INTRODUCTION
The World Health Organization estimates that more than 80% of the world’s population uses traditional medicine to prevent illness and that more than 70,000 plant species are utilized medicinally [1]. The Ayurvedic system of medicine in India plays an important role because of its therapeutic activities for the development of herbal plants [2]. Apart from Ayurveda, conventional medicine is also used as a home remedy [3]. Herbal remedies have achieved interest among people in the COVID-19 pandemic era due to their proven efficacy as preventatives and immunomodulatory. It is necessary to do extensive, evidence-based research on the therapeutic properties of Ayurvedic medicines and medicinal plants. In the Ayurvedic literature, it is suggested that dhupana karma and yagya karma should be carried out in janpadodhwamsa to defend against infection and clean up the contaminated atmosphere caused by the COVID-19 epidemic, which may be associated with janpadodhwamsa [4].
Native to the Himalayan region, Betula utilis D. Don is found in the subalpine zone and grows between 2,700 and 4,500 m above sea level [4] is also called Himalayan silver birch and Bhojpatra belongs to the Betulaceae family and is broadleaved deciduous angiosperm [5]. Betula utilis survives more than 400 years (long-lived species) [6]. Hindus used it in various religious rituals, and the outer bark is a most valuable part used for writing scriptures, mantras, and texts, especially in Sanskrit. It is a tie around the arm or worn around the neck to protect the kids from all the misfortunes and receive blessings. According to Hindu astrology, people collect the outer bark in a specific nakshatra (lunar mansion, specific day, and time are considered) and make yantras for a prosperous life. In addition, B. utilis wood is aromatic, and hence, it is used in havan for marriage, birth, and religious prayers [7]. The striking Himalayan birch, B. utilis, is known by a variety of local names in the Himalayan region, each of which reflects the distinctive cultural and regional ties that run through this varied atmosphere (Table 1) [8] and taxonomy positions of B. utilis mentioned in (Table 2) [9].
 | Table 1. Vernacular name of B. utilis. [Click here to view] |
 | Table 2. Taxonomy position of B. utilis. [Click here to view] |
A medium-sized tree, B.utilis, can grow to a height of 20 m. The bole of this multibranched tree is typically asymmetrical (Fig. 1A). Short, silky hairs cover its stalk, juvenile leaves, and bracts. It has alternately oriented, deciduous leaves that are oval and irregularly serrated. Its bracts continue to be wider than the nut’s wings. It blooms in May and June and produces lenticular winged nuts on a spike as fruit [10]. Its bark is smooth, shiny, and reddish-white or white. Its outer bark is composed of several smooth layers that can be peeled off in flakes and its inner cortex is reddish and moist. The soil should be well-drained, as the plant selects a variety of soil types, including light sandy, medium loamy, and heavy clay soils. The plant can thrive in soils that are acidic, neutral, or alkaline. It can grow well in semi-shade (light woods) or in the absence of shade [11].
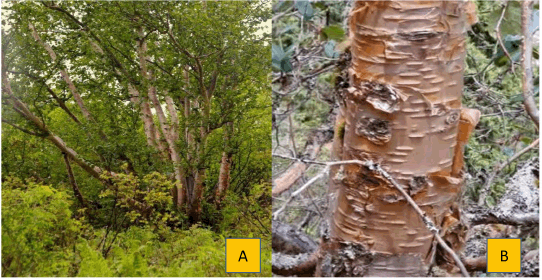 | Figure 1. (A) Plant of Betula utilis. (B) Bark of Betula utilis. [Click here to view] |
According to Ayurvedic Pharmacopoeia, therapeutic uses of B. utilis bark are karnaroga, raktapitta, kustharoga, raksoghnadhupana, vrana, aparapatana, garbhasanga, granthivisarpa, and balagraha [12]. The bark of plants is frequently used in various Ayurvedic medicines to cure several illnesses, such as ear and blood disorders, pneumonia, convulsions, leprosy, and skin infections [13]. Numerous biochemical components of the tree are also utilized for a range of therapeutic purposes. The main phytoconstituents of B. utilis are betulin, lupeol, acetyloleanolic acid, betulitc acid, lupenone, sitosterol, methyl betulonate, and methyl betulate. Various phytochemicals, such as betulinic aldehyde, ursolic acid (UA), and oleanolic acid, are also reported minorly [14].
SOURCES OF LITERATURE
The published information on B. utilis was collected from scientific datasets such as Google, Web of Science, Elsevier, PubMed, Google Scholar, and Semantic Scholar. Search terms were used in this review, “Betula utilis” or “bark of B. utilis,” or “Betulaceae,” “Bhojpatra” or “Birch,” or the genus of Betula along with phrases like “Botanical description,” or “Taxonomy or distribution” or “Phytochemistry,” or “Traditional uses,” “Pharmacology,” or “Toxicology” or “applications.” The search was conducted in any available language. Compound chemical structures were drawn using ChemDraw 15.0 software.
BOTANICAL AND GEOGRAPHICAL DESCRIPTION
Botanical description
The oval, 5–10 cm (2.0–3.9 in) long leaves with serrated margins and a light covering of hairs. Only a few male catkins and short, solitary (sometimes paired) female catkins are present throughout flowering, which lasts from May to July. In male flowers, the perianth has four components; it is missing in female flowers. From September to October, fruits ripen. The thin, paper-like bark has horizontal lenticels and is reddish brown, reddish-white, or white (Fig. 1B). It is extremely glossy. The timber is exceedingly heavy, brittle, and hard. The heartwood is pale reddish brown or pink [15].
Geographical description
Since B. utilis is well suited to cold climates, it can be found in the Himalayas (subalpine region) and is distributed up to high altitudes between 2,700 m and 4,500 m [4]. In the Nanda Devi Biosphere Reserve (Uttarakhand), B. utilis was discovered to be the predominant tree species, with the highest density (739 Ind/ha and 1525 Ind/ha), basal cover (between 22.13 and 28.61 m2/ha), and maximum IVI (between IVI: 217.31 and IVI: 266.56) [16]. Afghanistan, Kazakhstan, Bhutan, China, Nepal, India, Pakistan, Kyrgyzstan, Tajikistan, and Uzbekistan are among their native countries. Its growth ranges from 2,500 m to 4,300 m, and it can be found from the Afghan province of Nuristan to Hebei in northern China [17].
SPECIES OF BETULA
According to the literature and data that are currently available, the twelve most popular species of Betula have been traditionally utilized as medicines in various world regions given in Table 3.
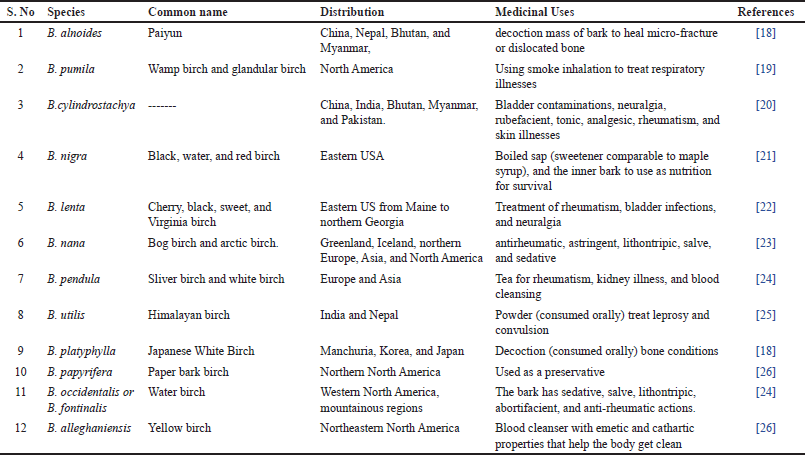 | Table 3. Species of Betula with their medicinal uses. [Click here to view] |
TRADITIONAL USES
Betula utilis is one of the most valuable tree species in Indian traditional medicine. It is used in the Tridosha, which includes “Vata” (air), “Pitta” (phlegm), and “Kapha” (cough). It prepares its herbal medicine as an infusion, powder, paste, and decoction for the treatment of ailments. In addition, plants produce food, gums, and resins. The bark, leaves, and resin are used to cure rheumatism, broken bones, joint stiffness, swellings, asthma, and blood purification [27,28]. The stem bark’s astringent properties make it effective for treating wounds, leprosy, and styptic [29,13]. Its resin is used to treat burns and external wounds as well as for contraception.
The bark is used to cure earaches, kidney, and bladder issues. It is used to treat insanity, epilepsy, and hysteria because it is thought to be a good therapeutic agent for treating psychological diseases. Jaundice, constipation, and cough are also treated with bark [30]. Bark infusion is used to treat flatulence [31]. The bark is also used to heal domesticated animals. The bark is reduced to ashes and its paste is used for the deep wounds and cuts of animals [28].
The leaves are used to treat kidney and bladder stones, and urinary tract infections, as diuretics, and a leaf decoction of B. utilis is frequently utilized. Fever can be treated and fear released with bark paper. To promote family peace, a part of papery bark is kept in households [32]. The bark paper is used to compress ghee, create binding ropes, shield wood from the elements, and relieve ear-related problems. Wood is a fuel source and is used to make spoons and plows [33].
The bark and roots of Betula provide a home for numerous insects and beetles, giving them a niche [34]. Betula leaf tea is used to treat hepatic, gastrointestinal, and respiratory ailments, and also stop hair loss and dandruff [35,36]. Other uses of B. utilis include the making of wrapping material, roof construction, home construction, and the construction of regional bridges by the locals of its distribution range (Fig. 2). The wood is tough and substantial. While living at high altitudes in the Himalayas, pastoral people utilize this wood to build shelters and firewood. These communities also make umbrella covers and rooftops out of the papery bark of B. utilis [8].
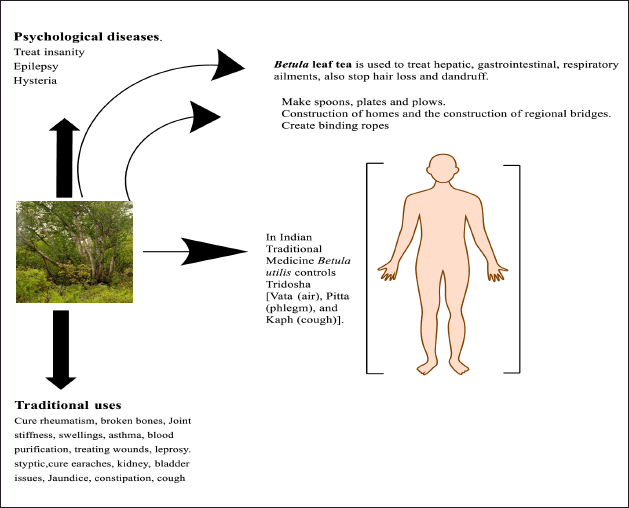 | Figure 2. Traditional uses of Betula utilis. [Click here to view] |
Perybark is used to make plates, while the leaves are used as fodder. Betula utilis trees are also used for landscape beautification and decorative purposes. Numerous wild animals, including musk deer, black bears, and monal, are frequently seen in the B. utilis forest. Additionally, it provides high-altitude wild herbivores food [37]. Ethnomedicinal uses of B. utilis are reported in (Table 4) and traditional uses of different dosage forms are reported in (Table 5).
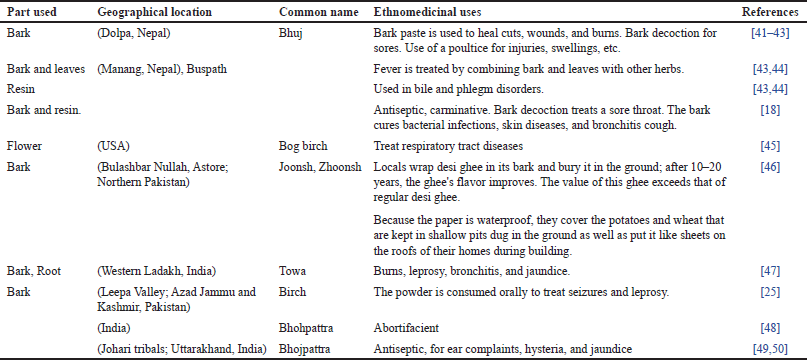 | Table 4. Ethnomedicinal uses of various parts of B. utilis. [Click here to view] |
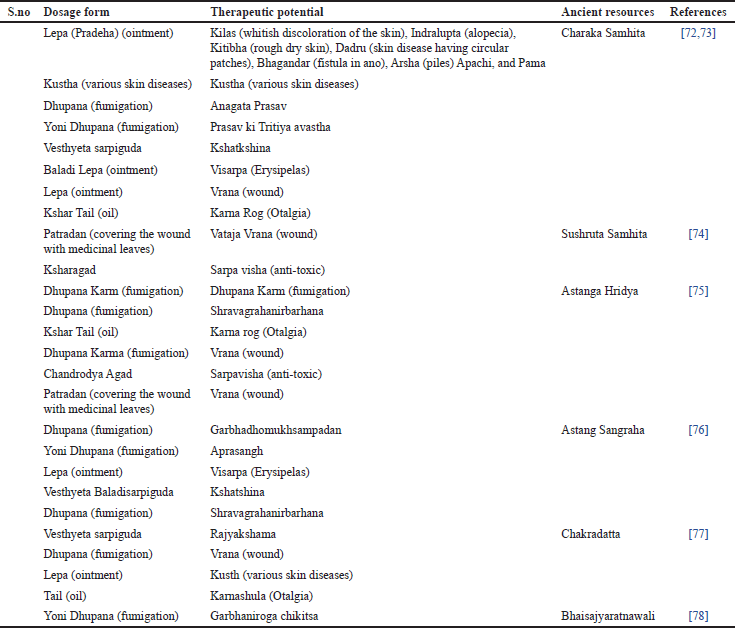 | Table 5. Traditional uses of different dosage forms reported in India. [Click here to view] |
CONVENTIONAL AND MODERN APPLICATION
Asanadi kashaya and Ayaskrithi are two significant herbal remedies used as prescriptions in Ayurveda. Asanadi kashaya is a liquid Ayurvedic medicine that treats various ailments such as diabetes, skin diseases, and obesity and also relieves intestinal worms. Ayaskrithi is also liquid Ayurvedic medicine and is used for the treatment of anemia, skin diseases, chronic urinary disorders, diabetes, hemorrhoids, leucoderma, anorexia, intestinal worm, malabsorption disorder, and obesity [12].
Bharat bhojpatra tablet (a herbal product) manufactured by Bharat and the product number is GHTYR76594. Intimate vaginal tablets (Veeon) manufactured by Veeon are a natural solution for leucorrhoea and vaginal contraction and help in contracting, toning up lax, and sagging vaginal muscles, HOO-IMM PLUS 750 mg for Functiono immune restorative, Jiva Ayurveda (SCIM detox tea) 75 mg for Functiono immune restorative, and aswagandharishtam (Ayurvedic medicine) effective for treating fainting, epilepsy, insanity, swelling, piles emaciation, and arthritis conditions.
PHYTOCHEMISTRY
A wide array of chemicals, including betulin, betulinic acid, acetyloleanolic acid, lupenone, methyl betulonate, sitosterol, and methyl betultriterpenoid, are found in the bark of B. utilis, along with tannins, flavonoids, phenolics, triterpenoids, and essential oils [55,56,57]. Numerous vitamins, minerals, sugars, and carbohydrates are also found in the sap of Himalayan birch trees. Additionally, monoterpenes, phenylpropenes, terpene alcohol, sesquiterpenes, alkenals, esters, acid, and sesquiterpenoid alcohols are found abundantly in the whole plant of B. utilis [51]. The stem bark of B. utilis contains essential oils such as geranic acid (11.38%), seleneol (10.98%), linalool (10.91%), terragon (10.61%), sesquiphellendrene (8.02%), champacol (6.33%), and 1,8-cineol (5.49%), which were also identified by GCMS [52]. The bark of B. utilis contains phenolic compounds such as ferulic acid, caffeic acid, and chlorogenic acid and estimated by UHPLC-ESI-MS/MS [53]. The stem bark of B. utilis contains flavonoids such as quercetin, kaempferol, apigenin, catechin, and luteolin identified by UHPLC-ESI-MS/MS [54,55]. The linoleic (17.66%), myristic (15.9%), palmitic (9.09%), and oleic (11.30%) fatty acid components are also found in B. utilis bark [56,55]. Various minerals, such as calcium, manganese, potassium, magnesium, iron, zinc, sodium, phosphorus, and copper [53], and apart from these minerals, various vitamins, such as B complex and Vitamin C, are also reported in plant [57]. The bark of B. utilis contains triterpenoids such as betulin, betulinic acid, lupeol, karachic acid and oleanolic acid-3-acetate (Fig. 3) [12,58]. Apart from that various modification in botulin and betulinic acid shows their potential as promising agents in various medical treatments [52,59].
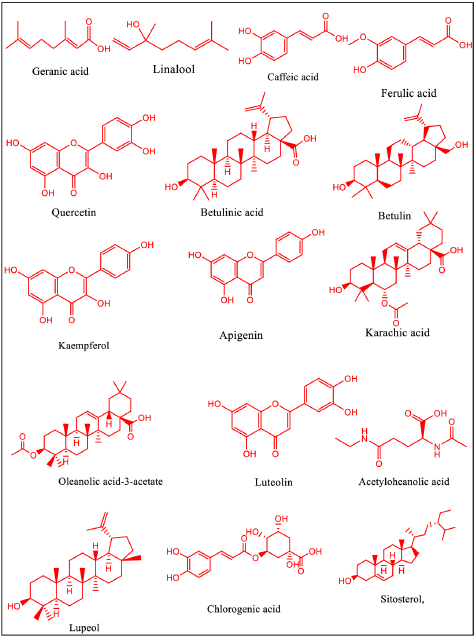 | Figure 3. Structures of chemical constituents present in B utilis. [Click here to view] |
The two most prevalent active compounds in B. utilis are betulin and betulinic acid [60]. Betulin is a pentacyclic triterpene (lupane series) natural compound with systematic name 3β, 28-dihydroxy-20(29)-lupen or lup-20(29)-en-3β, 28-diol, molecular formula C30H50O2, molecular weight 442.728 g.mol-1 (Fig. 3) [61,62]. Betulin is isolated from the various species of Betulaceae, Platanaceae, Dilleniaceae, Rhamnaceae, Rosaceae, and Fagaceae, mainly from the bark of different species of Betulaceae. Betulin was first observed by Lowitz in 1788, from the sublimation products of birch bark. Betulinic acid, the oxidation product of Betulin, was first discovered by Retzlaff in 1902 and was described as an unknown compound extracted from Gratiola officinalis (Plantaginaceae), which was further named as betulinic acid and identified as the oxidation product of betulin by Robertson and colleagues in 1939. This compound was first isolated as a pure chemical substance in 1788, being one of the first natural products identified from plants [63]. Birch bark’s white color is caused by the crystal clustering in the thick-walled, massive cells and betulin occurs in spring [64]. This wide range of betulin content in the dry weight, which ranges from 10% to even 45%, is the result of several elements, including tree type and age, climate patterns, and geographic locality [65,66]. The content of betulin in the root skin and leaves is also present; however, it is considerably lower than that in the outer bark [67]. Betulin exhibits various pharmacological activities, such as antitumor, anti-HIV, antiviral, antibacterial, anti-inflammatory, antileishmanial, antimalarial properties, and anticonvulsant [68–70].
Betulinic acid well-known triterpene, with the chemical formula C30H48O3, chemical name (3-beta-hydroxy-lup20(29)-en-28-oic acid (Fig. 3) is found in the bark of some trees, including the Betula pubescens, Ziziphus mauritiana, sycamore, and other members of the Platanus family (Platanus occidentalis, Platanus orientalis, and Platanus acerfolia). The pharmacological effects of betulinic acid are quite diverse, including the suppression of the human immunodeficiency virus (HIV), as well as antibacterial, antimalarial, anti-inflammatory, anthelmintic, antinociceptive, anti-HSV-1, and anti-cancer properties. Due to its specific cytotoxicity against tumor cells and good therapeutic index, even at doses up to 500-mg/kg body weight, betulinic acid is a very promising innovative chemotherapeutic agent for the treatment of HIV infection and cancer [71]. Betulinic acid is a new anticancer medication that promotes apoptosis, distinguishing it from “classical” anticancer medicines such as doxorubicin. Betulinic acid is a prototypical cytotoxic substance that causes apoptosis through a direct action on mitochondria. In isolated mitochondria, betulinic acid causes a decrease of transmembrane potential that is independent of a benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone inhibitable caspase. This is suppressed by bongkrekic acid, which stabilizes the permeability transition pore complex (PTPC). In a cell-free system, mitochondria are subjected to betulinic acid-induced PT-mediated cleavage of caspase-8 and caspase-3. Significant factors, such as cytochrome C or AIF (apoptosis-inducing factor) generated by betulinic acid-treated mitochondria, are sufficient for caspase cleavage and nuclear fragmentation. The addition of cytochrome C to cytosolic samples causes caspase-3 to be cleaved, but not caspase-8. However, supernatants of PT-treated mitochondria, as well as partly purified AIF, activate both caspase-8 and caspase-3 in cytosolic extracts and are sufficient to activate recombinant caspase-8. These findings suggest that the development of mitochondrial PT alone is inadequate to initiate the whole apoptotic mechanism and that betulinic acid may induce apoptosis by a direct action on mitochondria [79].
Pharmacological activities
This botanical treasure trove is noteworthy because it exhibits a wide range of pharmacological actions, including anti-inflammatory, anti-microbial, anti-cancer, hepatoprotective, anti-psoriatic, anti-obesity, anti-urolithiatic capabilities, anticonvulsant potential, anti-HIV, as well as antioxidant (Fig. 4).
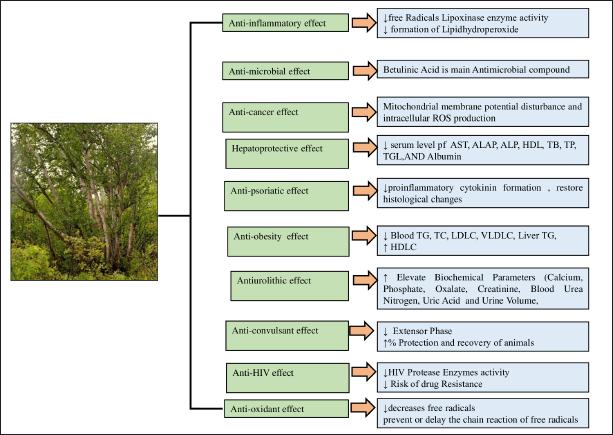 | Figure 4. Pharmacological effect of B. utilis. [Click here to view] |
Anti-inflammatory activity
Methanolic and water extracts were used to evaluate antioxidant and anti-inflammatory activity of B. utilis. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical assay and the 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay were used to assess the extracts’ antioxidant properties. The lipoxygenase inhibition assay was used to measure the anti-inflammatory activity. While indomethacin was utilized as the standard for the lipoxygenase inhibition experiment, ascorbic acid and gallic acid served as reference standards for the DPPH and ABTS assays. With IC50 values of 8.4 μg/ml and 35.08 μg/ml for the DPPH assay and 83.18 μg/ml and 37.14 μg/ml for the ABTS assay, the methanol and water extracts of B. utilis demonstrated strong antioxidant activity. The extracts also displayed anti-inflammatory activity, with 18.74% and 28.78% lipoxygenase inhibition at a dosage of 1.0 mg/ml for the methanol and water extracts, respectively [80,81].
Anti-microbial activity
Plant-based compounds having antibacterial properties are useful as new tools in the fight against antibiotic resistance. The current work determines the chemical components that give B. utilis, a plant grown at high altitudes in the Himalayas of India, its antibacterial properties, and evaluates the antimicrobial activity of the essential oil isolated from the fresh bark [40,82]. Bark of B. utilis has antimicrobial activity in contradiction of many significant human pathogenic bacteria. The chemical composition of the essential oil was analyzed using gas chromatography–mass spectrometry, which revealed the major constituents as geranic acid (11.38%), β-seleneol (10.98%), β-linalool (10.91%), terragon (10.61%), β-sesquiphellandrene (8.02%), champacol (6.33%), and 1,8-cineole (5.49%). The antimicrobial activity of the essential oil was assessed using a micro-dilution assay against human pathogenic bacteria and fungi. The results demonstrated that the essential oil exhibited strong antimicrobial activity, particularly against Candida albicans Gram-positive and Gram-negative human pathogenic bacteria, with minimum inhibitory concentrations ranging from 60.5 to 240 µg/ml [40].
Anti-cancer activity
The in vitro anticancer activity of different B. utilis fractions in nine different cancer cell lines and the ethyl acetate fraction proved to be one of the most effective fractions in terms of inducing cytotoxic activity against various cancer cell lines. Six triterpenes, including betulin, betulinic acid, lupeol, UA, oleanolic acid, and amyrin, have also been isolated from ethyl acetate extract. The in vitro cytotoxic activity of isolated triterpenes against six distinct cancer cell lines was then examined, and it was discovered that UA was selective for breast cancer cells over nontumorigenic breast epithelial cells (MCF 10A). The main cause of UA tumor cell-specific apoptotic effect was related to the upregulation of DR4, DR5, and PARP cleavage in MCF-7 cells over nontumorigenic MCF-10A cells, which activated the extrinsic apoptosis pathway. Additionally, the anti-cancer effects of UA are significantly influenced by mitochondrial membrane potential disturbance and intracellular ROS production caused by UA and also prevent the spread of breast cancer [78].
Hepatoprotective activity
The hepatoprotective potential of B. utilis ethanolic and aqueous extracts against D-galactosamine-induced liver damage was assessed in vitro and in vivo. The hepatoprotective efficacy of ethanolic and aqueous B. utilis extracts against D-galactosamine (D-galN) at various concentrations was evaluated in vitro on isolated rat hepatocytes. Then, against D-galN, which when administered intravenously at a dose of 400-mg/kg body weight caused liver damage in rats, in vivo hepatoprotective studies of BUE and BUA at doses of 100- and 200-mg/kg body weight were performed. The hepatocytes were tested with 50 μl of a 10-mM D-galN solution. When contrast to the control group, the group to which the toxicant was introduced displayed a significantly higher level of the ASAT, ALAT, and ALP enzymes. Significantly, more of all the biochemical indicators were restored to normal. The plant extract’s minimum restoration level was 62.5 g/ml in both cases. Additionally, in vivo administration of the BUE and BUA extract resulted in dose-dependent significant reductions in the serum levels of ASAT, ALAT, ALP, LDH, and TB and significant increases in the serum levels of TP, TGL, and albumin. However, the liver of the DgalN-treated group showed severe centrilobular necrosis, localized necrosis, and bile duct growth, entirely losing its normal architecture [83].
Anti-psoriatic activity
Evaluation of anti-psoriatic efficacy was done by imiquimod (IMQ 5%)-induced psoriasis-like skin inflammation model. The experimental groups assessed psoriasis area and severity index (PASI) scores, macroscopically and behavioral evaluation, splenomegaly, cytokine levels, and histological alterations in the in vivo screening models [52]. The outcome is consistent with the PASI score (57.14% and 61.9%), with rehabilitation rates of test BE solution (180 mg/kg) and standard Betamethasone dipropionate ointment (BD-oint.0.5 mg/g), respectively. By focusing on other factors, BE demonstrates related outcomes, including improved macroscopically with behavioral circumstances, decreased proinflammatory cytokine affirmation, and restored histological changes with those of BD. Phytoconstituents from B. utilis BE-isolated Betula are a promising agent and a first step in the treatment of psoriasis, according to results [84].
Anti-obesity activity
The bark of B. utilis has anti-obesity properties against cafeteria diet-induced obesity in mice. The cafeteria diet experimental model was used to evaluate body weight, glucose level, liver weight, and TG level in the serum. According to reported activity for the experimental models, in rats with cafeteria diet-induced obesity models, B. utilis bark extract showed dose-dependent significant anti-obesity action by reducing blood TG, TC, LDL-C, VLDL-C, and liver TG and rising serum HDL-C as well as by boosting locomotor activity. Body weight, liver weight, and food intake all decreased as well. According to the findings, animals with EBEB at 200 mg/kg are more significant than those at 100 mg/kg [84]. The anti-obesity activity of Betula utilis was observed to exhibit a dose-dependent effect. After 4 weeks of HFD treatment, BU (100–400 mg/kg/day p.o) was given along with a high-fat diet for further 6 weeks, which significantly reduced HFD-induced body weight gain and an increase in adipose tissue mass in a dose-dependent manner. Moreover, BU attenuated HFD-induced augmented serum glucose, TG, and TC. The anti-obesity potential of BU was comparable to a marketed drug orlistat. These results reflect that BU supplementation decreases body weight and improves obesity serum biomarkers (TG, TC, and LDL), and the weight-reducing activity of BU may be mediated by decreased fat absorption from the GIT [85].
Anti-urolithic activity
Alcoholic B. utilis extract has anti-urolithiatic activity in rats using an ethylene glycol model. Betula utilis alcohol extract (250 and 500 mg/kg) and common medications (750-mg/kg cystone) were utilized. Numerous criteria were examined, such as urinary volume, urine pH, urine analysis, serum analysis, kidney homogenate, and histology of the kidney. In comparison to the model control, the results showed that giving rats with ethylene glycol-induced lithiasis an alcoholic extract of B. utilis significantly decreased all of the elevated biochemical parameters (calcium, phosphate, oxalate, creatinine, blood urea nitrogen, and uric acid), returned the urine pH to normal, and increased the urine volume. According to this study, alcoholic B. utilis extract can treat urolithiasis [86].
Anticonvulsant activity
The hydroalcoholic extract of B. utilis was used for the anticonvulsant and anxiolytic activity. According to OECD 425 Guidelines, an acute oral toxicity study revealed that at a dose of 2,000 mg/kg, 63% of the animals perished. Therefore, 100 mg/kg and 200 mg/kg orally were chosen for their convulsant and anxiolytic activities. At doses of 100 and 200 mg/kg, the extract demonstrated a reduction in the duration of the extensor phase and an increase in the percentage of protection. All animals were entirely protected by phenytoin, which completely decreased the duration of the tonic extensor phase [87].
Anti-HIV activity
Betulinic acid prevents HIV-1 replication at several points throughout the viral life cycle [88]. The capacity including its capacity to interfere with viral entry into host cells and suppress the activity of the HIV protease enzyme, both of which are necessary for the virus to mature. Researchers have created betulinic acid compounds with improved anti-HIV efficacy [89].
Bioassay-guided fractionation of the ethanol extract of Betula species stems using chromatographic techniques yielded five pentacyclic triterpenoids: betulinic acid, betulin, lupeol, oleanolic acid, and UA. Among these, betulin demonstrated the most potent inhibitory activity against HIV-1 integrase (IN) with an IC50 value of 17.7 μM. Computational docking studies revealed interactions of the active compounds with key residues of the IN active site, including Asp64, involved in 3′-processing, and Thr66, His67, and Lys159, which participate in strand-transfer reactions during the integration process. Furthermore, all compounds exhibited significant anti-inflammatory effects by inhibiting LPS-induced nitric oxide production (IC50 < 68.7 μM). These findings provide additional scientific evidence supporting the traditional medicinal use of Betula species in the treatment of HIV and related inflammatory conditions [90,91].
Anti-oxidant activity
The antioxidant activity by using the DPPH, 2, 2’-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and lipoxygenase inhibition assays, and the antioxidant properties of the B. utilis extracts were assessed. The B. utilis methanolic and water extract demonstrated DPPH and ABS scavenging activities (8.4, 35.08 g/ml IC50 for DPPH and 83.18, 37.14 g/ml IC50 for ABS test), but very little activity against ABS and lipoxygenase inhibition (18.74% and 28.78% inhibition at 1.0 mg/ml). The findings of this investigation suggest that B. utilis may be a source of antioxidants. Although B. utilis possesses free radical scavenging activity, it decreases free radicals, which could prevent or delay the chain reaction of free radicals that slows the spread of the oxidation mechanism [79]. Antioxidant activity by using various in vitro techniques includes the DPPH free radical scavenging assay, SOS activity, hydroxyl radical scavenging, and ferric thiocyanate activity of B. utilis extracts. The ethyl acetate and methanolic extracts had a percent inhibition of 72% and an IC50 value of 30.16 g/ml and 83.86% and 27.62 g/ml for their ability to scavenge DPPH radicals. The reference substance utilized was ascorbic acid, which had an IC50 value of 25.82 g/ml and a percent inhibition of 91.81%. The SOS activity of the ethyl acetate extract indicated a percent inhibition of 72.49% and an IC50 value of 44.47 g/ml. Similar results were seen for the SOS scavenger activity of methanol extract, which showed a percent inhibition of 81.49% and an IC50 value of 20 g/ml. Ascorbic acid was utilized as a reference substance for SOS activity; it displayed a percent inhibition of 86.72% and an IC50 value of 12.01 g/ml. The extracts of ethyl acetate and methanol showed a percent inhibition of 76.93% and 81.62%, and their respective IC50 values were reported to be 48.003 and 27.14 g/ml. Similar to this, ethyl acetate and methanol extract’s ferric thiocyanate activity showed percent inhibition values of 74.68% and 81.27%, and their respective IC50 values were reported to be 40.112 and 23.79 g/ml. The B. utilis may be a valuable source of antioxidants that can be used to create effective therapeutic products in the future [92].
TOXICITY AND SIDE EFFECTS
Himalayan birch may be safe at therapeutic doses for the majority of humans, even for brief periods. Regarding the usage of Himalayan birch, while breastfeeding or during pregnancy, there is not enough trustworthy information available. Birch pollen is a major cause of airborne allergies, with many patients also hypersensitive to apples. This study investigated the role of HLA class II genes in birch pollen allergy with or without food allergies. Blood samples from 42 atopic patients and 42 healthy controls were analyzed. The antibody responses were assessed, and DNA was genotyped using PCR-RFLP. While no differences were found in DPB1 alleles, HLA-DR4 and/or DR7 alleles were present in 42.6% of patients but only 2.4% of controls. The study concludes that HLA-DR7 is linked to allergen presentation and atopy, rather than specific allergen responses [93].
The utilization of birch leaf extract by those with high blood pressure raises some questions because it may raise the quantity of Na (salt) that the body preserves, which can exacerbate the condition [28].
CONCLUSION
In conclusion, B. utilis, also known as Himalayan birch or Bhojpatra, is a medicinal plant with a rich phytochemical composition. This plant has been used traditionally in various cultures and has drawn attention in modern research for its potential pharmacological properties. Betula utilis is abundant in triterpenoids (betulin and betulinic acid), flavonoids, and essential oils, which are associated with their medicinal properties. Research has indicated that B. utilis and its constituents exhibit a wide range of pharmacological actions, including anticancer, antiviral (against influenza and HIV), anti-inflammatory, antibacterial, hepatoprotective, antioxidant, anti-psoriatic, anti-convulsant, and anti-urolithiatic effects. Betula species, including B. utilis, have a historical tradition of use in various cultures for conditions such as arthritis, pain relief, and bone-related disorders. Betula utilis is incorporated into Ayurvedic formulations, such as Asanadi kashaya and Ayaskrithi, for the management of various ailments. It is also utilized as an active ingredient in modern herbal products, including tablets and vaginal tablets, reflecting its potential in contemporary herbal medicine. While promising, further research is needed to uncover additional properties, conduct toxicity studies, and better understand the full scope of benefits and potential side effects associated with B. utilis.
FUTURE PERSPECTIVE
Future applications for B. utilis include medicine, pharmaceuticals, natural products, and conservation. Realizing its full potential and guaranteeing its sustainability in the wild will depend on continued research and ethical use. The nutraceutical and functional food sectors might use extracts from B. utilis. They might be used in food goods intended to improve health and well-being. Conservation efforts to safeguard the plant and its environment in the Himalayan region may become more urgent as interest in B. utilis develops. To guarantee a steady supply of this priceless resource, it could be possible to investigate sustainable harvesting methods and agricultural practices. The B. utilis tree might be important to the natural products sector. Making use of their potential health and aesthetic benefits, their extracts and derivatives could be added to skin care products, herbal treatments, and dietary supplements. In laboratory experiments, compounds produced from B. utilis, particularly betulinic acid and its derivatives, have demonstrated potential as therapeutic development prospects. Preclinical and clinical trials may be conducted in the future to further refine these molecules and produce novel medications to treat various ailments.
ACKNOWLEDGMENT
Author’s Acknowledge Central Library Facility, Internet Facility provided by Jamia Hamdard, New Delhi.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this review article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicine. Geneva, Switzerland: World Health Organization; 2000.
2. Chattergee A, Pakrashi SC. Introduction In: The treatise on Indian medicinal plants. New Delhi, India: CSIR; 1992.
3. Das M, Barua N. Pharmacological activities of Solanum melongena Linn.(Brinjal plant). Int J Green Pharm. 2013;7(4):274–7. CrossRef
4. Zobel DB, Singh SP. Himalayan forests and ecological generalizations. BioScience. 1997;47(11):735–45. CrossRef
5. Dasila K, Pandey A, Samant SS, Pande V. Endophytes associated with Himalayan silver birch (Betula utilis D. Don) roots in relation to season and soil parameters. Appl Soil Ecol. 2020;149:103513. CrossRef
6. Bhattacharyya A, Shah SK, Chaudhary V. Would tree ring data of Betula utilis be potential for the analysis of Himalayan glacial fluctuations. Curr Sci. 2006;25:754–61.
7. Kala CP. Uses, population status and management of Betula utilis. Appl Ecol Environ Res. 2018;6(3):79–83. CrossRef
8. Verma D, Ajgaonkar S, Sahu N, Rane M, Teli N. Pharmacological and phytochemical properties of Betula utilis: an overview. Res J Pharm Biol Chem Sci. 2014;5(5):284–8.
9. Joshi H, Saxena GK, Singh V, Arya E, Singh RP. Phytochemical investigation, isolation and characterization of betulin from bark of Betula utilis. J Pharmacogn Phytochem. 2013;2(1):145–51.
10. Hooker JD. The flora of British India. London, UK: L. Reeve; 1890.
11. Wani ZA, Pant S. Betula utilis D. Don: an ecologically and economically important timberline species of Himalayan Region in Jeopardy. Bot Rev. 2021;87(3):377–91. CrossRef
12. The Ayurvedic Pharmacopoeia. National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, India: Pharmacopoeia Commission for Indian Medicine & Homoeopathy, Ministry of AYUSH, Government of India, 2016.
13. Chauhan NS. Medicinal and aromatic plants of Himachal Pradesh. New Delhi, India: Indus Publishes; 1999.
14. Kumaraswamy MV, Kavitha HU, Satish S. Antibacterial evaluation and phytochemical analysis of Betula utilis D. Don against some human pathogenic bacteria. World J Agric Sci. 2008;4(5):661–4.
15. Rastogi RP, Mehrotra BN 2004. Indian medicinal plants: compendium of indian medicinal plants. Niscair. 2004;1:57.
16. Maletha A, Maikhuri RK, Bargali SS. Criteria and indicator for assessing threat on Himalayan birch (B. utilis) at timberline ecotone of Nanda Devi Biosphere Reserve: a world heritage site, Western Himalaya, India. Environ Sustain Indic. 2020;8:100086. CrossRef
17. Shaw K, Roy S, Wilson B. Betula utilis. The IUCN Red list of threatened species 2014:e.T194535A2346136. [cited 2023 Oct 04]. CrossRef
18. Moerman D. Native American Ethnobotany.1st edition. Portland, OR: Timber Press; 1998. pp 1098.
19. Singla C, Ali M. Antidandruff activity and chemical constituents of the leaves of Betula cylindrostachya Lindl. ex Wall. J Med Plants Stud. 2018;6(4):18993.
20. Harlow WM, Harrar ES. Textbook of dendrology 5th edition. New York City, NY: McGraw Hill Book Company; 1969.
21. Woods KE, Chhetri BK, Jones CD, Goel N, Setzer WN. Bioactivities and compositions of Betula nigra essential oils. J Med Plant. 2013;2(1-2):1–9. CrossRef
22. Murphy BJ, Carlson RE, Howa JD, Wilson TM, Buch RM. Determining the authenticity of methyl salicylate in Gaultheria procumbens L. and Betula lenta L. essential oils using isotope ratio mass spectrometry. J Essent Oil Res. 2021;33(5):442–51.
23. Saric-Kundalic B, Dobes C, Klatte-Asselmeyer V, Saukel J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J Ethnopharmacol. 2010;131(1):33–55. CrossRef
24. Kim H, Song MJ. Analysis and recordings of orally transmitted knowledge about medicinal plants in the southern mountainous region of Korea. J Ethnopharmacol. 2011;134(3):676–96. CrossRef
25. Malhi BS, Trivedi VP. Vegetable antifertility drugs of India. Int J Crude Drug Res. 1972;12(3):1922–8. CrossRef
26. Quality standards of Indian medicinal plants. Medicinal plants unit Indian council of medical research, New Delhi, India: ICMR; 2012.
27. Phondani PC. A study on prioritization and categorization of specific ailments in different high altitude tribal and non-tribal communities and their traditional plant based treatments in Central Himalaya. Unpublished Ph. D. Thesis submitted to HNB Garhwal Central University, Srinagar (Garhwal) Uttarakhand, India. 2010.
28. Sharma N. Conservation and utilization of medicinal and aromatic plants in Dhauladhar mountain range of Himachal Pradesh. Ph.D. Thesis. Forest Research Institute (Deemed University), Dehradun, India: 2017.
29. Selvam A. Inventory of vegetable crude drug samples housed in botanical survey of India, Howrah. Pharmacogn Rev. 2008;2(3):61.
30. Jain SK. Dictionary of Indian folk medicine and ethnobotany. 1991.
31. Anonymous. The Wealth of India: Raw Materials. National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, India: Publications and Information Directorate, CSIR; 1989.
32. Khan N, Ahmed M, Ahmed A, Shaukat SS, Wahab M, Ajaib M, et al. Important medicinal plants of chitral gol National park (cgnp) Pakistan. Pak J Bot. 2011;2:797–809.
33. Zaki M, Sofi MS, Kaloo ZA. A reproducible protocol for raising clonal plants from leaf segments excised from mature trees of Betula utilis a threatened tree species of Kashmir Himalayas. Int J Adv Multidiscip Res. 2011;1(5):07–13.
34. Khanday AL, Buhroo AA. Shothole borer (Scolytus nitidus Schedl) (Coleoptera: Curculionidae: Scolytinae): a new host-Himalayan birch (Betula utilis)-Short Communication. Nat Sci. 2015;13:15–26.
35. Mir NA, Masoodi TH, Geelani SM, Wani AA, Parrey GN, Mugloo JA. Floristic diversity along altitudinal gradient under Betula utilis in North Western Himalayas of Kashmir, India. Acta Ecologica Sinica. 2019;39(5):362–71. CrossRef
36. Dawadi B, Liang E, Tian L, Devkota LP, Yao T. Pre-monsoon precipitation signal in tree rings of timberline Betula utilis in the central Himalayas. Quat Int. 2013;283:72–7. CrossRef
37. Manandhar NP. Plants and people of Nepal. Portland, OR: Timber Press; 2002.
38. Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14(6):2016–31. CrossRef
39. Shukla S, Mishra T, Pal M, Meena B, Rana TS, Upreti DK. Comparative analysis of fatty acids and antioxidant activity of Betula utilis bark collected from different geographical region of India. Free Radic Antioxid. 2017;7(1):80–5. CrossRef
40. Pal M, Mishra T, Kumar A, Baleshwar, Upreti DK, Rana TS. Chemical constituents and antimicrobial potential of essential oil from Betula utilis growing in high altitude of Himalaya (India) J Essent Oil-Bear Plants. 2015;18(5):1078–82. CrossRef
41. Rajbhandari KR. Ethnobotany of Nepal Ethnobotanical Society of Nepal. Kathmandu, Nepal: Ethnobotanical Society of Nepal; 2001.
42. Ghimire SK, Sapkota IB, Oli BR, Parajuli RR. Non-timber forest products of Nepal Himalaya: database of some important species found in the mountain protected areas and surrounding regions. Kathmandu, Nepal: WWF Nepal; 2008.
43. Gewali MB. Aspects of Traditional Medicine in Nepal. Toyama, Japan: Institute of Natural Medicine; 2008.
44. Baral SR, Kurmi P. A Compendium of Medicinal Plants in Nepal. Kathmandu, Nepal: Mass Printing Press; International Union for the Conservation of Nature and Natural Resources; 2006.
45. Shinwari ZK, Gilani SS. Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (northern Pakistan). J Ethnopharmacol. 2003;84(2-3):289–98. CrossRef
46. Angmo K, Adhikari BS, Rawat GS. Changing aspects of traditional healthcare system in Western Ladakh, India. J Ethnopharmacol. 2012;143(2):621–30. CrossRef
47. Mahmood A, Mahmood A, Malik RN. Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. J Ethnopharmacol. 2012;143(1):338–46. CrossRef
48. Malhotra CL, Balodi B. Wild medicinal plants in the use of Johari tribals. J Econ Taxon Bot. 1984;5:841–3.
49. Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants (including the supplement). New Delhi, India: CSIR; 1986.
50. Negi KS, Tiwari JK, Gaur RD. Economic importance of some common trees in Garhwal Himalaya: an ethnobotanical study. Indian J For. 1985;8:276–89.
51. Khan MA. Karachic acid: a new triterpenoid from Betula utilis. Phytochem. 1975;14(3):789–91. CrossRef
52. Mishra T, Chandra P, Kumar B, Baleshwar M, Joshi P, Rana TS, et al. Phytochemical profiling of the stem bark of Betula utilis from different geographical regions of India using UHPLC-ESI-MS/MS. Anal Sci Adv T. 2021;11:497–504. CrossRef
53. Fulda S. Betulinic acid for cancer treatment and prevention. Int J Mol Sci. 2008 Jun 27;9(6):1096–107. CrossRef
54. Jonnalagadda SC, Suman P, Morgan DC, Seay JN. Recent developments on the synthesis and applications of betulin and betulinic acid derivatives as therapeutic agents. Stud Nat Prod Chem. 2017;53:45–84. CrossRef
55. Amiri S, Dastghaib S, Ahmadi M, Mehrbod P, Khadem F, Behrouj H, et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol Adv. 2020;38:107409. CrossRef
56. Pal M, Mishra T, Kumar A, Baleshwar, Upreti DK, Rana TS. Characterization of fatty acids in the bark of Betula utilis growing in high altitudes of himalaya. Chem Nat Compd. 2015;51:326–7. CrossRef
57. Zdzisi?ska B, Szuster-Ciesielska AM, Rzeski W, Kanderfer-Szerszen M. Therapeutic properties of betulin and betulinic acid, components of birch bark extract. Farmaceutyczny Przeglad Naukowy. 2010;7(3):33–9.
58. Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12(6):657–66. CrossRef
59. Markert B, Steinbeck R. Einige Aspekte der Elementverteilung in Betula alba, ein Beitrag zur repräsentativen Probenahme von terrestrischen Pflanzen für die Multielement-Analyse. Fresenius Z Anal Chem. 1988;331:616–9. CrossRef
60. Shehzad MR, Hanif MA, Rehman R, Bhatti IA, Bhatta KR. Himalayan birch. In: Medicinal plants of South Asia. Elsevier 2020. pp 369–79.
61. Kuznetsova SA, Skvortsova GP, Maliar IN, Skurydina ES, Veselova OF. Extraction of betulin from birch bark and study of its physico-chemical and pharmacological properties. Russ. J Bioorganic Chem. 2014;40:742–7. CrossRef
62. Šiman P, Filipová A, Tichá A, Niang M, Bezrouk A, Havelek R. Effective method of purification of betulin from birch bark: the importance of its purity for scientific and medicinal use. PLoS One. 2016;11(5):e0154933. CrossRef
63. Tsepaeva OV, Nemtarev AV, Abdullin TI, Grigor’eva LR, Kuznetsova EV, Akhmadishina RA, et al. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J Nat Prod. 2017;80(8):2232–9. CrossRef
64. Chrobak E, Jastrz?bska M, B?benek E, Kadela-Tomanek M, Marciniec K, Latocha M, et al. Molecular structure, in vitro anticancer study and molecular docking of new phosphate derivatives of betulin. Molecules. 2021 Jan 31;26(3):737. CrossRef
65. Wang J, Wu J, Han Y, Zhang J, Lin Y, Wang H, et al. Synthesis and biological evaluation of novel betulin derivatives with aromatic hydrazone side chain as potential anticancer agents. J Braz Chem Soc. 2022;33(03):227–37. CrossRef
66. Bebenek E, Chodurek E, Orchel A, Dzieroewicz Z, Boryczka SL. Antiproliferative activity of novel acetylenic derivatives of betulin against g-361 human melanoma cells. Acta Pol Pharm. 2015;72(4):699–703.
67. Santos RC, Salvador JA, Marín S, Cascante M, Moreira JN, Dinis TC. Synthesis and structure–activity relationship study of novel cytotoxic carbamate and N-acylheterocyclic bearing derivatives of betulin and betulinic acid. Bioorg Med Chem. 2010;18(12):4385–96. CrossRef
68. Tang J, Jones SA, Jeffery JL, Miranda SR, Galardi CM, Irlbeck DM, et al. Synthesis and biological evaluation of macrocyclized betulin derivatives as a novel class of anti-HIV-1 maturation inhibitors. Open J Med Chem. 2014;8:23. doi: https://doi.org/10.2174/1874104501408010023
69. Csuk R. Targeting cancer by betulin and betulinic acid. In novel apoptotic regulators in carcinogenesis. Dordrecht, The Netherlands: Springer Netherlands; 2012. pp 267–87.
70. Rastogi S, Pandey MM, Rawat AK. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J Ethnopharmacol. 2015;159:62–83. CrossRef
71. Aiken C, Chen CH. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med. 2005 Jan 1;11(1):31–6. CrossRef
72. Pandey KP, Chaturvedi G. Charak Samhita. Varanasi, India: Chaukhamba Bhartiya Academy; 2015.
73. Shastri AK. Sushruta chikitsa and Sushruta Kalpa. Vol-I. Varanasi, India: Chaukhamba Sanskrit Sansthana; 2017.
74. Gupta KSA. Astanga Hridya. Varanasi, India: Chaukhamba Sanskrit sansthana; 1980.
75. Shrikantamurthya KR. Astanga samgraha. Vol-II and Vol-III. Bengaluru, India: Chaukhamba Orientalia; 2005.
76. Tripathi I. Chakradatta Samhita. Varanasi, India: Chaukhamba Sanskrit Sansthana; 2005.
77. Shastri AK. Varanasi, India: Chaukhamba Sanskrit Sansthana; 2010.
78. Mishra T, Arya RK, Meena S, Joshi P, Pal M, Meena B, et al. Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. PLoS One. 2016;11(7):e0159430. CrossRef
79. Singh S, Yadav S, Sharma P, Thapliyal A. Betula utilis: a potential herbal medicine. Int J Pharm Biol Arch. 2012;3(3):493–8.
80. Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflamm. 2007;4(1):1–7.
81. Kumaraswamy MV, Satish S. Free radical scavenging activity and lipoxygenase inhibition of Woodfordia fructicosa Kurz and Betula utilis Wall. Afr J Biotechnol. 2008;7(12):2013–6.
82. Sareen A, Ahirwar R, Gautam A, Bhadauria R. Fungal contamination of some common medicinal plant samples of Himachal Pradesh. Sci Cult. 2010;76:118–20.
83. Duraiswamy B, Satishkumar MN, Gupta S, Rawat M, Porwal O, Murugan R. Hepatoprotective activity of Betula Utilis bark on D-galactosamine induced hepatic insult. World J Pharm Pharma Sci. 2012;1:456–71.
84. Biswasroy P, Pradhan D, Sahu DK, Rai V, Halder J, Rajwar TK, et al. Phytochemical investigation, structural elucidation, in silico study and anti-psoriatic activity of potent bioactive from Betula utilis. J Biomol Struct Dyn. 2023;41(17):8093–108. CrossRef
85. VS A, Satish S, Shabaraya AR. Effect of Betula utilis bark extract on cafeteria diet-induced obesity in rats. European J Biomed Pharm Sci. 2019;6(5):441–5.
86. Goyal A, Kaur R, Sharma D, Sharma M. Protective effect of Betula utilis bark extract on high fat diet induced obesity in Wistar rats. Obes Med. 2019;15:100123. CrossRef
87. Shah SK, Patel KM, Vaviya PM. Evaluation of antiurolithiatic activity of Betula utilis in rats using ethylene glycol model. Asian J Pharm Res Dev. 2017;7(2):81–7.
88. Patley C, Bhadhkariya S, Sahu K, Kohli S, Sagar R. Betula utilis D. don: study of anticonvulsant and anxiolytic agent. Eur J Biomed Pharm Sci. 2022;9:305–9.
89. Xu HX, Zeng FQ, Wan M, Sim KY. Anti-HIV triterpene acids from Geum japonicum. J Nat Prod. 1996;59(7):643–5.
90. Mayaux JF, Bousseau A, Pauwels R, Huet T, Henin Y, Dereu N, et al. Activation and inhibition of proteasomes by betulinic acid and its derivatives. Natl Acad Sci. 1994;91:3564.
91. Chaniad P, Sudsai T, Septama AW, Chukaew A, Tewtrakul S. Evaluation of anti-HIV-1 integrase and anti-inflammatory activities of compounds from Betula alnoides Buch-Ham. Adv Pharmacol Pharm Sci. 2019;1:2573965.
92. Bhat AM, Qureshi R, Yaseen M. Phytochemical investigation and antioxidant activity of methanolic extract of Betula Utilis Bark. J Pharm Innov. 2022;2(2):4–12.
93. Sénéchal H, Geny S, Desvaux FX, Busson M, Mayera C, Arond Y, et al. Genetics and specific immune response in allergy to birch pollen and food: evidence of a strong, positive association between atopy and the HLA class II allele HLA-DR7. J Clin Immunol. 1991;104(2):395–401.