INTRODUCTION
Diabetes mellitus, a chronic disorder marked by hyperglycaemia, ranks among the top 10 causes of death globally. According to the International Diabetes Federation, diabetes currently affects over 537 million people. Diabetes mellitus in adults aged 20 years and over in Indonesia reached 12.5% in 2021 and is predicted to increase to 28.6% in 2045, positioning Indonesia as the fifth country with the highest number of diabetes sufferers in the world. Over 90% of diabetic patients have type 2 diabetes mellitus or T2DM [1]. T2DM is defined by persistently high blood glucose levels, increased blood insulin, dyslipidaemia, and low-grade inflammation [2–4]. A treatment for T2DM involves controlling postprandial hyperglycaemia to reduce glucose absorption by blocking carbohydrate-hydrolyzing enzymes in the digestive system, including α-glucosidase [5].
The α-glucosidase enzyme is a hydrolase that catalyses the hydrolysis of non-reducing terminal carbohydrates into α-glucose [6]. Glucosidase inhibitors impede carbohydrate absorption in the small intestine, unabsorbed complex carbohydrates into absorbable simple carbohydrates by competitively blocking glucosidase. By postponing carbohydrate absorption, they mitigate the postprandial metabolic acceleration in blood glucose levels. The α-glucosidase inhibitors may postpone the onset of diabetes in people with diminished glucose tolerance or impaired fasting plasma glucose level [7]. Therefore, a crucial technique for managing postprandial hyperglycemia associated with diabetes mellitus is the suppression of intestinal α-glucosidase.
Phyllanthus niruri Linn. (Euphorbiaceae), also referred to as Meniran, as traditional medicine, extensive utilization has been observed in various regions of Indonesia. These therapeutic plants encompass several phytochemical elements, including flavonoids, alkaloids, terpenoids, lignans, polyphenols, tannins, coumarins, saponins, steroids, phenols, and glycosides [8,9]. Preclinical studies demonstrated that extracts of P. niruri possess antidiabetic properties [10–13]. A recent study on the antidiabetic potential of Phyllanthus plants has focused on the α-glucosidase inhibition. The component of corilagin and repanducinic acid A present in the aqueous extract of P. niruri showed activity of glucosidase inhibitory by using high-performance liquid chromatography (HPLC)–high-resolution mass spectrometry and nuclear magnetic resonance (NMR) investigations [14]. A study by Mediani et al [15] found that flavonoids, phenolic acids, and strong α-glucosidase inhibition were all linked in ethanolic extracts of P. niruri at different ratios and water extracts.
The measurement of constituents in P. niruri by HPLC has been published, indicating that the phenolics in P. niruri may exert antidiabetic activity through α-glucosidase inhibitory pathways [12]. Phyllanthus urinaria, a different type of Phyllanthus, has been shown to help treat diabetes using α-glucosidase inhibitors. The NMR analysis showed that corilagin, mallotinin, and repandusinic acid A were the active ingredients in the water extract [11]. The α-glucosidase inhibition method has been successfully found using bioassay-guided fractionation. However, it is a time-consuming process that needs a lot of preparative-scale separations, which could mean that small parts with important bioactivity are missed [16].
This study aims to evaluate the potential of P. niruri fractions (PNFs) as an antidiabetic agent using an in vitro α-glucosidase enzyme inhibition approach. The partitioning of P. niruri ethanolic extract was performed using medium-pressure liquid chromatography (MPLC). The examination and identification of chemical and biological substances in each fraction employed ultra-HPLC with quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS). The UPLC-QTOF/MS delivers good mass accuracy during a brief run, establishing itself as a preferred approach for impartial compound screening and yielding substantial global constituent and metabolite profiling data [17]. The molecular docking simulation method was employed to predict the bioactive inhibitory potential of these drugs in silico. We performed molecular docking studies to elucidate the precise interactions between the bioactive components of P. niruri and the α-glucosidase enzyme model.
MATERIALS AND METHODS
Plant material and reagents
Phyllanthus niruri herb were sourced from Bogor, West Java, Indonesia. The plant was identified at the Research Centre for Biology, Indonesia Institute for the Science, with number identified 2094/IPH.1.01/if.07/XII/2018. Ethanol food grade for the extraction process (PT Acidatama, Indonesia). Dimethyl sulfoxide, p-nitrophenyl-alpha-D-glucopyranoside (pNPG), α-glucosidase derived from Saccharomyces cerevisiae, sodium phosphate monobasic dihydrate, sodium phosphate dibasic, corosolic acid, gallic acid, quercetin, aluminium chloride, sodium carbonate, sodium hydroxide, and Folin-Ciocalteu reagent (Sigma-Aldrich, USA). The waters were purified utilizing Milli-Q Academic manufactured by Merck Millipore (Burlington, MA). Methanol for MPLC and acetonitrile, along with formic acid for UPLC-DAD (Diode Array Detector)-QToF/MS analysis (Merck Millipore and Sigma-Aldrich, USA).
Extraction
Phyllanthus niruri herbs powder (1,000 g) was mixed with 70% ethanol (10 l) and extracted using the maceration method for 4 hours at a temperature of 60ºC with shaking. The filtrate was evaporated until a semisolid crude extract (200.39 g) was obtained.
Characterization of extract
Organoleptic qualities
We observed the colour, aroma, and shape of the leaves of the 70% ethanol extract of P. niruri.
The water-soluble extract content
In a glass-stopped flask, 5 g of ethanol extract of P. niruri were macerated for 24 hours with 100 ml of chloroform-saturated water, while being shaken for the first 6 hours, left for 18 hours. Filtered and subsequently dried in a shallow flat-bottomed dish at 105ºC. Heat the remainder at 105ºC until the weight remains constant. Calculate the content in % of air-soluble extract [18].
The ethanol-soluble extract content
In a glass-stopped flask, 5 g of ethanol extract of P. niruri were macerated for 24 hours with 100 ml of 96% ethanol, while being shaken for the first 6 hours, left for 18 hours. Filtered and subsequently dried in a flat-bottomed dish at 105ºC. Heat the remainder at 105ºC until the weight remains constant. Calculate the content in % of ethanol-soluble extract [18].
Determination of water content
The water content of P. niruri extract was determined using Karl–Fischer titration method. An amount of 50 mg of extract with glass weight and the transfer into the titration chamber, which contains combiMethanol-5. Titrate the extract using the CombiTitran-5 reagent automatically. Water content expressed as a percentage of the P. niruri extract weight [19].
Determination of loss on drying
The loss on drying of P. niruri extract was determined using a water balance. Approximately 0.5 g of extract was accurately weighed into an aluminium weighing pan and heated at 105°C. The extract was evenly distributed over the entire surface of the pan, then placed into the moisture balance chamber. Drying was carried out at a predetermined temperature until the instrument automatically displayed a stabil final reading [19].
Total ash content
Approximately 1 g of extract was added to the crucible. It was then placed in a furnace and heated at 450ºC until the weight was constant. The sample was removed, cooled in a desiccator, and weighed. If the charcoal could not be removed, boiling water should be added and filtered through ash-free filter paper. The residue and filter paper in the same crucible were burned. The filtrate was placed in a crucible, evaporated, and burned until constant, then weighed. The total ash content was calculated against the weight of the extract [18].
Acid insoluble ash content
The ash obtained in the determination of the ash total content, heated with 25 ml of diluted sulfuric acid solution for 5 minutes. The insoluble component was collected, filtered through a glass crucible or ash-free filter paper, rinsed in hot water, and burned until the weight constant, weigh. The acid-insoluble ash content was calculated against the dried material [18].
Total phenol content
The total phenol in P. niruri extract was measured using the Folin–Ciocalteu method of Abd-Ghafar et al. [20], with slight modifications. The extract of P. niruri was dissolved in ethanol (1,000 μg/ml). The assay was conducted by adding 25 μl of sample to the 96-well microplate. To this, 75 μl of Folin–Ciocalteu reagent (10-fold dilution with deionized water) was added and mixed thoroughly at room temperature for 5 minutes. Then, 75 μl of sodium carbonate (6%) solution was added to the mixture. The solution was incubated for 90 min in the dark at room temperature. Absorbance was measured at 725 nm using a microplate reader (BioTek Instruments, Germany). The total phenolic content was determined using a standard curve of gallic acid at 6.25–200 μg/ml concentrations. Total phenolic content was calculated and expressed as mg of gallic acid equivalent in 1 g of the extract (mg GAE/g extract).
Total flavonoid content
The flavonoid content of P. niruri extract was measured using the aluminium chloride colorimetry method as described by Singh et al. [21], with slight modifications. Briefly, 50 μl of P. niruri extract (1,000 μg/ml) was added into a microplate containing 50 μl of 95% ethanol. Then, 20 μl of 10% aluminium chloride hexahydrate, 20 μl of 1 M sodium acetate, and 100 μl of water were added to the 96-well microplate. The mixture was incubated for 30 minutes at room temperature, and the absorbance was read at 425 nm using a microplate reader (BioTek Instrument, Germany). Quercetin was used as the standard to determine the total flavonoid content values of the samples. A standard curve was used with concentrations of quercetin in the 10–160 μg/ml range, and the total flavonoid content values are presented as mg quercetin equivalent in 1 g of the extract (mg QE/g extract).
Fractionation
The extract (1.02 g) was fractionated by MPLC, which was equipped with an ultraviolet (UV) and evaporative light scattering detector (ELSD). The sample was run in the YMC-Pack ODS column AQ-HG 250 × 20 mm, 10 µm, Kyoto, Japan with the water (A) and methanol (B) gradient elution as follows: 0%–100% (B) for 105 minutes, flow rate = 20 ml/min, and injection volume = 5 ml.
UPLC-QTOF/MS examination
The sample of PNFs was studied using a Waters Acquity UPLC system that has a photodiode array and a QTOF mass detector (Waters, Milford, MA). The system has an Acquity UPLC® BEH C18 column that is 1.7 µm, 2.1 × 100 mm, and is kept at 40ºC. The mobile phase eluent system consisted of A (0.1% formic acid in demineralized water) and B (0.1% formic acid in acetonitrile) at a flow rate = 0.4 ml/min, injection volume = 2 µl, and concentration of sample of 1,000 µg/ml. The sample analysis concentrated on phenolic and tannin chemicals, including flavonoids, utilizing gradient elution as follows: 5% (B) for 0.0–1.0 minutes, 5%–100% (B) for 1.0–20.0 minutes, 100% (B) for 20.0–22.3 minutes, 100%–5% (B) for 22.3–22.4 minutes, and 5% (B) for 22.4–25.0 minutes.
In vitro of α-glucosidase inhibition assay
The α-glucosidase inhibition assay was measured using the method adopted previously by Hou et al. and Ni et al. [22,23], with minor modifications. The assay utilized pure enzymes obtained from yeast (S. cerevisiae). To do the test, 50 μl of 100 mM phosphate buffer (pH 6.8), 10 μl of α-glucosidase (1 U/ml), and 20 μl of P. niruri extract or PNF at different concentrations (0.3, 1, 3, 10, 13, and 100 μl/ml) were put into a 96-well plate. We incubated the reaction mixture at 37°C for 15 minutes. Subsequently, 20 μl of 5 mM pNPG was introduced, and then incubated for 20 minutes at 37ºC. The reaction was stopped by introducing 50 μl of 0.1 M sodium carbonate. At 405 nm, the absorbance was measured using a microplate reader (Epoch, BioTek Instruments, Germany). The control utilized dimethyl sulfoxide, while the positive control employed corosolic acid. To determine α-glucosidase inhibition, the following equation was used: Inhibitory activity (%) = (1 − A405 of sample/A405 of control) × 100. We determined the IC50 value using linear regression analysis.
Molecular docking simulation
The simulation of molecular docking was performed using Molegro Virtual Docker (MVD) ver. 6.0 to estimate the inhibitory action mechanism of P. niruri bioactive compounds against α-glucosidase. For the 3D structure of α-glucosidase from the Saccharomyces model, homology modelling was constructed using the Swiss model (swissmodel.expasy.org) as the structure is not available in the Protein Data Bank (www.rscb.org). The α-glucosidase structure used in the assay is an enzyme with a sigma product number G0660 and commission number 3.2.1.20. Based on this information, the appropriate sequence was selected in the UniProt P53341-MAAL12-yeast, which consists of 584 amino acids. The model was built by copying and pasting the fasta of the sequence using the Swiss model (swissmodel.com) with the 3AXH.1A as a template, which has a sequence identity of 72.12% and a GMQE of 0.94. The 3D crystal structure of α-glucosidase model was used as a receptor in the docking study then it was imported to the MVD program. As an initial approach, validation of docking protocol settings was done through isomaltase (ligand reference of 3AXH.1A) at some cavities of the enzyme structure. We are confirming the confidence in our study used the docking protocol reproduces the bound structure closely with root mean square deviation (RMSD) values less than 2Å [24]. Then, active compounds of P. niruri herb were selected to be docked into the receptor at the position, and the interactions were investigated.
Statistical analysis
The data are presented as mean ± SD of three observations. The IC50 values of were statistically analyzed using the slope of the scatter plot in Excel.
RESULTS AND DISCUSSION
Extraction and characterization of P. niruri extract
We assessed the extraction and characterization of P. niruri extract (Table 1). The organoleptic evaluation of the ethanol extract of P. niruri indicated a blackish-green hue, absence of odor, and a bitter flavor, with a yield of 20.03%. The subsequent characterization parameter involved quantifying the concentrations of the chemical dissolved in water and ethanol. This parameter represents the amount of chemicals that a specific solvent can extract or dissolve. The results indicated that the extract containing a water-soluble solvent produced higher quantities than the ethanol-soluble extract. The solubility of P. niruri extract in water and ethanol was 37.86% ± 0.08% and 17.16% ± 0.28%, respectively. In this finding, P. nirurri extract was predicted to contain high levels of polar compounds. Meanwhile, based on the research results of Jayani et al. [25], the levels of water-soluble and ethanol extracts of P. nirurri leaves powder showed values of 17.65% ± 0.93% and 11.10% ± 0.16%, respectively.
Table 1. Characterization of Phyllanthus niruri extract.
| Parameters | Result obtained |
|---|---|
| Water soluble extract content | 37.86% ± 0.08% |
| Ethanol soluble extract content | 17.16% ± 0.28% |
| Water content | 2.72% ± 0.14% |
| Loss on drying | 33.39% ± 0.14% |
| Total ash | 4.01% ± 0.41% |
| Acid insoluble ash | 0.42% ± 0.01% |
| Total phenolic content | 167.65 ± 4.80 mg GAE/g of extract |
| Total flavonoid content | 10.07 ± 0.22 mg QE/g of extract |
GAE = gallic acid equivalent; QE = quercetin equivalent.
Based on analysis, P. niruri extract had a water content of 2.72% ± 0.14%. Phyllanthus niruri extract is a thick extract preparation that is in accordance with standards for P. niruri herbs, which must have a moisture content of less than 17% [18]. The water content in the extract can vary depending on the humidity of the storage area and the amount of water still present in the extract. The water content is critical for preserving the extract’s quality, inhibiting the proliferation of bacteria, fungus, or insects, as well as preventing degradation from hydrolysis [26].
The drying loss aims to determine the maximum limit of the number of compounds lost in the form of water and volatile components in the drying process. To calculate the loss on drying, we must weigh the substance after it has dried at 105°C to a constant weight and express it as a percentage. The assessment of loss on drying of the P. niruri extract yielded a value of 33.39% ± 0.14%. The loss on drying of the extract shows that the remaining volatile substances in the P. niruri extract are a maximum of 33.39% ± 0.14% [19].
The determination of ash content entails heating the extract until organic molecules and their derivatives decompose and evaporate, resulting in residual mineral and inorganic elements. This provides a comprehensive overview of the internal and exterior mineral composition from the early stages of the process until extract generation. The extract of P. niruri contains a total ash concentration of 4.0% ± 0.41% and an acid-insoluble ash level of 0.42% ± 0.01%. The amount of acid-insoluble ash shows that there are extra contaminants such as soil and sand, while the amount of total ash in the extract shows that overall minerals, including plant minerals and possible external residues, are present during the maceration process. The maximum ash content range indicates the level of pollution and cleanliness. It is utilized to assess the mineral composition in extracts and the mineral content that is insoluble in acids [26].
According to the study, the total phenol content of the 70% ethanol extract of P. niruri herb has a phenol content of 167.65 ± 4.80 mg GAE/g extract. Phenolic compounds are secondary metabolite and have anti-diabetes and antioxidant activity. The analysis of phenol levels showed higher results than previous studies. Research by Rusmana et al. [27] reported that 70% ethanol extract of P. niruri extracted for 24 hours using the maceration method obtained a total phenol content of 61.36 ± 0.42 μg EGA/mg extract. Mediani et al. [15] studied changes in metabolites of 70% ethanol extract of P. niruri after three freeze drying, oven drying, and air drying, showing phenol content values of 0.145 ± 0.006, 0.107 ± 0.003, and 0.113 ± 0.004 mg GAE/mg extract, respectively.
In our research results, the total flavonoid content of the 70% ethanol extract of P. niruri was obtained at 10.07 ± 0.22 mg QE / g extract. Several researchers have reported the flavonoid content of P. niruri, Carmagnani et al. [28] found that the extract obtained by the maceration method for 7 days using 50% and 96% ethanol solvents obtained total flavonoid values of 35.67 ± 6.139 and 55.72 ± 3.137 mg QE/g extract. Phyllanthus niruri extracted with 80% ethanol for 3 × 24 hours obtained a value of 60.1 ± 1.95 mg QE/g extract [29]. Meanwhile, the flavonoid content in the 70% ethanol extract of P. niruri obtained through maceration is 12.32 ± 0.53 mg of rutin equivalent per gram of extract (mg RE/g extract) [30]. Phenolic compounds are known to have antidiabetic activity by inhibiting the α-glucosidase enzyme, which plays a role in breaking down carbohydrates into glucose. Inhibition of this enzyme can slow down the absorption of glucose in the intestine, so that it can reduce postprandial blood glucose levels. Some phenolic compounds, such as flavonoids and phenolic acids, show a high affinity for the α-glucosidase enzyme and have the potential as natural therapeutic agents for type 2 diabetes [31–33].
Inhibitory activities of the extract P. niruri against α-glucosidase
The evaluation of inhibitory activities of the extract of P. niruri was examined using yeast glucosidase. The strongest inhibitory effect of the ethanolic extract of P. niruri was 98.85% at 10 μg/ml, while the strongest effect of the positive control, corosolic acid, was 99.25% at 30 μg/ml. Figure 1 shows that the percentage inhibition profiles relative to sample concentration indicate that the extract of P. niruri exhibited the most potent glucosidase inhibition with an IC50 value of 1.48 ± 0.51 µg/ml, than corosolic acid (IC50 = 2.72 ± 0.72 µg/ml).
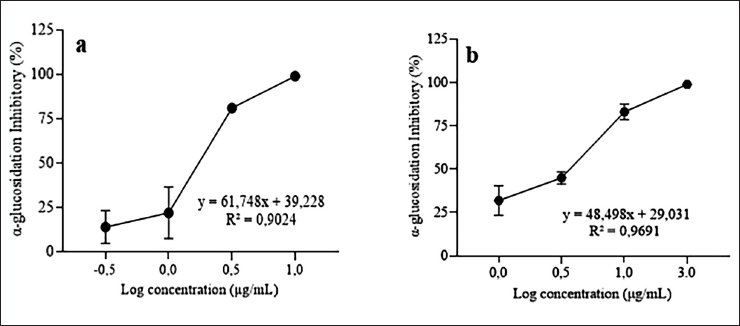 | Figure 1. Profile of concentration inhibition of α-glucosidase activity. Phyllanthus niruri extract (a) and corosolic acid (b). The IC50 values for the P. niruri extract and corosolic acid on α-glucosidase inhibition are 1.48 ± 0.51 and 2.72 ± 0.72 µg/ml, respectively. [Click here to view] |
According to previous research, the α-glucosidase inhibitory activity of P. niruri in our study was higher than that of a number of known extracts. The ethanol extract of Phyllanthus amarus, obtained through maceration, demonstrated activity with an IC50 value of 362.33 μg/ml [34]. After 30 minutes of ultrasonication and overnight maceration, the IC50 value of 6.3 ± 4.8 mg/ml of P. niruri ethanol extract was found to have α-glucosidase inhibitory [35]. The 70% ethanol extract of P. niruri was made by freeze drying, oven drying, and air drying, had α-glucosidase inhibitory effects with the IC50 values of 11.43 ± 1.06, 12.89 ± 1.41, and 11.62 ± 1.32 µg/ml, respectively [15]. The ethanol extracts of P. amarus and P. urinaria, which were made by sonicating the plants for 30 minutes and then macerating them overnight, had IC50 values of 926.5 ± 39.0 and 39.7 ± 97 μg/ml, respectively, for glucosidase inhibition [11]. Nevertheless, compared to other extract types, our result’s efficacy was marginally inferior. Phyllanthus niruri methanol extract exhibited α-glucosidase inhibition with an IC50 value of 0.2 ± 0.02 mg/ml [12]. The IC50 of Phyllanthus emblica was found to be 0.66 ± 0.01 µg/ml for α-glucosidase inhibition [36]. Corosolic acid inhibited α-glucosidase with an IC50 value of 1.35 × 10−5 mol/l [23]. Corosolic acid is a triterpenoid molecule with significant potential for glucosidase inhibitory action. Corosolic acid is present in Lagerstroemia speciosa leaves, ethyl acetate fraction with an IC50 = 3.53 pg/ml and in Psidium guajava leaves, methanol extract with an IC50 = 1.33 µg/ml [37,38]. Meanwhile, acarbose was used as a standard control of the α-glucosidase inhibitor, with an IC50 value of 41.23 µg/ml [39]. Acarbose, a common drug prescribed an α-glucosidase inhibitor, has been approved for the management of T2DM [39,40]. These results indicate that the extract of P. niruri exhibited stronger α-glucosidase inhibitory activity, as reflected by its lower IC50 value compared to both acarbose and gallic acid.
Fractionation and characterization of the phytochemicals in P. niruri extract
Phyllanthus niruri extract was fractionated utilizing a gradient methanol–water system by MPLC. The gradient system was rendered highly polar during the initial 10 minutes to isolate all chemicals present in the aqueous fraction. Subsequently, the polarity level transitions gradually to the non-polar phase. Compounds with analogous polarity will reside in the same fraction. Subsequently, eight fractions (PNF-1 to PNF-8) were derived, exhibiting a dispersion of components with varying polarity.
UPLC was employed to analyze the phytochemicals in the ethanol extract of P. niruri to ascertain its chemical makeup and potential active constituents. Figure 2 illustrates the performance of profiling mass spectrometry on the fraction using negative ion modes. The UV and MS spectrum data primarily demonstrated the presence of derivatives of phenolic acid, tannin, and flavonoids in the fraction (Table 2). The primary constituents identified were geraniin (6), corilagin (7), gallic acid dimethyl ester (8), quercetin derivatives (9), quercetin (10), hydroxyflavonol (11), and galangin-8-sulfonate (12). In addition, there are unidentified compounds (4), (13), and (14). However, according to the molecular formula and fragmentation pattern derived from UPLC-QTOF-MS data, the finding is ellagitannin class (4), phenolic acid derivatives (13), and flavonoids (14) are found.
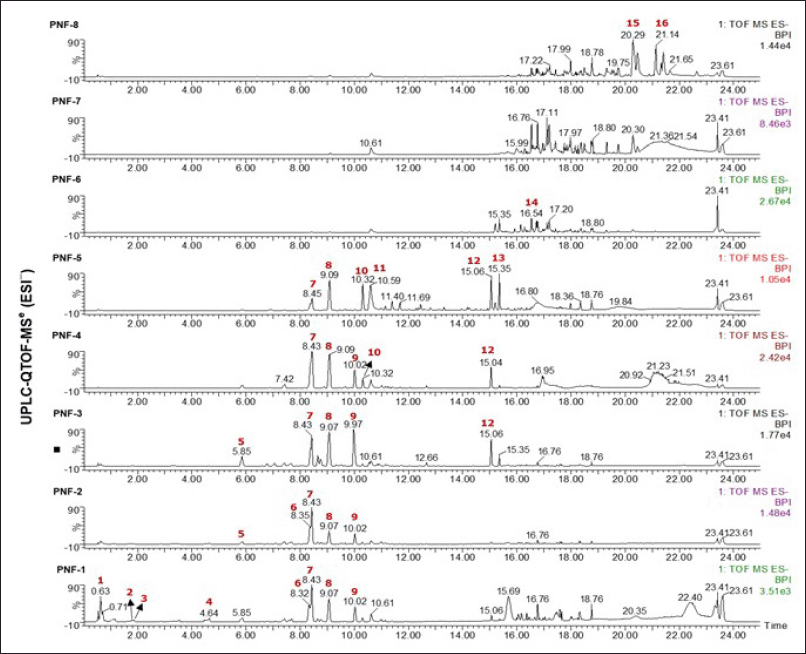 | Figure 2. The MS chromatograms of the Phyllanthus niruri fractions in negative mode. [Click here to view] |
Table 2. Tentative identification of Phyllanthus niruri components using UPLC-QTOF/MS.
| No. | tR, min | Molecular formula | UV | Negative | Tentative ID | Class | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MS RT(min) | Detectedion [M-H]¯ | Calculated ion [M-H]¯ | Error(µg/ml) | Major fragment ions | |||||||
| 1 | 0.63 | C12H14O10 | 201 | 0.63 | 317.0487 | 317.0509 | −6.9 | 317, 209, 191, 162 | Mucic acid derivatives | Galactaric acid | [72] |
| 2 | 1.75 | C13H16O10 | 207, 275 | 1.76 | 331.0661 | 661.0665 | −1.2 | 331, 169 | Gallic acid hexoside | Phenolic acid | [58,59] |
| 3 | 1.82 | C7H6O5 | 207, 270 | 1.86 | 169.1021 | 169.0137 | −9.5 | 169, 125 | Gallic acid | Phenolic acid | [57–59] |
| 4 | 4.55 | C18H14O13 | 207, 300 | 4.57 | 437.0337 | 437.0356 | −4.3 | 437, 409, 137 | Unknown | Ellagitannin | * |
| 5 | 5.81 | C8H8O5 | 208, 275 | 5.85 | 184.0290 | 184.0400 | −1.1 | 183, 125 | Gallic acid methyl ester | Phenolic acid | [57–59] |
| 6 | 8.32 | C41H28O27 | 215, 265 | 8.32 | 951.0736 | 951.0740 | −0.4 | 951, 633 | Geraniin | Tannin | [58,73,74] |
| 7 | 8.38 | C27H22O18 | 215, 270 | 8.41 | 633.0681 | 633.0728 | −7.4 | 633, 631, 387, 137 | Corilagin | Ellagitannin | [58,72] |
| 8 | 9.02 | C9H10O5 | 211, 272 | 9.04 | 197.0434 | 197.0450 | −8.1 | 197, 169, 125 | Gallic acid dimethyl ester | Phenolic acid | [58,72] |
| 9 | 9.86 | C24H16O13 | 211 | 9.92 | 511.0510 | 511.0513 | −0.6 | 511, 301, 137 | Quercetin derivatives | Flavonoid | [69,75] |
| 10 | 10.28 | C15H10O7 | 211 | 10.29 | 301.0374 | 301.0348 | 8.6 | 301, 271, 255, 229 | Quercetin | Flavonoid | [60] |
| 11 | 10.56 | C15H10O7 | 211, 253 | 10.61 | 317.0026 | 317.0041 | −2.0 | 635 [2M-H], 317, 301 | Hydroxyflavonol | Flavonoid | [76] |
| 12 | 14.96 | C15H9O8S | 218 | 14.99 | 348.9966 | 348.9987 | −7.7 | 348 [M-Na], 269 | Galangin-8-sulfonate | Flavonol Sulfonates | [77] |
| 13 | 15.32 | C6H20N2O4 | 218 | 15.35 | 183.1343 | 183.1345 | −1.1 | 183 | Unknown | Phenolic acid | * |
| 14 | 16.50 | C30H60O16 | 220 | 16.53 | 675.3790 | 675.3803 | −1.9 | 721 [M+FA], 675, 397 | Unknown | Flavonoid | * |
*Based on fragmentation pattern.
In vitro inhibition of α-glucosidase by PNFs
In vitro investigations demonstrated that the inhibitory potency of the α-glucosidase enzyme is closely correlated with the compound’s polarity, as seen by the peak in the UPLC chromatogram (Table 3). The results showed that fractions PNF-1 to PNF-6 significantly blocked α-glucosidase, with doses of 1 and 3 µg/ml showing more than 90% inhibition. The inhibitions were markedly elevated (p < 0.05) in comparison to fractions PNF-7 and PNF-8. The UPLC study reveals that the primary constituents of the active fraction of P. niruri include geraniin, corilagin, gallic acid dimethyl ester, and quercetin derivatives. Corilagin and gallic acid dimethyl ester were identified as consistent components in fractions PNF-1 to PNF-5. While fraction PNF-6 to PNF-8 lacks these chemicals, it remains unclear whether the drug inhibits the glucosidase enzyme. Fraction PNF-6 to PNF-8 showed a decrease of α-glucosidase inhibitory activity when compared to fraction PNF-1 to PNF-5, which could be due to the synergistic effect of active compounds present in fraction PNF-1 to PNF5 in inhibiting α-glucosidase [40–42].
Table 3. The α-glucosidase inhibitory activity and tentative compounds of Phyllanthus niruri fractions
| Fractions | Weight (mg) | α-glucosidase inhibitory (%) | Tentative compounds of P. niruri fractions | |
|---|---|---|---|---|
| 1 µg/ml | 3 µg/ml | |||
| PNF-1 | 301.0 | 99.25 ± 0.19 | 98.36 ± 0.00 | Mucic acid derivatives (1), gallic acid hexoside (2), gallic acid (3), geraniin (6), corilagin (7), gallic acid dimethyl ester (8), quercetin derivatives (9) |
| PNF-2 | 384.6 | 99.25 ± 0.19 | 99.69 ± 0.05 | Gallic acid methyl ester (5), geraniin (6), corilagin (7), gallic acid dimethyl ester (8), quercetin derivatives (9) |
| PNF-3 | 10.1 | 96.21 ± 0.15 | 99.52 ± 0.00 | Gallic acid methyl ester (5), corilagin (7), gallic acid dimethyl ester (8), quercetin derivatives (9), galangin-8-sulfonate (12) |
| PNF-4 | 24.2 | 96.55 ±0.05 | 99.59 ± 0.00 | Corilagin (7), gallic acid dimethyl ester (8), quercetin derivatives (9), quercetin (10), galangin-8-sulfonate (12) |
| PNF-5 | 36.4 | 96.51 ± 0.00 | 99.49 ± 0.15 | Corilagin (7), gallic acid dimethyl ester (8), quercetin (10), hydroxyflavonol (11), galangin-8-sulfonate (12) |
| PNF-6 | 17.5 | 94.10 ± 1.64 | 99.45 ± 0.00 | Unknown |
| PNF-7 | 12.9 | 47.83 ± 3.56 | 75.15 ± 5.85 | Unknown |
| PNF-8 | 9.2 | 7.62 ± 1.55 | 21.16 ± 0.68 | Unknown |
The loss of synergy in the fraction reported in studies of α-glucosidase inhibition activity. Mugaranja et al. [40] founded that the α-glucosidase inhibition activity of the purified fraction-14 from Simarouba glauca was slightly lower than that of the crude extract, likely because the phenolic compounds in the crude extract work together to enhance the activity. The ethanol extract of S. glauca and fraction-14 had activity of α-glucosidase inhibition with an IC50 value of 0.5 ± 0.04 and 2.4 ± 0.4 μg/ml. Black tea and green tea crude extracts exhibited lower α-glucosidase inhibition activity than semi-purified extracts. The black tea of 70% ethanol extract and semi-purified extract was observed to have activity of α-glucosidase inhibition with an IC50 value of 30.01 ± 1.50 and 46.02 ± 1.71 µg/ml. Meanwhile, a small reduction in α-glucosidase inhibition activity was detected in 70% ethanol of green tea and semi-purified extract, with IC50 values of 26.04 ± 1.20 µg/ml and 38.07 ± 1.40 µg/ml, respectively [41]. Suzlin Sulaiman et al. [42], reported the alkaloid separation process from Uncaria longiflora, showing pteropodine and isopteropodine inhibited α-glucosidase with IC50 values of 226.70 ± 2.82 and 98.06 ± 1.98 μg/ml, while the crude extract methanol IC50 value was 138.10 ± 1.32 μg/ml. This pattern suggests that isopteropodine and its other chemical constituents could synergistically contribute to the extract’s activity. In addition, the presence of antagonistic compounds in a mixture of natural ingredients can result in decreased α-glucosidase enzyme activity. Researchers Rahayu et al. [43] reported that the combined infusion extract of bay leaves and soursop or guava and soursop showed antagonistic effects. The combination of bay and soursop and guava and soursop infusions had IC50 values of 0.063 ± 0.005 microgram gallic acid equivalent per ml (µg GAE/ml) and 0.094 ± 0.002 μg GAE/ml. But, the infusion of bay, guava, and soursop showed lower 0.025± 0.007, 0.083 ± 0.01, and 0.533 ± 0.039 μg GAE/ml of α-glucosidase activity inhibitory. These findings correlated with their total phenolic content, where bay, guava, and soursop infusions contained 18.48 ± 2.27, 17.84 ± 2.48, and 21.43 ± 0.13 µg GAE/ml. In addition, the effect of the active ingredient is covered by other compounds in a complex mixture, which also occurs in natural ingredient mixtures. Complexity can also occur because the levels of compounds contained are very small, causing the active compound to have low activity, which results in decreased activity [43,44].
Our investigation suggests that the four principal substances (geraniin, corilagin, gallic acid dimethyl ester, and a quercetin derivative) substantially inhibited the α-glucosidase enzyme. Our findings are corroborated by prior research, which demonstrated that these chemicals can block α-glucosidase [11,22,38,45–66].
Geraniin is an ellagitannin, a category of hydrolyzable tannin, that constitutes a significant component in various plant species, including Phyllanthus, Geranium, Nephelium lappaceum, and Erodium glaucophyllum. Geraniin has been recognized for its antioxidant, anti-hyperglycemic, antiviral, and anticancer properties [46,48,49,66]. According to a study by Agyare et al. [50], the primary component was an aqueous extract of Phyllanthus muellerianus leaves, which contained 2.9% geraniin. LC-MS analysis of a cocktail extract of Phyllanthus species (a mixed of Phyllanthus watsonii, P. amarus, P. niruri, and P. urinaria in a 2:2:1:1 ratio) revealed geraniin as a predominant constituent. The rind extracts of N. lappaceum identified geraniin as a significant constituent, accounting for 56.8% of the methanolic extract [51]. According to Palanisamy et al. [49], geraniin in N. lappaceum rind ethanol extract was around 37.9 mg/g. This extract demonstrated the ability to inhibit the α-glucosidase enzyme with an IC50 value of 0.92 µg/ml, while geraniin had an IC50 value of 16.12 µg/ml [46].
Corilagin, a gallotannin, is predominantly located in the families Aceraceae, Combretaceae, Euphorbiaceae, Geraniaceae, and Sapindaceae. It is a significant active constituent of numerous plants, including P. emblica, P. niruri, and P. urinaria [52]. Notka et al. [53], reported isolating corilagin and geraniin from the water–alcohol extract of P. amarus, achieving concentrations of 2.28% and 1.10%, respectively, through HPLC analysis. Zheng et al. [54], found corilagin in plant alcoholic extracts. The amounts found were 67.9, 159, and 619 µg/g in Phyllanthus debilis, Phyllanthus tenellus, and P. urinaria, respectively. A study by Hou et al. [22], investigated extracts of P. amarus, P. niruri, P. tenellus, and P. urinaria utilizing an aqueous ionic liquid in conjunction with preparative HPLC and precipitation techniques; the corilagin concentrations were determined to be 1111.68, 1157.87, 1684.08, and 144.09 µg/g, respectively. The inhibitory of α-glucosidase activity of corilagin derived from water extract of P. urinaria exhibited IC50 values of 1.70 and 0.9 µM [11,35]. A separate study identified corilagin in Nymphaea stellata flowers, methanol extract exhibited 24% (at a concentration of 1 mg/ml) of α-glucosidase inhibition. Corilagin, derived from Macaranga tanarius leaves ethyl acetate extract, has the ability to inhibit mammalian intestinal glucosidase, exhibiting an IC50 value of 2.63 mM [55]. Corilagin, also found derived from Terminalia chebula aqueous extract of was documented with an IC50 value of 2.64 µM [45].
Gallic acid and its methyl ester (gallic acid dimethyl ester), belonging to the phenolic acid class, are naturally occurring secondary metabolites present in several plants and herbs [56]. Gallic acid is abundantly sourced from medicinal plants within the Phyllanthus genus, including P. amarus, P. urinaria, P. myrtifolius, P. multiflorus, P. debilis, P. embergeri, P. tenellus, P. emblica, and P. fraternus [57–59]. Kumar et al. [58], demonstrated that P. amarus contained gallic acid at levels ranging from 0.45 to 32.80 mg/g and quercetin from 0.01 to 5.22 mg/g, as ascertained by UPLC-MRM. The gallic acid concentration in the aqueous extract of P. amarus is 135.08 mg/g. Moreover, gallic acid is found in other therapeutic plants, including Momordica charantia with 97.35 mg/g, Achillea schischkinii with 11.2 mg/g, and Rhodiola crenulata with 8.12 mg/g [60–62].
Gallic acid and alkyl gallates derived from winery by-products exhibited anti-α-glucosidase activity, with grape stems, grape pomace, and wine leaves demonstrating α-glucosidase inhibitory activity levels of 1.58, 1.46, and 123 units/l, respectively [63]. Simultaneously, 4-O-methyl gallic acid was identified in Syzygium myrtifolium ethyl acetate fraction, with an IC50 value of 25.19 µg/ml [64].
Quercetin is a flavonoid subclass seen in plants, fruits, vegetables, and leaves. Quercetin possesses multiple pharmacological characteristics, including the suppression of α-glucosidase. P. urinaria and P.acidus extract were obtained using 60% aqueous acetone and 50% ethanol, respectively, and were identified to contain the quercetin compound [67]. Quercetin is present in P. emblica fruit and P. amarus leaves extract at a measured level of 10.20 and 5.84 mg/g, respectively [60,68]. The callus culture of P. niruri comprises 1.72% quercetin [47]. At other plants, M. charantia, Morus alba, and Ginkgo biloba extracts contain quercetin at concentrations of 50.39, 6.29, and 3.49 mg/g, respectively [60,68]. According to Li et al. [69], reported quercetin and its derivative isoquercetin were identified as α-glucosidase activity inhibitors, IC50 = 0.017 and 0.185 mmol/l, respectively. Another report, as presented by Sofa et al. [65], quercetin derivatives (3’,4’-dimethoxy quercetin, quercetin 3-O-α-L-rhamnoside, and quercetin-3-O-α-L-arabinopyranosyl (1→2) α-L rhamnopyranoside) from Bryophyllum pinnatum exhibited α-glucosidase inhibitory, IC50 = 103.20, 83.83, and 110.52 μg/ml, respectively.
Molecular docking analysis
A molecular docking study yields crucial insights into the framework of interactions between identified compounds and enzymes. Additionally, it provides binding energy data that can be used to assess the viability of specific substances as α-glucosidase inhibitors. Validation of the docking simulation was done by redocking the native ligand and then investigating its superimposition. The RMSD value obtained from the superimposition is 1.16 Å. The coordinates for docking at the specified place are x = −19.80, y = −7.74, and z = −21.47, with a radius of 15 ?. The simulation of molecular docking was conducted for the bioactive chemicals found in P. niruri plants, including mucic acid (1), gallic acid hexoside (2), gallic acid (3), gallic acid methyl ester (5), geraniin (6), corilagin (7), gallic acid dimethyl ester (8), quercetin (10), hydroxyflavonol (11), galangin-8-sulfonate (12). Each molecule was classified as either a ligand or an inhibitor, which had an impact on the receptor’s functionality. The docking scores for each compound and amino acid that interacted were provided in Table 4, employing the MVD version 6.0 program.
Table 4. MolDock score and hydrogen bonding with amino acid after docked target α-glucosidase and ligands.
| Ligand | MolDock score | Hydrogen bonding with amino acid | Steric interactions with amino acid |
|---|---|---|---|
| Isomaltase | −131 | Asp 68, His 111, Gln 181, Arg 212, Asp 214, Glu 276, His 348, Asp 349, Gln 350, Arg 439 | Asp214, Asp 349 |
| Mucic acid | −87 | Asp 68, Gln 181, Arg 212, Asp 214, Glu 276, His 348, Asp 349, Arg 439 | Asp 68, Asp 349 |
| Gallic acid hexoside | −120 | Lys 155, Phe 157, Gly 217, His 239, Glu 276 | Phe 157, Leu 218 |
| Gallic acid | −83 | Asp 68, His 111, Gln 181, Asp 214, Glu 276, Asp 314, Arg 439 | Asp 68, Tyr 71, Thr 215, Glu 276 |
| Gallic acid methyl ester | −82 | Lys 155, Phe 157, Arg 312, Asp 408, Asn 412 | Phe 157, Phe 311, Arg 312 |
| Geraniin | −43 | Lys 155, Asn 241, His 279, Tyr 313, Phe 310, Arg 312, Asp 349, Gln 350, Asp 408, Asn 412 | Phe 157, Phe 158, Thr 215, His 239, Asn 241, Glu 276, Ala 278, His 279, Phe 300, Phe 310, Arg 312, Arg 439 |
| Corilagin | −152 | Asn 241, Ser 244, His 245, His 279, Glu 304, Pro 309, Gln 350 | Phe 157, Thr 215, Leu 218, His 279, Phe 300, Asp 349 |
| Gallic dimethyl ester | −81 | His 245, Glu 276 | Ser 156, Leu 218, Asn 241 |
| Quercetin | −115 | Asp 68, Asp 214, Glu 276, Tyr 313, Gln 350, Asp 408, Arg 439 | Phe 300, Asp 349, Arg 439 |
| 3-hydroxyflavone | −93 | Glu 276, Asp 349, Arg 439 | Phe 157, Phe 177, Phe 300, Asp 349, Arg 439 |
| 7-hydroxyflavone | −104 | Phe 157, Leu 176, Asn 241 | Phe 157, Asn 241, Glu 276 |
| Galangin-8-sulfonate | −103 | Lys 155, Phe 157, Glu 304, Arg 312, Asp 408, Asn 412 | Phe 157, Arg 312, Asp 408 |
| Corosolic acid | −87 | His 279, Pro 309, Arg 439 | Phe 157, Thr 215, Leu 218, His 279, Phe 300, Glu 304, Asp 349 |
Bold residues indicate same interaction with the native ligand.
In the present study, it was found that the α-glucosidase inhibitory potential of P. niruri extract reported previously could be attributed to geraniin, corilagin, gallic acid, and quercetin. Our study showed that the docking score of the corilagin tested showed a consistent trend with the in vitro α-glucosidase inhibition data. Corilagin showed a stronger binding affinity with a MolDock score of −152, suggesting it plays an important role in inhibiting α-glucosidase. Fractions containing Corilagin, such as PNF-1 to PNF-5, appear to have inhibitory activity. Meanwhile, fractions PNF-6 to PNF-8, which do not show the presence of Corilagin, exhibit weak inhibitory activity as shown in Tables 3 and 4.
The interaction between compounds and α-glucosidase involves various binding forces, including hydrogen bonding and steric interactions, which are essential in molecular recognition and stability. In Table 4, corilagin provides hydrogen bonds with amino acids with glucosidase, namely Asn241, Ser244, His245, His279, Glu304, Pro309, and Gln350. Amino acid Gln350 is found only in corilagin, geraniin, and quercetin. In addition, Gln350 is found in isomaltose, which is a native ligand in α-glucosidase. Hydrogen bonds are crucial for the protein stability of ligand-protein complexes, and the number of hydrogen bonds is positively correlated with the binding energy and interaction specificity [42,70]. In addition, steric interactions with amino acids observed in corilagin, namely Phe157, Thr215, Leu218, His279, Phe300, and Asp349, contribute to the inhibition of activity, which isomaltose has steric interactions with amino acids Asp214 and Asp349. Furthermore, the corosolic acid exhibited steric interactions with specific amino acids such as Phe157, Thr215, Leu218, His279, Phe300, Glu304, and Asp349. Steric interactions are interactions between atoms or groups of the ligand that occur due to physical size (volume space), where the shape and size fit with the amino acids in the binding pocket of the protein [71].
The MolDock score represented the binding energy between the target receptor, α-glucosidase, and ligands. Corilagin had the most negative Moldock score of −152, which was lower than that of isomaltose, corosolic acid, and other ligands. Negative values imply a higher degree of bond stability, suggesting that the contact between the receptor and the ligand may impede the enzyme’s performance. Figure 3 illustrates the interaction between ligands and amino acid residues of the enzyme. Corilagin established hydrogen bonds, electrostatic contacts, and steric interactions with various amino acids present in the enzyme residues, including those located in the active sites.
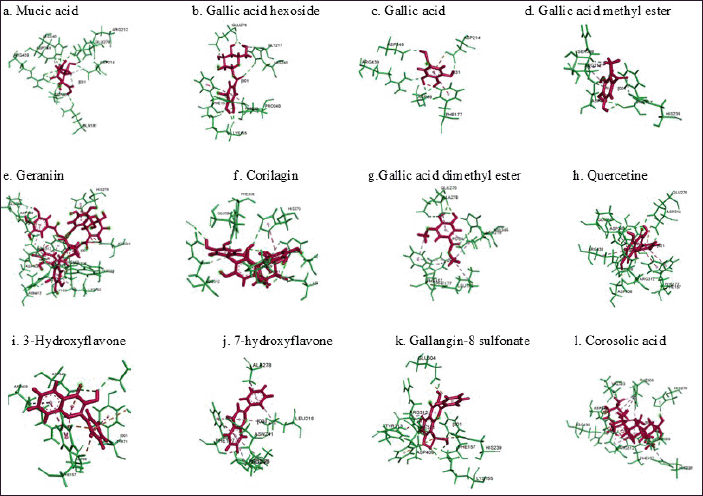 | Fig. 3. Predicted binding modes, H-bond interactions of α-glucosidase with mucic acid (a), gallic acid hexoside (b), gallic acid (c), gallic acid methyl ester (d), geraniin (e), corilagin (f), gallic dimethyl ester (g), quercetin (h), 3-hydroxiflavone (i), 7-hydroxyflavone (j), galangin-8-sulphonate (k), and corosolic acid (l). Green is α-glucosidase and red is target of ligand. [Click here to view] |
CONCLUSIONS
The bio-guided fractionation of P. niruri extract showed that fractions PNF-1 to PNF-6 had higher α-glucosidase inhibition activity compared to PNF-7 and PNF-8. This analysis revealed that the major compounds of consistently in PNF-1 and PNF-5 were tannin (corilagin) and phenolic acid (gallic acid dimethyl ester) class compounds that can be used as chemical marker candidates to control the quality. According to the results of the docking simulation, it is projected that corilagin, which contains several phenol groups, plays a significant role in inhibiting the enzyme. Further research is required to validate this, which should involve the isolation of the active chemical, led by bioassay.
ACKNOWLEDGMENTS
We gratefully acknowledge for Korea Research Institute of Bioscience and Biotechnology (KRIBB) for the support and research facilities provided.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was support by Korea Research Institute of Bioscience and Biotechnology (KRIBB) and BRIN research collaboration program in Scientist Exchange Program.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Magliano D, Boyko E. IDF diabetes atlas 2021. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021.
2. Bodaghi AB, Ebadi E, Gholami MJ, Azizi R, Shariati A. A decreased level of high-density lipoprotein is a possible risk factor for type 2 diabetes mellitus: a review. Health Sci Rep. 2023;6:e1779. CrossRef
3. Kartika R, Wibowo H. Impaired function of regulatory T cells in type 2 diabetes mellitus. Mol Cell Biomed Sci. 2020;4:1. CrossRef
4. Westman EC. Type 2 diabetes mellitus: A pathophysiologic perspective Eric C. Westman. Front Nutr. 2021;8:707371. CrossRef
5. Kashtoh H, Baek K-H. Recent updates on phytoconstituent alpha-glucosidase inhibitors: an approach towards the treatment of type two diabetes. Plants (Basel) 2022;11:2722. CrossRef
6. Ernawati T, Mun’Im A, Hanafi M, Yanuar A. In silico evaluation of molecular interactions between known α-glucosidase inhibitors and homologous α-glucosidase enzymes from Saccharomyces cerevisiae, Rattus norvegicus, and GANC-human. J Pharm Sci. 2018;42:14–20. CrossRef
7. Van de Laar FA, Lucassen P, Akkermans RP, Van de Lisdonk EH, Rutten G, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003639. CrossRef
8. Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559–70. CrossRef
9. Widiadnyani NKE, Astawa NM, Yasa WPS, Sukrama IDM. Phytochemical test and identification of active compounds with LC-MS/MS in green meniran leaf (Phyllanthus niruri Linn). Bali Med. J. 2021;10(1):132–6.
10. Domínguez-Perles R, García-Viguera C, Medina S. New anti-α-glucosidase and antioxidant ingredients from winery byproducts: contribution of alkyl gallates. J Agric Food Chem. 2023;71:14615–25. CrossRef
11. Trinh BTD, Staerk D, Jäger AK. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. J Ethnopharmacol. 2016;186:189–95. CrossRef
12. Okoli CO, Obidike IC, Ezike AC, Akah PA, Salawu OA. Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm Biol. 2011;49:248–55. CrossRef
13. Bavarva JH, Narasimhacharya AVRL. Comparative antidiabetic, hypolipidemic, and antioxidant properties of Phyllanthus niruri. in normal and diabetic rats. Pharm Biol. 2007;45:569–74. CrossRef
14. Najari Beidokhti M, Andersen MV, Eid HM, Sanchez Villavicencio ML, Staerk D, Haddad PS, et al. Investigation of antidiabetic potential of Phyllanthus niruri L. using assays for α-glucosidase, muscle glucose transport, liver glucose production, and adipogenesis. Biochem Biophys Res Commun. 2017;493:869–74. CrossRef
15. Mediani A, Abas F, Khatib A, Tan CP, Ismail IS, Shaari K, et al. Relationship between metabolites composition and biological activities of Phyllanthus niruri extracts prepared by different drying methods and solvents extraction. Plant Foods Hum Nutr. 2015;70:184–92. CrossRef
16. Pieters L, Vlietinck AJ. Bioguided isolation of pharmacologically active plant components, still a valuable strategy for the finding of new lead compounds? J Ethnopharmacol. 2005;100:57–60. CrossRef
17. Sousa AD, Maia IV, Ribeiro PRV, Canuto KM, Zocolo GJ, Sousa de Brito E. UPLC-QTOF-MS E -based chemometric approach driving the choice of the best extraction process for Phyllanthus niruri. Sep Sci Technol. 2017;52:1696–706. CrossRef
18. Ministry of Health Republic of Indonesia. Farmakope herbal Indonesia. Jakarta, Indonesia: Ministry of Health Republic of Indonesia; 2017.
19. Rosidah I, Zainuddin Z, Agustini K, Bunga O, Pudjiastuti L. Standardization of 70% ethanol extract chayote fruit (Sechium edule (Jacq.) Sw.). Farmasains 2020;7:13–20. CrossRef
20. Abd Ghafar MF, Nagendra Prasad K, Kin Weng K, Ismail A. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr J Biotechnol. 2010;9:326–30.
21. Singh M, Thrimawithana T, Shukla R, Adhikari B. Extraction and characterization of polyphenolic compounds and potassium hydroxycitrate from Hibiscus sabdariffa. Future Foods 2021;4:100087. CrossRef
22. Hou X, Cheng Z, Wang J. Preparative purification of corilagin from Phyllanthus by combining ionic liquid extraction, prep-HPLC, and precipitation. Anal Methods 2020;12:3382–9. CrossRef
23. Ni M, Pan J, Hu X, Gong D, Zhang G. Inhibitory effect of corosolic acid on α -glucosidase: kinetics, interaction mechanism, and molecular simulation. J Sci Food Agric. 2019;99:5881–9. CrossRef
24. Hevener KE, Zhao W, Ball DM, Babaoglu K, Qi J, White SW, et al. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J Chem Inf Model. 2009;49:444–60. CrossRef
25. Jayani NIE, Krisnawan AH, Oktaviyanti ND, Kartini. Standardization of Phyllanthus niruri and Sonchus arvensis as components of scientific Jamu. Maj Obat Tradis. 2020;25:7–14. CrossRef
26. WHO. Quality control methods for medicinal plant materials. Geneva, Switzerland World Health Organization; 1998.
27. Rusmana D, Wahyudianingsih R, Elisabeth M, Balqis B, Maesaroh M, Widowati W. Antioxidant activity of Phyllanthus niruri extract, rutin and quercetin. Indones Biomed J. 2017;9:84. CrossRef
28. Carmagnani HJ, Bucciarelli Mansano G, Sobreira F. Otimização do processo extrativo de Phyllanthus niruri L. Mundo Saúde 2020;44:134–43. CrossRef
29. Shoeb M, Islam MdN, Nahar N. Biological activity studies of the aerial parts of Phyllanthus niruri L. Curr Res Biosci Biotechnol. 2022;4:251–5. CrossRef
30. Luliana S, Desnita R, Martien R, Nurrochmad A. Total flavonoid contents and in silico study of flavonoid compounds from Meniran (Phyllanthus niruri L.) towards alpha-amylase and alpha-glucosidase enzyme. Pharmaciana 2019;9:1–10. CrossRef%vi%i.10416
31. Artanti N, Dewijanti ID, Muzdalifah D, Windarsih A, Suratno S, Handayani S. Alpha-glucosidase inhibitory activity of the combination of Caesalpinia sappan L. and Garcinia mangostana extract. J Appl Pharm Sci. 2023;13(5):189–98. CrossRef
32. Indrianingsi AW, Prihantini AI, Tachibana S. α-Glucosidase inhibitor and antioxidant activity of procyanidin, an isolated compound from Quercus gilva Blume leaves. J Appl Pharm Sci. 2022;12(5):213–8. CrossRef
33. Adera KT, Inami YM, Akamatsu KT, Atsuoka TM. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52(2):149–53.
34. Limcharoen T, Chaniad P, Chonsut P, Punsawad C, Juckmeta T, Konyanee A, et al. Alpha-glucosidase inhibition, antioxidant activities, and molecular docking study of krom luang chumphon khet udomsak, a Thai traditional remedy. Adv Pharmacol Pharm Sci. 2024;2024:1–18. CrossRef
35. Beidokhti M, Andersen M, Eid HM, Sanchez Villavicencio ML, Staerk D, Haddad PS, et al. Investigation of antidiabetic potential of Phyllanthus niruri L. using assays for alpha-glucosidase, muscle glucose transport, liver glucose production, and adipogenesis. Biochem Biophys Res Commun. 2017;493:869–74. CrossRef
36. Sabuhom P, Subin P, Luecha P, Nualkaew S, Nualkaew N. Effects of plant part substitution in a Thai traditional recipe on α-glucosidase inhibition. Trop J Nat Prod Res. 2023;7:2919–25. CrossRef
37. Chao IC, Chen Y, Gao MH, Lin LG, Zhang XQ, Ye WC, et al. Simultaneous determination of alpha-glucosidase inhibitory triterpenoids in Psidium guajava using HPLC-DAD-ELSD and pressurized liquid extraction. Molecules 2020;25:1278. CrossRef
38. Hou W, Li Y, Zhang Q, Wei X, Peng A, Chen L, et al. Triterpene acids isolated from Lagerstroemia speciosa leaves as alpha-glucosidase inhibitors. Phytother Res. 2009;23:614–8. CrossRef
39. Siddiqui Z, Khan MI, Badruddeen B, Mohammad Ahmad, MA, Manvi M, Fatima G. Isolation and characterization of α-glucosidase inhibitors from Phyllanthus acidus (L.) Skeels stem bark. Ann Phytomed. 2024;13:1100–10. CrossRef
40. Mugaranja KP, Kulal A. Alpha glucosidase inhibition activity of phenolic fraction from Simarouba glauca: an in-vitro, in-silico and kinetic study. Heliyon 2020;6:e04392. CrossRef
41. Tan Y, Chang SKC. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J Funct Foods 2017;38:644–55. CrossRef
42. Suzlin Sulaiman NA, Syakir Nor Azman MF, Fasihi Mohd Aluwi MF, Zakaria ZA, Mohammad Ridhwana MJ, Salim F. Stereospecific α-glucosidase inhibition, kinetics, and molecular docking studies on isolated diastereomeric alkaloids from Uncaria longiflora. Results Chem. 2025;13:101926. CrossRef
43. Rahayu I, Heng PH, Timotius KH. In vitro antioxidant properties and α-glucosidase inhibition of combined leaf infusions from Psidium guajava L., Syzygium polyanthum L., and Annona muricata L. Pharmacog J. 2019;11:1269–77. CrossRef
44. Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36:869–88. CrossRef
45. Li DQ, Zhao J, Xie J, Li SP. A novel sample preparation and on-line HPLC–DAD–MS/MS–BCD analysis for rapid screening and characterization of specific enzyme inhibitors in herbal extracts: case study of α-glucosidase. J Pharm Biomed Anal. 2014;88:130–5. CrossRef
46. Widowati W, Maesaroh M, Fauziah N, Erawijantari PP, Sandra F. Free radical scavenging and alpha/beta-glucosidases inhibitory activities of rambutan (Nephelium lappaceum L.) peel extract. Indones Biomed J. 2015;7:157. CrossRef
47. Anuar N, Markom M, Khairedin S, Johari NA. Production and extraction of quercetin and (+)-catechin from Phyllanthus niruri callus culture. Int J Biol Sci. 2012;6:1240–3.
48. Yang Y, Zhang L, Fan X, Qin C, Liu J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg Med Chem Lett. 2012;22:2209–11. CrossRef
49. Palanisamy UD, Ling LT, Manaharan T, Appleton D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011;127:21–7. CrossRef
50. Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011;18:617–24. CrossRef
51. Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules 2010;15:1453–65. CrossRef
52. Li X, Deng Y, Zheng Z, Huang W, Chen L, Tong Q, et al. Corilagin, a promising medicinal herbal agent. Biomed Pharm. 2018;99:43–50. CrossRef
53. Notka F, Meier G, Wagner R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antiviral Res. 2004;64:93–102. CrossRef
54. Zheng ZZ, Chen LH, Liu SS, Deng Y, Zheng GH, Gu Y, et al. Bioguided fraction and isolation of the antitumor components from Phyllanthus niruri L. Biomed Res Int. 2016;2016:9729275. CrossRef
55. Gunawan-Puteri MDPT, Kawabata J. Novel α-glucosidase inhibitors from Macaranga tanarius leaves. Food Chem. 2010;123:384–9. CrossRef
56. Abdullah H, Ismail I, Suppian R, Zakaria NM. Natural gallic acid and methyl gallate induces apoptosis in hela cells through regulation of intrinsic and extrinsic protein expression. Int J Mol Sci. 2023;24:8495. CrossRef
57. Kumar S, Singh A, Kumar B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J Pharm Anal. 2017;7:214–22. CrossRef
58. Kumar S, Chandra P, Bajpai V, Singh A, Srivastava M, Mishra DK, et al. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind Crops Prod. 2015;69:143–52. CrossRef
59. Lee C, Chiu T, Tsai S. Quantitative HPLC methods for gallic acids of Phyllanthus (Euphorbiaceae). J Liq Chromatogr Relat Technol. 2005;28:2965–77. CrossRef
60. Saliu JA, Oyeleye SI, Olasehinde TA, Oboh G. Modulatory effects of stonebreaker (Phyllanthus amarus) and bitter gourd (Momordica charantia) on enzymes linked with cardiac function in heart tissue of doxorubicin-stressed rats. Drug Chem Toxicol. 2022;45:331–9. CrossRef
61. Wang X, Hou Y, Li Q, Li X, Wang W, Ai X, et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1alpha/microRNA 210/ISCU1/2(COX10) signaling pathway. J Ethnopharmacol. 2019;241:111801. CrossRef
62. Türkan F, Atalar MN, Aras A, Gülçin I, Bursal E. ICP-MS and HPLC analyses, enzyme inhibition and antioxidant potential of Achillea schischkinii Sosn. Bioorg Chem. 2020;94:103333. CrossRef
63. Dominguez-Perles R, Garcia-Viguera C, Medina S. New anti-alpha-glucosidase and antioxidant ingredients from winery byproducts: contribution of alkyl gallates. J Agric Food Chem. 2023;71:14615–25. CrossRef
64. Nor I, Wirasutisna KR, Hartati R, Insanu M. The α-glucosidase inhibitory activity of avicularin and 4-O-methyl gallic acid isolated from Syzygium myrtifolium leaves. Saudi Pharm J. 2023;31:101677. CrossRef
65. Sofa F, Akhmad D, Megawati M, Muhammad H. Isolation and identification of quercetin derivatives and their α-glucosidase inhibitory acitivities from Bryophyllum pinnatum. Res J Chem Environ. 2018;22:114–9.
66. Ren Z, Zou W, Cui J, Liu L, Qing Y, Li Y. Geraniin suppresses tumor cell growth and triggers apoptosis in human glioma via inhibition of STAT3 signaling. Cytotechnology 2017;69:765–73. CrossRef
67. Abd Ghafar SZ, Mediani A, Maulidiani, Ramli NS, Abas F. Antioxidant, α-glucosidase, and nitric oxide inhibitory activities of Phyllanthus acidus and LC–MS/MS profile of the active extract. Food Biosci. 2018;25:134–40. CrossRef
68. Chawansuntati K, Hongjaisee S, Sirita K, Kingkaew K, Rattanathammethee K, Kumrapich B, et al. Effects of quercetin and extracts from Phyllanthus emblica, Morus alba, and Ginkgo biloba on platelet recovery in a rat model of chemotherapy-induced thrombocytopenia. Heliyon 2024;10:e25013. CrossRef
69. Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J Agric Food Chem. 2009;57:11463–8. CrossRef
70. Firdayani, Arsianti A, Churiyah, Yanuar A. Molecular docking and dynamic simulation studies of benzoylated emodin into HBV core protein. J Young Pharm. 2018;10:s20–4. CrossRef
71. Zhang B, Zaric SD, Zrilic SS, Gofman I, Heck B, Reiter G. London dispersion forces and steric effects within nanocomposites tune interaction energies and chain conformation. Commun Chem. 2025;8:21. CrossRef
72. Olennikov DN, Kashchenko NI, Schwabl H, Vennos C, Loepfe C. New mucic acid gallates from Phyllanthus emblica. Chem Nat Compd. 2015;51:666–70. CrossRef
73. Londhe JS, Devasagayam TP, Foo LY, Shastry P, Ghaskadbi SS. Geraniin and amariin, ellagitannins from Phyllanthus amarus, protect liver cells against ethanol induced cytotoxicity. Fitoterapia 2012;83:1562–8. CrossRef
74. Matou M, Bercion S, Marianne-Pepin T, Haddad P, Merciris P. Phenolic profiles and biological properties of traditional Phyllanthus amarus aqueous extracts used for diabetes. J Funct Foods 2021;83:104571. CrossRef
75. Xu M, Zha ZJ, Qin XL, Zhang XL, Yang CR, Zhang YJ. Phenolic antioxidants from the whole plant of Phyllanthus urinaria. Chem Biodivers. 2007;4:2246–52.
76. Husnunnisa H, Hartati R, Mauludin R, Insanu M. A review of the Phyllanthus genus plants: their phytochemistry, traditional uses, and potential inhibition of xanthine oxidase. Pharmacia. 2022;69:681–7. CrossRef
77. Huang YL, Chen CC, Hsu FL, Chen CF. Tannins, flavonol sulfonates, and a norlignan from Phyllanthus virgatus. J Nat Prod 1998;61:1194–7. CrossRef