C. Research progress on the antineoplastic pharmacological effects and mechanisms of litchi seeds. Chin Med. 2015;6:20–6. CrossRef
26. Li W, Zhu YT, Huang ZY, He JJ, Pei J, Song JP. Experimental studies on anti-fluvirus effect of Litchi seed in vivo. Chin J Ethnomed Ethnopharm. 2011;18:34–6.
27. Zhao HX, Guo K, Cui YD, Wu XG, Shang YZ. Effect of Scutellaria barbata flavonoids on abnormal changes of Bcl-2, Bax, Bcl-xL and Bak protein expression in mitochondrial membrane induced by composite Aβ(25–35). Chin J Pathophysiol. 2014;30:2262–6.
28. Ye HM, Zhong CY, Huang MX, Wang CY, Fang X, Chen XY, et al. Effect of litchi seed aqueous extracts on learning and memory obstacles induced by d-galactose in mice and its mechanism. J Chin Med Mater. 2013;36:438–40.
29. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–78. CrossRef
30. Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities of polyphenols of Orthosiphon stamineus and evaluation of the free radical scavenging activity. Food Chem. 2005;93(2):311–7. CrossRef
31. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem. 1996;239(1):70–6. CrossRef
32. Rajakrishnan V, Menon VP. Protective role of curcumin in ethanol toxicity. Phytotherapy Res. 1998;12:55–6. CrossRef
33. Gotz ME, Janetzky B, Pohli S, Gottschalk A, Gsell W, Tatschner T, et al. Chronic alcohol consumption and cerebral indices of oxidative stress: is there a link? Alcohol Clin Exp Res. 2001;25:717–25. CrossRef
34. Tongun T, Phachonpai W. Cognitive booster of wampee peel extract on chronic restraint stress-induced memory dysfunction in rats. J Appl Pharm Sci. 2020;10(7):19–26. CrossRef
35. Jeefoo WP, Phachonpai W, Duangjai A, Ontawong A, Amornlerdpison D. Purple eggplant (Solanum melongena L.) ameliorates D-galactose-induced cognitive impairment through inhibition of oxidative stress and acetylcholinesterase in the hippocampus of an aging rat model. Trends Sci. 2023;21(1):7245. CrossRef
36. Phachonpai W. Tongun T. Neuroprotective and cognitive enhancing effects of Clausena lansium (Lour.) skeels peels extract in ischemic rat brains. J Appl Pharm Sci. 2021;11(9):1–8.
37. Moron MA, Depierre JW, Mannervick B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. CrossRef
38. Pohsa S, Hanchang W, Singpoonga N, Chaiprasart P, Taepavarapruk P. Effects of cultured Cordycep militarison sexual performance and erectile function in streptozotocin-induced diabetic male rats. Biomed Res Int. 2020;2020:4198397. CrossRef
39. Nakdook W, Khongsombat O, Taepavarapruk P, Taepavarapruk N, Ingkaninan K. The effects of Tabernaemontana divaricata root extract on amyloid β-peptide 25-35 peptides induced cognitive deficits in mice. J Ethnopharmacol. 2010;130:122–6. CrossRef
40. Shoaib S, Ansari MA, Fatease AA, Safhi AY, Hani U, Jahan R, et al. Plant-derived bioactive compounds in the management of neurodegenerative disorders: challenges, future directions and molecular mechanisms involved in neuroprotection. Pharmaceutics. 2023;15(3):749. CrossRef
41. Costin BN, Miles MF. Molecular and neurologic responses to chronic alcohol use. Handb Clin Neurol. 2014;125:157–71. CrossRef
42. Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2013;369(1635):20120510. CrossRef
43. Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci U S A. 1998;95(13):7721–6. CrossRef
44. Pérez-Cervera L, De Santis S, Marcos E, Ghorbanzad-Ghaziany Z, Trouvé-Carpena A, Selim MK, et al. Alcohol-induced damage to the fimbria/fornix reduces hippocampal-prefrontal cortex connection during early abstinence. Acta Neuropathol Commun. 2023;11(1):101. CrossRef
45. Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, et al. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiol Learn Mem. 2010;94(4):538–46. CrossRef
46. Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, et al. Alcohol use disorder, neurodegeneration, Alzheimer’s and Parkinson’s disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front Cell Neurosci. 2020;14:282. CrossRef
47. Rico EP, Rosemberg D, Dias RD, Bogo MR, Bonan CD. Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol Lett. 2007;174:25–30. CrossRef
48. Phachonpai W, Wattanathorn J, Wannanon P, Thipkaew C, Sripanidkulchai B, Muchimapura S. Coscinium Fenestratum protects against ethanol-induced neurodegeneration in adult rat brain. Am J Pharmacol Toxicol. 2012;7(3):81–8. CrossRef
49. Macieira MS, Almeida WG, Silva EA, Schenberg LC, Nakamura-Palacios EM. Alcohol dependence induced in rats by semi voluntary intermittent intake. Braz J Med Biol Res. 1997;30(9):1107–11. CrossRef
50. Bruijniks SJE, van Grootheest G, Cuijpers P, de Kluiver H, Vinkers CH, Peeters F, et al. Working memory moderates the relation between the brain-derived neurotropic factor (BDNF) and psychotherapy outcome for depression. J Psychiatr Res. 2020;130:424–32. CrossRef
51. Cichon N, Saluk-Bijak J, Gorniak L, Przyslo L, Bijak M. Flavonoids as a natural enhancer of neuroplasticity-an overview of the mechanism of neurorestorative action. Antioxidants. 2020;9:1035. CrossRef
52. AL-Qahtani AA, Shati AA, Al-Doaiss AA. Elsaid FG. Mitigating alcohol-induced neurohepatotoxicity in male albino rats with avocado and mustard. J Umm Al-Qura Univ. Appll Sci. 2024;10:530–40. CrossRef
53. Hossain S, Chowdhury I, Basunia M, Nahar T, Rahaman A, Choudhury B, et al. Syzygium cumini seed extract protects the liver against lipid peroxidation with concurrent amelioration of hepatic enzymes and lipid profile of alcoholic rats. JCIM. 2011;8(1):1–17. CrossRef
54. Bera D, Lahiri D, Nag A. Studies on a natural antioxidant for stabilization of edible oil and comparison with synthetic antioxidants. J Food Eng. 2006;74:542–5. CrossRef
55. Potisate Y, Pintha K. Optimum extraction condition and dehydration of lychee seeds extracted. Health Sci Tech Rev. 2021;14(2):105–15
56. Omena CMB, Valentim IB, Guedes GS, Rabelo LA, Mano CM, Bechara EJH, et al. Antioxidant, anti-acetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seeds of exotic Brazilian fruits. Food Res Int. 2012;49:334–44. CrossRef
57. Findik BT, Yildiz H, Akdeniz M, Yener I, Yilmaz MA, Cakir O, et al. Phytochemical profile, enzyme inhibition, antioxidant, and antibacterial activity of Rosa pimpinellifolia L.: a comprehensive study to investigate the bioactivity of different parts (whole fruit, pulp, and seed part) of the fruit. Food Chem. 2024;15;455:139921. CrossRef
58. Shin CY, Kim HS, Cha KH, Won DH, Lee JY, Jang SW, et al. The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. Biomol Ther (Seoul). 2018;26(3):274–81. CrossRef