INTRODUCTION
Globally, high-quality healthcare should be accessible in a timely manner, which is equitable, safe, efficient, and patient-centered [1]. During treatment, patients may experience delays in effective treatment due to substandard drugs and inaccessibility to standard and necessary medication [2]. According to the most recent definition by the World Health Organization (WHO), substandard medicines are “authorized medical products that do not meet either their quality standards or specification, or both, falsified medicines are medical products that intentionally or falsely misrepresent their identity, composition or source” [3]. In addition, falsified medications are intentionally misbranded, may contain zero active ingredients, and tend to mislead the consumer in various ways (quality, ingredients, source, and so on) [4].
Patients’ use of substandard and falsified medicines (SFM) and widespread availability in nations threaten to advance the Sustainable Development Goals [5]. According to WHO estimates, one in every 10 drugs in the global market are of inferior quality [6]. The issue of poor and fake medical items keeps growing as global manufacturing and distribution networks get more complex [5], and the prevalence has continued to be 25%, as per the study [7]. One reason is that low and middle-income countries (LMICs) worldwide cannot afford to monitor the quality of production, importation, and supply of medicines [8]. In addition to causing adverse effects and developing drug resistance, fraudulent medications may hinder the progress of clinical efficacy, which may have financial consequences on patients and their families [9].
WHO categorizes children into different age groups, and the Convention on the Rights of the Child defines a “child” as an individual under 18 [10]. Every child has the right to a healthy life and access to safe and effective medical care. However, millions of children worldwide are denied these rights and necessities due to their birthplace, ethnicity, family origin, gender, and race [11]. As reported, drugs like antibiotics and antimicrobials for children are vulnerable to SFM and are primarily found in LMICs [12]. About 12.4% of pediatric antibiotics tested in Asia and Africa were either falsified or substandard. As per the WHO list, amoxicillin-clavulanic acid, ampicillin, and ceftriaxone are the most affected categories [12,13]. Several instances of analgesic samples had an issue with active pharmaceutical ingredients (APIs) levels that were both above and below the standard range. One such incident occurred in Indonesia, where at least 195 children died after consuming substandard cough and fever syrups containing a higher limit of diethylene glycols (DEGs) and (mono) ethylene glycol (EG), resulting in a public health emergency [14]. In August 2024, the WHO alert regarding the falsified DOW US Pharmacopeial (USP)/EP propylene glycol was detected in Pakistan in its five locally produced oral liquid medicines [15]. The issue of SFM has also extended to the treatment of cancer associated with substandard asparaginase products [16].
Approximately 64 million people worldwide, particularly children, are affected by counterfeit medical supplies, vaccines, and in vitro diagnostic tests. Table 1 presents key highlights from WHO alerts, identifying these products as unsafe [15]. Global organizations have worked to gather comprehensive data on the most vulnerable areas to provide them with high-quality medications. In 2021, the United Nations International Children’s Emergency Fund (UNICEF) allocated US$99.1 million to supply quality medicines for the most vulnerable children, primarily targeting infectious diseases in LMICs [17]. Similarly, Tanzania’s Accredited Drug Dispensing Outlets program is critical in improving the quality of drugs through the regulation of the dispensing of only those drugs approved by the local regulator [18]. This program was effective and implemented in other nations such as Bangladesh, Liberia, and Uganda, but under different labels [4]. Pan American Health Organization Strategic Fund also assists the fight against SFM through the provision of optimal quality drugs at affordable rates to the vulnerable regions of LMICs [17]. The common SFM categories, with the antibiotics being the top rated followed by pain and fever drugs, are depicted in Figure 1. Drugs such as antimalarials, anti-diabetics, dietary supplements, anti-allergy, cardiac, anti-cancer, and other drugs were of poor quality products as per the study report [19].
Table 1. Global WHO reports on SFM for children over the past 5 years.
| WHO alert no | Region identified | Category | API | Issues |
|---|---|---|---|---|
| N°1/2020 [15] | Chad, Cameroon, Nigeria | Falsified antimalarials | Quinine sulphate | Under dose of expected API |
| N°4/2020 [15] | Burkina, Faso, Cameroon, Democratic Republic of Congo, France, and Nigeria | Falsified antimalarials | Chloroquine | Required amount of API not found |
| N°1/2021 [15] | Chad | Falsified Vitamin A | Retinol | Falsified drug with low API |
| N°6/2021 [15] | Uganda, India and Myanmar | Falsified COVID-19 vaccine (COVISHIELD) | (ChAdOx1 nCoV-19 corona virus vaccines (recombinant) | Counterfeit drug with wrong spelling of the drug product |
| N°6/2022 [15] | Gambia | Substandard baby cough and cold syrup | Paracetamol phenylephrine HCl and chlorphenamine maleate | Diethylene glycol and ethylene glycol, more than specified limit |
| N°7/2022 [15] | Indonesia | Substandard analgesic and antipyretic syrup and drops | Paracetamol, guaifenesin, and chlorphenamine maleate | Diethylene glycol and ethylene glycol, more than specified limit |
| N°1/2023 [15] | Uzbekistan and Cambodia | Substandard analgesic and antipyretic syrup and drops | Paracetamol BP, guaifenesin BP, and phenylephrine hydrochloride B | Unacceptable amounts of diethylene glycol and ethylene glycol |
| N°6/2023 [15] | Republic of Iraq | Substandard analgesic and antipyretic syrup and drops | Paracetamol, and chlorphenamine maleate | Unacceptable amounts of diethylene glycol and ethylene glycol |
| N°8/2023 [15] | Maldives and Pakistan | Substandard antihistaminic | Cetirizine | Substandard drug with low API |
| N°1/2024 [15] | Pakistan | Falsified DOW USP/EP propylene glycol | Propylene Glycol | Under dose and substandard |
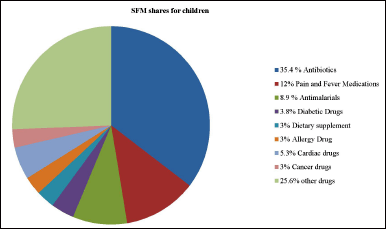 | Figure 1. Most substandard and falsified medical products. [Click here to view] |
This narrative review aims to highlight the impacts and interventions to combat the issues of various classes of SFM for children in LMICs. It also incorporates the recent regulatory advancements and the international Pharmacopoeial harmonization initiatives such as the Pharmacopoeial Discussion Group (PDG).
METHODS
A comprehensive English literature search was conducted in this study from 2004 to 2024 through scientific databases and search engines, such as PubMed, Scopus, Web of Science, and Science Direct, to find relevant literature using the words “substandard drug,” “falsified drug,” “pediatric drugs,” “regulations,” “drug policies,” “low middle-income countries,” “poor quality drugs,” “low-quality medicines,” Boolean operators like “AND” and “OR” were used to refine the literature. To gather thorough information, a search of grey literature, including numerous public and official reports, regulatory guidelines, and theses, was included.
Eligibility criteria
The inclusion factors based on the review of pediatric drugs included substandard and falsified drugs. Additionally, reviews of studies conducted in other contexts were included to enhance comprehension of the effects of strategies in pharmaceutical settings; the inclusion was limited to pediatric studies published in English. Observational and analytical research on SFM is included in this review, resulting in a deeper comprehension of the issues and their solutions.
Studies on herbal remedies, cosmetics, food, and veterinary drugs were excluded. Additionally, conference abstracts and those that address the pharmaceutical industries’ audit or inspection results or reports are excluded.
GLOBAL IMPACTS OF SUBSTANDARD AND FALSIFIED ANTIMALARIAL DRUGS
According to the United Nations Sustainable Development Goals, everyone has the right to “access safe, effective, efficient, quality, and cost-effective essential medicines.” However, the prevalence of substandard, counterfeit, and falsified medicines hinders this goal. Different countries affected by SFM are illustrated in Figure 2. Mortality cases were reported from these nations, with Nigeria reporting the highest rate (64%). Despite having a committed national regulatory body, such as the National Agency for Food and Drug Administration and Control (NAFDAC), Nigeria faces significant challenges in pharmaceutical quality control [20]. Similarly, in the Democratic Republic of Congo (DRC), counterfeit and substandard antimalarials contribute significantly to the country’s highest malaria death rates [21]. In 2015, over 1,000 patients, including children, were hospitalized due to drug-falsified intoxication. Between 2017 and 2021, West Africa seized around 605 tonnes of illegally manufactured counterfeit medical supplies, adding to the region’s economic burden [22]. In Tanzania, between 2005 and 2015, the government incurred significant losses due to SFM, with USD 13.65 million for substandard medicines and USD 149,369 for falsified medicines [23]. The WHO’s latest report (2023) states that Africa accounts for 94% of global malaria cases (246 million) and 95% of malaria deaths (569,000), with children under five representing 76% of these deaths. SFM further adds to worsen the scenario [24]. A WHO study found that up to 90% of antimalarial drugs in Africa were substandard. Chloroquine is identified as the most common substandard or falsified antimalarial, with a failure rate of 73%. Following chloroquine, quinine had a failure rate of 32% [12]. These low-quality drugs are often highlighted on the WHO website as an alert globally [25]. One such instance was related to counterfeit antimalarial drugs that were visually identical to the original but adulterated with starch. They may not cause immediate toxicity but fail to achieve the desired therapeutic effect [26]. The identification of inadequate antimalarial compounds increases the rate of the challenge for poor children and their nations. Poor-quality antimalarials (PQAs) lead to drug resistance, increased mortality, and complicated medical treatment. Counterfeit drugs contribute to treatment failure, reduced adherence, and higher morbidity and mortality rates within affected populations, undermining public health efforts [27]. There is an urgent need for the availability of high-quality antimalarials and strong drug control regulations in each country [28].
 | Figure 2. Countries affected with substandard and falsified drugs. [Click here to view] |
Potential effects of substandard antibiotics
Poor-quality and counterfeit antibiotics for children can significantly reduce clinical efficacy while increasing healthcare costs [27]. An infection may remain untreated if the prescribed antibiotic dose is below the recommended therapeutic amount, leading to the prescription of a different broad-spectrum antibiotic, exacerbating antibiotic resistance [28]. Counterfeit medications, even those with a low therapeutic index, can contain hazardous contaminants or excessive amounts of APIs; increasing toxicity can cause the patient’s condition to worsen rapidly, leading to life-threatening complications or death, particularly if counterfeit drugs are not swiftly identified and replaced with effective treatments [26]. The global antibiotic supply rates are about 17.4% under SFM [29]. Various study reports from Ghana, Nigeria, and the United Kingdom highlight the cases of antibiotic tablets that failed to meet the quality standards set by the United States Pharmacopoeia [28]. A survey in Bangladesh revealed that samples of ciprofloxacin and cephadrine contained just 1.5% and 1% of the specified levels, respectively. Research in the Western Pacific Islands found cloxacillin pills containing only 6.9% of the stated amount. Additionally, in India, Azithromycin pills contained 160% of the indicated amount, while in Kenya, parenteral ampicillin contained 190% of the specified quantity. The highest failure rate was seen with sulfamethoxazole-trimethoprim, followed by tetracycline, ampicillin, amoxicillin, and ciprofloxacin [29]. As seen in Table 2, there are different cases of poor-quality antibiotics in LMICs. Studies conducted in Africa highlight the presence of poor-quality amoxicillin formulations, which are most commonly prescribed as one of the economical drugs. One such study from Nairobi reports that 37.7% of the drugs analyzed failed to comply with the Pharmacopoeial standards [30]. More importantly, the scenario is still worse as LMICs do not comply with the WHO Model List of Essential Medicines for Children [31].
Table 2. Reported cases of antibiotics from LMICs.
| Product/drug name | Region identified/year | API | Issues |
|---|---|---|---|
| Amoxycillin DT IP 125 mg | 2015/Uganda | Amoxycillin | Absence of stated API [40] |
| Amoxyverse 250 m | 2017/Democratic Republic of Congo (DR Congo) | Amoxicillin | Absence of stated API and sample does not conform to label claim [41] |
| Metronyl tablets BP | Metronidazole | ||
| Ampiverse 250 mg | Ampicillin | ||
| Amoxyverse 250 mg | 2017/Kenya | Amoxicillin | |
Mirzpan suspension 100mg/100ml | 2017/Pakistan | Cefixime | Absence of stated API, declared as spurious, unregistered and misbranded [42] |
| Cefixime DT IP 100 mg | 2024/India | Cifixime | The assay and disintegration test does not conform [43] |
| Amoxycillin DT IP 125 mg | Amoxycillin | Sample does not conform to IP specification with respect to uniformity of dispersion and disintegration [43] | |
| Vrizarox 100 DT | Cefpodoxime Proxetil | Misbranded as the drug labeled is not in proper manner as per IP [43] | |
| Vithrocin suspension | Azithromycin | As sample failed with respect to test in pH as per IP [43] | |
| Megapure-TZ IU suspension | Ciprofloxacin Hydrochloride and Tinidazole | Sample does not conform to label claim [43] | |
| Petsow starnicillin capsule | 2025/Africa | Ampicillin | Counterfeit as the drug labeled is not in proper manner as per the original code [44] |
A thorough assessment of antibiotic effectiveness, safety, and cost-effectiveness is crucial, especially in regions where irrational use of medications and substandard drugs exacerbate health issues. WHO reports that over 50% of medications in LMICs are incorrectly prescribed, delivered, or sold, leading to widespread health hazards [32]. The prevalence of substandard and counterfeit antibiotics significantly contributes to increased illness and mortality, undermining public health [33].
Consequences of substandard analgesic and antipyretic syrup
Paracetamol (Acetaminophen) is commonly used as an analgesic and antipyretic for children; it helps to reduce severe pain and fever when taken with other strong analgesics. Paracetamol is listed in the essential medicines category by WHO, but the quality is highly compromised in LMICs [31]. According to a study, paracetamol is the most encountered SFM in these regions, with the highest consumption rate of approximately 30 million daily doses, highlighting both the widespread use of paracetamol and the serious concerns regarding safety in LMICs [34].
As depicted in Table 1, most WHO alert cases are related to paracetamol due to the higher concentration of excipients reported from major LMICs. The excipient up to 2.8 g may be consumed by a patient above, which can prove fatal [35]. Several instances have been reported in Nigeria where 74% of drugs failed laboratory testing and were not suitable for consumption [36]. Similarly, in India, drugs like cough expectorants and sedative agent cases were reported [37]. To mitigate fatality risk, the Indian Pharmacopoeia (IP) sets limits on DEG and EG concentration at 0.10% [38]. Consumption of drug products with a higher range of concentration can be fatal, causing hypertension, cardiac arrhythmias, pancreatitis, disturbances in potassium and sodium levels (hyperkalemia or hyponatremia), and finally, delayed neurological effects [39]. Much attention was gained when an unexplained acute kidney injury in children occurred, which had a mortality rate of 43.9% in Nigeria and 14.4% in the US due to DEG [37]. Table 3 represents the pediatric SFM risk matrix, highlighting the region and detection complexity to understand SFM threats. The hope for healing ill children often results in more harm than good in such a scenario [49]. The strategies for the above-mentioned issues will be discussed later in this review paper.
Table 3. Pediatric SFM risk matrix.
| Pharmaceutical Formulation | Severity of associated risk | Testing complexity | Affected regions |
|---|---|---|---|
| Liquid dosage forms | |||
| Antibiotics and antimalarials oral suspensions | High | Medium(stability, reconstitution) | Nigeria, Bangladesh, and Pakistan [45] |
| Paracetamol and cough syrups | High | Low(visual/organoleptic, excipient ID) | Gambia, Uzbekistan, Indonesia, and India [25,46] |
| Multivitamin syrups | Moderate | High (requires micronutrient assay) | Southeast Asia and India [47] |
| Antihistamine syrups | Moderate | Medium (HPLC, label verification) | Sub-Saharan Africa [48] |
| Solid dosage forms | |||
| Dispersible tablets | Low to Moderate | Medium(solubility, dissolution) | India and Nigeria [47] |
| Antibiotics and antimalarials tablets | Moderate | High(requires sterility, microbial testing) | Southeast Asia and Sub-Saharan Africa [25,48] |
| Iron and folic acid tablets | Moderate | Medium (active content assay, oxidation test) | Bangladesh, India, and Nepal [47] |
| Vitamin C and zinc chewable tablets | Low | Low (organoleptic, label check) | LMICs with school nutrition programs [47] |
REASONS FOR LIMITED ACCESS TO QUALITY DRUGS AND RISE IN SFM
The limited services provided in an area may be due to factors including ineffective health systems, lack of competent professionals, and an increase in the burden of disease [50]. In many LMICs, the manufacturing and distribution of SFM are hardly illegal [51]. Figures 3 and 4 highlight the distinct reasons for the rise of SSFFC cases [1]. The survey highlights that the counterfeiting of pharmaceuticals is aimed at essential and more established medications and targets more recent and new-generation pharmaceuticals [10]. Despite receiving WHO prequalification, the quality of drugs in LMICs is still influenced by the country of procurement. Inadequate licensing procedures and a lack of regular outlet inspections were most probably the causes of fake and poor-quality drugs [52]. Recently, the WHO faced significant challenges due to insufficient resources, differing concerns among member states, conflicts between public health goals, and the constraints of its member-state-driven governance structures. Additionally, continuous reforms within the WHO have further complicated its ability to tackle the issue [53].
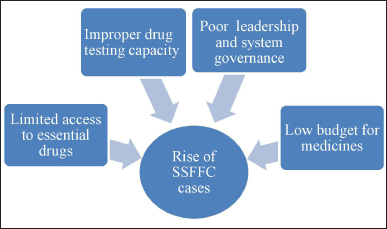 | Figure 3. Reasons for rise of SSFFC cases. [Click here to view] |
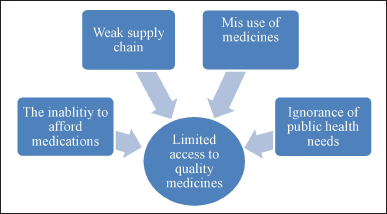 | Figure 4. Reasons for limited access to quality medicines. [Click here to view] |
Stronger political will and concrete action are essential to ensure that all individuals, regardless of their background or location. They must have access to safe, effective, and affordable medicines. Without this commitment, progress in healthcare will remain uneven, leaving vulnerable populations without the treatments they need to survive and thrive [54].
STRATEGIES TO INCREASE THE QUALITY OF MEDICINES FOR CHILDREN
Addressing the issue of SFM requires the active involvement of child health professionals and stakeholders. A global effort is needed to document medicine quality and monitor effectiveness. Improving the quality of medicines for children requires cooperation among nations, regulatory bodies, health ministries, purchasing organizations, and wholesalers. Additionally, sharing detailed and strengthened information is crucial to support these efforts [55,56].
Healthcare leadership and governance structure
A well-integrated leadership and management process is key to enhancing access to medicines by fostering a positive work environment [1]. Additionally, the law should be well established with implemented penalties [4] to criminalize and punish those who deal in SFM for children intentionally or carelessly [51]. In response to the numerous reported cases of SFM in Nigeria, the government approved the Counterfeit and Fake Drugs and Unwholesome Processed Foods Amendment Bill 2015 in November 2016. This bill imposed more severe penalties, such as life sentences and significant charges [4]. To further combat the spread of PQAs, the US President’s Malaria Initiative collaborates with various domestic and international partners globally [26]. The global distribution chain and the extent of the fake drugs racket depend heavily on cross-border cooperation and alerting neighboring nations by collaborating with regulatory bodies to address the issue [54,55]. As per the 2015 Access to Medicine Index Methodology, institutions should report issues to national authorities and “WHO Rapid Alert” to reduce the prevalence of SFM products [56]. The Regulatory Standards Assistance Program, established under the USP Convention, offers resources to LMICs to enhance their ability to test the quality of medicines [26].
Additionally, Attaran recently released a Model Law on Medicine Crime designed to serve as an adaptable framework for a nation seeking to strengthen its laws regarding SFM. This model law upholds key principles and includes provisions about online “pharmacies,” “whistleblowers,” and unregistered medications. These actions emphasize that governments are giving priority to the critical issue of SFM as a priority manner [56].
Pharmaceutical supply chain and advanced technology
To reduce the risk of patients receiving SFM, it is crucial to establish a reliable drug tracking and tracing system within the supply chain in LMICs [57]. Identifying the best logistics service provider that has global and local regulations is essential for improving pharmaceutical access and ensuring the industry’s growth by reducing the production and manufacturing of fake drugs [58,59]. According to the 2016 Access to Medicine Index, pharmaceutical companies should adopt internal verification procedures to minimize risks [60]. A promising advancement in ensuring drug authenticity is mobile verification systems, which assign unique product codes to medicines, enabling consumers to access detailed information [57]. These systems and technologies that improve packaging security strengthen the entire supply chain. While effective, some systems may not be feasible for many LMICs due to costs. For instance, cost-effective technologies like color-shift inks and holograms allow consumers to verify packaging authenticity [2].
In India, the technique has come into widespread use in the pharmaceutical sector, with holograms appearing on packaging materials for both Indian and export markets.
In addition, 2D Data Matrix code-based technologies are increasingly applied when dispensing to detect counterfeit drugs [5]. Radio-frequency identification (RFID) and barcodes also help track products in point-of-sale systems, coupled with cell phones, and hold immense potential for enhancing supply chain management [2]. RFID tags are passive transmitters attached to bottles of medicines, providing near-permanent tracking [61]. In 2023, government-recognized blockchain technology proved to be a system that assisted the FDA in verifying data, allowing for transparent tracking of drug distribution [6]. This technology prevents unauthorized drugs from entering the supply chain and offers a secure tracking and tracing system [62]. Organisations such as Pfizer, Amgen, and Sanofi have made investments in blockchain to enhance drug testing, transparency, and commercialization [6]. Blockchain deployment, as effective as it may be in enhancing supply chain transparency, requires strong digital infrastructure, cooperation from stakeholders, and ongoing financial investment, which can be unaffordable in resource-limited environments in LMICs [48,63].
Thus, these technologies are promising; their existing scalability and usability in LMICs must be thoroughly assessed and preferably tested through donor-funded or public–private ventures before large-scale adoption.
The Medicines Quality Database also tracks drugs that are being tested for quality in various regions, such as South America, Asia, and Africa [51]. Agencies such as the Bill & Melinda Gates Foundation, UNICEF, and the Global Fund are backing innovative technologies for product traceability and are supporting LMICs [64]. Electrochemical sensors, such as paper-based analytical devices (ePADs), have emerged as a major force in identifying SFM, providing a cost-effective tool for detecting harmful medications [34,65].
An anti-counterfeiting technology like 3D screen printing (3DSP) provides greater security and offers promising avenues to increase the quality of medicines. Additionally, supported by international organizations, 3DSP can produce concealed features in oral drug products that are hard to copy using conventional techniques [66]. It also promises to deliver personalized low-dose drugs for children, enhance the supply chain, and lower the risk of counterfeiting [67], but it is not cost-effective for LMICs as they require capital investment in equipment, skilled staff and regulations [66]. Owing to these factors 3DSP are generally underdeveloped in LMICs [67]. In addition, regulatory mechanisms for the approval of 3D-printed medicines are yet to evolve even in developed nations, which adds further difficulties for LMICs [68]. The absence of guidelines for 3D-printed pharmaceuticals highlights the immediate need for broad regulatory requirements to guarantee safety, potency, and quality [69].
Pharmaceutical cost-effective analysis
The number of pharmaceutical screening equipment is increasing, focusing on cost-effective methods for detecting inferior and counterfeit medicines [70]. Physical profiling, such as mass, thickness, and color, is an initial and cost-effective step. Digital micrometers and analytical balances are used for mass and dimensions, while a colorimeter helps assess tablet color compared to genuine ones [71]. Nondestructive technologies such as Raman spectroscopy, near-infrared (NIR) spectroscopy, X-ray diffraction, and nuclear magnetic resonance spectroscopy are used for identifying APIs and excipients in tablets [72]. Furthermore, dynamic thermal analysis is another quick, affordable method for distinguishing between genuine and counterfeit tablets. It requires minimal equipment and sample preparation, with thermokinetic properties evaluated in the temperature range of 60°C–22.2°C, distinguishing manufacturing differences and drug compositions [73]. A study in Laos evaluated portable screening devices such as handheld NIR, Raman, and FTIR spectrometers, as well as paper analytical devices, finding that devices like MicroPHAZIR RX and 4500a FTIR were cost-effective for detecting counterfeit artemisinin-based combination therapies (ACTs) [70]. Additionally, Portable NIR devices were found to have less than 6% error for screening ACT, antiretroviral therapy, and tuberculosis therapy [74].
Global Pharma Health Fund minilab
The Global Pharma Health Fund (GPHF), based in Frankfurt, funded by Merck KGaA Darmstadt, provides the GPHF-Minilab™ at a reasonable price for the verification of the quality of medicines in LMICs. This device has been used for more than 25 years and assists over 100 countries with little training involved [72]. The minilab uses thin-layer chromatography (TLC), an inexpensive method that accurately detects SFM. The GPHF-minilab was employed by African and Asian local organizations to screen branded and generic medicines. Physical inspection, color reaction testing, TLC assays, and disintegration tests in solid oral drug forms part of the process [75]. This method has been found to be highly effective for the evaluation of antimalarial drugs in a resource-constrained environment [71]. If significant quality issues are identified, samples are shipped out for confirmatory analysis. Integration of the minilab with other technologies such as NIR or Raman spectroscopy and high-performance liquid chromatography (HPLC) would make it more effective [76]. The GPHF-minilab has been extensively employed in nations such as Ethiopia, Indonesia, Ghana, and Vietnam owing to its affordability and simplicity to use, and thus used as a sound screening for drug quality [73].
Pharmaceutical education system, training, and support
The aim of education and training is to enhance the important role of chemists in the pharmaceutical and healthcare network [77] towards preventing the distribution of SFM in the community [78]. Pharmacists are educated to identify counterfeit and substandard drug products and to warn the relevant authorities of the varied sources of these drugs. However, studies show a lower perception of the effectiveness of training in SFM, particularly regarding its impact on individual pharmacists’ practices and skills [75]. Despite this, it is highly skilled pharmacists who are needed to rapidly identify and report suspicious pharmaceutical products to regulatory authorities. Their expertise is vital for developing tools and frameworks to enhance the monitoring of pharmaceutical supply chains, contributing to better oversight and safety [79]. The result of a knowledge, attitude, and practice survey that took place at the Iranian Pharmacist Association congress showed a significant knowledge gap amongst pharmacists about counterfeit medications. The results stress the need for creating and implementing specific educational programs [78]. Similarly, a study conducted in Nigeria found that pharmacists with less than 5 years of experience struggled to manage counterfeit medications due to their limited exposure and lack of practical training during their professional development [52]. The American Pharmacists Association has emphasized the need for continuous updates in the knowledge and skills of the pharmacist is a need [80]. In response, the WHO and the International Pharmaceutical Federation worked with pilot universities to create an undergraduate pharmacy course on medication quality that the European Commission financed between 2019 and 2021 [70]. Professional experts and regulatory agencies can successfully implement this recommendation by emphasizing the participation of pharmacy schools and professionals in academics towards the conduct of refresher courses and upgrading their knowledge [75].
Furthermore, chemists must work closely with patients and other medical professionals to provide necessary information and highlight the risk factors associated with SFM [81]. In partnership with the International Intellectual Property Crime Investigators College, the international police (Interpol) offers webinars and online training programs addressing the criminal activities of fraudulent and fake drug products [65]. Additionally, the Regional Council of Pharmacy of Sao Paulo has implemented initiatives to regulate the actions of every pharmacist, providing a contact number and email address for reporting information about SFM, pharmacies, or organizations involved in selling such products [25]. A chemist with the right skills, education, and experience can offer essential primary care services to children and infants and advice on the quality of medications for treating chronic conditions, which require a high degree of skill and close patient interaction [82].
Raising public awareness: mode and system
Public education campaigns are essential for raising awareness about the dangers of SFM. Access to information should be extended to all stakeholders, including pediatricians, infant nurses, and others involved in child healthcare, to highlight the potential risks [28]. Pharmaceutical industries and various regulatory and global bodies are making efforts to combat the issue; WHO: As a global alert, WHO regularly publishes the various classes of SFM on their websites, focusing on expediting the identification and prevention of the spread of SFM by facilitating prompt and coordinated responses across national borders. Details about particular events and the associated health risks are also shared. As an outcome, such transparency enhances global vigilance, improves risk communication among stakeholders, and strengthens regulatory action against the distribution and use of SFM [16].
CDSCO: A “Whistleblower scheme” was introduced to report SFM cases. Additionally, they release a monthly list on their official website, providing detailed information accessible to the public [82]. This alert system ensures that only the best goods are supplied to the market by preventing the sale and distribution of SFM. It also serves as a reminder of the pharmaceutical industry’s requirement for stringent quality control. Patients, consumers, and healthcare professionals must stay updated on drug safety alerts to ensure that only safe and effective medications are used. Manufacturers may contribute to restoring consumer confidence in their goods and improving patient outcomes by resolving the issues that have been identified [83].
ANVISA: They have initiated a report on the labeling and batch number of falsified medicines in the Brazilian Official Diary of the Union, resulting in strengthened safeguards, such as improved inspection procedures and labeling standards, to guarantee the safety and quality of medications [26].
NAFDAC: In Nigeria, “Operation Shine Your Eyes” was established to educate the public about the hazards of SFM, leading to a significant change in consumer behavior, with customers routinely requesting the NAFDAC registration number as proof of a medicine’s authenticity and quality [4].
Pharmaceutical companies like Pfizer have launched a campaign to identify, dissuade, and discourage manufacturers and distributors involved in producing and distributing counterfeit Pfizer medicine. Over 12 million tablets of counterfeit Pfizer medications were seized by police in 49 countries in 2017. To make the public aware that the seized products come under the category of SFM, Pfizer Global Security has increased the number of raids to safeguard the authenticity of its drug products [84].
According to a Tanzanian Health and Safety Department study on public knowledge and counterfeit drug detection, 55.6% of participants could tell the difference between real and fake medications after the awareness and education program [85]. Additionally, a study in Egypt reported that pharmacists actively support the efforts to combat SFM by purchasing medications from approved sources, indicating the importance of education and awareness campaigns [86].
Despite these efforts, there is still a need for training and programs in LMICs to strengthen the capacity of medical professionals and pharmacists.
Global organizations and funds
Numerous organizations and funds are dedicated to focusing on various fronts, from essential health services to education for children. WHO has developed its investigation against counterfeit medications to the UN Office of Drugs and Crime and Interpol to address the issue and bridge the policy gap [53]. Also, The World Wide Antimalarial Resistance Network has created the Antimalarial Quality Literature Surveyor to improve the understanding of the existing evidence and clarify data to inform public health [9]. Children’s Investment Fund Foundation is one of the major philanthropic foundations with a goal to achieve high-impact sectors like health, education, and child protection to make long-term change. By collaborating with other organizations like governments, NGOs, and other stakeholders, they enhance the availability of quality education and improve health for children. Similarly, the International Medical Products Anti-Counterfeiting Taskforce established by the WHO seeks to protect public health through the prevention of production, distribution, and sale of counterfeit products [26]. Following a resolution endorsed by WHO Member States, a uniform framework was established, initiating the WHO/Health Action International Project on Medicine Prices and Availability. The project aims to contribute towards the accomplishment of target 17 of the Millennium Development Goals, with emphasis on collaborating with pharmaceutical organizations to provide access to affordable, essential medicines in LMICs [87]. The Global Malaria Programme leads and coordinates for malaria elimination. It operates in line with the “Global Technical Strategy for Malaria 2016–2030,” adopted by the World Health Assembly, thus decreasing the malaria burden globally and the incidence of SFM among children [88]. In 2022, the WHO-hosted multistakeholder initiative, the Global Accelerator for Pediatric Formulations, expanded its target medicines to include antibiotics. This step came in response to the urgent demand for pediatric formulations of crucial antibiotics. In March 2023, the network stepped forward by publishing its priority list of antibiotics towards pediatric medicine optimization. The list mentions major medicines such as oral azithromycin, amoxicillin–clavulanic acid, nitrofurantoin, and parenteral cefiderocol, which is a major step towards better medications for children globally [89].
Other initiatives
Several countries have pursued extra measures to counter SFM. For instance, the NAFDAC has implemented strong security measures to assist in port inspection and address operational interference [26]. Similarly, China’s National Institute for the Control of Pharmaceutical and Biological Products created a mobile laboratory quality assessment system. The activity was implemented to conduct onsite testing of quality and extend pharmaceutical drug surveillance to remote rural regions, thus expanding the monitoring scope for SFM [90].
Adherence to strict regulations and compliance with Good Manufacturing Practices (GMPs) are essential to ensuring the production of high-quality medicines, a key component in achieving Universal Health Coverage (UHC), which is particularly important for Kenya, which has set a target to attain UHC by 2030 [28]. Furthermore, regional initiatives to harmonize pharmaceutical regulations have successfully contributed to regulatory improvements. With the DRC joining the East African Community (EAC) in 2022, there is growing optimism that the country will benefit from the ongoing progress of the EAC Regulatory Harmonization initiative [89].
Similarly, in India, during the latest revision of IP monographs, the associated method from other pharmacopoeias was also considered. To avoid and prevent EG/DEG substandard issues, revisions recommend limiting the levels of EG and DEG to ≤0.10% and adding EG/DEG tests to identification procedures. Currently, manufacturers are required to estimate EG/DEG during production and transfers. cGMP Guidelines also require identification testing of representative samples from each shipment before use [43].
As a response to the numerous incidences involving substandard and spurious drugs around the world, regulatory bodies have proactively moved to improve drug safety and avoid such events in the future. An example of such efforts is the coordination between the United States, Japanese, and European Pharmacopoeias under the PDG, which have played an important role in developing the harmonization of general chapters and compendial monographs concerning excipients [91].
LIMITATIONS OF THE STUDY
The main drawback of this review lies in the formal selection of the included studies. Additionally, in several instances, only a few interventions were reported, which lack a firm conclusion. Furthermore, we only considered English-language studies, which might have resulted in the exclusion of pertinent papers written in other languages.
A robust methodological framework is needed, like systematic reviews, considering better study criteria and outcomes.
FUTURE PROSPECTS
A multifaceted, evolutionary approach involving ongoing training, supervision initiatives, and the involvement of numerous stakeholders is necessary to find a solution to this “wicked problem,” as law enforcement measures alone are insufficient. The prequalification methods of pharmaceutical goods and their producers must be adopted in procuring medicines by commercial, nonprofit, and public entities. To enhance the coverage of all geographic regions, an optimal approach for assessing the health, social, and economic effects of SFM should be developed and maintained. More information should be acquired to estimate the costs of implementing regulations, quality assurance, and education to lessen the SFM effects on children’s health and finances in all the major LMICs. Although a total solution is unlikely to disappear entirely, children’s risk can be reduced through collaboration and professional and national commitment, which needs global support. Similarly, deficiencies in pharmacists’ ability to recognize poor-quality medications can be addressed by strengthening pharmacy education programs offered by various educational institutions and through ongoing professional development. Additionally, two key innovative solutions are proposed; first is the development of Global Pediatric Drug Quality Index, that aims to rank countries based on the regulatory effectiveness, testing capability, and incident reporting mechanisms for pediatric drugs, helping to identify gaps, encourage responsibility, and guide global efforts to enhance medicine safety and quality for children, particularly in LMICs. Second, the implementation of Zero-Tolerance Supply Chain Protocol integrating blockchain technology, minilabs, and community surveillance to enhance supply chain transparency and prevent the spread of substandard and falsified pediatric drugs. Ultimately, achieving safe and quality medicines is possible through unified action, and the victory is achievable for all children who rely on medications for their well-being.
CONCLUSION
In conclusion, this review highlights the WHO alerts and cases of compromised quality of antibiotics that cause a serious threat to global health, especially for vulnerable groups like children, due to the widespread use of SFM in LMICs. Antibiotic effectiveness, safety, and cost-effectiveness are crucial for pediatric exacerbating antibiotic resistance. The commonly used medicines, such as analgesics and antipyretics for children, proved fatal due to exceeding the limit of DEG and EG concentration. Several global instances of the same were reported.
Well-established healthcare leadership and governance were established, and a Model Law on Medicine Crime was signed for a nation seeking to strengthen its laws on SFM. Technology like tracking and tracing systems within the supply chain and anti-counterfeiting technologies like 3DSP offer a higher level of protection, but are not cost-effective.
However, cost-effective pharmaceutical analyses are discussed as an intervention for monitoring the quality of the marketed products, including the Frankfurt-based GPHF minilab, which precisely identifies SFM as being widely used in many countries for reliable and quality drug screening. In addition, public education and awareness campaigns, numerous global organizations, and dedicated funds. Finally, other initiatives like the mobile laboratory quality inspection, UHC policy for all, and harmonizing pharmaceutical regulations shall benefit from the ongoing issues. Significant obstacles include regulatory frameworks, poor healthcare infrastructure, and restricted access to high-quality medications that continue to exist despite continuous international attempts to address this problem. Therefore, collaborating with nongovernmental organizations, national drug regulatory authorities, and key stakeholders presents a promising strategy for advancing the Sustainable Development Goal of ensuring access to quality medicines. Achieving this goal would be a significant step in safeguarding the health and well-being of all children who depend on essential medications.
ACKNOWLEDGMENTS
We thank Manipal College of Pharmaceutical Sciences and Manipal Academy of Higher Education for the library access and support provided for this literature search.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sorato MM, Davari M, Kebriaeezadeh A. Improving access to medicines to reduce marketing and use of substandard and falsified medicines in Africa: scoping review. J Med Access. 2024;8:27550834241236598. CrossRef
2. Hamilton WL, Doyle C, Halliwell-Ewen M, Lambert G. Public health interventions to protect against falsified medicines: a systematic review of international, national and local policies. Health Policy Plan. 2016;31(10):1448–66. CrossRef
3. Salami RK, Valente De Almeida S, Gheorghe A, Njenga S, Silva W, Hauck K. Health, economic, and social impacts of substandard and falsified medicines in low- and middle-income countries: a systematic review of methodological approaches. Am J Trop Med Hyg. 2023;109(2):228–40. CrossRef
4. Yakhkind A, Lang AE, Brophy G, Tesoro E, Levasseur-Franklin KE, Maldonado N. Substandard and falsified medications: a barrier to global health equity exemplified in Ecuador. Neurocrit Care. 2023;38(1):1–6. CrossRef
5. Tefera G, Bekele A, Getahun H, Tefera Y. Impact of substandard and falsified medicines on public health: a review. Acta Sci Pharm Sci. 2022;6(6):4–10.
6. Taylor D. RFID in the pharmaceutical industry: addressing counterfeits with technology. J Med Syst. 2014;38(11):141. CrossRef
7. McManus D, Naughton BD. A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: a focus on context, prevalence, and quality. BMJ Glob Health. 2020;5(8):e002393. CrossRef
8. Renschler JP, Walters KM, Newton PN, Laxminarayan R. Estimated under-five deaths associated with poor-quality antimalarials in Sub-Saharan Africa. Am J Trop Med Hyg. 2015;92(6_Suppl):119–26. CrossRef
9. Bassat Q, Tanner M, Guerin P, Stricker K, Hamed K. Combating poor-quality antimalarial medicines: a call to action. Malar J. 2016;15:302. CrossRef
10. The convention on the rights of the child: the children’s version UNICEF [Internet]. [cited 2024 Nov 22]. Available from: https://www.unicef.org/child-rights-convention/convention-text-childrens-version
11. Children United Nations [Internet]. [cited 2024 Nov 20]. Available from: https://www.un.org/en/global-issues/children
12. Tegegne AA, Feissa AB, Godena GH, Tefera Y, Hassen HK, Ozalp Y, et al. Substandard and falsified antimicrobials in selected east African countries: a systematic review. PLoS One. 2024;19(1):e0295956. CrossRef
13. Higgins CR, Kobia B, Ozawa S. Comparing the return on investment of technologies to detect substandard and falsified amoxicillin: a Kenya case study. PLoS One. 2023;18(1):e0268661. CrossRef
14. Yahoo News. Indonesia bans cough syrups after nearly 200 child deaths [Internet]. 2022 [cited 2024 Nov 4]. Available from: https://www.yahoo.com/news/indonesia-bans-cough-syrups-nearly-174411645.html
15. Full List of WHO Medical Product Alerts [Internet]. [cited 2024 Jun 29]. Available from: https://www.who.int/teams/regulation-prequalification/incidents-and-SF/full-list-of-who-medical-product-alerts
16. Barr RD, Furneaux R, Margottini L, Eden TOB. The international scandal of defective asparaginase: a blight on children with cancer. Pediatr Blood Cancer. 2023;70(8):e30403. CrossRef
17. Gray NJ, Barr RD, Karekezi C, Chanoine JP. Substandard and falsified medicines for children: UHC has a preventive role. Lancet Child Adolesc Health. 2023;7(12):821–3. CrossRef
18. Rutta E, Liana J, Embrey M, Johnson K, Kimatta S, Valimba R, et al. Accrediting retail drug shops to strengthen Tanzania’s public health system: an ADDO case study. J Pharm Policy Pract. 2015;8(1):23. CrossRef
19. Roncone A, Kelly S, Giannioti Z, Hauk C, Caillet C, Newton P, et al. Stable isotope ratio analysis: an emerging tool to trace the origin of falsified medicines. TrAC-Trends Anal Chem. 2024;174:117666. CrossRef
20. UNODC World Drug Report 2024: Harms of world drug problem continue to mount amid expansions in drug use and markets United Nations in North Macedonia [Internet]. [cited 2024 Jun 29]. Available from: https://northmacedonia.un.org/en/272585-unodc-world-drug-report-2024-harms-world-drug-problem-continue-mount-amid-expansions-drug
21. Newton PN, Caillet C, Guerin PJ. A link between poor quality antimalarials and malaria drug resistance?. Expert Rev Anti Infect Ther. 2016;14(6):531–3. CrossRef
22. Kusynová Z, Bais Y, Van Den Ham HA, Mantel-Teeuwisse AK, Etame-Loe G, Kaale E, et al. Improved knowledge of substandard and falsified (SF) medical products through a dedicated course for pharmacy students at three universities in sub-Saharan Africa. BMJ Glob Health. 2023;6(Suppl 3):e009367. CrossRef
23. Adhikari B, Bayo M, Peto TJ, Callery JJ, Tripura R, Dysoley L, et al. Comparing the roles of community health workers for malaria control and elimination in Cambodia and Tanzania. BMJ Glob Health. 2023;8(12):e013593. CrossRef
24. World Health Organization. Full list of WHO medical product alerts [Internet]. Geneva, Switzerland: WHO; [cited 2024 Jul 29]. Available from: https://www.who.int/teams/regulation-prequalification/incidents-and-sf/full-list-of-who-medical-product-alerts
25. Lima MBA, Yonamine M. Counterfeit medicines: relevance, consequences, and strategies to combat the global crisis. Braz J Pharm Sci. 2023;59:e20402. CrossRef
26. Walker EJ, Peterson GM, Grech J, Paragalli E, Thomas J. Are we doing enough to prevent poor-quality antimalarial medicines in the developing world? BMC Public Health. 2018;18(1):630. CrossRef
27. Evans DR, Higgins CR, Laing SK, Awor P, Ozawa S. Poor-quality antimalarials further health inequities in Uganda. Health Policy Plan. 2019;34(Supplement_3):iii36–47. CrossRef
28. Irungu BN, Koech LC, Ondicho JM, Keter LK. Quality assessment of selected co-trimoxazole suspension brands marketed in Nairobi County, Kenya. PLoS One. 2021;16(9):e0257625. CrossRef
29. Zabala G, Bellingham K, Vidhamaly V, Boupha P, Boutsamay K, Newton P, et al. Substandard and falsified antibiotics: neglected drivers of antimicrobial resistance? BMJ Glob Health. 2022;7(8):e008587. CrossRef
30. Koech LC, Irungu BN, Ng’ang’a MM, Ondicho JM, Keter LK. Quality and brands of amoxicillin formulations in Nairobi, Kenya. BioMed Res Int. 2020;2020(1):7091278. CrossRef
31. Kelesidis T, Falagas ME. Substandard/Counterfeit antimicrobial drugs. Clin Microbiol Rev. 2015;28(2):443–64. CrossRef
32. Assi S. Identification of counterfeit drugs using dual laser handheld Raman. Eur Pharm Rev. 2015;20(5):20–6.
33. Vandy A, Conteh E, Lahai M, Kolipha-Kamara M, Marah M, Marah F, et al. Physicochemical quality assessment of various brands of paracetamol tablets sold in Freetown Municipality. Heliyon. 2024;10(3):e25502. CrossRef
34. Silva MKLD, Barreto FC, Sousa GDS, Simões RP, Ahuja G, Dutta S, et al. Development of an electrochemical paper-based device modified with functionalized biochar for the screening of paracetamol in substandard medicines. Molecules. 2024;29(22):5468. CrossRef
35. Cavany S, Nanyonga S, Hauk C, Lim C, Tarning J, Sartorius B, et al. The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance. Nat Commun. 2023;14(1):6153. CrossRef
36. Gabel J, Lächele M, Sander K, Gnegel G, Sunny-Abarikwu N, Ohazulike RE, et al. Quality of essential medicines from different sources in Enugu and Anambra, Nigeria. Am J Trop Med Hyg. 2024;111(1):179–95. CrossRef
37. Soleman S, Adnan M, Sudiarto H, Mahathma S, Tazkia A, Firdaus H, et al. Effects of diethylene glycol contamination of pharmaceutical products on unexplained acute kidney injury in children: a systematic review. Clin Exp Pediatr [Internet]. 2024 [cited 2024 Nov 23];67(8):395–402. Available from: http://e-cep.org/journal/view.php?doi=10.3345/cep.2023.01039
38. Kumar P, Rastogi S, Saini PK, Sahoo S, Raghuvanshi RS, Jadaun GPS. Minimizing the risk of ethylene glycol and diethylene glycol poisoning in medications: a regulatory and pharmacopoeial response. Regul Toxicol Pharmacol. 2025;155:105741. CrossRef
39. Aronson JK. When I use a word . . . medicines regulation—diethylene glycol. BMJ. 2024;384:q356. CrossRef
40. Petersen A, Held N, Heide L, Difäm-EPN Minilab Survey Group. Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab. PLoS One. 2017;12(9):e0184165. CrossRef
41. Riaz MK, Riaz M, Latif A. Medication errors and strategies for their prevention. Pak J Pharma Sci. 2017;30(3):921–8.
42. Latest Alerts. Central drugs standard control organization [Internet]. [cited 2024 Jun 29]. Available from: https://cdsco.gov.in/opencms/opencms/en/Latest-Alerts/
43. Public Alert No. 03/2025 – Alert on the Sale of Counterfeit/Falsified Petsow Starnicillin (Ampicillin) 500mg found in Cameroon and Central African Republic - NAFDAC [Internet]. [cited 2025 Mar 5]. Available from: https://nafdac.gov.ng/public-alert-no-03-2025-alert-on-the-sale-of-counterfeit-falsified-petsow-starnicillin-ampicillin-500mg-found-in-cameroon-and-central-african-republic/
44. Health products policy and standards [Internet]. [cited 2024 Nov 29]. Available from: https://www.who.int/teams/health-product-policy-and-standards/medicines-selection-ip-and-affordability/medicines-policy/rational-use
45. Ozawa S, Evans DR, Bessias S, Haynie DG, Yemeke TT, Laing SK, et al. Prevalence and estimated economic burden of substandard and falsified medicines in low- and middle-income countries. JAMA Netw Open. 2018;1(4):e181662. CrossRef
46. Nayyar GML, Breman JG, Mackey TK, Clark JP. Falsified and substandard drugs: stopping the pandemic. Am J Trop Med Hyg. 2019;100(5):1058–65. CrossRef
47. UNICEF Supply Division. Annual report 2022. Available from: https://www.unicef.org/supply/media/13741/file/UNICEF-Supply-Annual-Report-2022.pdf
48. Mackey TK, Nayyar GML. A review of existing and emerging digital technologies to combat the global trade in fake medicines. Expert Opin Drug Saf. 2017;16(5):587–602. CrossRef
49. Wirtz VJ, Hogerzeil HV, Gray AL, Bigdeli M, de Joncheere CP, Ewen MA, et al. Essential medicines for universal health coverage. Lancet. 2017;389:403–76. CrossRef
50. Clark F. Rise in online pharmacies sees counterfeit drugs go global. Lancet. 2015;386 (10001):1327–8. CrossRef
51. Adigwe OP, Onavbavba G, Wilson DO. Challenges associated with addressing counterfeit medicines in Nigeria: an exploration of pharmacists’ knowledge, practices, and perceptions. IPRP. 2022;11:177–86. CrossRef
52. Worku MC, Mitku ML, Ayenew W, Limenh LW, Ergena AE, Geremew DT, et al. Assessment of knowledge, attitude, and practice on substandard and counterfeit pharmaceutical products among pharmacy professionals in Gondar City, North-West Ethiopia. Curr Pharm Teach Learn. 2024;16(10):102140. CrossRef
53. Mackey T, Liang B. Improving global health governance to combat counterfeit medicines: a proposal for a UNODC-WHO-Interpol trilateral mechanism. BMC Med. 2013;11:233. CrossRef
54. Adepoju P. African nations to criminalize falsified medicine trafficking. Lancet. 2020;395(10221):324. CrossRef
55. Bolla AS, Patel AR, Priefer R. The silent development of counterfeit medications in developing countries: a systematic review of detection technologies. Int J Pharm [Internet]. 2020;587:119634. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2007335737&from=export
56. Attaran A. Stopping murder by medicine: introducing the model law on medicine crime. Am J Trop Med Hyg. 2015;92(6):127–32. CrossRef
57. Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005;2(4):302–8.
58. El-Jardali F, Akl EA, Fadlallah R, Oliver S, Saleh N, El-Bawab L, et al. Interventions to combat or prevent drug counterfeiting: a systematic review. BMJ Open. 2015;5(3):e006290. CrossRef
59. Islam I, Islam M. A blockchain based medicine production and distribution framework to prevent medicine counterfeit. J King Saud Univ-Comput Inf Sci. 2024;36(1):101851. CrossRef
60. Adepoju P. Kenya sounds alarm over counterfeit cancer drugs. Lancet Oncol. 2024;25(7):834. CrossRef
61. Glass BD. Counterfeit drugs and medical devices in developing countries. Res Rep Trop Med. 2014;5:11–22. CrossRef
62. Sylim P, Liu F, Marcelo A, Fontelo P. Blockchain technology for detecting falsified and substandard drugs in distribution: pharmaceutical supply chain intervention. JMIR Res Prot [Internet]. 2018;7(9):e10163. CrossRef
63. Torkzadeh L, Tavana M, Maroufi S, Ghasemi P. Blockchain applications in combating counterfeit drugs: a systematic review. Technol Soc. 2022;68:101866.
64. Hajjou M, Krech L, Lane-Barlow C, Roth L, Pribluda VS, Phanouvong S, et al. Monitoring the quality of medicines: results from Africa, Asia, and South America. Am J Trop Med Hyg. 2015;92(6_Suppl):68–74. CrossRef
65. Pal T, Mathai T, Mukherji S. Colorimetric chemosensor for rapid detection of fluoroquinolone load in environmental water bodies, urine, and counterfeit drug testing. Biosens Bioelectron X [Internet]. 2023;14:100384. Available from: https://www.scopus.com/inward/record.uri
66. Schwarz SW, Decristoforo C. US and EU radiopharmaceutical diagnostic and therapeutic nonclinical study requirements for clinical trial and marketing authorizations. EJNMMI Radiopharm Chem [Internet]. 2019;4(1):10. Available from: https://www.embase.com/search/results
67. Jamróz W, Kurek M, Lyszczarz E, Szafraniec J, Jachowicz R. Application of 3D printing technology in pharmaceuticals. Pharm Res. 2022;39(2):289–302
68. Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50. CrossRef
69. Wang Y, Liu X. Safety signals of albumin-bound paclitaxel: data mining of the Food and Drug Administration adverse event reporting system. Indian J Pharmacol. 2023;55(3):167–73. CrossRef
70. Luangasanatip N, Khonputsa P, Caillet C, Vickers S, Zambrzycki S, Fernández FM, et al. Implementation of field detection devices for antimalarial quality screening in Lao PDR—a cost-effectiveness analysis. PLoS Negl Trop Dis. 2021;15(9):e0009539. CrossRef
71. Khuluza F, Kigera S, Jähnke RWO, Heide L. Use of thin-layer chromatography to detect counterfeit sulfadoxine/pyrimethamine tablets with the wrong active ingredient in Malawi. Malar J [Internet]. 2016;15(1):215. Available from: https://www.embase.com/search/results
72. GPHF The GPHF-MinilabTM [Internet]. [cited 2024 Jul 3]. Available from: https://www.gphf.org/en/minilab/
73. Pan H, Ba-Thein W. Diagnostic accuracy of global pharma health fund MinilabTM in assessing pharmacopoeial quality of antimicrobials. Am J Trop Med Hyg. 2018;98(1):344–8. CrossRef
74. Visser BJ, Meerveld-Gerrits J, Kroon D, Mougoula J, Vingerling R, Bache E, et al. Assessing the quality of antimalarial drugs from Gabonese pharmacies using the MiniLab®: a field study. Malar J [Internet]. 2015;14(1):273. Available from: https://www.embase.com/search/results?Subaction=viewrecord&id=L605176591&from=export
75. Gnegel G, Häfele-Abah C, Neci R, Heide L. Surveillance for substandard and falsified medicines by local faith-based organizations in 13 low- and middle-income countries using the GPHF Minilab. Sci Rep. 2022;12(1):13095. CrossRef
76. Ozawa S, Haynie DG, Bessias S, Laing SK, Ngamasana EL, Yemeke TT, et al. Modeling the economic impact of substandard and falsified antimalarials in the democratic Republic of the Congo. Am J Trop Med Hyg. 2019;100(5):1149–57. CrossRef
77. Shahverdi S, Hajimiri M, Pourmalek F, Torkamandi H, Gholami K, Hanafi S, et al. Iranian pharmacists’ knowledge, attitude and practice regarding counterfeit drugs. Iran J Pharm Res. 2012;11(3):963–8.
78. Jack A. Can anyone stop the illegal sale of medicines online? BMJ. 2016;352:i1317. CrossRef
79. Adigwe OP. The role of pharmacists in eliminating counterfeit medicines in Nigeria. Front Public Health. 2023;11:1170929. CrossRef
80. Chambliss WG, Carroll WA, Kennedy D, Levine D, Moné MA, Douglas Ried L, et al. Role of the pharmacist in preventing the distribution of counterfeit medications. J Am Pharm Assoc. 2012;52(2):195–9. CrossRef
81. Persson A, Troein M, Lundin S, Midlöv P, Lenander C. Exploring pharmacists’ perspectives about substandard and falsified medical products through interviews. Explor Res Clin Soc Pharm. 2024;13:100421. CrossRef
82. Mani G, Danasekaran R, Annadurai K. Substandard, spurious, falsely-labelled, falsified and counterfeit (SSFFC) drugs: time to take a bitter pill. J Krishna Inst Med Sci Univ. 2016;5(4):122–24.
83. CDSCO Drug Alert: 48 Medicine Batches Flagged as Not of Standard Quality in August 2024 – The Doctorpreneur Academy [Internet]. [cited 2025 Jun 3]. Available from: https://thedoctorpreneuracademy.com/2024/09/23/cdsco-drug-alert-48-medicine-batches-flagged-as-not-of-standard-quality-in-august-2024/
84. Counterfeit awareness campaign. [cited 2023 Mar 1]. Available from: https://www.Pfizer.com/counterfeiting/counterfeitawarenesscampaign
85. El-Dahiyat F, Fahelelbom KM, Jairoun AA, Al-Hemyari. Combatting substandard and falsified medicines: public awareness and identification of counterfeit medications. Front Public Health. 2021;9;1–8 CrossRef
86. Bashir A, Galal S, Ramadan A, Wahdan A, El-Khordagui L. Community pharmacists’ perceptions, awareness and practices regarding counterfeit medicines: a cross-sectional survey in Alexandria, Egypt. East Mediterr Health J. 2020;26(5):556–64. CrossRef
87. Le Doare K, Barker CIS, Irwin A, Sharland M. Improving antibiotic prescribing for children in the resource-poor setting. Br J Clin Pharm. 2015;79(3):446–55. CrossRef
88. Global Malaria Programme [Internet]. [cited 2024 Dec 21]. Available from: https://www.who.int/teams/global-malaria-programme
89. Tack B, Vita D, Ntangu E, Ngina J, Mukoko P, Lutumba A, et al. Challenges of antibiotic formulations and administration in the treatment of bloodstream infections in children under five admitted to Kisantu Hospital, Democratic Republic of Congo. Am J Trop Med Hyg. 2023;109(6):1245–59. CrossRef
90. Fadlallah R, El-Jardali F, Annan F, Azzam H, Akl E. Strategies and systems-level interventions to combat or prevent drug counterfeiting: a systematic review of evidence beyond effectiveness. Pharm Med. 2016;30(5):263–76. CrossRef
91. Press Information Bureau. Government of India issues warning on counterfeit drugs [Internet]. 2025 [cited 2025 Jul 3]. Available from: https://www.pib.gov.in/PressReleasePage.aspx?PRID=1966349