INTRODUCTION
One of the most significant flagellated protozoan parasites in the family Trypanosomatide and order Kinetoplastida is Leishmania. The intracellular protozoan of the genus Leishmania causes the parasitic disease Leishmaniasis, which has a serious impact on human health [1]. Approximately 350 million individuals are at risk for various forms of Leishmania protozoa, and about 12 million people in 98 countries worldwide contracted the disease each year from 1990 to 2018 [2]. There are two subgenera of Leishmania: the subgenera that cause the self-healing cutaneous leishmaniasis (CL) (such as L. major) and the deadly visceral leishmaniasis (VL) (L. infantum in the new world and Leishmania donovani in the old world are two examples). The Leishmania viannia subgenus is frequently linked to either CL or muco cutaneous (MCL) forms (L. braziliensis). Leishmania species develop into externally flagellated promastigotes inside the sand flies’ digestive tracts as part of a digenetic life cycle. They subsequently differentiate within the phagolysosome of the host macrophage to become internal rudimentary-flagellated amastigotes [3]. In accordance with the World Health Organization’s leishmaniasis information sheet, in seven countries (Somalia, India Kenya, South Sudan, Brazil, Sudan, and Ethiopia) in 2020, (VL) accounted for more than 90% of cases. Over 84% of patients that were recorded in 10 countries in 2016 had (CL): Tunisia, Afghanistan, Pakistan, Brazil, Peru, Colombia, Iraq, the Syrian Arab Republic, Yemen, and Algeria [4,5]. Moreover, Bolivia, Brazil, Ethiopia, and Peru account for approximately 90% of cases of (MCL). With an estimated 4,500–5,000 new cases of (VL) annually, Ethiopia is highly affected by both MCL and VL [6]. The currently available drugs lead to drug resistance and are cost ineffective [7]. Hence, more affordable drugs need to be developed and tested.
Betula utilis (Betulaceae) can be referred to as the “Himalayan Silver Birch.” and is a significant medicinal plant native to the high-altitude regions of the Himalayas in India [8]. Previous studies on B. utilis have revealed the presence of triterpenoids, flavonoids, and Phenolics. Betula utilis bark extract and their isolated compounds have been demonstrated to display remarkable biological activities, anti-microbial, anti-cancer, anti-inflammatory, and hepatoprotective activity [8]. Betulin, betulinic acid, and lupeol are the triterpenoids found in the plant bark [9], Betulin (Fig. 1) is a pentacyclic triterpene natural product that belongs to the lupane family and has the systematic name 3, 28-dihydroxy-20(29) lupen or Lup-20(29)-en-3, 28-diol, also called as betulinol, betulinic alcohol, or betulin [10,11]. It is reported for various biological activities, like anti-HIV, anti-fungal, anti-bacterial, anti-inflammatory, anti-tumor, anti-leishmaniasis, and immunological regulatory effects [12–14]. The derivatives of betulin including imidazole carboxylic ester, hemisuccinate, hemiphthalate, nicotinate, acetylbetulin-28-O-hemiphthalate, mono- and dimethacrylate, acetylenate, oxime, oligomeric ester, phosphate, triphenylphosphonium, succinyl, and 3-substituted glutaryl, exhibit various pharmacological activities such as anti-viral, anti-inflammatory, anti-cancer, and hepatoprotective effects [15–20].
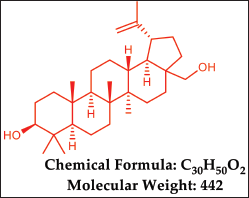 | Figure 1. Chemical properties of Betulin. [Click here to view] |
The literature has reported the activity of betulin and its derivatives in Leishmania. Alakurtti and collaborators [21] investigated the effect of heterocyclic betulin derivatives on L. donovani amastigotes. Sousa et al. [22] investigated the antileishmanial action of imidazole betulin derivatives. Chowdhury et al. [23] focused on targeting DNA topoisomerases in L. donovani and synthesized betulin derivatives, including disuccinyl betulin, diglutaryl dihydrobetulin, and disuccinyl dihydrobetulin. These compounds were found to inhibit both parasite growth and the relaxation activity of L. donovani-type IB topoisomerase. Zhang et al. [24] predicted target identification for betulin derivatives as L. donovani inhibitors using in silico target fishing with structure-based pharmacophore searching and compound-pharmacophore-target pathway network analysis.
The present work evaluates the potential of B. utilis D. Don ethanolic extract, isolated Betulin. and its ester derivative for the anti-leishmanial effect using in-vitro and ex-vivo assay. The ethanolic extract was obtained by Soxhlet extraction, and betulin was isolated by column chromatography. The semisynthetic ester derivative was synthesized by the reaction of betulin (75 mg, 0.17 mmol) and 3, 5 dinitrobenzoic acid (38 mg, 0.18 mmol) in dicyclohexylcarbodiimide (DCC) (32 mg, 0.15 mmol) and 4-dimethylaminopyridine (DMAP) (19 mg, 0.15 mmol) in dry dichloromethane (50 ml) by stirring at room temperature for 4 hours. The synthesized compound was characterized by NMR, IR, and mass spectroscopy. The molecular docking study was performed using the Schrodinger Suite (Maestro 11.8), to explore the binding affinities and interactions of amphotericin B, betulin, and its semisynthetic derivative with the active site of Pteridine reductase 1 (PTR1), proposed as a potential target for parasitic diseases. The Betula utilis ethanolic extract (EEBU), isolated betulin (VA1), and synthesized compound (VA2) were tested for their cytotoxicity and in-vitro anti-leishmanial activity (anti-promastigote and anti-amastigote) against L. donovani. Anti-promastigote activity and cytotoxicity tests were conducted using the (3-(4,5- dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) (MTT) cell viability method on modified THP-1 cells infected with L. donovani. Anti-amastigote activity was conducted by the culture of macrophage-lineage THP1 cells (105 cells. µM) on a 24-well plate.
MATERIAL AND METHODS
Plant material
A 1 kg of fresh bark from B. utilis was collected from Himachal Pradesh, in the month of September and was authenticated by Prof. Vidhu Aeri, and the voucher specimen (JH/PCOG/371.STBK.BT) was submitted for record in the Department of Pharmacognosy & Phytochemistry, Jamia Hamdard, New Delhi, India.
Chemicals
All the chemicals used were of analytical grade: dichloromethane (Merck), ethanol (Merck), petroleum ether (Merck), DMAP (SRL), DCC (Sigma Aldrich), amphotericin B, MTT, Alamar blue®, THP 1cells (Sigma Aldrich), Dimethyl sulfoxide (99% DMSO) (Merck), and Silica gel 60–120 mesh (Merck).
Preparation of extracts
A 250 g of powdered B. utilis bark was defatted in a Soxhlet apparatus with 3.25 L of petroleum ether, and the defatted marc was subsequently extracted with 3.25 L of 95% ethanol for approximately 72 hours at 40°C. The ethanolic extract was concentrated in a vacuum rotary evaporator and was subjected to further investigations after drying for 4–5 days in a vacuum desiccator [25,26].
Isolation of betulin using column chromatography
The column was packed with silica gel (60–120 mesh size) using a wet technique and left overnight. The mixture of 5 g of ethanolic extract and silica gel was added to an open silica gel column chromatography system, and the column was eluted in pure toluene (100%) initially and followed by toluene and ethyl acetate in a gradient manner (90:10), (80:20), and (70:30). The 50 ml of fractions were collected, concentrated in rota evaporator, and left for crystallization. A total of 210 fractions were collected. The fractions with the same Rf value as that of standard betulin were grouped together and concentrated using a rotary evaporator. Betulin was isolated from fractions 77–104 in the mobile phase (toluene: ethylacetate 70:30). The concentrated fraction was crystallized/ recrystallized to obtain pure betulin. The isolated betulin was characterized by spectroscopy methods (NMR, IR, and Mass).
Quantitative estimation of betulin in the bark extract of B. utilis D.Don by using UV spectroscopy
A standard curve was developed by preparing different dilutions of the standard and measuring the absorbance at 210 nm. The concentration of betulin present in the extract was calculated by using the standard calibration curve of betulin.
Preparation of standard
A standard stock solution of 1 mg/ml betulin was prepared by using methanol as a solvent and working solutions were drawn from the stock solution at concentrations of 2, 4, 6, 8, and 10 µg/ml. The absorbance of the working solution was measured at 210 nm and the standard calibration curve was plotted.
Preparation of sample
A stock solution of 1 mg/ml of extract was prepared by using methanol as a solvent and absorbance was measured at 210 nm.
Molecular docking
Molecular docking studies play an important role in computational biology and drug discovery, enabling the prediction of the interaction between molecules of interest (ligands) and their macromolecular targets (proteins). The molecular docking studies were conducted using Schrodinger Suite (Maestro 11.8) software. The protocol included the steps, focussing on protein selection and preparation, grid building, ligand preparation, the docking process, and result analysis of Betulin, amphotericin B, and synthesized compound with the enzyme PTR1. The PTR1 is a crucial enzyme for pterin salvage in the Leishmania parasite, making it a possible target for improved therapy. PTR1 is a homotetramer with a subunit molecular mass around 30 kDa. PTR1 catalyzes the two-step reduction of folate to dihydrofolate (H2) and then tetrahydrofolate (H4). The dihydrofolate reductase (DHFR) component of the DHFR-thymidylate synthase bifunctional polypeptide (DHFR-TS) reduces folate and is the major cell target for antiprotozoal activity [27].
Protein selection and preparation
The first step in molecular docking studies is selecting and preparing the target protein. For this study, the crystal structure of PTR1 (PDB ID: 2XOX) [28] was obtained from the RCSB Protein Data Bank (http://www.pdb.org). Once the protein structure was retrieved, it was prepared using the Protein Preparation Wizard in Maestro. This process involved several steps to ensure that the protein structure was ready for docking studies. Initially, the PDB file was imported into Maestro, hydrogens were added, bond orders were assigned, and formal charges were treated. The protein’s protonation states were adjusted, termini were capped, and water molecules were removed beyond 5 Å. Next, the structure is optimized to relieve any steric clashes, followed by restrained minimization to refine the hydrogen bond network. These steps ensure the protein is in its most stable and relevant conformation for docking.
Grid formation
The next step was to generate a receptor grid, defining the docking space around the protein’s active site. In Maestro, the “Receptor grid generation” tool was used to create this grid. The grid box is centered on the active site of LM-PTR1, ensuring it encompasses the entire binding region and potential interaction sites. The size of the grid box is 20 ×20 ×20 Å set to balance the inclusion of all relevant binding regions without making it excessively large, which could decrease the precision of the docking results.
Ligand preparation
Ligand preparation is a vital component of molecular docking studies. For this protocol, the ligands betulin, amphotericin B, and the designed compound were first drawn using ChemDraw software. These structures were then saved in pdb formats compatible with Maestro. The ligand structures were imported into Maestro, they were prepared using the LigPrep tool. The 3D conformations of the ligands using the LigPrep dialogue box. Three-dimensional structures were ionized at pH 7.0 and tautomerized. Before energy minimization, each chemical generated 32 stereoisomers. This preparation ensured that the ligands were in the appropriate shape and energy state for docking.
Docking process
In Maestro, the Glide dock was used for docking studies. The prepared receptor grid and ligands were imported into the Glide module. Docking parameters were set, including choosing the docking precision standard precision, and the 5 number of poses for each ligand were generated.
Preparation of betulin-derived esters
The designed compound (VA2) was synthesized using betulin (75 mg, 0.17 mmol) and 3, 5 dinitrobenzoic acid (38 mg, 0.18 mmol) in DCC (32 mg, 0.15 mmol) and DMAP (19 mg, 0.15 mmol) in dry dichloromethane (50 ml) by stirring at room temperature for 4 hours. The progress of the reaction was monitored by TLC using [(n-hexane: ethyl acetate) 6:4 v/v]. After completion, the reaction mixture was quenched in cold water, filtered, and was subsequently with dichloromethane. The resulting filtrate was then evaporated under reduced pressure and subjected to purification via column chromatography using mobile phase [(n-hexane: ethyl acetate) 6:4 v/v] [29,30].
Structural identification
The isolated and synthesized compound was structurally characterized by melting point and spectral analyses. The spectral analyses were performed using a Bruker DPX-400 spectrometer for both 1H (500 MHz) and 13C (400 MHz) NMR spectra, recorded in CDCl3, where TMS served as the internal standard. The KBr pellet IR spectra were acquired using a Thermo Scientific Nicolet 380 FTIR spectrometer, while mass spectra were obtained utilizing a Shimadzu mass spectrometer.
Culture conditions/Leishmania strain and growth
The studies employed the L. donovani strain MHOM/SD/62/1S-C12D (SD), obtained from FDA, USA with due approvals was used [31,32]. Promastigotes were cultivated in M199 medium with pH 6.8 at 260C [31].
In vitro promastigote assay (anti-leishmanial activity)
Experiments were conducted in 96-well plates using promastigotes in the mid-log phase (10^6 cells/ml) in M199 media. The test samples EEBU, VA1, and VA2 were dissolved in 3% v/v (DMSO, Vetec®) and sterile-filtered through 0.22 µm Millipore membranes [33,34]. These compounds were assessed at concentrations ranging from 32 to 500 µM. The untreated control and amphotericin B, used as a reference, also contained 3% v/v DMSO. The antileishmanial activity was determined by measuring growth inhibition and mortality over a 72-hour period at 25°C. Parasite viability was assessed using the MTT assay, with absorbance measured at 570 nm. All assays were performed in triplicate in three independent experiments.
Assay for ex vivo cytotoxicity
THP1 cells (Sigma Aldrich) grown in RPMI 1640 media supplemented with 10% FBS, were subjected to escalating doses of test compounds (5–50µM) at 37°C and 5% CO2 for 48 hours in order to evaluate the adverse effects of test compounds VA1, VA2, and EEBU on mammalian cells. The reference drug was amphotericin B, while the controls were untreated macrophages. The MTT (3-{4, 5-dimethylthiazol-2- yl}-2, 5- diphenyltetrazoliumbromide) assay was used to measure the proportion of viable cells to assessed cell viability [35].
Amastigote-macrophages assay
Late-log promastigotes (10 × 106) were added to PMA-differentiated THP-1 macrophage-lineage cells (1 × 106 cells/ml) cultured on glass coverslips, at a cell-to-parasite ratio of 1:10. After a six-hour incubation period, uninternalized parasites were removed by washing with 1× PBS. Cells were then replenished with fresh RPMI medium and incubated for an additional 48 hours to allow amastigote differentiation and multiplication within the macrophages. Following this incubation, infected cells were treated with VA1, VA2, and EEBU, compounds previously shown to exhibit minimal cytotoxicity while enhancing anti-leishmanial activity against L. donovani promastigotes. Infected cells were then fixed and stained with Giemsa (Laborclin®). The glass coverslips were removed, and the number of infected macrophages (200 per slide/replicate) and the number of amastigotes per macrophage were quantified using a bright field inverted microscope; EVOS-Thermo (Invitrogen) under 40× magnification [36,37].
Statistical analysis
The cytotoxicity and anti-leishmanial activity were assessed using one-way ANOVA, with a statistical significance level of ****p < 0.0001. The latest version of the GraphPad Prism software was used to calculate the CC50 and IC50 values.
RESULTS
Preparation of extracts
The petroleum ether extract yield was found to be 30.68% and the ethanolic extract yield was found to be 57.91% (yellowish brown).
Isolation of betulin by column chromatography
The betulin was isolated and purified by column chromatography from the ethanolic extract of B. utilis bark. The compound was isolated from fractions 77–104 using mobile phase (toluene: ethyl acetate 70:30). Methanol was used to purify the compound. The compound was identified by anisaldehyde sulphuric acid reagent. The melting range of the isolated compound, as tested by the open capillary method, was found to be 242°C–246°C. The maximum absorbance (λmax) of the compound in concentrated sulfuric acid was found to be 326 nm, this is quite close to the 316 nm standard value, and the Rf is 0.41. Betulin (15%) was successfully extracted from the bark of B. utilis using an optimized ethanolic extraction process and column chromatography. The UV spectrum of Betulin is mentioned in (Supplementary Fig. S1).
Quantitative estimation of betulin in the bark extract of B. utilis D.Don by using UV spectroscopy
The amount of betulin present (12.36 µg/ml) in the extract of B. utilis D.Don bark was calculated by using the y = 0.1364 x – 0.033 equation. The standard calibration curve of betulin is indicated in Figure 2.
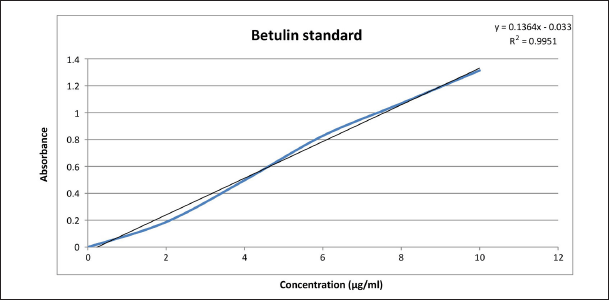 | Figure 2. The standard calibration curve of betulin. [Click here to view] |
Molecular docking
In the present study, molecular docking studies were conducted to explore the binding affinities and interactions of betulin, amphotericin B, and a semisynthetic derivative with the active site of PTR1. The docking studies were performed using the Schrodinger Suite [Maestro 11.8] and employed a well-defined protocol encompassing protein and ligand preparation, receptor grid generation, and subsequent docking processes. The docking results indicated that amphotericin B forms specific hydrogen bond interactions with THR5, 165, 174, and GLN169 with a docking score of –6.216 kCal/mol. This suggests that hydrophobic interactions and van der Waals forces primarily contribute to its moderate binding affinity, highlighting its potential to effectively occupy the active site (Fig. 3). Betulin displayed a specific hydrogen bond interaction with GLU 123 with a docking score of –3.578 kCal/mol. This specific binding suggests that directed interactions are crucial for its stable binding within the PTR1 active site, thereby enhancing its binding specificity and stability (Fig. 4). The good binding affinity was observed for the designed compound (VA2) as compared to Betulin (Parent moiety), which achieved a docking score of –5.724 kCal/mol. It formed multiple hydrogen bonds with THR-146, and GLU123, indicating a strong and stable binding mode. The extensive hydrogen bonding network suggests the compound’s high potential as a potent inhibitor of PTR1, making it a promising candidate for further development (Fig. 5).
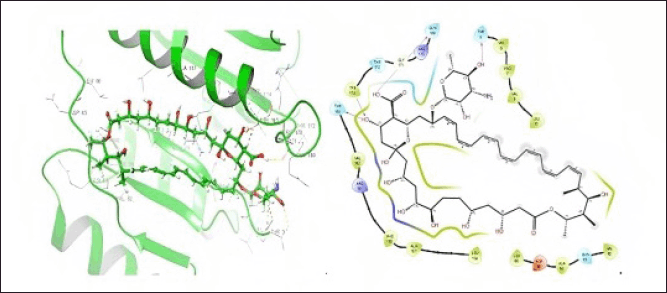 | Figure 3. 3D and 2D representation of protein-ligand interaction of amphotericin B at PTR1 active site. [Click here to view] |
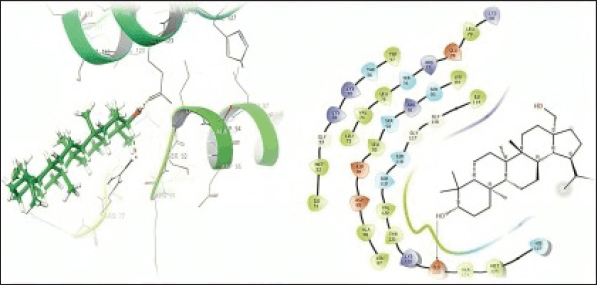 | Figure 4. 3D and 2D representation of the protein-ligand interactions of the betulin (VA1) at the PTR1 active site. [Click here to view] |
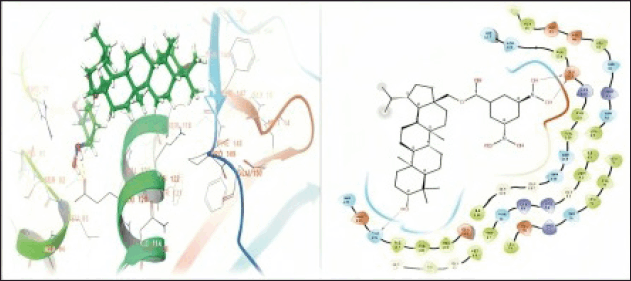 | Figure 5. 3D and 2D representations of the protein-ligand interactions of the compound (VA2) at the PTR1 active site. [Click here to view] |
Characterization of betulin and 3, 5- dinitrobenzoate betulin
Spectral identification (isolated betulin)
C30H50O2, Rf 0.41, Melting point 240°C–245°C,1H NMR (500 MHz, CDCl3) δ value δ 4.70 (s, 1H, OH), 4.60 (s, 1H, O-H), 3.83–3.80 (dd, 1H, =CH2), 3.36–3.33 (dd, 1H, =CH2), 3.22–3.20 (dd, 1H, -CH2), 2.41–2.37 (m, 1H, -CH2), 1.96–1.86 (m, 3H, =CH, -CH2), 1.70–1.69 (m, 5H, -CH2), 1.62–1.59 (m, 9H, -CH2), 1.45–1.39 (m, 6H, -CH2), 1.28–1.27 (t, 3H, -CH2), 1.23–1.22 (d, J = 5.25 Hz, 1H, -CH2), 1.20–1.19 (d, J =5.3 Hz, -CH2), 1.04 (s, 3H, -CH3), 1.00–0.98 (d, J = 6.25 Hz, 6H, -CH3), 0.84 (s, 3H, -CH3), 0.78 (s, 3H, -CH3), 0.71–0.69 (dd, 1H, -CH3).13C NMR (400 MHz, CDCl3) δ 150.4 (C-20),109.72 (C-29), 77.23 (C-3), 60.57 (C-28), 55.28 (C-5), 50.39 (C-9), 47.79 (C-17), 48.75 (C-18), 48 (C-19), 42.72 (C-14), 40.91 (C-8), 38.70 (C-4), 38.87 (C-1), 37.30 (C-13), 37.16 (C-10), 34.23 (C-7), 33.98 (C-22), 29.74 (C-21), 29.17 (C-16), 27.99 (C-2), 27.39 (C-15), 25.20 (C-12), 20.83 (C-24), 20.83 (C-23), 16.12 (C-11), 18.30 (C-30), 19.09 (C-26), 15.98 (C-6), 15.38 (C-25), 14.77 (C-27). IR (KBr, cm−1): 3359 (OH), 3,078, 2,944, 2,870 (CH), 1,695, 1,644, 1,457, 1,457, 1,374, 1,029, 884, 759. MS (EI, 70 eV) m/z: 442 [M] + (Supplementary Figs. S2–S5).
Spectral identification (3, 5- dinitrobenzoate betulin)
C37H52N2O7, % yield 32.39, Rf 0.53, Melting point 230°C–234°C,1H NMR (500 MHz, CDCl3) δ 0.77–0.76 (d, J = 8.2 Hz, 3H, -CH3), 0.84–0.82 (d, J = 9.3 Hz, 3H, -CH3), 0.98–0.96 (m, 6H, -CH 3), 1.02 (s, 7H, -CH3), 1.07 (s, 6H, -CH2), 1.25-1.24 (m, 2H, -CH2), 1.40-1.39 (d, J = 6.7 Hz, 3H, -CH2), 1.68–1.67 (m, 4H, -CH2), 1.72–1.71 (m, 7H, -CH2), 2.17 (s, 1H, -CH2), 3.34–3.32 (s, 1H, -CH2), 4.24–4.22 (d,1H, -CH2), 4.57 (s, 1H, -CH2), 4.63 (s, 1H, =CH2), 4.67 (s, 1H, =CH2), 4.73 (s, 1H, OH), 9.10 (s, 1H, Ar-H), 9.14 (s, 1H, Ar-H), 9.23 (s, 1H, Ar-H).13C NMR (400 MHz, CDCl3) δ 13.73, 15.09, 19.80, 24.79, 26.97, 28.44, 31.36, 34.88, 38.21 48.13, 49.32, 54.86, 59.46, 64.57, 77.92, 121.35, 128.48, 133.08, 148.67, 161.87. IR (KBr, cm−1): 3644 (OH) free, 1751 (C=O), 1583 (C=C) aromatic, 1412 (N=O), 1132 (C-O). MS (EI, 70 eV) m/z: 639 [M] +. The compound structure is shown in Figure 6 (Supplementary Figs. S6–S9).
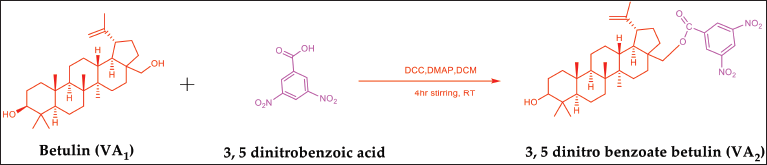 | Figure 6. Scheme for synthesis of betulin ester derivative. [Click here to view] |
In vitro anti-leishmanial evaluation
The test samples were evaluated for their ability to treat L. donovani; VA1, VA2, and EEBU were compared for their anti-leishmanial efficacy. The IC50 values of VA1 were 58.39 ± 0.4977 μM, and the IC50 of amphotericin B was 6.463 ± 0.5220 μM (Fig 7A). The IC50 values of compound VA2 and amphotericin B were found to be 25.20 ± 0.4459μM and 5.062 ± 0.4325 μM, respectively (Fig. 7B). EEBU had an IC50 value of 58.28 ± 0.4986 μM, and amphotericin B had an IC50 value of 6.46 ± 0.5220 μM (Fig. 7C). Therefore, VA2 exhibited more potent results than did VA1 and EEBU. Additionally, the anti-leishmanial assay revealed that VA2 was more active than VA1 and EEBU.
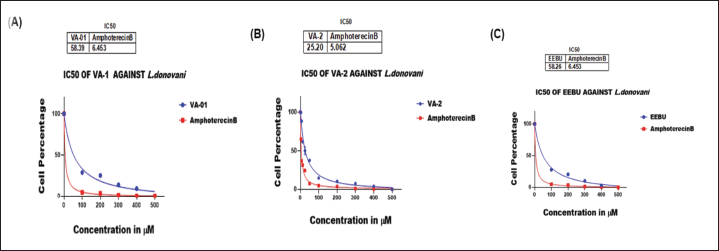 | Figure 7. Anti-promastigote activity as determined by cell percentage and concentration of (A) VA1 and amphotericin B; (B) VA2 and amphotericin B; (C) EEBU and amphotericin B. The significance level was ****p < 0.0001. [Click here to view] |
Ex-vivo cytotoxicity evaluation
The cytotoxic effects of VA1, VA2, and EEBU on THP-1 differentiated macrophages were evaluated via incubation at twofold serial dilutions (100–400 μM), and viability was assessed via the MTT assay. The cytotoxicity IC50 values of VA1 and VA2 were found to be 72.9 ± 0.9765 and 110.2 ± 0.6858 μM, respectively. The standard drug (control) amphotericin B had a cytotoxicity IC50 value of 171.2 ± 0.9389 μM (Fig. 8A and B). The cytotoxicities of EEBU and the standard drug amphotericin B were found to be 52.45 ± 0.1124 and 109.4 ± 0.3835 μM, respectively (Fig. 9 A and B). VA2 is safer and more noncytotoxic to human macrophages than VA1 and EEBU.
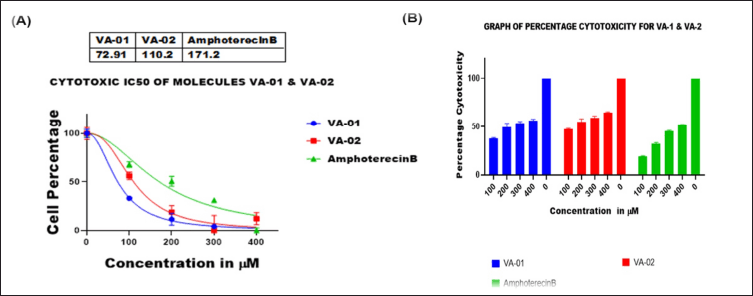 | Figure 8. (A) Cytotoxicity activity as determined by the cell percentage vs. the concentration of VA1, VA2, and amphotericin B; (B) Comparative bar graph of the percentage cytotoxicity of VA1, VA2, and amphotericin B. The significance level was ****p < 0.0001. [Click here to view] |
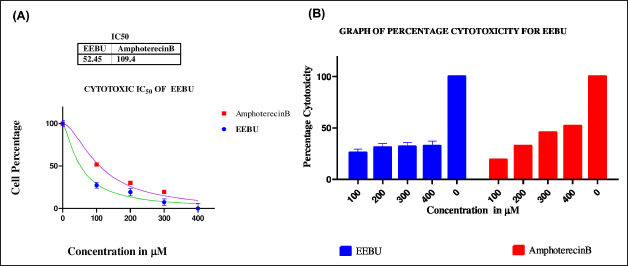 | Figure 9. (A) Cytotoxicity activity graph showing the differences in the percentages and the percentages of cells treated with EEBU and amphotericin B; (B) Bar graph of the percentage cytotoxicity versus the total concentration of EEBU and amphotericin B. The significance level was ****p < 0.0001. [Click here to view] |
Anti-amastigote activity
The effect of various concentrations of each molecule on parasite-infected macrophages was examined. The test sample VA2 reduced the infection of macrophages, and a substantial effect was observed at 5 µM as compared to EEBU and VA1 (Fig. 10, Table 1).
The selectivity indices table no. 2 describes amongst all test samples, VA2 is more selective than VA1 and EEBU for L. donovani than the host macrophages.
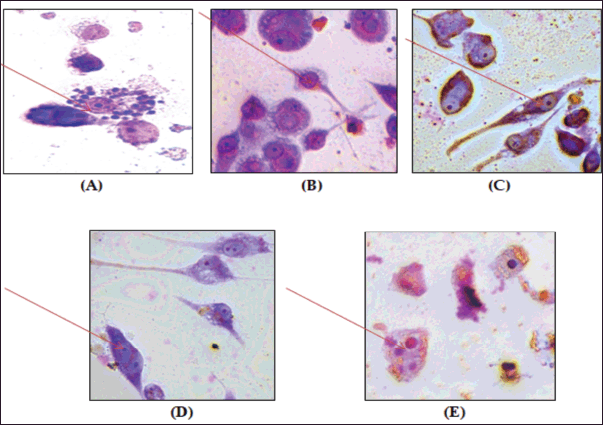 | Figure 10. Anti-amastigote activity of betulin, the semisynthetic derivative, and the ethanolic extract. (A) Untreated control, (B) amphotericin B (50 μM), (C) VA1 (betulin) (50 μM), (D) V2 semisynthetic derivative (50 μM), and (E) EEBU ethanolic extract of B. utilis (50 μM) increase of 100x. The arrow indicates internalized parasites. [Click here to view] |
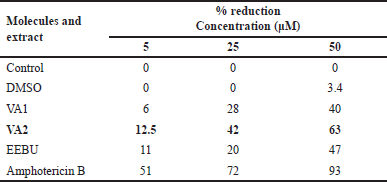 | Table 1. Anti-amastigote activity of the molecules and EEBU. [Click here to view] |
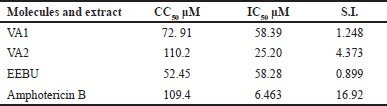 | Table 2. Selectivity index of the molecules and EEBU. [Click here to view] |
DISCUSSION
As per the literature survey, naturally occurring triterpenes from the lupane class, such as betulinic acid, betulin, and betulonic acid, exhibit leishmanicidal properties [37]. Numerous triterpenoid derivatives were synthesized using the lupane skeleton as a basis because of its adaptable positions, which included the hydroxy groups at C-3 and C-28 and the carbon–carbon double bond at C-20–C-29. These derivatives were specifically designed with the aim of potentially exhibiting anti-leishmanial activity. The compounds were examined against L. donovani axenic amastigotes at a concentration of 50 μM using a microplate assay. In the microplate assay, betulin demonstrated considerable anti-leishmanial activity against L. donovani axenic amastigotes, with 35% inhibition at 50 μM. Anti-leishmanial activity was often retained when the hydroxy groups at C-3 or C-28 were acetylated, esterified, or etherified. Additionally, 3-O-acetyl betulin showed anti-leishmanial inhibitory action that was similar to that of the starting material, betulin. Nevertheless, it was discovered that 3, 28-di-O-acetylbetulin and 3, 28- di-O-levulinoylbetulin were totally ineffective against leishmanial inhibition. The efficacy of derivatives of betulin and betulinic acid against L. donovani amastigotes has not been extensively reported; IC50 values have been found to be around 8.9 and 30 mM [38]. Additionally, research on L. amazonensis promastigotes has revealed IC50 values ranging from 44.9 to 69.9 mM [39]. 3β-Hydroxy-(20R)-lupan-29-oxo-28-yl-1H-imidazole-1-carboxylate, the betulin derivative BT06, showed demonstrated notable effectiveness against L. infantum, with an IC50 value of 50.8 mM.
In the present study, the overall yield of ethanolic extract was 57.91% and the compound (Betulin) was isolated and purified by column chromatography by using mobile phase (toluene: ethyl acetate 90:10). The melting range of the isolated compound (Betulin) was found to be 242°C–246°C. The molecular docking study revealed favorable interactions between amphotericin B, VA1, and VA2 compounds and the PTR1 enzyme of L. donovani. The ester derivative was synthesized keeping in view that Leishmania parasite: PTR1 is a crucial enzyme for pterin salvage in the Leishmania parasite, making it a possible target for an improved anti-leishmanial activity. Good binding affinity was observed for the semisynthetic derivative (VA2) THR-146, and GLU-123 with a docking score of −5.724 kCal/mol as compared to Betulin (Parent moiety) binding affinity was observed GLU-123 with a docking score of −3.578 kCal/mol. The in vitro anti-leishmanial activity against L. donovani and IC50 values were found to be as follows: VA1 58.39 ± 0.4977 μM, VA2 25.20 ± 0.4459 μM, and EEBU 58.26 ± 0.4325 μM, showed significant level ****p < 0.0001 against L. donovani. However, VA2 exhibited strong anti-leishmanial activity, as well as was found to be active against promastigote cells.
In the pursuit of discovering new drugs, it is crucial that molecules VA1, VA2, and EEBU cytotoxicity IC50 values were found to be 72. 91 ± 0.9765, 110.2 ± 0.6858, and 52.45 ± 0.1124 μM showed significant levels ****p < 0.0001 exhibiting anti-leishmanial activity and at the same time do not induce significant toxicity in host cells. This study employed a macrophage cell line to evaluate the toxicity of the most active derivative. We found that VA2 was not cytotoxic to the macrophage cell line, suggesting that it is safe for use in mammalian cells and a promising molecule for the discovery of new anti-leishmanial medicines against Leishmania infections. It is imperative to mention that VA1 and EEBU are also not toxic to the macrophage cell line, but comparatively exhibit less anti-leishmanial activity as compared to VA2. After the determination of the IC50, the effect of VA1, VA2, and EEBU was examined on parasite-infected macrophages. The VA2 at 5 µM significantly reduced the infection of macrophages.
On the basis of activity data and molecular docking analysis, it was found that the synthesized compound had the potential to inhibit PTR1enzyme.
CONCLUSION
The present study is the first to report anti-leishmanial activity of ethanolic extract of B. utilis and semisynthetic ester derivative (3, 5- dinitro benzoate betulin). The semisynthetic derivative (VA2) of isolated betulin tested against L. donovani was found to be more potent than isolated betulin (VA1) or the ethanolic plant extract (EEBU) of B. utilis. The amphotericin B was taken as a standard in the present study. Docking studies revealed that VA2 interacts more strongly with the receptor site and has the potential to inhibit L. donovani PTR1enzyme. In the future, novel ester derivatives of betulin need to be designed, synthesized, and evaluated against anti-leishmanial activity with less toxicity in macrophage cell lines or in human host cells. Furthermore, animal studies are required to support in vitro evaluation against L. donovani.
ACKNOWLEDGMENTS
The facilities provided by SPER, Jamia Hamdard are duely acknowledged. The authors are thankful for the financial support provided by the Hamdard National Foundation Doctorate fellowship (HNF).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Kaufer A, Ellis J, Stark D, Barratt J. The evolution of trypanosomatid taxonomy. Parasit Vect. 2017;10:1–7. CrossRef
2. Lockard RD, Wilson ME, Rodríguez NE. Sex-related differences in immune response and symptomatic manifestations to infection with Leishmania species. J Immunol Res. 2019;(1):4103819. CrossRef
3. Ahuja K, Beg MA, Sharma R, Saxena A, Naqvi N, Puri N, et al. A novel signal sequence negative multimeric glycosomal protein required for cell cycle progression of Leishmania donovani parasites. BBA Adv. 2018;1865(8):1148–59. CrossRef
4. World Health Organization. Health sector bulletin. Geneva, Switzerland: World Health Organization; 2020. [cited 2023 December 29]. Available from: https://healthcluster.who.int/docs/librariesprovider16/meeting-reports/syria-health-sector- bulletin-jan-2020.pdf? CrossRef
5. Scarpini S, Dondi A, Totaro C, Biagi C, Melchionda F, Zama D, et al. Visceral leishmaniasis: epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms. 2022;10(10):1887. CrossRef
6. Leta S, Dao TH, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. 2014;8(9):e3131. CrossRef
7. Selvapandiyan A, Croft SL, Rijal S, Nakhasi HL, Ganguly NK. Innovations for the elimination and control of visceral leishmaniasis. PLoS Negl Trop Dis. 2019;13(9):e0007616. CrossRef
8. Rastogi S, Pandey MM, Rawat AK. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J Ethnopharmacol. 2015;159:62–83. CrossRef
9. Verma D, Ajgaonkar S, Sahu N, Rane M, Teli N. Pharmacological and phytochemical properties of Betula utilis: an overview. Res J Pharm Biol Chem Sci. 2014;5:284–8. CrossRef
10. Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006;29(1):1–3. CrossRef
11. Cichewicz RH, Kouzi SA. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev. 2004;24(1):90–114. CrossRef
12. Amiri S, Dastghaib S, Ahmadi M, Mehrbod P, Khadem F, Behrouj H, et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol Adv. 2020;38:107409. CrossRef
13. Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev. 2019;18:929–51. CrossRef
14. Siman P, Filipova A, Ticha A, Niang M, Bezrouk A, Havelek R. Effective method of purification of betulin from birch bark: the importance of its purity for scientific and medicinal use. PLoS One. 2016;11(5):e0154933. CrossRef
15. Chrobak E, Jastrz?bska M, B?benek E, Kadela-Tomanek M, Marciniec K, Latocha M, et al. Molecular structure, in vitro anticancer study and molecular docking of new phosphate derivatives of betulin. Molecules. 2021;26(3):737. CrossRef
16. Wang J, Wu J, Han Y, Zhang J, Lin Y, Wang H, et al. Synthesis and biological evaluation of novel betulin derivatives with aromatic hydrazone side chain as potential anticancer agents. J Braz Chem Soc. 2022;33(03):227–37. CrossRef
17. Bebenek E, Chodurek E, Orchel A, Dzierzewicz Z, Boryczka S. Antiproliferative activity of novel acetylenic derivatives of betulin against G-361 human melanoma cells. Acta Pol Pharm. 2015;72(4):699–703. CrossRef
18. Santos RC, Salvador JA, Marin S, Cascante M, Moreira JN, Dinis TC. Synthesis and structure–activity relationship study of novel cytotoxic carbamate and N-acylheterocyclic bearing derivatives of betulin and betulinic acid. Bioorg Med Chem. 2010;18(12):4385–96. CrossRef
19. Tang J, Jones SA, Jeffery JL, Miranda SR, Galardi CM, Irlbeck DM, et al. Synthesis and biological evaluation of macrocyclized betulin derivatives as a novel class of anti-HIV-1 maturation inhibitors. Open J Med Chem. 2014;8:23. CrossRef
20. Krol SK, Kie?bus M, Rivero-Müller A, Stepulak A. Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int. 2015;(1):584189. CrossRef
21. Alakurtti S, Heiska T, Kiriazis A, Sacerdoti-Sierra N, Jaffe CL, Yli-Kauhaluoma J. Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. Bioorg Med Chem. 2010;18(4):1573–82. CrossRef
22. Sousa MC, Varandas R, Santos RC, Santos-Rosa M, Alves V, Salvador JA. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine. PLoS One 2014;9(3):e89939. CrossRef
23. Chowdhury S, Mukherjee T, Sengupta S, Chowdhury SR, Mukhopadhyay S, Majumder HK. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Mol Pharmacol. 2011;80(4):694–703. CrossRef
24. Zhang Y, Xhaard H, Ghemtio L. Predictive classification models and targets identification for betulin derivatives as Leishmania donovani inhibitors. J Cheminf. 2018;10:1–6. CrossRef
25. Joshi H, Saxena GK, Singh V, Arya E, Singh RP. Phytochemical investigation, isolation and characterization of betulin from bark of Betula utilis. J Pharmacogn Phytochem. 2013;2(1):145–51. CrossRef
26. Li TS, Wang JX, Zheng XJ. Simple synthesis of allobetulin, 28-oxyallobetulin and related biomarkers from betulin and betulinic acid catalysed by solid acids. J Chem Soc Perkin Transactions 1. 1998;23:3957–66. CrossRef
27. Patil SR, Asrondkar A, Patil V, Sangshetti JN, Kalam Khan FA, Damale MG, et al. Antileishmanial potential of fused 5-(pyrazin-2-yl)-4H-1, 2, 4-triazole-3-thiols: synthesis, biological evaluations and computational studies. Bioorg Med Chem Lett. 2017;27(16):3845–50. CrossRef
28. Gao J, Liang L, Zhu Y, Qiu S, Wang T, Zhang L. Ligand and structure-based approaches for the identification of peptide deformylase inhibitors as antibacterial drugs. Int J Mol Sci. 2016;17(7):1141. CrossRef
29. Reddy KP, Singh AB, Puri A, Srivastava AK, Narender T. Synthesis of novel triterpenoid (lupeol) derivatives and their in vivo antihyperglycemic and antidyslipidemic activity. Bioorg Med Chem Lett. 2009;19(15):4463–6. CrossRef
30. P?cak P, Orzechowska B, Chrobak E, Boryczka S. Novel betulin dicarboxylic acid ester derivatives as potent antiviral agents: design, synthesis, biological evaluation, structure-activity relationship and in-silico study. Eur J Med Chem 2021;225:113738. CrossRef
31. Joshi M, Dwyer DM, Nakhasi HL. Cloning and characterization of differentially expressed genes from in vitro-grown ‘amastigotes’ of Leishmania donovani. Mol Biochem Parasitol. 1993;58(2):345–54. CrossRef
32. Ahuja K, Arora G, Khare P, Selvapandiyan A. Selective elimination of Leptomonas from the in vitro co-culture with Leishmania, Parasitol Int. 2015;64:1–5. CrossRef
33. Fumarola L, Spinelli R, Brandonisio O. In vitro assays for evaluation of drug activity against Leishmania spp. Res Microbiol. 2004;155(4):224–30. CrossRef
34. Sharma S, Anjaneyulu Yakkala P, Beg MA, Tanwar S, Latief I, Khan A, et al. 5-Arylidene-2, 4-thiazolidinediones as cysteine protease inhibitors against Leishmania donovani. Chem Select. 2023;8(29):e202302415. CrossRef
35. Das R, Roy A, Dutta N, Majumder HK. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis. 2008;13:867–82. CrossRef
36. Comandolli-Wyrepkowski CD, Jensen BB, Grafova I, Santos PA, Barros AM, Soares FV, et al. Antileishmanial activity of extracts from Libidibia ferrea: development of in vitro and in vivo tests. Acta Amazonica. 2017;47:331–40. CrossRef
37. Vats K, Tandon R, Beg MA, Corrales RM, Yagoubat A, Reyaz E, et al. Interaction of novel proteins, centrin4 and protein of centriole in Leishmania parasite and their effects on the parasite growth. Biochim Biophys Acta, Mol Cell Res. 2023;1870(3):119416. CrossRef
38. Alakurtti S, Bergström P, Sacerdoti-Sierra N, Jaffe CL, Yli-Kauhaluoma J. Anti-leishmanial activity of betulin derivatives. J Antibiot. 2010;63(3):123–6. CrossRef
39. Domínguez-Carmona DB, Escalante-Erosa F, García-Sosa K, Ruiz-Pinell G, Gutierrez-Yapu D, Chan-Bacab MJ, et al. Antiprotozoal activity of betulinic acid derivatives. Pytoey. 2010;17(5):379–82. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4567_pdf.pdf]