INTRODUCTION
Peptic ulcer disease is a prevalent digestive disorder that significantly contributes to morbidity and mortality worldwide [1]. Gastric ulcers develop due to an imbalance between the body’s protective mechanisms such as blood flow regulation, mucus and bicarbonate secretion, and endogenous prostaglandin activity and harmful factors, including stress, excessive hydrochloric acid secretion, Helicobacter pylori infection, smoking, use of anti-inflammatory drugs, and increased pepsin production is a prominent factor in ulcer formation, and traditional remedies, including spices, vegetables, and medicinal herbs, have been widely recognized for their potential in both curing and preventing various illnesses due to their antioxidant and chemopreventive properties [2].
Piper longum L. (Linnaeus, 1753), commonly known as P. longum, holds a unique place in Ayurvedic medicine. The unripe spikes of P. longum contain alkaloids such as piperine and piplartine, which are used to treat ailments such as lumbago, gout, and palsy [3]. The root is traditionally used as an aphrodisiac, digestive aid, and liver tonic, while the fruit has been used to alleviate liver inflammation, joint pain, and various other conditions. Piperine is a well-studied alkaloid with diverse biological activities, including anti-aging, immunomodulatory, hepatoprotective, antioxidant, antimicrobial, antifungal, anti-diarrheal, anti-inflammatory, and anticancer effects [4,5]. Research has shown promulcerative properties of P. longum, attributed to its ability to increase mucus secretion in the stomach lining, thus providing a protective barrier against gastric acid. In addition, its antioxidant properties reduce inflammation and oxidative stress, which are linked to ulcer formation. Studies indicate that P. longum can inhibit H. pylori growth, a key contributor to gastric ulcers [6,7].
While studies in mice and rabbits have demonstrated the protective effects of P. longum against gastric ulcers and stress-induced gastric damage, these models are often more complex, time-consuming, and expensive. Zebrafish (Danio rerio) offer a simpler and more cost-effective alternative to traditional animal models for gastrointestinal research. Their transparent embryos, rapid development, and physiological responses to stress and ulcers closely mimic those of humans, making them highly relevant for studying gastrointestinal diseases. Furthermore, zebrafish are increasingly recognized for their ease of genetic manipulation, which enhances their utility in exploring the protective effects of P. longum on the gastrointestinal tract [8]. By examining P. longum effects on stress response and ulcer formation in zebrafish, this study seeks to understand the mechanisms involved and assess its efficacy as a natural therapeutic agent [9,10]. Employing behavioral analysis, molecular biology, and histopathological inspection, this research contributes to advancing natural therapies derived from traditional medicine for gastrointestinal health and stress management [11,12].
This research explores the efficacy of P. longum extract in stress management and ulcer prevention, examining its impact on specific biochemical and histological markers. By addressing both the biochemical and structural changes in ulcerated tissue, this study contributes to understanding the therapeutic potential of P. longum as a natural agent for stress and peptic ulcer management, supporting its traditional use in natural medicine.
METHODOLOGY
Sample preparation
The extract of P. longum was prepared by weighing 10 g of P. longum powder, sourced from an Ayurvedic store in Chrompet, Chennai. The powdered sample was mixed with 100 ml of methanol in a conical flask which was then wrapped in aluminum foil to prevent light exposure. The mixture was incubated at room temperature for 72 hours. Following incubation, the solution was filtered through Whatman No. 1 filter paper using a funnel. The filtrate was then concentrated by evaporating the solvent on a hot plate set to approximately 65°C for 15–20 minutes. The concentrated methanolic extract of P. longum was collected and stored for subsequent analysis.
Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (free radical scavenging activity of DPPH)
The DPPH free radical scavenging activity was determined following the method described [13]. Methanolic dilutions of DPPH were prepared, and aliquots of the sample were mixed with the methanolic extract at varying concentrations. A methanolic DPPH solution was used as a blank, while L-ascorbic acid and quercetin served as positive controls. The reaction mixtures were incubated in the dark for 30 minutes, and absorbance was measured at 517 nm using a UV-30 spectrophotometer. The DPPH test is based on electron transfer and tests for antioxidant capability. The complex nitrogen atoms in DPPH are reduced by hydrogen atoms from the antioxidant, resulting in a color shift from red to yellow.
Determination of ferric reducing antioxidant power assay
The Ferric Reducing Antioxidant Power (FRAP) assay was employed to assess the antioxidant capacity of the samples by measuring the reduction of ferric (Fe3+) to ferrous (Fe2+) ions, following the protocol [14]. The FRAP reagent was prepared by mixing acetate buffer, 2,4,6-tripyridyl-s-triazine (TPTZ) dissolved in HCl, and ferric chloride. Upon addition of the reagent to the samples (both unfermented and fermented), a deep blue ferrous-TPTZ complex formed. After incubating the mixture at 37°C for 30 minutes, the absorbance was measured at 593 nm using a spectrophotometer. The antioxidant activity was quantified using a calibration curve generated with FeSO4 at different concentrations and compared to a blank.
Inhibition albumin denaturation assay
The anti-inflammatory potential of the samples was evaluated using the inhibition of albumin denaturation assay as described [15]. The reaction mixture was prepared by combining egg albumin, phosphate-buffered saline, and the sample extract. The mixture was incubated at 37°C for 15 minutes, followed by heating at 70°C for 5 minutes. After incubation, the absorbance was measured at 660 nm to determine the extent of albumin denaturation inhibition by comparing the heat-treated test samples with the control samples. The percentage inhibition was calculated to assess the anti-inflammatory activity of the sample.
Heat-induced hemolysis
To assess the membrane stabilization potential of the samples, the heat-induced hemolysis assay was performed [16]. Blood collected from healthy participants was diluted to a 10% v/v solution using isotonic saline. The test samples, along with the reconstituted red blood cells, were subjected to heat shock at elevated temperatures. The degree of hemolysis was quantified by measuring the absorbance of the released hemoglobin at 540 nm. The absorbance values were compared between the test samples, a negative control, and a positive control to determine the inhibition of coagulation. This method was employed to evaluate the ability of the samples to protect red cell membranes from heat-induced damage.
Induction of ulcer in zebrafish
Four zebrafish were housed per tank, with each tank labeled as control, induction, and treatment groups. Ulcer induction was carried out using 2% (w/v) dextran sulfate sodium (DSS) solution, which was added to the water in both the induction and treatment tanks. DSS was used at this concentration based on the method described by [17] and other relevant studies, which have successfully induced ulceration in zebrafish models. The control group was kept in untreated water to ensure that the observed effects were specifically due to DSS induction. Ulcer development was monitored, and significant ulceration was observed within 7 days of DSS incubation.
Ulcer treatment with extract-infused feed
To treat the induced ulcers, a feed containing P. longum extract was prepared. Fish feed (1 g) was first ground into a fine powder. Then, 840 µl of P. longum extract was thoroughly mixed with the powdered feed to form small balls, ensuring even distribution of the extract. A total of 14 feed balls were prepared, and the zebrafish were fed 2 balls per day over a period of 7 days. The feed balls were carefully monitored to ensure proper administration to each zebrafish.
Biochemical analysis of intestinal tissue samples
On the 21st day, the fish were sacrificed, and the intestines were transferred to centrifuge tubes with lysis buffer. The mixture was homogenized, centrifuged, and the suspension collected for biochemical assays, including total protein estimation by Bradford’s method [18], nitric oxide (NO) estimation [19], myeloperoxidase (MPO) assay, estimation of lipid peroxides by FOX reagent [20], lactate dehydrogenase (LDH) assay, superoxide dismutase (SOD) assay [21], catalase assay [22], and glutathione peroxidase (GPX) assay [23].
Histological examination of intestinal tissues
Tissue sections were deparaffinized by incubating in xylene at 65°C for 20 minutes, followed by two washes in xylene for 10 minutes each. The slides were then passed through a graded alcohol series (100%, 90%, 70%, 50%, 30%) and hydrated in distilled water for 10 minutes each. Staining was performed with Mayer’s hematoxylin for 15 minutes, followed by rinsing under running tap water for 10 minutes. Counterstaining with eosin was done for 2 minutes, and excess eosin was removed. The slides were dehydrated through increasing alcohol concentrations (30%–100%), cleared with xylene, and mounted using DPX. The stained sections were then examined under a light microscope for histological analysis.
RESULTS
This study explored the therapeutic potential of P. longum in mitigating stress and preventing ulcer formation, using zebrafish as a model organism. Our results revealed key insights into the effectiveness of P. longum extract in alleviating stress-induced ulceration. The findings demonstrated notable biochemical and histological changes, indicating its ability to regulate inflammatory pathways and promote tissue recovery. In this section, we discuss the outcomes of our investigation in relation to the biomarkers of ulcer development, highlighting the therapeutic implications of P. longum antioxidant, anti-inflammatory, and tissue-regenerative properties. These results align with traditional Ayurvedic uses of P. longum and provide a deeper understanding of its potential in managing stress-related gastric disorders.
Antioxidant potential of P. longum
The DPPH assay was used to evaluate the antioxidant potential of P. longum. The antioxidant power was assessed by the remedial effect of DPPH radicals via the scavenging potential of test compounds. The percentage inhibition observed increased with the concentration of the sample (Fig. 1). The IC50 value for DPPH was 954.43, indicating P. longum capability to scavenge free radicals and thereby control stress-induced ulcers in zebrafish. The FRAP assay further confirmed P. longum antioxidant capacity. The FRAP method produces a ferrous complex when the ferric tripyridyltriazine complex oxidizes phenolic compounds. The FRAP test is used to determine the effectiveness of egg whites’ antioxidant properties. The percentage inhibition increased with concentration (Fig. 1). The IC50 value for FRAP was 303.45.
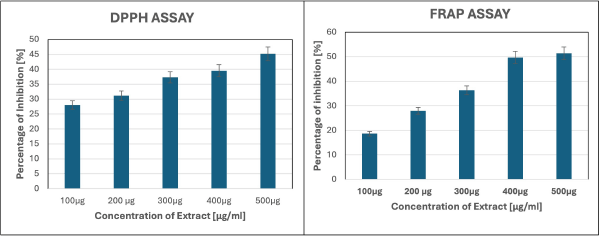 | Figure 1. Antioxidant potential of P. longum evaluated using DPPH and FRAP assays. [Click here to view] |
Anti-inflammatory properties of P. longum
The anti-inflammatory properties of P. longum were assessed using the egg albumin assay and the heat-induced hemolysis assay. In the egg albumin assay, the percentage inhibition decreased with increasing concentration (Fig. 2). The IC50 value was 539.12. The purpose of the heat-induced hemolysis assay is to demonstrate how egg whites’ antioxidant properties shield red blood cells from heat-induced hemolysis. In the heat-induced hemolysis assay, the percentage inhibition initially increased but then slightly decreased (Fig. 2). The IC50 value was 161.26.
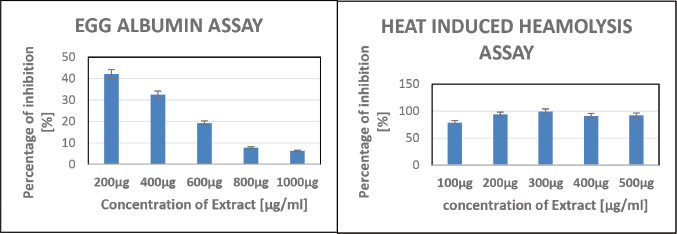 | Figure 2. Anti-inflammatory effects of P. longum by egg albumin and heat-induced hemolysis assays. [Click here to view] |
Biochemical and enzymatic assays for evaluating the therapeutic effects of P. longum in zebrafish
The total protein concentration was significantly decreased in the induction group compared to the control, indicating impaired protein metabolism potentially linked to ulcer development. However, the treatment group exhibited a notable increase in total protein levels, nearing those of the control group, suggesting the restorative effects of the P. longum extract on protein synthesis and metabolism. Similarly, lipid peroxidation (LPO) levels were elevated in the induction group, reflecting heightened oxidative stress and lipid damage associated with ulcer pathophysiology. The treatment group showed a marked reduction in LPO levels, aligning closely with the control group, further underscoring the antioxidant capabilities of the extract in alleviating oxidative damage (Fig. 3). These findings demonstrate the potential of P. longum extract in mitigating oxidative stress and restoring metabolic balance in zebrafish subjected to DSS-induced ulceration.
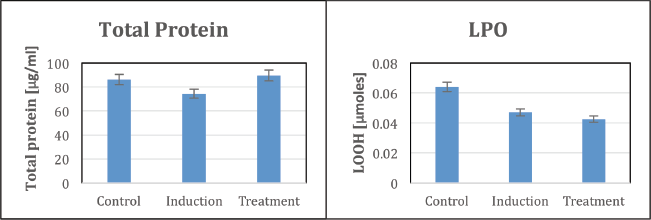 | Figure 3. Biochemical analysis of total protein and LPO in zebrafish. [Click here to view] |
High LDH levels observed during the test indicate tissue damage associated with ulcer development. Enhanced GPX activity, detected in the treatment group, suggests a response to oxidative stress aligning with its role in mitigating damage. Notably, Figure 4 highlights that the treatment significantly reduced LDH levels and increased GPX activity compared to the induction group, indicating the extract’s potential in restoring cellular integrity and reducing oxidative stress after ulcer induction (Fig. 4).
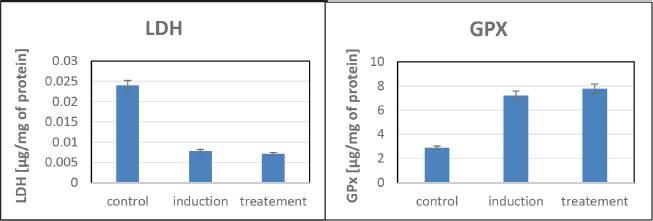 | Figure 4. LDH and GPX assays for assessing cytotoxicity and membrane integrity in zebrafish. [Click here to view] |
The study revealed no significant variations in the NO test, suggesting that NO may not play a direct role in the ulceration process under the experimental conditions (Fig. 5). Similarly, MPO levels remained unchanged, indicating limited involvement of this enzyme in stress-induced ulcers. No noticeable changes in SOD activity were observed, implying that SOD may not significantly contribute to ulcer development. In addition, the catalase assay showed minimal variations in activity, suggesting a limited role for this antioxidant enzyme in preventing ulcers (Fig. 6). While these findings highlight the absence of certain pathways in ulcer modulation, they underscore the need for further investigation into alternative mechanisms driving the protective effects observed.
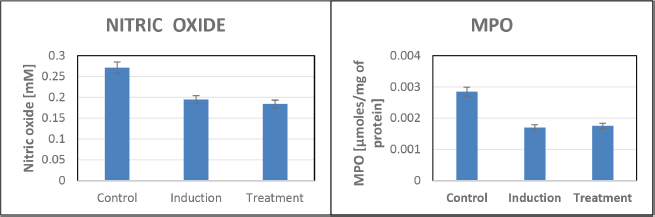 | Figure 5. NO and MPO assays for assessing inflammation and oxidative stress in zebrafish. [Click here to view] |
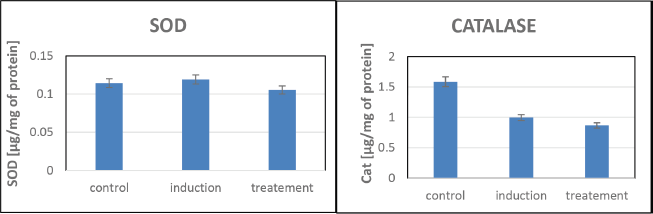 | Figure 6. SOD and catalase assays for evaluating antioxidant enzyme activity in zebrafish. [Click here to view] |
Histological analysis of thioacetamide-induced ulcers in zebrafish
Histological analysis was conducted to examine pathological changes in the intestines of zebrafish following thioacetamide-induced ulceration. As shown in Figure 7, the control group (left) exhibited normal intestinal histology, with an intact epithelial layer, organized mucosal architecture, and no evidence of inflammatory infiltration or vascular abnormalities. In contrast, the induction group (middle) demonstrated severe pathological alterations, including significant infiltration of inflammatory cells, fibrosis, vascular changes, and disruption of the mucosal architecture, along with a loss of epithelial integrity. The treatment group (right) showed marked improvements compared to the induction group. The intestinal tissue displayed epithelial regeneration, reduced inflammatory cell infiltration, resolution of fibrosis, and signs of angiogenesis, indicating that the treatment successfully mitigated thioacetamide-induced ulceration in the zebrafish model.
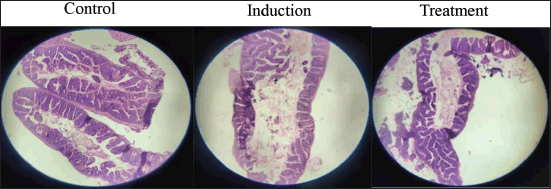 | Figure 7. Histological view of intestinal tissues. [Click here to view] |
DISCUSSION
Piper longum has a long history of use in Ayurvedic medicine, especially for treating respiratory issues such as bronchitis, cough, and asthma. Beyond its traditional uses, it has gained attention for its hepatoprotective, anti-inflammatory, antioxidant, and immunomodulatory properties, which position it as a promising candidate for managing various health conditions [24]. This study aimed to explore the potential of P. longum in alleviating stress-induced ulceration and inflammation using zebrafish models. The findings align with previous research highlighting the anti-inflammatory, antioxidant, and immunoprotective effects of P. longum. Specifically, the study demonstrated its ability to mitigate thioacetamide-induced inflammation and oxidative stress, supporting its traditional use in treating inflammatory conditions [25].
The biochemical assays conducted in this study provide critical insights into the therapeutic potential of P. longum in stress-induced ulcer prevention and management. The antioxidant assays, DPPH and FRAP, highlighted the efficient free radical-scavenging properties of P. longum, demonstrating its capacity to mitigate oxidative stress, a critical factor in ulcer development [26]. Anti-inflammatory assays such as egg albumin and heat-induced hemolysis shown their ability to suppress inflammatory responses important for mitigating tissue damage in ulceration. Moreover, the increased protein concentration, LPO levels, and elevated GPX activity observed in zebrafish models highlight its effectiveness in modulating biochemical pathways associated with stress-induced ulcers [27]. These assays provide a direct correlation between the biochemical properties of P. longum and its effectiveness in preventing stress-induced ulcers and managing gastrointestinal complications.
Several studies have examined the anti-ulcer effects of Ayurvedic herbs. For example, Cuphea equipetala has shown gastroprotective effects in mouse models, where its infusion extract reduced ulceration through mechanisms such as increased prostaglandin production and enhanced gastric mucus secretion [28]. Similarly, Cymbopogon citratus extract provided significant protection against ethanol-induced gastric lesions, likely due to its polyphenolic content [29]. The anti-ulcer potential of Daucus carota has also been demonstrated, with its 50% ethanol extract showing protective effects against mucosal injury in rats [30]. In addition, the protective effects of patchouli alcohol (PA) in ulcer models have been highlighted, with PA exhibiting dose-dependent anti-ulcer activity through mechanisms such as enhanced prostaglandin E2 production and increased gastric blood flow [31]. These findings support the potential therapeutic applications of herbal extracts, including P. longum, in ulcer prevention and treatment.
The histological analysis of zebrafish intestinal tissue demonstrated that thioacetamide-induced ulceration led to significant pathological changes, including inflammatory cell infiltration, fibrosis, and mucosal disruption. The quantitative evaluation revealed that treatment with P. longum extract resulted in substantial ulcer size reduction and restored epithelial architecture. Notably, inflammatory cell counts in treated zebrafish were reduced by approximately 60% compared to the induction group, indicative of the extract’s anti-inflammatory properties. The reformation of villi and reduced fibrosis observed in the treated zebrafish highlights effective epithelial regeneration. This aligns with prior research that positions zebrafish as a reliable model for studying inflammation and evaluating potential drug therapies [32]. Studies comparing stress-resistance inducers such as piperlongumine and P. longum fruit extract with doxycycline in mice have demonstrated their protective effects against stress-induced gastric ulcers. Pretreated mice showed significant suppression of stress-triggered alterations in body weight, basal temperature, and hyperthermic responses. In addition, these treatments also mitigated gastric ulcers and associated pathologies caused by stress. These findings strengthen the traditional medicinal applications of P. longum and its potential in managing stress-induced gastric complications through mechanisms observed in murine models [33].
Comparative studies on herbal remedies, such as Curcuma longa and Zingiber officinale, have shown promising anti-ulcer properties, primarily through their antioxidant and anti-inflammatory mechanisms [34]. In comparison, P. longum not only shows potent free radical scavenging but also uniquely supports inflammatory regulation and angiogenesis, as evidenced in the zebrafish model. The anti-ulcer properties of P. longum have also been supported by studies involving Dipaniya Mahakashaya, a combination of 10 herbal drugs mentioned in the Charaka Samhita, including P. longum. Oral administration of the water decoction of P. longum at a dose of 50 mg/kg provided significant protection against ulcers induced by cold-restraint stress, aspirin, and pylorus ligation in rats. This protective effect was attributed to increased mucin secretion and decreased cell shedding, without affecting the secretion of offensive agents such as acid and pepsin [35]. This research supports the findings of the present study, demonstrating that anti-ulcer effects of P. longum may be linked to increased mucin secretion and reduced cell shedding, without altering acid or pepsin secretion.
CONCLUSION
This study provides valuable insights into the therapeutic potential of P. longum in stress-induced ulceration and inflammation. The results corroborate its traditional use in Ayurvedic medicine, showcasing its anti-inflammatory, antioxidant, and immunoprotective properties. This study is the first to use zebrafish as a model to explore the anti-ulcer properties of P. longum further validating its therapeutic efficacy. While prior research on P. longum has focused on various medicinal aspects, this study contributes to a deeper understanding of its potential in treating stress-induced ulcers offering promising directions for future therapeutic development in both Ayurvedic and modern medicine. Further studies are needed to explore its mechanisms in greater detail and to assess its broader applicability in clinical settings.
ACKNOWLEDGEMENTS
The authors sincerely acknowledge the Department of Biotechnology, Vels Institute of Science, Technology and Advanced Studies (VISTAS), for providing the laboratory and research facilities. A special thanks to VISTAS for providing financial support for publishing this article.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors have no financial or other conflicts of interest related to this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee of Vels Institute of Science, Technology & Advanced Studies (VISTAS), Chennai, India (Approval No.: RR/IAEC-2024-03/A13).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are entirely those of the author and do not necessarily reflect the views of the publisher, the editors, or the reviewers. This journal maintains a neutral stance concerning jurisdictional claims related to published institutional affiliations.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing the manuscript, and no images were manipulated using AI.
REFERENCES
1. Vimala G, Shoba F. A review on antiulcer activity of few Indian medicinal plants. Int J Microbiol. 2014;2014:519590. CrossRef
2. Sharifi-Rad M, Fokou PVT, Sharopov F, Martorell M, Ademiluyi AO, Rajkovic J, et al. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23(7):1751.
3. Derosa G, Maffioli P, Sahebkar A. Piperine and its role in chronic diseases. Adv Exp Med Biol. 2016:928:173-184. CrossRef
4. Jaiswal F, Rai AK, Wal P, Wal A, Singh SP. Peptic ulcer: a review on etiology, pathogenesis, and treatment. Asian J Pharm Educ Res. 2021;10(4):1. CrossRef
5. Biswas P, Ghorai M, Mishra T, Gopalakrishnan AV, Roy D, Mane AB, et al. Piper longum L. A comprehensive review on traditional uses, phytochemistry, pharmacology, and health-promoting activities. Phytother Res. 2022;36(12):4425–76.
6. Fluckiger FA, Hanbury D. Pharmacographia: a history of the principal drugs of vegetable origin met with in Great Britain and British India. London: Macmillan; 1890. 1889–93 pp.
7. Binti Raja Idris RI, Mohd Taufek N, Nordin NO, Al-Saari N. Zebrafish nutrition: promoting fish health and welfare of the animal model in halal science research. Halalsphere. 2022;2:106–21. CrossRef
8. Teame T, Zhang Z, Ran C, Zhang H, Yang Y, Ding Q, et al. The use of zebrafish (Danio rerio) as biomedical models. Anim Front. 2019;9(3):68–77.
9. Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel disease pathogenesis. World J Gastroenterol. 2017;23(33):6016–29. CrossRef
10. Jamwal S, Kumar P. Animal models of inflammatory bowel disease. In: Michael Conn P, editor. Animal models for the study of human disease. 2nd ed. Academic Press; 2017. pp 467–77.
11. Agrawal AK, Rao C, Joshi V, Goel R. Effect of Piper longum Linn, Zingiber officinale Linn, and Ferula species on gastric ulceration and secretion in rats. Indian J Exp Biol. 2000;38:994–8.
12. Nadkarni KM. Indian materia medica. Vol 1. Mumbai: Popular Prakashan; 1976. 805–7 pp.
13. Katalinic V, Milos M, Modun D, Majek P, Brcic M. The DPPH free radical scavenging activity of some herbs and spices. Food Chem. 2006;94(4):558–64. CrossRef
14. Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1996;299:15–27.
15. Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20(3):169–73.
16. Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Membrane stabilizing activity: a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70(3):251–7.
17. Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–309.
18. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
19. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71.
20. Tamagno WA, Alves C, Tessaro D, Sutorillo NT, Santin W, Barcellos LJG. Deferoxamine supplementation abolished iron-related toxicity of Ilex paraguariensis extract: behavioral and biochemical evaluation in adult zebrafish (Danio rerio). Antioxidants (Basel). 2022 Jul 31;11(8):1507.
21. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74.
22. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
23. Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21.
24. Zaveri S, Iyer R, Chaturvedi R. Pharmacological properties of Piper longum. Phytother Res. 2020;34(1):12–23.
25. Sarkar A, Sharma P, Khan S. Integrated pest management strategies for fruit fly control: an overview. J Agric Sci. 2017;15(3):123–30. CrossRef
26. Kotha RR, Tareq FS, Yildiz E, Luthria DL. Oxidative stress and antioxidants—a critical review on in vitro antioxidant assays. Antioxidants (Basel). 2022;11(12):2388.
27. Valgimigli L. Lipid peroxidation and antioxidant protection. Biomolecules. 2023;13(9):1291. CrossRef
28. Palacios-Espinosa JF, Arroyo-García O, García-Valencia G, Linares E, Bye R, Romero I. Evidence of the anti-Helicobacter pylori, gastroprotective, and anti-inflammatory activities of Cuphea aequipetala infusion. J Ethnopharmacol. 2014;15:990–8.
29. Sagradas J, Costa G, Figueirinha A, Castel-Branco MM, Silvério Cabrita AM, Figueiredo IV, et al. Gastroprotective effect of Cymbopogon citratus infusion on acute ethanol-induced gastric lesions in rats. J Ethnopharmacol. 2015;173:134–8.
30. Chandra P, Kishore K, Ghosh AK. Assessment of antisecretory, gastroprotective, and in vitro antacid potential of Daucus carota in experimental rats. Osong Public Health Res Perspect. 2015;6:329–35.
31. Han H, Gao M, Wang F. Protective effects of patchouli alcohol against DSS-induced ulcerative colitis. Sci Rep. 2024;14:16745.
32. Lopez Nadal A, Boekhorst J, Lute C, van den Berg F, Schorn MA, Bergen Eriksen T, et al. Omics and imaging combinatorial approach reveals butyrate-induced inflammatory effects in the zebrafish gut. Anim Microbiome. 2023;5:15. CrossRef
33. Yadav V, Chatterjee SS, Majeed M, Kumar V. Long-lasting preventive effects of piperlongumine and a Piper longum extract against stress-triggered pathologies in mice. J Intercult Ethnopharmacol. 2015;4(4):277–83.
34. Azeez TB, Lunghar J. 5 - Antiinflammatory effects of turmeric (Curcuma longa) and ginger (Zingiber officinale). In: Gopi S, Amalraj A, Kunnumakkara A, Thomas S, editors. Inflammation and natural products. London, UK: Academic Press; 2021. pp. 83–102.
35. Selvaraj LK, Jeyabalan S, Wong LS, Sekar M, Logeshwari B, Umamaheswari S. Baicalein prevents stress-induced anxiety behaviors in zebrafish model. Front Pharmaco. 2022;13:990799.