INTRODUCTION
Artocarpus lakoocha, commonly known as monkey jack, is a medicinal tree widely used in traditional Thai medicine. Its heartwood is a key ingredient in “powdered Puag-Haad,” an anthelmintic remedy. Decoctions derived from the heartwood have been traditionally used to treat colic, dyspepsia, tendon ailments, and constipation. Additionally, the roots are used as an antipyretic, demonstrating broad therapeutic applications [1].
Acne vulgaris is a prevalent dermatological disorder that primarily affects adolescents, characterized by clogged hair follicles, inflammation, and the formation of pimples. In addition to its physical manifestations, acne has significant psychological and social implications. Standard treatments include antibiotics, such as clindamycin, vitamins, antioxidants, hormones, and anti-inflammatory agents. However, these therapies frequently result in side effects, such as skin irritation and dryness. Moreover, prolonged use of antibiotics contributed to the emergence of bacterial resistance, highlighting the need for safer and more effective natural alternatives [2].
Recent studies have highlighted the pharmacological potential of A. lakoocha, particularly its antibacterial, antioxidant, cytotoxic, and anti-inflammatory activities [3]. Artocarpus lakoocha extracts demonstrated significant antibacterial activity, including activity against Shigella sonnei, Escherichia coli, Bacillus pumilus, Proteus mirabilis, and Bacillus subtilis [4]. Furthermore, A. lakoocha extracts exhibit bactericidal activity against methicillin-resistant Staphylococcus aureus along with potent antioxidant properties [5].
Oxyresveratrol is a major bioactive compound comprising 70%–80% of A. lakoocha heartwood extract [1]. It can inhibit tyrosinase activity, an enzyme critical for melanin synthesis. This inhibition contributes to skin-lightening effects and enhances antioxidant activity, making oxyresveratrol a popular ingredient in cosmeceutical products [6]. Additionally, oxyresveratrol exerts immunomodulatory and anti-inflammatory effects because of its ability to downregulate interleukin-6, tumor necrosis factor-alpha, nuclear factor kappa B (NF-kB), and cyclooxygenase-2 while upregulating trefoil factor 2 in mouse models [7]. Oxyresveratrol also exhibits anti-inflammatory effects in vitro and in vivo by regulating NF-kB signaling and alleviating eczematous lesions in dermatitis models [8]. Although the antibacterial properties of oxyresveratrol against other bacteria have been established [3], its activity against Cutibacterium acnes, a key acne-causing bacterium, remains underexplored.
In the present study, we aimed to evaluate the therapeutic potential of oxyresveratrol isolated from A. lakoocha. Specifically, we focused on the antibacterial efficacy of the compound against C. acnes and Staphylococcus epidermidis, its antioxidant and anti-inflammatory properties, and its cytotoxicity in human skin cells. This study provides novel insights into the therapeutic potential of oxyresveratrol derived from A. lakoocha, contributing to the development of new and natural treatments for acne and inflammation and offering a safer alternative to conventional antibiotics.
MATERIALS AND METHODS
Artocarpus lakoocha extracts
The heartwood of A. lakoocha (DMSC 5237) was collected during October–November 2022 and authenticated by comparing it with plant specimens in the Herbarium Laboratory of the Medicinal Plant Research Institute, Nonthaburi, Thailand. A total of 4.5 kg of fresh heartwood was processed to obtain 950 g of dried powdered material, which was used for extraction. Methanol extracts of A. lakoocha heartwood were extracted and provided by the Medicinal Plant Research Institute as crude (crude AR), partially purified (AR06), and purified (AR061) extracts. The extracts were analyzed using high-performance liquid chromatography (HPLC).
Bacterial strains
The bacterial strains C. acnes DMST 14916, S. epidermidis DMST 3547, and S. epidermidis DMST 4343 were obtained from the Department of Medical Sciences of the Ministry of Public Health, Thailand. Bacteria were cultured in brain heart infusion broth at 37°C for 24 hours. Moreover, C. acnes was cultured under anaerobic conditions.
Quantitative analysis of oxyresveratrol using HPLC
Oxyresveratrol concentration was quantified using Scion HPLC-DAD LC6000 (SCION Instruments, the Netherlands). Oxyresveratrol standard (Chengdu Biopurify Phytochemical Ltd, PRC) and sample solutions from A. lakoocha heartwood were analyzed using a COSMOSIL column (C18 4.6 mm. I.D. × 250 mm). The mobile phase consisted of 0.1% orthophosphoric acid in water and acetonitrile (80:20) at a flow rate of 1.0 ml/min. The column temperature was set to 40°C, with detection at 326 nm and an injection volume of 5 µl. This method was used to quantify oxyresveratrol concentration in extract samples of crude AR, AR06, and AR061.
Antibacterial susceptibility testing
Agar disc diffusion method
Antibacterial activity was assessed according to the Clinical and Laboratory Standards Institute standards (2009) [9]. Test bacterial cultures were prepared to match the McFarland standard No. 0.5 and spread onto Mueller–Hinton agar (MHA) plates. Extract solutions (64 mg/ml) were pipetted (10 µl) onto sterile discs, which were then placed on inoculated MHA plates and incubated at 37°C for 24 hours. Subsequently, the inhibition zones (mm) were measured. Tests were conducted in triplicate, with 30 µg of tetracycline disk (Oxoid, Basingstoke, United Kingdom) used as a positive control and 1% dimethyl sulfoxide (DMSO) as a negative control.
Microdilution broth method
The microdilution broth dilution method was performed as described previously [10]. Briefly, extracts (16 mg/ml) were serially diluted two-fold in 96-well microplates. The test bacterial cultures were diluted to match McFarland standard No. 0.5 and then diluted 100-fold. The diluted bacterial suspension (100 µl) was added to each well with controls included. The microplates were incubated at 37°C for 24 hours. The minimum inhibitory concentration (MIC) was defined as the lowest concentration at which no visible bacterial growth was observed. To determine the minimal bactericidal concentration (MBC), samples from wells with no growth were cultured on brain heart infusion agar (BHA) and incubated at 37°C for 24 hours. MBC was defined as the lowest concentration with no bacterial colony formation.
Time-kill assays
A time-kill assay was performed to evaluate the bactericidal activity following a previously reported method, with some modifications [11]. Briefly, test bacterial cultures were prepared to match the McFarland standard No. 0.5. The bacterial suspension was mixed with A. lakoocha extracts at concentrations of 0.5, 1, 2, 4, and 8 times the MIC, followed by incubation at 37°C. Samples were collected at 0, 24, 48, and 72 hours, diluted, and cultured on BHA plates. The plates were incubated at 37°C for 24 hours. Colonies were counted, and time-kill curves were plotted to determine the relationship between surviving bacteria (log CFU/ml) and time (hour). Tests were performed in triplicate.
Thin-layer chromatography and bioautography
Thin-layer chromatography (TLC) was performed to separate the chemical components followed by bioautography for the antibacterial compounds. Silica gel 60 F254 was used as the stationary phase; the mobile phase comprised chloroform:methanol:H2O (8:2:0.2). The extract solutions were spotted onto TLC plates and placed in a mobile phase chamber. After separation, TLC plates were used for antibacterial activity testing by spreading BHA medium containing bacterial cultures, allowing it to solidify, and incubating it at 37°C for 24 hours. The plates were sprayed with thiazolyl blue tetrazolium bromide to visualize the inhibition zones indicating antibacterial activity [11].
Antioxidant activity and total phenolic content assays
The antioxidant potential of the extracts was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity assay [12]. Briefly, various concentrations of the extract (0.1–500 µg/ml) were mixed with 0.004% DPPH solution in methanol, and the absorbance at 517 nm was measured after 30 minutes of incubation in the dark. The percentage inhibition of DPPH radicals was calculated for each concentration. The half-maximal inhibitory concentration (IC50) values, which represent the concentration at which 50% of the DPPH radicals were scavenged, were determined.
The total phenolic content of the extracts was determined using the Folin–Ciocalteu method [13]. Gallic acid (0.002–1.0 mg/ml) was used as the standard. Briefly, 100 µl of a 10% (v/v) Folin–Ciocalteu solution was mixed with 20 µl of the extract at 1 mg/ml and 80 µl of 7.5% (w/v) Na2CO3. Absorbance was measured at 765 nm using a spectrophotometer (BMG Labtech, Spectrostar Nano, Germany) after 30 minutes of incubation in the dark.
Cytotoxicity test
The extract was dissolved in DMSO and diluted with Dulbecco’s modified Eagle’s medium to obtain concentrations ranging from 0.07 to 10.00 mg/ml. Human dermal fibroblast normal (HDFn) cells were seeded in 96-well plates at a density of 2 × 105 cells/well and incubated with varying concentrations of the AR extract for 24 hours at 37°C with 5% CO2. Following incubation, the WST-1 reagent was added to each well, and the optical density at 450 nm was measured after 2 hours using a microplate reader to determine cell viability. Morphological changes in HDFn cells were observed under an inverted microscope and evaluated according to ISO 10993-5 standards [14].
Anti-inflammatory activity
HDFn cells were treated with non-toxic concentrations of the AR extract (0.07, 0.15, and 0.31 mg/ml) for 24 hours, followed by UVA+B irradiation (1.5 J/cm²). Prostaglandin E2 (PGE2) levels were measured using an enzyme-linked immunosorbent assay kit (Cayman Chemical, Ann Arbor, MI). The inhibition rates of PGE2 production were calculated to assess the anti-inflammatory activity of the AR extract.
Statistical analysis
Data are presented as the mean ± SD. Statistical analyses were conducted using analysis of variance to evaluate the variance among groups, followed by Duncan’s multiple range test to determine significant differences between means at a 95% confidence level. All statistical analyses were performed using SPSS for Windows, version 16.0 (SPSS Inc, San Diego, CA). A p-value of < 0.05 was considered significant.
RESULTS
Quantitative analysis of oxyresveratrol using HPLC
Quantitative analysis revealed that the extract samples crude AR, AR06, and AR061 contained oxyresveratrol at concentrations of 253.72 ± 0.60, 357.09 ± 0.36, and 424.01 ± 0.16 µg/ml, respectively. The percentages recoveries of oxyresveratrol were 50.6%, 71.4% and 84.8%, respectively (Fig. 1 and Table 1).
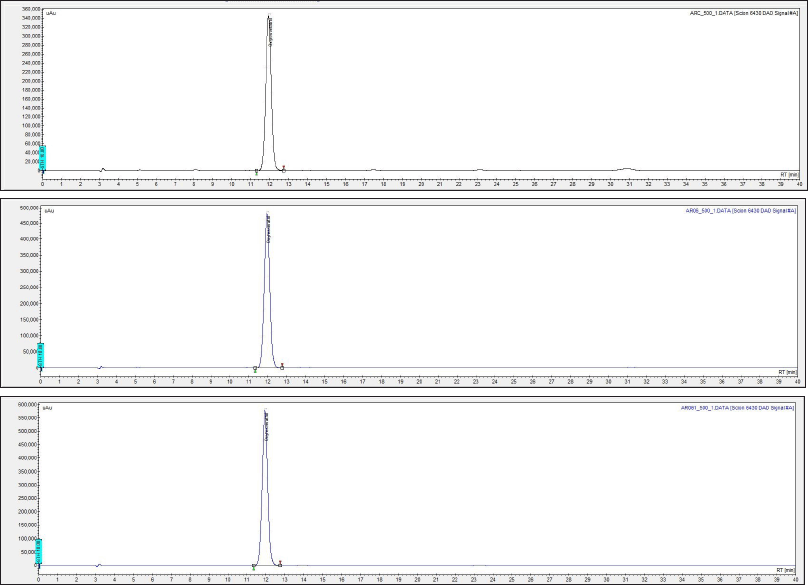 | Figure 1. High-performance liquid chromatography showing oxyresveratrol concentrations in crude (crude AR, A), partially purified (AR06, B), and purified (AR061, C) extracts of Artocarpus lakoocha. Peaks indicate oxyresveratrol levels quantified via standard calibration. [Click here to view] |
 | Table 1. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha extracts using HPLC. [Click here to view] |
Antibacterial activity against acne-causing bacteria
The antibacterial activity of A. lakoocha extracts was assessed using the agar disc diffusion method. All extracts demonstrated inhibitory effects against the tested bacterial strains. However, the purified extract (AR061) exhibited significantly greater antibacterial activity than both AR06 and crude AR (p < 0.05). The inhibition zones for the crude AR, AR06, and AR061 ranged from 12.17–19.58, 13.69–21.90, and 16.37–31.90 mm, respectively. Notably, purified oxyresveratrol resulted in large inhibition zones for all the tested bacteria, with the largest zone observed for C. acnes (31.90 mm). The absence of an inhibition zone for tetracycline against S. epidermidis DMST 4343 suggests the potential use of A. lakoocha extracts as natural and effective antibacterial agents (Table 2).
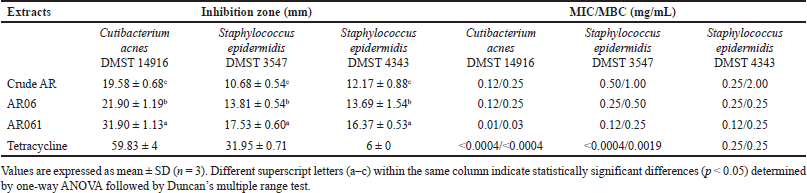 | Table 2. Antibacterial activity of Artocarpus lakoocha extracts (crude AR, AR06, and AR061) against acne-associated bacteria. [Click here to view] |
The antibacterial efficacy of the extracts was further evaluated using the microdilution broth method to determine the MIC and MBC (Table 2). Both MIC and MBC values decreased significantly with increasing extract purity (p < 0.05) for C. acnes DMST 14916 and S. epidermidis DMST 3547. The purified extract exhibited the greatest antibacterial activity with MIC values ranging from 0.01 to 0.12 mg/ml. Specifically, the MIC values of AR061 were 10-, 5-, and 2-fold lower than those of crude AR for C. acnes DMST 14916, S. epidermidis DMST 3547, and S. epidermidis DMST 4343, respectively. These differences were statistically significant (p < 0.05), reinforcing the conclusion that the extract purity directly correlates with antibacterial efficacy (Table 2).
Time-kill assay analysis
The time-kill assay results demonstrated that all A. lakoocha extracts exhibited bactericidal activity over time (Fig. 2). Significant growth inhibitory effects were observed against the tested bacteria within 24 hours at all extract concentrations. Bactericidal activity increased with an increase in extract concentration and exposure duration.
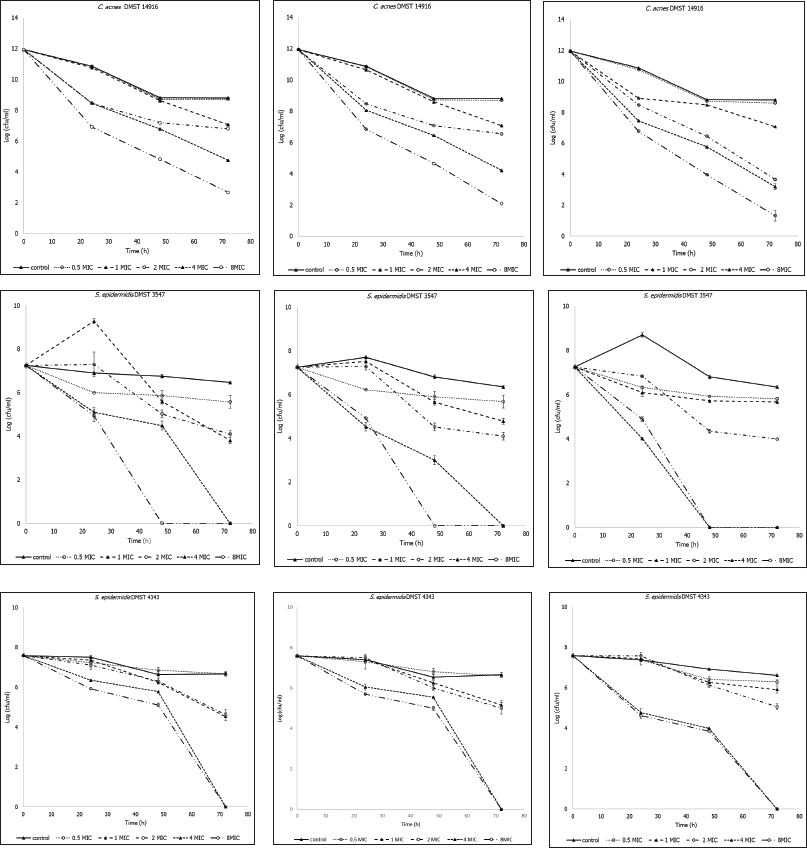 | Figure 2. Time-kill curves of Artocarpus lakoocha extracts at various concentrations against the tested bacteria (A: Cutibacterium acnes DMST 14916, B: Staphylococcus epidermidis DMST 3547, C: S. epidermidis DMST 4343, 1: crude AR, 2: AR06, 3: AR061). Data represent bacterial survival over time (hours). MIC = minimum inhibitory concentration. [Click here to view] |
Antibacterial activity based on TLC bioautography
The crude AR, AR06, and AR061 were separated using TLC (Fig. 3). Bioautography results indicated that the active component in the crude AR did not migrate on the TLC silica gel 60 when tested against C. acnes DMST 14916. The AR06 contained multiple components with antibacterial activity against all tested bacteria. The AR061 displayed strong antibacterial activity against all strains, confirming oxyresveratrol as the key active compound in A. lakoocha exhibiting potent antibacterial activity against acne-causing bacteria.
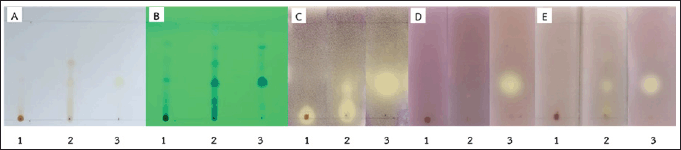 | Figure 3. Thin-layer chromatography (TLC)-bioautography analysis of crude AR, AR06, and AR061 extracts. Panels show (A) visible TLC, (B) UV-detected TLC, and antibacterial activity zones against (C) Cutibacterium acnes DMST 14916, (D) Staphylococcus epidermidis DMST 3547, and (E) S. epidermidis DMST 4343. 1 = crude AR, 2 = AR06, 3 = AR061. [Click here to view] |
Antioxidant properties of A. lakoocha extracts
The antioxidant activity of crude AR, AR06, and AR061 was evaluated using the DPPH radical-scavenging assay. The IC50 values were 49.00±1.02 µg/ml for crude AR, 31.00±0.85 µg/ml for AR06, and 30.00±0.77 µg/ml for AR061, indicating significantly stronger antioxidant activity in the purified extracts compared to crude AR (p < 0.05). Although AR06 and AR061 showed similar IC50 values, their antioxidant capacity was significantly superior to that of crude AR. This result indicates that the purification process enhanced the antioxidant properties of the extract.
Additionally, the total phenolic content was higher in the AR061 than in the crude AR. Specifically, the total phenolic contents were 0.21±0.01 mg GAE/g for AR, 1.12±0.03 mg GAE/g for AR06, and 1.25±0.02 mg GAE/g for AR061, showing statistically significant differences among all groups (p < 0.05). These findings suggest a strong correlation between increased phenolic content and enhanced antioxidant activity, supporting oxyresveratrol as the primary active compound responsible for this effect (Table 3).
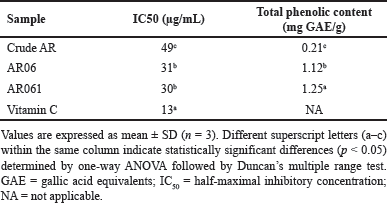 | Table 3. Antioxidant properties of Artocarpus lakoocha extracts. [Click here to view] |
Cytotoxicity
The cytotoxicity of crude AR was assessed using the WST-1 assay, revealing an IC50 value of 6.08 ± 1.28 mg/ml, which indicates moderate cytotoxicity. At concentrations below 0.31 mg/ml, the extract maintained cell viability above 80%, suggesting low toxicity at doses relevant to anti-inflammatory activity (Table 4).
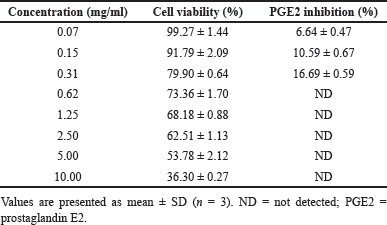 | Table 4. Cytotoxicity (cell viability) and anti-inflammatory (PGE2 inhibition) activity of Artocarpus lakoocha crude extracts (crude AR). [Click here to view] |
Anti-inflammatory activity
The crude AR exhibited significant dose-dependent inhibition of PGE2 production at concentrations that maintained cell viability above 80%. At concentrations ranging from 0.07–0.31 mg/ml, the extract inhibited PGE2 production by 6.64%–16.69% (Table 4). These results suggest that crude AR exerts potential anti-inflammatory properties by inhibiting PGE2 production in a dose-dependent manner at non-cytotoxic concentrations.
DISCUSSION
The present study highlights the potential of oxyresveratrol-containing A. lakoocha extracts in treating acne and associated skin inflammation. The extracts exerted significant antibacterial activity against C. acnes and S. epidermidis, with the purified extract (AR061) exhibiting superior efficacy. Notably, the inhibition zone of S. epidermidis DMST 4343 obtained using AR061 was greater than that obtained using tetracycline, highlighting the potential of this extract as a natural alternative to conventional antibiotics. Similar antibacterial activity of oxyresveratrol has also been reported in recent studies, including enhanced effects when chemically modified or delivered through optimized formulations [15,16].
The strong antioxidant activity of the extracts was closely linked to their oxyresveratrol content, which increased with purification. This increase emphasizes the contribution of oxyresveratrol to the overall therapeutic potential of the extract. Antioxidants are crucial for mitigating oxidative stress, which is a key factor in skin inflammation and damage [17]. In agreement with our findings, Thaweesest et al. [15] demonstrated that oxyresveratrol derivatives exhibit strong antioxidant and anti-inflammatory activities in macrophage cells. Therefore, these findings underscore the role of natural antioxidants in skincare formulations, particularly in acne treatments.
In the present study, cytotoxicity assays revealed an IC50 value of 6.08 ± 1.28 mg/ml for crude AR, indicating moderate cytotoxicity. Notably, concentrations at which optimal antibacterial and anti-inflammatory activities were observed were below the cytotoxic levels, suggesting a favorable safety profile for topical applications. However, its moderate cytotoxicity at higher concentrations necessitates careful dose optimization for potential applications in anti-inflammatory and anti-acne skincare formulations.
Furthermore, oxyresveratrol significantly inhibited PGE2 production in a dose-dependent manner at non-cytotoxic concentrations (0.07–0.31 mg/ml). This inhibition is crucial as PGE2 is a key inflammatory mediator that promotes redness, swelling, and pain associated with acne lesions. By inhibiting PGE2 production, oxyresveratrol suppresses these inflammatory symptoms and downregulates a broader inflammatory cascade, including the production of other inflammatory cytokines and enzymes [18]. Additionally, its antioxidant properties likely complement these effects by neutralizing reactive species and further reducing inflammation. Oxyresveratrol also inhibits the conversion of arachidonic acid to prostaglandins and regulates NF-kB signaling, which broadly downregulates inflammatory genes [4]. This is consistent with recent mechanistic studies showing that oxyresveratrol modulates MAPK and NF-kB pathways, thereby reducing pro-inflammatory mediators [16]. Furthermore, oxyresveratrol can regulate NF-kB signaling and reduce skin inflammation, resulting in the amelioration of eczematous lesions in dermatitis mouse models [8].
In this study, TLC bioautography and time-kill assay results confirmed that oxyresveratrol provides a multifaceted approach to acne treatment. The AR06 contained multiple antibacterial components; the AR061 displayed potent activity against all tested bacterial strains. These results establish oxyresveratrol as the primary active compound in A. lakoocha, corroborating previous findings regarding its bactericidal and antioxidant properties [3].
The present study had a few limitations. First, the in vitro nature of these assays does not fully replicate the complex conditions present in human skin. Further, in vivo studies on animal models and clinical trials are essential to validate the safety and efficacy of oxyresveratrol-based treatments. Second, the molecular mechanisms underlying the antibacterial and anti-inflammatory effects require further investigation. Investigating the potential synergistic effects of oxyresveratrol with other natural compounds or antibiotics that may enhance its therapeutic efficacy will broaden its applications. Finally, the long-term safety assessments of topical use are critical to ensure its suitability for cosmeceutical development.
CONCLUSION
This study provides evidence that oxyresveratrol extracted from A. lakoocha exhibits significant antibacterial activity against C. acnes, a major contributor to acne vulgaris, along with notable antioxidant and anti-inflammatory properties. Among the tested extracts, the purified fraction (AR061) demonstrated superior efficacy, as indicated by its low MIC and MBC values, potent radical-scavenging activity, and effective inhibition of PGE2 production at non-cytotoxic concentrations. These findings suggest that purified oxyresveratrol holds promise as a natural alternative to conventional antimicrobial and anti-inflammatory agents in topical applications for acne and related skin disorders.
Moreover, this study underscores the broader potential of A. lakoocha-derived oxyresveratrol in cosmeceutical development. However, the findings are based on in vitro assays, which may not fully reflect in vivo conditions. Further animal studies and clinical trials are necessary to confirm its efficacy, safety, pharmacokinetics, and underlying mechanisms. Future investigations should also evaluate formulation stability, delivery systems, and potential synergistic interactions with standard treatments to support its clinical translation.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was supported by grants from Naresuan University and Maejo University in Thailand.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Maneechai S, Likhitwitayawuid K, Sritularak B, Palanuvej C, Ruangrungsi N, Sirisa-ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and ‘Puag-Haad’. Med Princ Pract. 2009;18(3):223–27. CrossRef
2. Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20(3):335–44. CrossRef
3. Sitorus P, Keliat JM, Asfianti V, Muhammad M, Satria D. A literature review of Artocarpus lacucha focusing on phytochemical constituents and pharmacological properties of the plant. Molecules 2022;27(20):6940. CrossRef
4. Phoolcharoen W, Sooampon S, Sritularak B, Likhitwitayawuid K, Kuvatanasuchati J, Pavasant P. Anti-periodontal pathogen and anti-inflammatory activities of oxyresveratrol. Nat Prod Commun. 2013;8(5):641–44. CrossRef
5. Charirak P, Ratananikom K. Anti-methicillin-resistant Staphylococcus aureus activities of Artocarpus lakoocha Roxb extract and its mode of action. Sci World J. 2022;2022(1):1–6. CrossRef
6. Saesue K, Thanomrak P, Prompan W, Punan W, Khorana N, Juprasert W, et al. Development of a ready-to-use oxyresveratrol-enriched extract from Artocarpus lakoocha Roxb. using greener solvents and deep eutectic solvents for a whitening agent. Cosmetics 2024;1(2):58. CrossRef
7. Aziz RS, Siddiqua A, Shahzad M, Shabbir A, Naseem N. Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α, NF-kB, and COX-2 levels, and upregulation of TFF-2 levels. Biomed Pharmacother. 2019;110:554–60. CrossRef
8. Tran HG, Shuayprom A, Kueanjinda P, Leelahavanichkul A, Wongsinkongman P, Chaisomboonpan S, et al. Oxyresveratrol attenuates inflammation in human keratinocyte via regulating NF-kB signaling and ameliorates eczematous lesion in DNCB-induced dermatitis mice. Pharmaceutics 2023;15(6):1709. CrossRef
9. Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed. CLSI standard M07. Wayne, PA: CLSI; 2018.
10. Sanguansermsri D, Sanguansermsri P, Buaban K, Kawaree R, Fraser I, Wongkattiya N. Oral antipathogenic and antiviral properties of Boesenbergia rotunda (L.) Mansf. extracts with panduratin A as active compound. J Appl Pharm Sci. 2024;14(07):59–64. CrossRef
11. Sanguansermsri D, Sanguansermsri P, Buaban K, Kawaree R, Wongkattiya N. Antimicrobial activity and time-kill kinetics of Boesenbergia rotunda essential oil and geraniol alcohol against oral bacterial pathogens. J Appl Pharm Sci. 2024;14(02):215–21. CrossRef
12. Takahashi M, Hirose N, Ohno S, Arakaki M, Wada K. Flavor characteristics and antioxidant capacities of hihatsumodoki (Piper retrofractum Vahl) fresh fruit at three edible maturity stages. J Food Sci Technol. 2018;55:1295–305. CrossRef
13. Derakhshan Z, Ferrante M, Tadi M, Ansari F, Heydari A, Hosseini MS, et al. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice, and seeds. Food Chem Toxicol. 2018;114:108–11. CrossRef
14. Pratoomsoot C, Wongkattiya N, Sanguansermsri D. Synergistic antimicrobial and antioxidant properties of Coccinia grandis (L.) Voigt, Clerodendrum inerme (L.) Gaertn. and Acanthus ebracteatus Vahl. extracts and their potential as a treatment for xerosis cutis. Complement Med Ther. 2020;27(6):410–20. CrossRef
15. Thaweesest W, Buranasudja V, Phumsuay R, Muangnoi C, Vajragupta O, Sritularak B, et al. Anti-inflammatory activity of oxyresveratrol tetraacetate, an ester prodrug of oxyresveratrol, on lipopolysaccharide-stimulated RAW264.7 macrophage cells. Molecules 2022;27(12):3922. CrossRef
16. Likhitwitayawuid K. Oxyresveratrol: sources, production, biological activities, pharmacokinetics, and delivery systems. Molecules 2021;26(14):4212. CrossRef
17. Singhatong S, Leelarungrayub D, Chaiyasut C. Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. heartwood extract. J Med Plant Res. 2010;4(10):947–53.
18. Wongwat T, Srihaphon K, Pitaksutheepong C, Boonyo W, Pitaksuteepong T. Suppression of inflammatory mediators and matrix metalloproteinase (MMP)-13 by Morus alba stem extract and oxyresveratrol in RAW 264.7 cells and C28/I2 human chondrocytes. J Tradit Complement Med. 2019;10(1):132–40. CrossRef