INTRODUCTION
One of the most common movement illnesses is Parkinson’s disease (PD), which primarily affects the nigrostriatal pathway [1,2]. According to estimates, the number of persons with PD will increase dramatically, from 7 million in 2015 to 13 million in 2040 [3]. The predominant symptoms of PD are loss of gait, rigidity of muscles, postural imbalance, bradykinesia, and tremors [4,5]. Abnormal deposition of alpha-synuclein was primarily observed in the PD-affected person, which leads to the generation of oxidative stress, disturbance in calcium homeostasis, and mitochondrial dysfunction [6–9]. Mitochondria are a well-established player involved in the pathophysiology of PD. Based on the previous observations, alterations in the mitochondria may lead to neuronal death in various human and animal models [10]. It has been suggested that disruption in the striatal dopamine homeostasis due to deficiency of ATP is an early consequence of mitochondrial dysfunction [11,12]. A vital part of the cellular response against oxidative stress is the redox-sensitive transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2). In ordinary circumstances, Kelch-like ECH-associated protein 1 (Keap1) sequesters Nrf2 in the cytoplasm, facilitating its ubiquitination and proteasomal breakdown. In response to electrophilic challenge or oxidative stress, Nrf2 breaks away from Keap1 and translocates into the nucleus, where it attaches itself to antioxidant response elements in the target genes’ promoter regions and synthesis antioxidant proteins and phase II detoxifying enzymes like glutamate-cysteine ligase, heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), and glutathione S-transferases (GSTs), which work together to restore redox homeostasis and shield neurons from oxidative stress. Recently, it has been proposed that Nrf2 controls mitochondrial functions associated with the pathogenesis of PD [13,14]. If Nrf2 activity is decreased, it is more likely to increase the susceptibility of dopaminergic neuronal loss, whereas increasing the activity of the same molecule increases the protection of neurons [15]. Therefore, the appropriate management of mitochondrial dysfunction via the Nrf2 mechanism could be a therapeutic target in PD.
Various hormones, neuroendocrine, and molecular factors are involved in the pathogenesis of PD. Brain-renin angiotensin system (RAS) is involved in several neurodegenerative diseases, including PD [16]. Several studies of Parkinsonism revealed that the classical renin-angiotensin axis (Ang-II/AT-1R; pro-oxidative and pro-inflammatory) exacerbates dopaminergic neuronal death, while another component of brain-RAS, i.e., Ang (1–7), a vasoprotective heptapeptide of the renin-angiotensin system, counteracts the classical brain RAS axis via Mas receptor (MasR) [17,18]. Previously, it has been observed that Ang (1–7) was found more abundantly in the isolated mitochondria in the striatal region of rats, suggesting that maintaining mitochondrial function [16]. Furthermore, Ang (1–7) suppressed the generation of reactive oxygen species (ROS) and reactive nitrogen species via Mas receptor involvement, which was induced by the mitochondrial AT-1 receptor [16]. Furthermore, the Ang II/AT-1R cascade increased ROS production, leading to oxidative stress and neuronal apoptosis, ultimately resulting in the loss of dopaminergic neurons [19,20]. Excessive production of ROS was diminished by A779 [19]. Nevertheless, the use of A779 has been limited due to its ability to disrupt the blood-brain barrier [21]. Previously, the Ang (1–7)/Mas axis has been shown to exhibit neuroprotection in neurodegenerative conditions, such as PD and Huntington’s disease [22,23]. Furthermore, in rotenone-induced neuronal damage, Ang (1–7) markedly decreased oxidative stress and enhanced cell survival [24]. It also modulates the function of mitochondria in various neurodegenerative conditions through an Nrf2-based mechanism. Conversely, A779 has been routinely utilized to validate the receptor-mediated responses of Ang (1–7) and prevent its beneficial outcomes. Research has indicated that A779 modulates the redox state, mitochondrial integrity, and neuronal survival by reversing the neuroprotective effects of Ang (1–7) [16,25]. Moreover, it has been estimated that Ang (1–7) plasma levels were reduced in PD-affected patients [26]. Although Ang (1–7) does not easily cross the blood–brain barrier, its ability to act in the brain can be improved using methods like nanoparticles [27]. Since both MasR and Nrf2 are proven targets in humans and Nrf2 activators are already used in some treatments, targeting the Ang (1–7)/MasR/Nrf2 pathway appears to be a promising strategy for protecting the brain in PD [28,29].
Trigonelline, a naturally occurring alkaloid with anti-inflammatory, neuroprotective, and antioxidant properties, can be found in fenugreek and coffee beans. According to recent research, trigonelline suppresses oxidative stress by interfering with the Nrf2 signaling pathway, a master regulator of antioxidant defence. Trigonelline modifies Nrf2 activity, depending on the dose and experimental paradigm [30,31]. In this study, Trigonelline was employed as a pharmacological tool to investigate the function of Nrf2 in mediating the neuroprotective effects of angiotensin-1–7. If Trigonelline reverses the behavioral and biochemical parameters of Ang (1–7), it would indicate that the Nrf2 pathway is a key mediator of the Ang (1–7) effect in rotenone-induced Parkinson-like pathology.
Hence, it can be assumed that the Ang (1–7)/Nrf2-mediated pathway modulates the severity of the pathophysiology in rotenone-induced PD-like symptoms. Hence, using motor and metabolic tests, the current investigation examined the neuroprotective effects of Ang (1–7) alongside or excluding A779 and Trigonelline (an Nrf2 inhibitor) on motor impairment associated with rotenone-induced Parkinson-like symptoms.
MATERIALS AND METHODS
Experimental animals
The animal house facility of IPR, GLAU Mathura, provided 36 male Wistar rats (200 ± 20 g). The selection of male rats in this study is based on the fact that female rats exhibit estrogen-mediated neuroprotection, which can result in reduced lesion severity and increased variability in behavioral outcomes [32]. These animals were divided into six experimental sets, each containing six subjects at random. The rodents were maintained under ambient conditions, with a temperature of approximately 25°C ± 1°C and a relative humidity of between 45% and 55%, during their daily circadian rhythm. These animals were provided with unrestricted access to water and food. The research proposal was approved by the Institutional Animal Ethics Committee (IAEC) with the reference number (GLAIPR/IAEC/CPCSEA/09/22/PhD./R04), and the experimental procedure was directed as per the principle of the NRC Committee (USA) to update the protocol for research animals’ care and use 2011 [33].
Chemicals and reagents
The sources of Rotenone, Ang (1–7), A779, Trigonelline, and Selegiline were Sigma Aldrich (USA). The remaining analytical-grade chemicals and reagents were purchased from Himedia.
Stereotaxic injection of rotenone
The animals were placed on a stereotaxic frame manufactured by Stereotaxic Manufacturer Cooperation, Ambala Cantt, India, and anesthetized with the intraperitoneal injection of sodium pentobarbital (40 mg/kg). A solution of rotenone [DMSO: PEG (1:1)] was administered at the rate of 2 µl/min up to 2 µl in substantia nigra pars compacta (SNpc) with coordinates lateral = 0.20; antero-posterior = 0.53 and dorso-ventral = 0.75 from the bregma point for the stereotaxic frame [34]. A solution of DMSO and PEG was prepared in the same ratio, and 2 µl was injected into sham animals. Furthermore, animals received the necessary aftercare until they had fully recovered, which included placing the rats in individual recovery cages under a warm environment until they regained consciousness. Daily monitoring for infection or distress was made at the surgery site during the recovery phase; the animals were also observed for typical feeding, grooming, and movement behaviors.
Experimental protocol
Figure 1 depicts the experimental schedule for fourteen days. Animals were familiarized for seven days and then divided into seven groups Group I- Control, Group-II- Sham (DMSO and PEG in 1:1 ratio), Group III- ROT treatment (Rotenone was administered for Day 1 only), Group-IV- ROT + Angiotensin (1– 7) (50 μg per kg; i.p.), Group -V- ROT + Angiotensin (1–7) + A779 (10 mg per kg, i.p.), Group-VI- ROT +Angiotensin (1–7) + Trig (10 mg per kg, i.p.), Group-VII- ROT + Selegiline (10 mg per kg; i.p.) [34–38]. Angiotensin (1–7), A779, and selegiline were administered for all days (Day 1–Day 14) intraperitoneally during the experimental schedule in their respective groups. A779 and Trigonelline were administered for 30 minutes. before the Angiotensin (1–7) treatment in the [ROT + Angiotensin (1–7) + A779] and [ROT + Angiotensin (1–7) + Trig] groups. The dosages of A779 and Angiotensin-(1–7) were selected based on previous studies of Pawlik et al. [35] that demonstrated their pharmacological efficacy in rodent models of inflammation and neurodegenerative disorders through the Mas receptor axis modulation. Additionally, Nakhate et al. found that trigonelline (10 mg/kg, i.p.) significantly influenced the Nrf2 pathway and decreased oxidative damage in mouse models within a non-toxic, pharmacologically efficacious range for systemic administration and was well tolerated, guiding the choice of dosage. Behavioral parameters were accessed on the last day (Day 14) of the experimental plan. After behavioral observation, cervical dislocation was used to sacrifice all the animals. Brains were quickly extracted, and the striatum and SNpc were carefully dissected on an ice-cold glass plate. Dissected tissues were immediately placed on dry ice.
 | Figure 1. Pictorial representation of the detailed experimental schedule. [Click here to view] |
Behavioral tests
Narrow beam walk test
Rats were examined on the final day to determine how many times their hind paws slipped and how long it took them to cross the beam. The hind limb impairment was assessed using the Henderson et al. [39] approach. All the animals were preliminarily trained twice on the first day of the plan before receiving 2 hours of ROT injection cross on an elevated wooden beam. The animals were positioned on a beam that measured 120 cm in length, 3 cm in diameter, and 60 cm above the ground on the final day of the experiment. A darkened goal box (25 × 20 × 18 cm) was present on another beam end. The narrow beam test maximum timing was 2 minutes.
Open field test
Using wooden base equipment (60 cm W, 60 cm L, and 30 cm H) with the floor divided into equal squares using black lines, utilized to assess emotional reaction, as well as locomotor function. All the behavioral data, including distance traveled, immobility period, rearing count, and the count of lines traversed by each rat’s hind limb after being placed in the center of the equipment of each rat, were video-recorded for 5 minutes. Total distance traveled and rearing were considered only when the grid crossed by the hind limb under moderate illumination was later counted using video recording and analyzed with Any Maze tracking software [38].
Rotarod test
A digital rotarod apparatus (Orchid Scientific) was used to monitor the motor and grip strength of the rats. They were acclimated through pre-training sessions conducted over five consecutive days on a rotating rod at speeds ranging from 4 to 40 rpm for 10 minutes. The animals were placed carefully on top of the moving rod so they could learn how to avoid falls. The cutoff time (maximum period rats can stay on the rod without fail) was 1 minute [40].
Catalepsy bar test
Rat muscle rigidity can be measured with a commonly used tool called the bar test. In this test, the rats must hold their forelimbs on a 10 cm high bar while using their two hind limbs to support their posture. The total number of latencies, or the time taken by the rats to remove either of their forelimbs from the bar, is recorded across three repeated trials [41].
Grip strength test
A standard method was used to determine neuromuscular strength on Day 14 of the experimental plan using equipment consisting of a wire (90 cm L; 1 cm D) made of metal, fixed between upstanding supports [42]. The rat was hung on the wire at 50 cm height from the surface, and the forelimb reached the center of the wire. The neuromuscular strength was evaluated using a scoring system based on the rats’ performance: Score 0 if the rat falls off the wire, Score 1 if the rat hangs onto the wire using only its frontal paws, Score 2 if the rat hangs onto the wire using its frontal paws and also tries to pull back on the wire, Score 3 if the rat hangs onto the wire using its frontal paws and one or both hind paws, Score 4 if the rat hangs onto the wire using its frontal paws and tail, and Score 5 if the rat escapes and then falls off the wire [38].
Actophotometer test
An actophotometer is used to evaluate locomotor activity via a cutoff of the light that emerges from the light source to the photocell. This cutoff was recorded and displayed by the instrument. The locomotor activity test was performed for 5 min on Day 14 of the experimental plan. The rat’s activity was recorded by total photo beam counts every 5 minutes [43].
Footprint analysis
Walking patterns represent the animal’s gait, which has been calculated via footprint analysis. Rodents walked along the 100 cm long, 10 cm wide, as well as 20 cm high walls of a small track coated with white sheeting, leaving a trace of their footsteps. The rat’s feet (fore in red and hind in green) were painted. Rats were trained to walk down a white sheet-covered path to collect the footprint pattern. The investigation of the animals’ footprints was conducted by measuring the base width of their front and rear paws together with their respective stride lengths [44].
Biochemical measurement
Measurement of dopamine level
The level of dopamine was estimated in the striatum of all experimental rats. The level of dopamine was measured using HPLC, which consisted of an electrochemical detector [45,46]. Protein content was determined by a colorimetric assay [47].
Measurement of mitochondrial functions
Pedersen [48] used a conventional method to separate the mitochondria from the SNpc of treated and untreated rats. Mitochondrial function was estimated by the reduction of MTT at 595 nm wavelength [49]. The amount of mitochondrial protein was calculated using the conventional method, and these findings were reported as the amount of formazan formed per minute per milligram of protein.
Statistical investigation
All the results were statistically evaluated using GraphPad Prism (Version 8.0.4) and displayed as mean ± SEM. To determine the level of significance, a post hoc examination using Student Newman-Keuls is performed after an Analysis of Variance (ANOVA). Every finding with a p-value of less than 0.05 was considered significant.
RESULTS
Protective effect of Ang (1–7) on the alterations of hind limb functions by the treatment of rotenone during the narrow beam walk test
Figure 2 shows how Ang (1–7) affected the rats’ hind limb function alterations, considering the duration it took to arrive at the target box (A), the slip count of the left hind paw (B), and the slip count of the right hind paw (C) during the beam walk assessment. The treatment animals’ transfer time [F (6, 35) = 64.3] and the number of left hind paw slips [F (6, 35) = 55.1] varied significantly, according to a one-way ANOVA, right hind paw slip count [F (6, 35) = 2.5] did not vary significantly. In contrast to control and sham groups, post hoc assessment revealed that rotenone-exposed animals required more time to traverse the narrow beam, as well as showed a significant increase in left hind paw slippage. A dosage of Ang (1–7) every day significantly decreased the slip count of the left hind paw and decreased the duration it took to cross the narrow beam. Moreover, selegiline improved these behavioral observations more effectively than Ang (1–7) during the narrow beam walk test. Although A779 and Trigonelline treatment prevented Ang (1–7) from having a therapeutic effect on these behavioral findings.
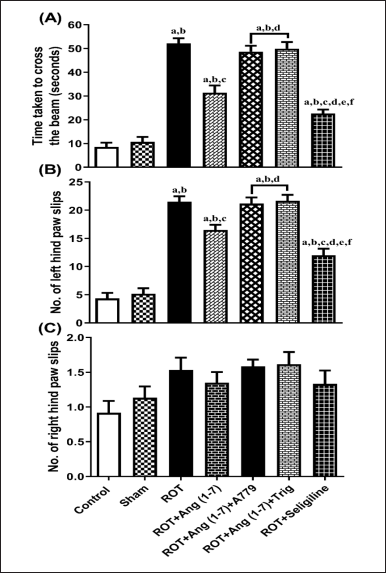 | Figure 2. Impact of rotenone-induced variation on (A) time taken to reach the goal box, (B) number of left hindpaw slips, and (C) number of slips of the right hindpaw in the narrow beam walk test. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
Protective effect of Ang (1–7) on the alteration of behavioral parameters by the treatment of rotenone during the open field test
Figure 3 depicts the influence of Ang (1–7) on variations during behavioral observations about the following: distance traveled (A), no. of lines transverse by the hindlimb (B), no. of rearing (C), and immobility period (D). One-way ANOVA showed a notable variation in distance traveled [F (6, 35) = 44.3], lines transversed by the hindlimb [F (6, 35) = 47.9], no. of rearing [F (6, 35) = 44.7], and immobility period [F (6, 35) = 52.6] among the all the treated animals. In comparison to the control and sham groups, post hoc analysis revealed that the rotenone-influenced group displayed a substantial decrease in distance traveled, the number of lines crossed, the number of rearing episodes, and a dramatic increase in immobility duration. Daily administration of Ang (1–7) improved these behavioral parameters, in contrast to that of rotenone, as observed in the open field test. Moreover, the selegiline-treated group showed improved behavioral observations compared to Ang (1–7). Although A779, as well as Trigonelline treatment, prevents Ang (1–7) from affecting the open-field test.
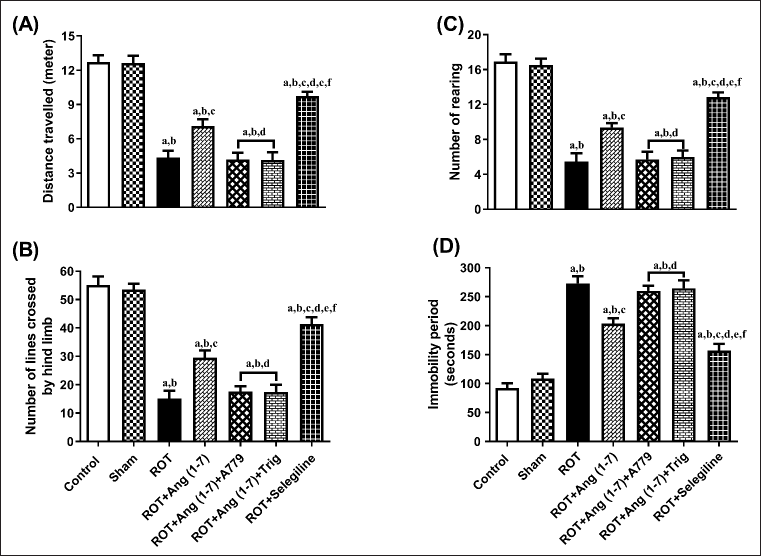 | Figure 3. Impact of rotenone-induced alterations in (A) overall distance covered, (B) lines crossed by rat’s hindlimb, (C) rearing count, along with (D) duration of inactivity during the open field assessment. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
Protective effect of Ang (1–7) on the alterations of motor changes by the treatment of rotenone during the time of retention, locomotor activity, grip strength, and catalepsy bar test
Figure 4 illustrates the action of Ang (1–7) on variations during behavioral observations, specifically retention time (A), grip strength (B), immobility period (C), and sustaining an externally imposed posture (D). Notable variation has been observed in the retention time [F (6, 35) = 105.2], grip strength [F (6, 35) = 48.2], immobility period [F (6, 35) = 64.6], and sustaining externally imposed posture [F (6, 35) = 75.9] in the one-way analysis among all the animal sets. Post-hoc evaluation revealed that rotenone induced a significant reduction in retention duration and gripping power, and a corresponding increase in the immobility period and the ability to sustain an externally imposed posture, compared to the control and sham groups. Daily treatment with Ang (1–7) markedly improves all these behavioral observations, in contrast to that of rotenone. Moreover, selegiline demonstrated a better outcome than Ang (1–7) on the above-mentioned behavioral observations. Although the protective effect of Ang (1–7) on these behavioral findings is blocked by A779 and Trigonelline therapy.
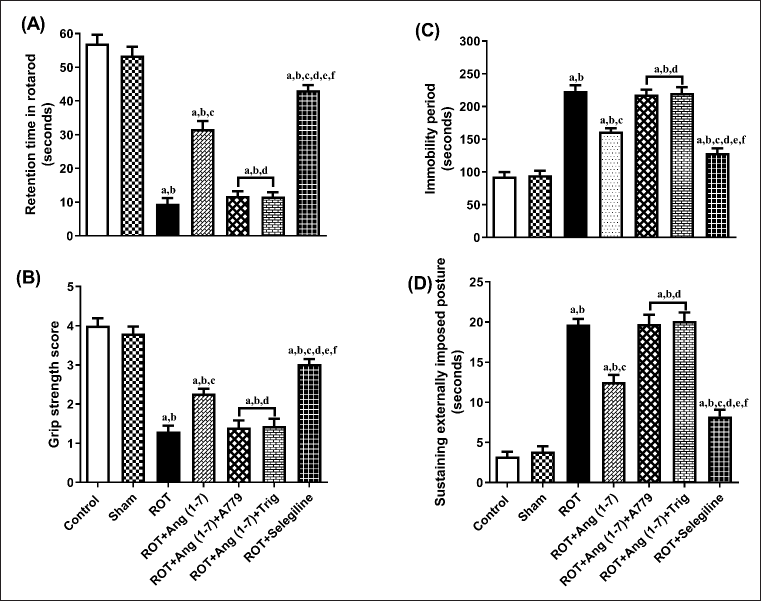 | Figure 4. Impact of rotenone-induced variations in (A) retention period in rotarod evaluation, (B) grip strength assessment score, (C) locomotor performance during actophotometer, as well as (D) cataleptic response in bar test. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
Protective effect of Ang (1–7) on the alterations of behavioral parameters by the treatment of rotenone during footprint test
Ang (1–7) effect on the change in behavioral data concerning the stride length of the left forelimb (A), the stride length of the left hindlimb (B), the forelimb base width (C), and the hindlimb base width (D) is depicted in Figure 5. A notable variation has been observed in the left forelimb stride length [F (6, 35) = 56.6] and left hindlimb stride length [F (6, 35) = 52.6]. However, no notable variation has been identified in the forelimb base width [F (6, 35) = 2.0] and hindlimb base width [F (6, 35) = 1.9] in one-way analysis among all groups. Post hoc evaluation demonstrated that rotenone induced a severe decrease in the stride length of the left forelimb and stride length of the left hindlimb and no effect on the forelimb base width and hindlimb base width as compared to control and sham. Daily treatment of Ang (1–7) markedly enhanced the stride length of the left forelimb and the stride length of the left hindlimb, opposite to rotenone, but it did not affect the forelimb base width and hindlimb base width. Moreover, selegiline-treated animals displayed a better outcome than Ang (1–7) on the stride length of the left forelimb and left hindlimb. Although the protective effect of Ang (1–7) on these behavioral findings is blocked by A779 and Trigonelline therapy.
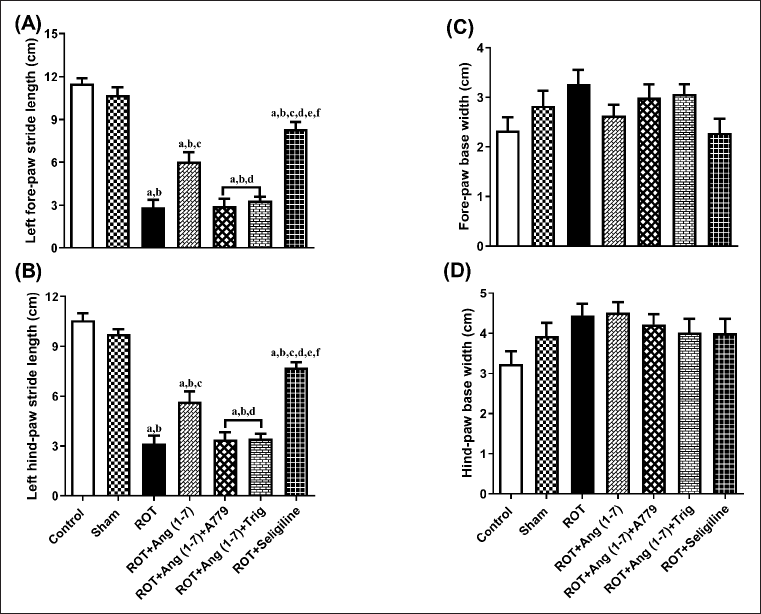 | Figure 5. Impact of rotenone-induced changes in (A) left fore limb stride-length, (B) left hind limb stride-length, (C) fore limb base-width, as well as (D) hind limb base-width. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
Protective effect of Ang (1–7) on the variations of concentration of striatal dopamine by the treatment of rotenone in rats
The action of Ang (1–7) on the alteration in concentration of striatal dopamine is depicted in Figure 6. A notable variation has been observed in the striatal dopamine concentration [F (6, 35) = 139.4] during one-way analysis among all the groupings. Moreover, post hoc evaluation revealed that rotenone induced a sharp drop in the level of dopamine concentration in the striatal region. Daily therapy with Ang (1–7) markedly enhanced dopamine concentration, in contrast to rotenone. Moreover, selegiline displayed a better outcome than Ang (1–7) on this estimated parameter. However, A779 and Trigonelline treatment block Ang (1–7)’s protective effect on the dopamine level.
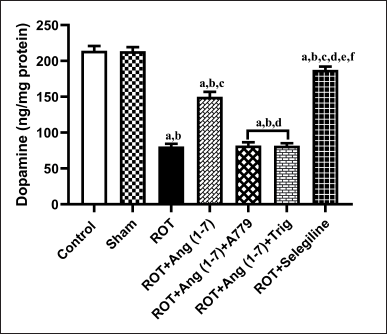 | Figure 6. Impact of rotenone-induced alterations in striatal dopamine levels. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
Protective effect of Ang (1–7) on the alterations of mitochondrial functions by the treatment of rotenone in rats
Figure 7 illustrates Ang (1–7) ‘s effect on the change in the functions of mitochondria via MTT assay. A notable variation has been observed in the mitochondrial functions [F (6, 35) = 39.8] during one-way analysis among all the groupings. Furthermore, post hoc assessment revealed that rotenone induced a sharp decline in mitochondrial function compared to the control and sham groups. Daily therapy with Ang (1–7) markedly enhanced mitochondrial functions, in contrast to rotenone. Moreover, the group treated with selegiline had a superior outcome compared to the Ang (1–7) group. However, A779 and Trigonelline treatment blocks the protective effect of Ang (1–7) on this estimation.
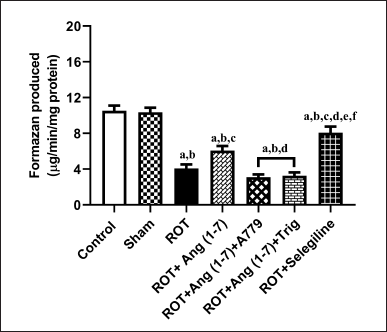 | Figure 7. Impact of rotenone-induced variations in mitochondrial function via MTT assay. Findings are presented as mean ± SEM (n = 6). ap < 0.05 versus control, bp < 0.05 versus Sham, cp < 0.05 versus ROT, dp < 0.05 versus ROT+Ang (1–7), ep < 0.05 versus ROT+Ang (1–7)+A779, fp < 0.05 versus ROT+Ang (1–7)+Trig. (One-way ANOVA accompanied by Student-Newman-Keuls multiple comparison test). [Click here to view] |
DISCUSSION
The present study provides evidence for the neuroprotective activity of Ang (1–7) against rotenone-induced PD-like symptoms in rodents while also highlighting the mechanistic involvement of the Mas receptor and the Nrf2 signaling pathway. The findings show that Ang (1–7) reduces the rotenone-caused impaired behavioral observations in rodents. Additionally, Ang (1–7) raised the dopamine levels in the striatum of rats given rotenone treatment. Furthermore, Ang (1–7) improved the mitochondrial functions in the SNpc of rotenone-treated animals. Importantly, A779 (a selective Mas receptor antagonist) and trigonelline (an Nrf2 modulator) reversed the therapeutic action of Ang (1–7) in the rotenone-treated PD animals. These results demonstrate that the Ang (1–7)/Nrf2-mediated signaling mechanism has a restorative impact against PD-like symptoms.
Rotenone is a well-known chemical that induces PD-like symptoms characterized by impaired behavioral activity, including deficits in the rotarod, open field, catalepsy, and footprint analysis, which are consistent with previously published studies. Rotenone significantly reduced the motor activities observed in the various tests conducted in the current investigation [38,45]. Ang (1–7) showed improvements in behavioral functions, including motor performance and muscle coordination, as well as symptoms of PD in rotenone-treated animals. In contrast, A779 reversed the impact of Ang (1–7) on the above-mentioned behavioral findings in this study. Additionally, trigonelline also exerted a nonsignificant result as A779, suggesting that Ang (1–7) improved these motor functions through the Nrf2 mediating pathway. These findings suggest that the interaction between Ang (1–7) and MasR/Nrf2 could potentially alleviate PD symptoms induced by rotenone.
The present investigation documents a notable reduction in dopamine content in rotenone-treated animals, consistent with earlier published reports [38]. Importantly, the administration of Ang (1–7) significantly restored striatal dopamine levels in rotenone-treated animals, suggesting a potent neurorestorative effect on the dopaminergic system. This aligns with other experimental PD models where Ang (1–7) has been shown to exert dopaminergic protective effects, either by promoting dopamine synthesis, reducing its catabolism, or preventing oxidative damage to dopaminergic neurons [50]. Some researchers also suggested that heptapeptide tends to decrease the activity of monoamine oxidase [51,52]. The restorative effect of Ang (1–7) on the dopaminergic system was reversed by the treatment of A779 and trigonelline separately. These results demonstrate that Ang (1–7) enhances the activity of dopamine in the targeted brain region, thereby improving the rodents’ behavioral functioning when they are administered rotenone. The limitations of the present study include the lack of direct molecular assays, such as qPCR or Western blotting, to confirm changes in Nrf2 or downstream antioxidant enzymes like HO-1 or NQO1. Additionally, the dopaminergic neuronal survival in the substantia nigra was not histopathologically investigated, which would have provided better structural evidence of neuroprotection.
In line with classical PD pathology, the current study also documented a significant reduction in striatal dopamine content in rotenone-treated rats. This finding supports previous reports describing the vulnerability of nigrostriatal dopaminergic neurons to rotenone-induced oxidative damage [38]. Ang (1–7) significantly improved the mitochondrial functions in rotenone-challenged rodents. Interestingly, these findings were consistent with other studies, where Ang (1–7)/Nrf2 improved mitochondrial function in rat brains, thereby enhancing learning and memory. Importantly, this restorative effect on the dopaminergic system was negated by both A779 and trigonelline, reaffirming the involvement of MasR and Nrf2 in maintaining dopaminergic neurotransmission. These findings suggest that the Ang (1–7)/MasR/Nrf2 axis may help preserve dopamine homeostasis under conditions of neurotoxic stress. Also, in the present study, we selected a dose of trigonelline (10 mg/kg) based on prior literature where it was shown to modulate Nrf2-related pathways in a downregulatory manner in rotenone model [38]. Future research should examine downstream molecular markers and evaluate the long-term neuroprotection without side effects resulting from persistent stimulation of the Ang (1–7)/MasR/Nrf2 axis. Testing Ang (1–7)’s effectiveness in conjunction with conventional dopaminergic treatments might also be beneficial.
CONCLUSION
Ang (1–7) prevented rotenone-influenced impairments in the animals’ motor functioning. Ang (1–7) improved the dopamine level and mitochondrial functions in the brain areas of rotenone-treated animals. A779 and trigonelline reverted the therapeutic effect of Ang (1–7) in rotenone-challenged animals. According to these findings, the Ang (1–7)/MasR/Nrf2 signaling pathway has neuroprotective effects on animals with rotenone-induced Parkinson’s symptoms. As a result, it seems reasonable that the Ang (1–7)/Nrf2 signaling pathway could be a different approach to treating PD.
LIMITATIONS OF THE STUDY
The absence of immunohistochemical analysis in the substantia nigra and the lack of respirometry or electron microscopy to assess ultrastructural mitochondrial changes represent key limitations of the present study.
ACKNOWLEDGMENTS
The author is grateful to the administration of GLA University, Mathura, for the financial assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals are given in the ‘Materials and Methods’ Section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article; we have none to disclose.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. CrossRef
2. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nature reviews. Disease primers. 2017;3:1–21. CrossRef
3. Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3–8. CrossRef
4. Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J Neurochem. 2016;139 Suppl 1:318–24. CrossRef
5. Jellinger KA. Morphological characteristics differentiate dementia with Lewy bodies from Parkinson disease with and without dementia. J Neural Transm. 2023;130(7):891–904. CrossRef
6. Henchcliffe C, Beal FM. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600–9. CrossRef
7. Hwang O. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 2013;22(1):11–17. CrossRef
8. Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47(2):495–508. CrossRef
9. Friedman JH. Dementia with lewy bodies and parkinson disease dementia: it is the same disease. Parkinsonism Relat Disord. 2018;46(Suppl 1):S6–9. CrossRef
10. Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31(14):3038–62. doi:https://doi.org/10.1038/EMBOJ.2012.170
11. Choi WS, Palmiter RD, Xia Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson’s disease model. J Cell Biol. 2011;192(5):873–82. CrossRef
12. Sterky FH, Hoffman AF, Milenkovic D, Bao B, Paganelli A, Edgar D, et al. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21(5):1078–89. CrossRef
13. Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta. 2018;1865(5):721–33. CrossRef
14. Ammal Kaidery N, Ahuja M, Thomas B. Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson’s disease. Mol Cell Neurosci. 2019;101:103413. CrossRef
15. Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58(5):588–98. CrossRef
16. Costa-Besada MA, Valenzuela R, Garrido-Gil P, Villar-Cheda B, Parga JA, Lanciego JL, et al. Paracrine and intracrine angiotensin 1-7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol Neurobiol. 2018;55(7):5847–67. CrossRef
17. Labandeira-García JL, Garrido-Gil P, Rodriguez-Pallares J, Valenzuela R, Borrajo A, Rodríguez-Perez AI. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:67. CrossRef
18. Labandeira-Garcia JL, Rodriguez-Pallares J, Dominguez-Meijide A, Valenzuela R, Villar-Cheda B, Rodríguez-Perez AI. Dopamine-angiotensin interactions in the basal ganglia and their relevance for Parkinson’s disease. Mov Disord. 2013;28(10):1337–42. CrossRef
19. Rodriguez-Pallares J, Rey P, Parga JA, Muñoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31(1):58–73. CrossRef
20. Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of the central renin-angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst. 2010;11(1):49–56. CrossRef
21. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance, and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. CrossRef
22. Rabie MA, Abd El Fattah MA, Nassar NN, Abdallah DM, El-Abhar HS. Correlation between angiotensin 1-7-mediated Mas receptor expression with motor improvement, activated STAT3/SOCS3 cascade, and suppressed HMGB-1/RAGE/NF-κB signaling in 6-hydroxydopamine hemiparkinsonian rats. Biochem Pharmacol. 2020;171:113681. doi:https://doi.org/10.1016/J.BCP.2019.113681
23. De Mello WC, Gerena Y, Ayala-Peña S. Angiotensins and Huntington’s disease: a study on immortalized progenitor striatal cell lines. Front Endocrinol. 2017;8:108. CrossRef
24. Zhou Y, Li M, Zhu DL, Jiang T, Gao Q, Tang XH, et al. Neuroprotective effect of angiotensin-(1-7) against rotenone-induced oxidative damage in CATH. a neurons. Toxicol In Vitro. 2018;50:373–82. CrossRef
25. Varshney V, Garabadu D. Ang (1-7) exerts Nrf2-mediated neuroprotection against amyloid beta-induced cognitive deficits in rodents. Mol Biol Rep. 2021;48(5):4319–31. CrossRef
26. Rocha NP, Scalzo PL, Barbosa IG, de Campos-Carli SM, Tavares LD, de Souza MS, et al. Peripheral levels of angiotensin are associated with depressive symptoms in Parkinson’s disease. J Neurol Sci. 2016;368:235–9. CrossRef
27. Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond). 2013;124(7):443–56. CrossRef
28. Mustafa AM, Rabie MA, Zaki HF, Shaheen AM. Inhibition of brain GTP cyclohydrolase I attenuates 3-nitropropionic acid-induced striatal toxicity: involvement of Mas Receptor/PI3k/Akt/CREB/ BDNF axis. Front Pharmacol. 2021;12:740966. CrossRef
29. Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18(4):295–317. CrossRef
30. Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem. 2012;19(21):3523–31. CrossRef
31. Nguyen V, Taine EG, Meng D, Cui T, Tan W. Pharmacological activities, therapeutic effects, and mechanistic actions of trigonelline. Int J Mol Sci. 2024;25(6):3385. CrossRef
32. Mathis V, Wegman-Points L, Pope B, Lee CJ, Mohamed M, Rhodes JS, et al. Estrogen-mediated individual differences in female rat voluntary running behavior. J Appl Physiol. 2024 Mar 1;136(3):592–605. CrossRef
33. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed. Washington, DC: National Academies Press. CrossRef
34. Paxinos G. Watson C. 1998. The rat brain in stereotaxic coordinates. San Diego: Acadamic Press. 4 pp.
35. Pawlik MW, Kwiecien S, Ptak-Belowska A, Pajdo R, Olszanecki R, Suski M, et al. The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J Physiol Pharmacol. 2016;67(1):75–91.
36. Nakhate KT, Bharne AP, Verma VS, Aru DN, Kokare DM. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β-secretase. Biomed Pharmacother. 2018;101:379–90. CrossRef
37. Schulz D, Mirrione MM, Henn FA. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiol Learn Mem. 2010;93(2):291–301. CrossRef
38. Garabadu D, Agrawal N. Naringin exhibits neuroprotection against rotenone-induced neurotoxicity in experimental rodents. Neuromolecular Med. 2020;22(2):314–30. CrossRef
39. Henderson JM, Stanic D, Tomas D, Patch J, Horne MK, Bourke D, et al. Postural changes after lesions of the substantia nigra pars reticulata in hemi parkinsonian monkeys. Behav Brain Res. 2005;160(2):267–76. CrossRef
40. Rozas G, Guerra MJ, Labandeira-García JL. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc. 1997;2(1):75–84. CrossRef
41. Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148(1):78–87. CrossRef
42. Moran PM, Higgins LS, Cordell B, Moser PC. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 1995;92(12):5341–5. CrossRef
43. Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging- and dizocilpine-induced learning impairment. Brain Res. 1998;799(2):215–29. CrossRef
44. Klapdor K, Dulfer BG, Hammann A, Van der Staay FJ. A low-cost method to analyse footprint patterns. J Neurosci Methods. 1997;75(1):49–54. CrossRef
45. Verma A, Goyal A. Reformative effect of daidzein on motor dysfunction following rotenone injection in ovariectomized rats. Rev Bras Farmacogn. 2022;32:563–74. CrossRef
46. Kim C, Speisky MB, Kharouba SN. Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high-performance liquid chromatography. Differential effect of probenecid, haloperidol and yohimbine on the concentrations of biogenic amines and metabolites in various regions of rat brain. J Chromatogr. 1987;386:25–35. CrossRef
47. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.
48. Pedersen PL, Greenawalt JW, Reynafarje B, Hullihen J, Decker GL, Soper JW, et al. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–81. CrossRef
49. Kamboj SS, Kumar V, Kamboj A, Sandhir R. Mitochondrial oxidative stress and dysfunction in rat brain induced by carbofuran exposure. Cell Mol Neurobiol. 2008;28(7):961–9. CrossRef
50. Rabie MA, Abd El Fattah MA, Nassar NN, El-Abhar HS, Abdallah DM. Angiotensin 1-7 ameliorates 6-hydroxydopamine lesions in hemi parkinsonian rats through activation of MAS receptor/PI3K/Akt/BDNF pathway and inhibition of angiotensin II type-1 receptor/NF-κB axis. Biochem Pharmacol. 2018;151:126–34. CrossRef
51. Fernández BE, Domínguez AE. Angiotensin II increases MAO activity in rat central nervous system. Rev Esp Fisiol. 1991;47(1):37–40.
52. Sumners C, Shalit SL, Kalberg CJ, Raizada MK. Norepinephrine metabolism in neuronal cultures is increased by angiotensin II. Am J Physiol. 1987;252(6 Pt 1):C650–6. CrossRef