INTRODUCTION
Flavonoids are a dominant class of polyphenols comprising almost 6,000 phenolic compounds. These metabolites have chemical structures consisting of two benzene rings A and B that are connected by a three-carbon heterocyclic pyran ring C. Together, they form the benzopyrone (C6–C3–C6) moiety [1,2]. Flavones, flavonols, flavanones, flavanonols, flavanols or catechins, anthocyanins, and chalcones are the sub-classes of flavonoids.
Flavanones are flavonoids with a C ring that lacks a C2−C3 double bond, do not have any substitution at C3, and have a chiral center at C2 [3,4]. Glycosylated flavanones have a sugar moiety bound to hydroxyl groups of the aglycone via an O-glycosidic linkage at C7 of the A ring. A total of 350 flavanone aglycones and 100 flavanone glycosides have been reported in nature [3]. Flavanones are commonly reported in plant species of the families Fabaceae, Compositae, and Rutaceae. The pharmacological properties of flavanones include antioxidant, anticancer, antimicrobial, anti-inflammatory, hypolipidemic, anti-atherosclerosis, anti-hypertension, cardiovascular protection, insulin sensitization, and anti-obesity activities [4−6].
Also known as 3,5,7-trihydroxyflavanone 7-rhamno glucoside or hesperetin-7-O-rutinoside, hesperidin is a major flavanone in fruits of Citrus species (Rutaceae) such as orange, grapefruit, tangerine, lime, and lemon [7,8]. In Citrus fruits, hesperidin also occurs in the juice and rind. It has also been reported in other plant families, e.g., Fabaceae, Betulaceae, Lamiaceae, and Papilionaceae. Hesperidin is endowed with diverse health-promoting pharmacological properties, e.g., antioxidant, antimicrobial, anti-diabetic, anticancer, anti-inflammatory, anti-hypertensive, cardiovascular protective, neuroprotective, hepatoprotective, analgesic, anti-arthritis, hypolipidemic, anti-fertility, and improving mental illness activities [7−9].
In this overview, the chemistry, pharmacology, safety studies, and clinical studies of glucosyl hesperidin or G-hesperidin (GH) are reviewed. To date, there are no reviews on GH, unlike hesperidin which is fairly well-documented. Besides having useful pharmacological properties, safety studies, and clinical studies, GH and its uses have been patented and its safety has been endorsed by safety authorities. GH is now a commercial product.
CHEMISTRY
GH or hesperetin rutinoside is a flavanone that has a molecular formula of C34H44O20, molecular weight of 772.7 Da, and CAS registry no. of 161713-86-6. In the chemical structure of GH, the B ring is connected to the C ring at positions 1' and 2, respectively [10]. There are two OH groups at C3' and C5, a carbonyl or keto moiety at C4, and an OCH3 group at C4' (Fig. 1). Like all flavanones, GH lacks the C2–C3 double bond. At C7, R is hesperetin-7-O-rutinoside + glucose (C6H12O6). GH has S- and R-configurations at C2 represented by (2S)-GH and (2R)-GH, respectively. Hence, C2 is the chiral center [11]. Hesperetin (C16H14O6) with R = OH is the aglycone of hesperidin [12].
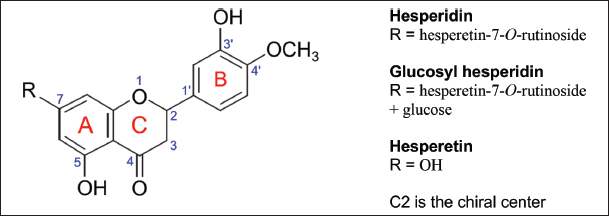 | Figure 1. Chemical structures of hesperidin, GH, and hesperetin. [Click here to view] |
GH can be synthesized by adding one glucose molecule to hesperidin. The water solubility of GH is significantly higher than that of hesperidin, and its absorption is greatly improved in the body and its effects are enhanced [13]. GH, a derivative of hesperidin, is a semi-synthetic flavanone. In 1997, the synthesis of GH by trans-glycosylation of cyclodextrin glucano-transferase (CGTase) using Bacillus stearothermophilus in alkaline pH was patented [13,14]. The water solubility of GH is excellent, i.e., 10,000 times greater than that of hesperidin. Hesperetin, the aglycone of hesperidin, can be synthesized from hesperidin via trans-glycosylation of CGTase using Bacillus sp. in alkaline pH of greater than 7.0 [15]. In 2000, another patent of GH involving the synthesis of a product of high GH content with extremely superior water solubility was published [16]. The product does not substantially contain hesperidin, glucosyl hesperetin, and hesperetin. Trans-glycosylation to GH was by CGTase using Bacillus A2-5a strain in alkaline pH. Recently, an efficient technique of producing GH by CGTase from Bacillus subtilis was described [17]. With a yield of 2.7 g/l, this technique has the potential for scaled-up production of GH.
Conversely, GH can be converted back to hesperidin via enzymatic hydrolysis by α-glucosidase and from hesperidin to hesperetin via β-glucosidase (GH → hesperidin → hesperetin) [13,15]. In rats administered with GH, hesperetin was found rapidly in the serum [13]. The amount of hesperetin was 3.7× greater than hesperidin.
GH and its uses have been patented as a United States Patent (No. 5,627,157) in 1997 by Hiromi Hijiya and Toshio Miyake as Inventors and by Hayashibara Biological Science Research Institute, Inc. as Assignee [14]. In 2000, another invention was patented by Toshio Miyake and Takashi Yumoto. The second patent involved the synthesis of a product of high GH content that does not substantially contain hesperidin, glucosyl hesperetin, and hesperetin, and has an extremely superior water solubility [16].
Hesperetin and hesperidin possess anticancer properties [18,19], but not GH, suggesting that the absence of the rhamnose and glucose groups as in the aglycone hesperetin or the presence of the rhamnose and glucose groups as in hesperidin are essential for the anticancer properties. Interestingly, the presence of the second glucose molecule as in GH results in the absence of the anticancer properties.
PHARMACOLOGY
The pharmacological activities of GH are diverse. In this article, bioactivities and their number of studies are anti-hypertension (6), antimicrobial (5), anti-inflammatory (3), and anti-obesity (3), (Table 1). Antioxidant, anti-cataract, anti-atherosclerosis, and improved blood flow are represented by two studies each. Activities with single studies are glucosidase inhibitory, hypolipidemic, ocular improvement, anti-diabetic, male reproductive protection, anxiolytic, anti-oral mucositis, renal fibrosis protection, anti-asthma, anti-allergy, anti-allergy, anti-arthritis, hypouricemic, improved exercise capacity, reduced bone loss, protection of DNA breakage, and anti-advanced glycation end-products (AGEs).
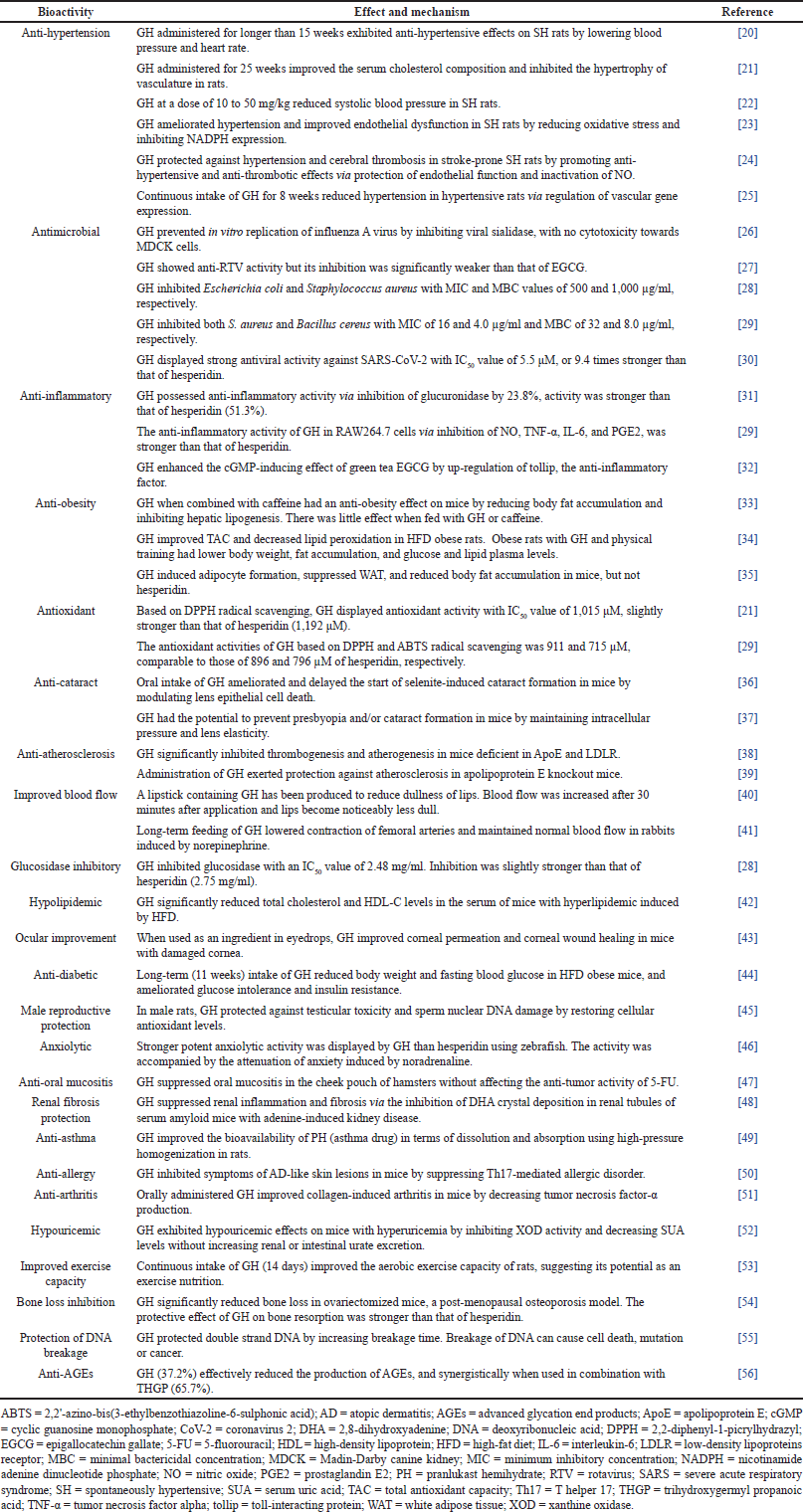 | Table 1. Pharmacological activities of GH. [Click here to view] |
USES
GH is being sold in Japan, Taiwan, and Korea as a food additive, dietary supplement, functional food, beverage, and cosmetics [57]. Currently, a blend of extracts of natural antioxidants comprising GH, lemon balm, and barley grass intended for the protection of the scalp and hair against deleterious urban pollutants is being sold in the market [58].
In Japan, GH has been accepted as an ingredient for functional foods under the Japanese Food Labeling Act [59]. In Korea, GH is monographed as Enzymatically Modified Hesperidin [60]. GH has been listed in the Food Ingredients List in Taiwan [61]. Recently, the European Food Safety Authority (EFSA) has endorsed GH as a novel food, pursuant to Regulation (EU) 2015/2283 [62]. EFSA has approved GH for use in food supplements and in drinks for human adults of up to 364 mg per day. The production method of HG was developed by Hayashibara Co. Ltd., in Japan in 1989, and has been marketed since 1999 [57].
SAFETY STUDIES
Three safety studies on GH have been reported, based on 4-week oral feeding of rats, 13-week sub-chronic toxicity on rats, and a teratogenicity study on rats.
In a 4-week oral feeding study, 20 female and 20 male rats were divided into four groups. GH of a placebo, 100, 2,000, and 15,000 ppm was mixed into the feed and fed to each group twice weekly for 28 days. Organ weights and organ/body weight ratios of female rats showed no significant differences between groups. In the male rats, only organ/body weight ratios at 100 ppm were significantly reduced. In organ/brain weight ratios, there were no significant differences in either the female or male groups. Data from this 4-week study, GH at 15,000 ppm showed no effect at 1,280 and 1,206 mg/kg/day in both male and female rats, respectively [57].
In a 13-week study, 10 male and 10 female rats were fed with feeds containing GH were placebo, 4,500, 15,000, and 50,000 ppm. No effect was observed at 3,428 and 3,084 mg/kg/day for the female and male rats, respectively. Data from this 13-week study, no effect was observed at 50,000 ppm or 3,428 and 3,084 mg/kg/day for the male and female rats, respectively [57].
In a teratogenicity study of GH in rats, successfully mated female rats were grouped into four (20 rats in each group). GH doses administered by gavage (forced feeding) were placebo, 100, 300, and 1,000 mg/kg. No adverse effects of GH at 1,000 mg/kg/day were observed for both maternal (dams) and fetal rats. There were no deaths nor aborted pregnancies among the dams, and no abnormalities among the fetuses. GH did not cause any teratogenicity or congenital disorders even at 1,000 mg/kg/day, i.e., the highest dose administered [57].
CLINICAL STUDIES
From 2004 to 2023, a total of 13 clinical studies involving GH were documented in the literature. These studies were all undertaken in Japan. Their venues, objectives, methodologies, and findings were highlighted. Their registration numbers if available were included.
The Health Science Laboratory (HSL) of Ezaki Glico Co. Ltd. in Osaka, Japan, conducted a clinical study on the effects of GH towards rheumatoid arthritis (RA) in humans [51]. Patients were given a beverage containing 3 g of HG (n = 9) or placebo (n = 10) every morning for 12 weeks. In addition, patients received RA therapy from a physician each month. The study revealed that HG was effective when administered with RA therapy. The study concluded that GH may improve the quality of life for RA patients [51].
The R & D Center (RDC) of Hayashibara Biochemical Laboratories (HBLs) in Okayama, Japan, carried out a clinical study aimed at assessing the effects of GH on serum lipid levels in humans, notably, the lowering of serum triglyceride (TG) [63]. Volunteers were 40 male adults (27−64 years of age) with serum total cholesterol (TC) level of 200 mg/dl were recruited. GH tablets were given to the subjects at 100 or 500 mg/day for 6 weeks. The percentage of subjects with reduced serum cholesterol levels was less than 20%. Subjects with a reduction in serum TG level were 45%−55%. TG level was significantly reduced in participants with >150 mg/dl. The study by RDC of HBL concluded that GH lowered serum TG in subjects [63].
This is a follow-up clinical study conducted by the RDC of HBL, Okayama, Japan. Its objectives were to examine the TG-lowering effect and its mechanisms [64]. Volunteers were 25 adult males (26−65 years of age) with fasting serum TG levels greater than 110 mg/dl. The subjects were administered 500 mg/day of GH tablets for 24 weeks. Results showed that the serum TG level significantly decreased in the high TG group (>150 mg/dl). In addition, remnant-like particle cholesterol, apolipoprotein (apo) B, apo C-II, apo C-III, and apo E were detected in the high TG group were also lower, suggesting an improvement in very low-density lipoprotein [64].
The HSL of Ezaki Glico Co. Ltd. in Osaka, Japan, carried out another clinical study on the effectiveness of GH in treating blood circulation disorders in women [65]. GH (250 mg/day) was given daily to 11 women (average age of 29.6 years) with coldness of the extremities. Subjects were given seven days of administration. After 40 minutes, the hand of each subject was exposed to cooling stress at 15°C for 1 minute. Then, the hand was measured for skin surface temperature, blood flow, and the diameter of blood vessels in the finger. After each dosage of GH, the recovery rates of hand temperature and blood flow in the finger were significantly higher. At the end of the study, the recovery rates of hand temperature and finger blood flow were also significantly higher. The HSL concluded that the administration of GH resulted in an increase in peripheral blood flow and recovery of skin surface temperature. This shows that GH may alleviate poor blood circulation in women [65].
A clinical study was carried out by the Department of Bioscience and Biotechnology, Faculty of Agriculture, Kyushu University in Fukuoka, Japan [66]. The objective of the study was to evaluate the effect of long-term ingestion of beverages containing GH on serum TG levels. Participants were 112 healthy adults with serum TG levels of 120−200 mg/dl. Each participant in the GH group was given a 500 ml drink per day (GH of 340 mg/day) for 12 weeks. Participants comprising 47 males and 52 females completed the study. In the GH group, serum TG levels were significantly reduced at weeks 4, 8, and 12. No adverse effects were found and the beverage containing GH was safe for ingestion [66].
A clinical trial (UMIN Clinical Trial Registry 000019241) was undertaken by the R & D Institute (RDI), House Wellness Foods Corp., Itami, Hyogo, Japan. The aim of the study was to assess the anti-obesity effects of GH plus caffeine on body fat and serum TG of healthy subjects with moderately high body mass index (BMI) and serum TG [67]. A total of 75 healthy subjects with moderately high BMI (24–30 kg/m2) and serum TG (100–250 mg/dl) were given a daily intake of 500 mg of GH for 12 weeks, with or without 25, 50, or 75 mg of caffeine. Results showed significantly lower subcutaneous abdominal fat area in the GH groups with 50 and 75 mg of caffeine. Fat-decreasing effects of GH were therefore enhanced by the addition of caffeine. The decrease in BMI was significantly greater in the GH group with 75 mg of caffeine. However, the GH groups with or without caffeine had no effect on serum TG. The overall data of the RDI study suggested that 500 mg of GH with 50 or 75 mg of caffeine was effective in preventing or treating obesity [67].
A clinical study was undertaken by the R & D Division, Nagase Viita Co., Ltd. Headquarters, Okayama, Japan, from February to September 2018. The objectives were to determine the effects of a beverage containing 100 mg of GH on the recovery of skin blood flow and temperature of the hand after cold-water loading [68]. The participants were 24 healthy adult men and women. Cold water at 15ºC was then applied onto the hand of each subject for 5 minutes, and the skin blood flow and temperature were measured at 5 minutes intervals for 30 minutes. These parameters were measured using a skin blood flow meter and a skin temperature sensor. Results showed that the intake of GH significantly promoted the recovery of skin blood flow and temperature. The effects of GH may be mediated by the improvement in peripheral blood flow. The study concluded showed that a single dose of 100 mg of GH significantly improved the recovery of skin blood flow and temperature that was decreased by cold water [68].
A clinical study (UMIN000043279) was carried out by the Medical Corporation of Hokubukai Utsukushigaoka Hospital in Tokyo Japan [69]. A total of 36 healthy male and female subjects (mean age of 53 years and mean BMI of 25.2 kg/m) were recruited and were given a beverage containing 165 mg GH with 387 mg of green tea catechin (GTC) daily for 4 weeks. Fasting serum TG and other lipids and glucose metabolites were analyzed. Results showed that continuous ingestion of GH and GTC significantly decreased fasting serum TG levels [69].
The objective of this clinical study was to determine the influence of intake of GH beverages on the lower leg swelling as a result of 6 hours of sitting by six healthy women [70]. Conducted by the Faculty of Sport Sciences, Nihon Fukushi University in Mihama, Japan, each subject ingested 100 ml of GH. The subjects were then required to sit on a chair for 6 hours, and were allowed two toilet breaks. The increase in ankle and calf girth was significantly less in the GH group compared to the placebo group. In the GH group, there was a gradual increase in skin temperature of the lower limb. The placebo group showed no change. The study concluded that gravity-induced calf and ankle swelling in women due to prolonged sitting can be reduced by ingesting GH which functions to lower the production of nitric oxide [70].
The Department of Bioscience and Biotechnology (DBB), Faculty of Agriculture, Kyushu University in Fukuoka, Japan conducted a clinical study (ID: UMIN000040109) to investigate the anti-obesity effects of a beverage of GH and epigallocatechin-3-gallate (EGCG) from green tea [71]. Participants comprising 60 healthy males and females, aged 30–75 years, consumed a beverage of 178 mg GH and 146 mg EGCG, daily for 12 weeks. Physical, hematological, blood biochemical, and urine examinations showed that the beverage was safe to drink. The beverage prevented weight gain and reduced BMI. For participants aged <50 years, TG and body fat decreased at week 6. At week 12, visceral fat, body fat, body weight, BMI, and blood low-density lipoprotein and high-density lipoprotein ratio decreased in this group. The study by DBB concluded that taking GH and green tea beverages prevented weight gain and that the anti-obesity effect was more pronounced in subjects <50 years of age [71].
The Nutrition Division of Taiyo Kagaku Co., Ltd., Mie, Japan, conducted a 12-week clinical study (Trial ID: UMIN000048342). The study assessed the effects of HG intake with cyclodextrin (HCD) on endothelial dysfunction, and on the mental and physical health of participants [72]. Healthy adult male and female subjects (59) consumed 150 or 300 mg of HCD for 12 weeks. ED was measured using flow-mediated vasodilation (FMD) scores. Mental and physical effects were assessed through changes in visual analog scale scores. Subjects who took 300 mg of HCD intake showed significant improvement in FMD at 12 weeks. These subjects also showed significantly alleviated weariness, dark circles under the eyes, and eyelid swelling. However, the effects on subjects who took 150 mg of HCD were not significant [72].
Conducted by the Department of Gastroenterology and Hepatology, Nara Medical University in Nara, Japan, this on-going clinical study (jRCTs051210210) evaluated the effects of GH on hepatic function [73]. Enrollment began in 2022 and results are expected in 2024. A total of 110 patients with primary biliary cholangitis and 20 years or older were expected to participate. PBC patients will be given either 500 or 1,000 mg of GH tablets per day. Analysis will be undertaken at 8, 16, and 24 weeks. The primary end-point is serum gamma-glutamyl transferase. Secondary end-points are serum alkaline phosphatase, transaminase, total bilirubin levels, and protein expression levels of nuclear factor erythroid 2-related factor 2. The role of GH in support of hepatic function is anticipated [73].
The beneficial effects of daily intake of GH (70 mg) and in combination with glucosyl rutin (GR) (140 mg) on improving vascular flexibility were studied by Toyo Sugar Refining Co., Ltd. Chuo-ku, Tokyo, Japan [74]. This clinical trial (UMIN 000046054) of 8 weeks involved 66 healthy males and females with a relatively high BMI and low vascular flexibility. The participants were assigned to a GH group, GH-GR group, or placebo group (n = 22). Participants took two tablets per day of either GH, GHGR, or placebo. Their vascular function indices, capillary flow, and inflammatory markers were analyzed after 4 and 8 weeks. Flow-mediated dilation was the primary outcome. Results showed that participants in the GHGR group had improved vascular flexibility which reduced their cardiovascular risks [74].
CONCLUSION
GH is a flavanone containing a glucose molecule that is derived from Citrus fruits. It has very high-water solubility and this dramatically widen its application as a food supplement. Promoted by Hayashibara Co. Ltd. and Toyo Sugar Refining Co. in Japan, the market size and growth opportunity (2024–2031) of GH are promising with major products in skin care, hair care, and cosmetics. Most of the health benefits of GH are not supported by scientific evidence for human use. Claims in the websites of some companies need to be verified via clinical trials. They include improving capillary flow, protecting arterial walls, promoting skin rejuvenation and skin care, improving bone density reducing anxiety, reducing dark eye circles and eyelid swelling, brightening dull complexion, providing more radiant look, and promoting healthier hair growth.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors have no financial or any other conflicts of interest with regard to this publication.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects. Sources of information used for this review are from databases such as Google Scholar Citations and ScienceDirect.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47.
2. Dias MC, Pinto DC, Silva AM. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26(17):5377.
3. Iwashina T. The structure and distribution of the flavonoids in plants. J Plant Res. 2000;113(3):287−99.
4. Barreca D, Gattuso G, Bellocco E, Calderaro A, Trombetta D, Smeriglio A, et al. Flavanones: citrus phytochemical with health-promoting properties. BioFactors. 2017;43(4):495−506. doi: CrossRef
5. Khan MK, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal. 2014;33(1):85−104. doi: CrossRef
6. Liu S, Lou Y, Li Y, Zhang J, Li P, Yang B, et al. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front Nutr. 2022;9:968604. doi: CrossRef
7. Garg A, Garg S, Zaneveld LJ, Singla A. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001;15(8):655−69. doi: CrossRef
8. Pyrzynska K. Hesperidin: a review on extraction methods, stability and biological activities. Nutrients. 2022;14(12):2387. doi: CrossRef
9. Ganeshpurkar A, Saluja A. The pharmacological potential of hesperidin. Indian J Biochem Biophys. 2019;56:287−300.
10. Chan EWC, Ng YK, Tan CY, Alessandro L, Wong SK, Chan HT. Diosmetin and tamarixetin (methylated flavonoids): a review on their chemistry, sources, pharmacology, and anticancer properties. J Appl Pharm Sci. 2021;11(3):22−8. doi: CrossRef
11. Li D, Mitsuhashi S, Ubukata M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pharm Biol. 2012;50(12):1531−5. doi: CrossRef
12. Khan A, Ikram M, Hahm JR, Kim MO. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants. 2020;9(7):609. doi: CrossRef
13. Yamada M, Tanabe F, Arai N, Mitsuzumi H, Miwa Y, Kubota M, et al. Bioavailability of glucosyl hesperidin in rats. Biosci Biotechnol Biochem. 2006;70(6):1386−94. doi: CrossRef
14. Hijiya H, Miyake T, inventors. Hayashibara Biological Science Research Institute, Inc., assignee. α-Glycosyl hesperidin and its uses. United States Patent US 5627157. 1997 May 6.
15. Kometani T, Terada Y, Nishimura T, Takii H, Okada S. Trans-glycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides. Biosci Biotechnol Biochem. 1994;58:1990–4. doi: CrossRef
16. Miyake T, Yumoto T, inventors. Hayashibara Seibutsu Kagaku Kenkyujo KK, assignee Process for producing α-monoglucosyl hesperidin-rich substance. United States Patent US 6048712. 2000 Apr 11.
17. Zhou J, Shi Y, Fang J, Gan T, Lu Y, Zhu L, et al. Efficient production of α-monoglucosyl hesperidin by cyclodextrin glucanotransferase from Bacillus subtilis. Appl Microbiol Biotechnol. 2023;107(15):4803−13.
18. Devi KP, Rajavel T, Nabavi SF, Setzer WN, Ahmadi A, Mansouri K, et al. Hesperidin: a promising anticancer agent from nature. Indust Crops Prod. 2015;76:582−9. doi: CrossRef
19. Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64−74. doi: CrossRef
20. Ohtsuki K, Abe A, Mitsuzumi H, Kondo M, Uemura K, Iwasaki Y, et al. Effects of long-term administration of hesperidin and glucosyl hesperidin to spontaneously hypertensive rats. J Nutr Sci Vitaminol. 2002;48(5):420−2. doi: CrossRef
21. Ohtsuki K, Abe A, Mitsuzumi H, Kondo M, Uemura K, Iwasaki Y, et al. Glucosyl hesperidin improves serum cholesterol composition and inhibits hypertrophy in vasculature. J Nutr Sci Vitaminol. 2003;49(6):447−50. doi: CrossRef
22. Yamamoto M, Suzuki A, Hase T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J Nutr Sci Vitaminol. 2008;54(1):95−8. doi: CrossRef
23. Yamamoto M, Suzuki A, Jokura H, Yamamoto N, Hase T. Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition. 2008;24(5):470−6. doi: CrossRef
24. Ikemura M, Sasaki Y, Giddings JC, Yamamoto J. Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke-prone spontaneously hypertensive rats. Phytother Res. 2012;26(9):1272−7. doi: CrossRef
25. Yamamoto M, Jokura H, Suzuki A, Hase T, Shimotoyodome A. Effects of continuous ingestion of hesperidin and glucosyl hesperidin on vascular gene expression in spontaneously hypertensive rats. J Nutr Sci Vitaminol. 2013;59(5):470−3. doi:
26. Saha RK, Takahashi T, Suzuki T. Glucosyl hesperidin prevents influenza a virus replication in vitro by inhibition of viral sialidase. Biol Pharm Bull. 2009;32(7):1188−92. doi: CrossRef
27. Lipson SM, Ozen FS, Louis S, Karthikeyan L. Comparison of α-glucosyl hesperidin of citrus fruits and epigallocatechin gallate of green tea on the loss of rotavirus infectivity in cell culture. Front Microbiol. 2015;6:359. doi: CrossRef
28. Chaisin T, Rudeekulthamrong P, Kaulpiboon J. Enzymatic synthesis, structural analysis, and evaluation of antibacterial activity and α-glucosidase inhibition of hesperidin glycosides. Catalysts. 2021;11(5):532. doi: CrossRef
29. Choi SS, Lee SH, Lee KA. A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants. 2022;11(8):1618. doi: CrossRef
30. Huang Y, Zhou W, Sun J, Ou G, Zhong NS, Liu Z. Exploring the potential pharmacological mechanism of hesperidin and glucosyl hesperidin against COVID-19 based on bioinformatics analyses and antiviral assays. Am J Chin Med. 2022;50(2):351−69. doi: CrossRef
31. Poomipark N, Chaisin T, Kaulpiboon J. Synthesis and evaluation of antioxidant and β-glucuronidase inhibitory activity of hesperidin glycosides. Agric Nat Resour. 2020;54(2):165−72. doi: CrossRef
32. Kumazoe M, Tanaka Y, Yoshitomi R, Marugame Y, Lee KW, Onda H, et al. Glucosyl-hesperidin enhances the cyclic guanosine monophosphate-inducing effect of a green tea polyphenol EGCG. J Nat Med. 2021;75(4):1037−42. doi: CrossRef
33. Ohara T, Muroyama K, Yamamoto Y, Murosaki S. A combination of glucosyl hesperidin and caffeine exhibits an anti-obesity effect by inhibition of hepatic lipogenesis in mice. Phytother Res. 2015;29(2):310−6. doi: CrossRef
34. Gonçalves TT, Lazaro CM, de Mateo FG, Campos MC, Mezencio JG, Claudino MA, et al. Effects of glucosyl-hesperidin and physical training on body weight, plasma lipids, oxidative status and vascular reactivity of rats fed with high-fat diet. Diabetes Metab Syndr Obes Targets Ther. 2018:321−32. doi: CrossRef
35. Nishikawa S, Hyodo T, Nagao T, Nakanishi A, Tandia M, Tsuda T. α-Monoglucosyl hesperidin but not hesperidin induces brown-like adipocyte formation and suppresses white adipose tissue accumulation in mice. J Agric Food Chem. 2019;67(7):1948−54. doi: CrossRef
36. Nakazawa Y, Naoki M, Ishiwa S, Morishita M, Endo S, Nagai N, et al. Oral intake of α?glucosyl?hesperidin ameliorates selenite?induced cataract formation. Mol Med Rep. 2020;21:1258−66. doi: CrossRef
37. Nakazawa Y, Doki Y, Sugiyama Y, Kobayashi R, Nagai N, Morisita N, et al. Effect of alpha-glucosyl-hesperidin consumption on lens sclerosis and presbyopia. Cells. 2021;10(2):382. doi: CrossRef
38. Sasaki Y, Hyodo K, Hoshino A, Kisa E, Matsuda K, Horikawa Y, et al. Myricetin and hesperidin inhibit cerebral thrombogenesis and atherogenesis in ApoE-/- and LDLR-/- mice. Food Nutr Sci. 2018;9(1):20−31. doi: CrossRef
39. Sugasawa N, Katagi A, Kurobe H, Nakayama T, Nishio C, Takumi H, et al. Inhibition of atherosclerotic plaque development by oral administration of α-glucosyl hesperidin and water-dispersible hesperetin in apolipoprotein E knockout mice. J Am Coll Nutr. 2019;38(1):15−22. doi: CrossRef
40. Iwai I, Yamashita T, Ochiai N, Masuda Y, Hosokawa K, Kohno Y. Can daily-use lipstick make lips more fresh and healthy −− A new lipstick containing α-glucosyl-hesperidin can remove the dull-color from lips. Proc Soc Cosmet Sci Korea (SCSK) Conf. 2003;162 −77 .
41. Naiki T, Kurose Y, Hayashi K, Takumi H, Kometani T. Effects of long-term feeding of α-glucosylhesperidin on the mechanical properties of rabbit femoral arteries. Biorheology. 2012;49(5−6):353−63. doi: CrossRef
42. Yamada M, Mitsuzumi H, Tsuzaki Y, Miwa Y, Chaen H, Yamamoto I. Antioxidant activity of glycosylated vitamin P and its suppressive effect on oxidative stress in hyperlipidemic mice. J Jpn Soc Nutr Food Sci. 2003;56:355–63.
43. Yu L, Zhang Q, Zhou L, Wei Y, Li M, Wu X, et al. Ocular topical application of alpha-glucosyl hesperidin as an active pharmaceutical excipient: in vitro and in vivo experimental evaluation. Drug Deliv Translat Res. 2024;14(2):373−85. doi: CrossRef
44. Yoshida H, Tsuhako R, Sugita C, Kurokawa M. Glucosyl hesperidin has an anti-diabetic effect in high-fat diet-induced obese mice. Biol Pharm Bull. 2021;44(3):422−30. doi: CrossRef
45. Bharathi BV, Jaya Prakash G, Krishna KM, Ravi Krishna CH, Sivanarayana T, Madan K, et al. Protective effect of alpha glucosyl hesperidin (G-hesperidin) on chronic vanadium induced testicular toxicity and sperm nuclear DNA damage in male Sprague Dawley rats. Andrologia. 2015;47(5):568−78. doi: CrossRef
46. Nishida T, Horita C, Imagawa M, Hibarino M, Tateno S, Kubo Y, et al. Glucosyl hesperidin exhibits more potent anxiolytic activity than hesperidin accompanied by the attenuation of noradrenaline induction in a zebrafish model. Front Pharmacol. 2023;14:1213252. doi: CrossRef
47. Yoshino F, Yoshida A, Toyama T, Wada-Takahashi S, Takahashi SS. α-Glucosyl hesperidin suppressed the exacerbation of 5-fluorouracil-induced oral mucositis in the hamster cheek pouch. J Funct Food. 2016;21:223−31. doi: CrossRef
48. Kumrungsee T, Kariya T, Hashimoto K, Koyano T, Yazawa N, Hashimoto T, et al. The serum amyloid A3 promoter-driven luciferase reporter mice is a valuable tool to image early renal fibrosis development and shows the therapeutic effect of glucosyl hesperidin treatment. Sci Rep. 2019;9(1):14101. doi: CrossRef
49. Uchiyama H, Tozuka Y, Asamoto F, Takeuchi H. α-Glucosyl hesperidin induced an improvement in the bioavailability of pranlukast hemihydrate using high-pressure homogenization. Int J Pharm. 2011;410(1−2):114−7. doi: CrossRef
50. Nagashio Y, Matsuura Y, Miyamoto J, Kometani T, Suzuki T, Tanabe S. Hesperidin inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th17 activity. J Funct Foods. 2013;5(4):1633−41. doi: CrossRef
51. Kometani T, Fukuda T, Kakuma T, Kawaguchi K, Tamura W, Kumazawa Y, et al. Effects of α-glucosylhesperidin, a bioactive food material, on collagen-induced arthritis in mice and rheumatoid arthritis in humans. Immunopharmacol Immunotoxicol. 2008;30(1):117−34. doi: CrossRef
52. Ota-Kontani A, Hirata H, Ogura M, Tsuchiya Y, Harada-Shiba M. Comprehensive analysis of mechanism underlying hypouricemic effect of glucosyl hesperidin. Biochem Biophys Res Commun. 2020;521(4):861−7. doi: CrossRef
53. Nagayama S, Aoki K, Komine S, Arai N, Endo S, Ohmori H. Improvement of low-intensity long-time running performance in rats by intake of glucosyl hesperidin. Physiol Rep. 2023;11(2):e15413. doi: CrossRef
54. Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, et al. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133(6):1892−7.
55. Yoshikawa Y, Suzuki M, Yamada N, Yoshikawa K. Double-strand break of giant DNA: protection by glucosyl-hesperidin as evidenced through direct observation on individual DNA molecules. FEBS Lett. 2004;566(1−3):39−42. doi: CrossRef
56. Masaki M, Shimada Y, Takeda T, Aso H, Nakamura T. Inhibitory effect of organo-germanium compound 3-(trihydroxygermyl) propanoic acid on fructose-induced glycation of amino compounds. Carbohydr Res. 2024;542:109191. doi: CrossRef
57. Matsumoto S, Hashimoto T, Ushio C, Namekawa K, Richards AB. Glucosyl hesperidin: safety studies. Fundam Toxicol Sci. 2019;6(8):299−317. doi: CrossRef
58. Gyenge EB, Hettwer S, Schoeffel L, Suter B, Obermayer B. The invisible threat for hair and scalp. SOFW J. 2022;148:1−6.
59. CAA. Foods with functional claims. Food Labeling Act No. 70, Consumer Affairs Agency of Japan; 2013. doi: CrossRef
60. MFDS. Enzymatically modified hesperidin. Food Additive Code, Regulation No. 2019-63. Ministry of Food and Drug Safety of Korea; 2019.
61. FDA. Glucosyl hesperidin. List of raw materials available for food use. Taiwan: Food and Drug Administration; 1975.
62. EFSA. Safety of glucosyl hesperidin as a novel food pursuant to Regulation (EU) 2015/2283. European Food Safety Authority. EFSA J. 2024;22(8):e8911. doi: 10.2903/j.efsa.2024.8911
63. Miwa Y, Yamada M, Sunayama T, Mitsuzumi H, Tsuzaki Y, Chaen H, et al. Effects of glucosyl hesperidin on serum lipids in hyperlipidemic subjects: preferential reduction in elevated serum triglyceride level. J Nutr Sci Vitaminol. 2004;50(3):211−8. doi: CrossRef
64. Miwa Y, Mitsuzumi H, Sunayama T, Yamada M, Okada K, Kubota M, et al. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J Nutr Sci Vitaminol. 2005;51(6):460−70. doi: CrossRef
65. Yoshitani K, Minami T, Takumi H, Kagami Y, Shiraishi K, Kometani T. Effect of α-glucosyl hesperidin on poor circulation in women. J Jpn Soc Nutr Food Sci. 2008;61(5):233−9.
66. Tanaka Y, Imatomi H, Takihara T, Abe Y, Takano K, Usuda S, et al. Effects of glucosyl hesperidin on serum triglyceride and its safety in beverage. Jpn Pharmacol Ther. 2010;38(6):553−68.
67. Ohara T, Muroyama K, Yamamoto Y, Murosaki S. Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutr J. 2016;15:6. doi: CrossRef
68. Morishita N, Ogihara S, Endo S, Mitsuzumi H, Ushio S. Effects of glucosyl hesperidin on skin blood flow and temperature: a randomized, double-blind, placebo-controlled, crossover study. Shinryo Shinyaku. 2020;57:129−34.
69. Katada S, Oishi S, Yanagawa K, Ishii S, Oki M, Matsui Y, et al. Concomitant use of tea catechins affects absorption and serum triglyceride-lowering effects of monoglucosyl hesperidin. Food Funct. 2021;12(19):9339−46.
70. Nishimura N, Iwase S, Takumi H, Yamamoto K. Gravity-induced lower-leg swelling can be ameliorated by ingestion of α-glucosyl hesperidin beverage. Front Physiol. 2021;12: 670640. doi: CrossRef
71. Yoshitomi R, Yamamoto M, Kumazoe M, Fujimura Y, Yonekura M, Shimamoto Y, et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: a randomized placebo-controlled clinical trial. Sci Rep. 2021;11(1):19067. doi: CrossRef
72. Moriwaki M, Abe A, Kapoor MP, Yamaguchi A, Okamoto S, Ozeki M. Hesperetin-7-glucoside-β-cyclodextrin inclusion complex is associated with improvement in vascular endothelial function, and mental and physical health in healthy subjects: a randomized, parallel, double-blind, and placebo-controlled study. Med Cons New − Remed. 2023;60(8):449−57.
73. Moriya K, Asada K, Suzuki S, Enomoto M, Fujinaga Y, Tsuji Y, et al. Benefit of glucosyl hesperidin in patients with primary biliary cholangitis: a multi-center, open-label, randomized control study. Medicine. 2022;101(48):e32127. doi: CrossRef
74. Hashizume Y, Tandia M. The beneficial effects of mono-glucosyl hesperidin and monoglucosyl rutin on vascular flexibility: a randomized, placebo-controlled, double-blind, parallel-group study. Funct Food Health Dis. 2024;14(5):346−65. doi: CrossRef