INTRODUCTION
Inflammation is the body’s response to adverse stimuli, such as pathogens, allergens, cellular damage, tissue injury, and toxic substances [1,2]. The immune system plays a crucial role in removing harmful stimuli and instigating the healing process, making it an essential defense [3,4]. At the tissue level, inflammation is characterized by redness, swelling, heat, discomfort, and impaired tissue function. These symptoms result from the cellular and vascular reactions to infection or injury, as well as local inflammatory cell activity [5]. Important microcirculatory events involved in the inflammatory process include abnormalities in vascular permeability, the attraction and accumulation of leukocytes in the damaged area, and the release of inflammatory mediators [3,6].
Unregulated inflammatory reactions are a primary factor underlying a range of diseases, such as allergies, cardiovascular dysfunction, metabolic syndrome, cancer, and autoimmune diseases [2,7]. Medicines that regulate and reduce inflammation include steroid drugs, nonsteroidal anti-inflammatory drugs, and immunosuppressants. However, these have various side effects. To maximize the pharmaceutical response and minimize side effects in the treatment of inflammatory conditions, new anti-inflammatory agents must be identified and explored, including those derived from natural sources [8]. Polyscias scutellaria (Burm.f) Fosberg leaves have potential as anti-inflammatory agents due to their saponin, tannin, flavonoid, alkaloid, and terpenoid content [8,9]. Research has demonstrated that the leaves possess significant quantities of total phenols and strong antioxidant properties.
Antioxidant effects, particularly those arising from the polyphenol group, correlate with other biological responses, such as anti-plasmodial, antimicrobial, anti-arthritis, anticancer, and anti-inflammatory reactions [10,11]. Hexane, ethyl acetate, and ethanol (96%) extracts of P. scutellaria leaves have been investigated for their phenol, flavonoid, and antioxidant contents. The study found that the ethanol extract contains the highest polyphenol level that is proportional to its antioxidant activity [12]. Polyscias scutellaria leaves ethanol extract at a concentration of 50 μg/ml showed higher anti-inflammatory activity than the ethyl acetate extract by inhibiting the levels of nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and interleukin-12 (IL-12) [13]. Moreover, previous in vitro and in silico studies have published the ability of P. scutellaria leaves extract to inhibit the activity of the pro-inflammatory enzyme, cyclooxigenase-2 (COX-2) [14].
Therefore, further study on P. scutellaria leaves ethanol extract is required to confirm its antioxidant potency and anti-inflammatory activity, especially focusing on the effect on pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). A metabolomic profile analysis using liquid chromatography-orbitrap high-resolution mass spectrometry (LC-HRMS) was also performed to identify the phytochemical constituents of the active extract.
MATERIAL AND METHODS
Chemicals
Liquid chromatography-mass spectrometry (LC-MS) grade methanol, acetonitrile, and water were acquired from Fisher Scientific (Hampton, NH, USA). Formic acid, hydrochloric acid, and methanol of high-performance liquid chromatography quality were acquired from Merck (Darmstadt, Germany). The positive ion solution contains caffeine, MRFA, and ultramark 1621, while the negative ion solution contains sodium dodecyl sulfate, sodium taurocholate, and ultramark 1621. Additionally, carrageenan (Sigma Aldrich, St. Louis, Mo., USA), enzyme-linked immunosorbent assay (ELISA) kit TNF-α (KTE9007-96well), IL-1β (KTE9001-96well), IL-6 (KTE9004-96well) (EliKine™ Rat), and 2,2-diphenyl-1-picrylhydrazyl crystal (DPPH) (Sigma Aldrich, St. Louis, Mo., 101 USA) were used in this study.
Plant collection
The leaves of P. scutellaria were collected from the Candimulyo district, Magelang regency in Central Java, Indonesia (7°30’59.6”S 110°16’22.5”E) during the dry season in April 2023. The plant material was identified and verified at the laboratory of the Department of Pharmaceutical Biology, Universitas Gadjah Mada, with the identification number 25.20.7/UN1/FFA.2/BF/PT/2023. The leaves of P. scutellaria were washed in tap water and then dried in the oven at 50°C for 48 hours. The dried leaves were powdered and filtered using a 40 mesh filter. The dried and file leaves powders were stored in air-tight containers at room temperature until use.
Plant extraction
The method used for the extraction of the plant material is maceration as previously described [15]. The dried leaves of P. scutellaria (1 kg) were macerated in 90% ethanol (10 l) for 3 days. The macerate was filtered and the plant material residue was re-macerated in 5 l of 90% ethanol for 2 days. The macerate was then filtered, dried in a rotary evaporator (60°C) followed by a water bath (60°C), and then stored in a refrigerator at 4°C. The extract was weighed and the yield of extraction was then calculated.
Free radical scavenging activity
Plant extract solution (0.2 ml) at the concentrations of 104.00, 83.20, 66.56, 53.25, and 42.59 µg/ml) was added to a solution containing 4.0 ml of 0.7 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 4.0 ml methanol. The mixture was vigorously agitated in a vortex mixer and then left undisturbed according to the operating time of each test solution. The sample solution’s absorbance was quantified at a wavelength of 517 nm and compared to the control [16].
where
Ac = absorbance of control (4.0 mL 0.7 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 4.0 ml methanol) and As = absorbance of sample at 517 nm.
Carrageenan-induced rat hind paw oedema
The method used in this study was the rat hind paw oedema model that was adopted from the previously described method [17]. Thirty male Wistar rats (180–200 g) were acclimated and housed in groups of five per box for 1 week before the experiment. The animals were given unrestricted access to water and a conventional diet. Six groups (n = 5) were used for the experiments. This included three groups orally administered different doses of the ethanol extract of P. scutellaria leaves (EEPS) at 100, 200, and 400 mg/kg, solvent group orally administered sodium carboxymethyl cellulose (Na-CMC; 0.1 ml/10 g), and diclofenac sodium group orally administered diclofenac sodium (13.65 mg/kg). Thirty minutes after receiving treatment according to their group, the rats in all groups received a sub-plantar injection of carrageenan 1% (0.1 ml) to induce swelling in the right hind paw. The paw oedema volume of each rat was assessed using a plethysmometer (Ugo Basile, Gemonio VA, Italy) both before carrageenan swelling induction and at 30-minute intervals for a total of 360 minutes post-induction. Based on paw oedema volume, the level of inflammation inhibition was estimated as a percentage. The Ethics Committee of LPPT, Universitas Gadjah Mada, Yogyakarta, Indonesia has approved all animal procedures conducted in this study (approval number: 00045/04/LPPT/IX/2023).
Blood sample collection
After 6 hours, the blood (2 ml) was collected from retro-orbital of each rat using a plain vacutainer without anticoagulant. The blood was centrifuged at 3500 rpm for 5 minutes to obtain serum and put into a new microtube, and then stored in a deep freezer at −80°C for further analysis of pro-inflammatory cytokine levels.
Cytokines level measurement
The cytokine levels in the supernatant were measured using ELISA kits for TNF-α (KTE9007-96well), IL-1β (KTE9001-96well), and IL-6 (KTE9004-96well) (EliKine™ Rat) in the ELISA microplate reader (Biorad Imark). The protocol for cytokines level analysis followed the protocol recommended by the manufacturer’s.
Liquid chromatography-orbitrap high-resolution mass spectrometry analysis
The metabolite content of EEPS was determined by extracting the raw data from a total ion chromatogram acquired in an LC-HRMS metabolomic study from three updated online databases: Predicted Compositions, mzCloud Search, and ChemSpider Search. Thermo Scientific™ Accucore™ Phenyl-Hexyl 100 mm × 2.1 mm ID × 2.6 μm was used as a column; whereas the mobile phases were MS-grade water with 0.1% formic acid (A) and MS-grade methanol with 0.1% formic acid (B) with methanol gradient at a flow rate of 0.3 ml/minute. The mobile phase B was first set at 5%, increased to 90% over a duration of 16 minutes, and then maintained at 90% for 4 minutes before reverted to the previous state (5% B) until 25 minutes. The column temperature was set at 40°C with the volume of the injection at 3 μl. The mass spectrometry was run in both positive and negative ionization modes, with full MS/dd-MS2. Nitrogen was employed for sheath, auxiliary, and sweep gas, configured at 32, 8, and 4 arbitrary units, respectively. The capillary temperature was maintained at 320°C while a spray voltage of 3.30 kV was applied. The scanning range was 66.7–1,000 m/z, with a resolution of 70,000 and 17,500 for complete MS and dd-MS2, respectively. Compound Discoverer® software developed by Thermo Scientific (USA) was used to analyze the data including blank containing methanol. The results were then filtered based on the relative abundance of metabolite compounds.
Statistical analysis
The data were analyzed using GraphPad Prism version 10.2.3. Comparisons of the outcomes of the solvent group and treatment groups were made using one-way analyses of variance (ANOVA) followed by Tukey’s multiple-comparison post-hoc test. P-values below 0.05 (p < 0.05) were deemed statistically significant
RESULTS AND DISCUSSION
Radical scavenging activity
The antioxidant effect of the ethanol extract of P. scutellaria leaves were analyzed using the commonly used method, DPPH free radical scavenging assay. The antioxidant effects of the extract on DPPH free radicals were expressed as % inhibition. The IC50 values (µg/ml) of the extract were presented in Table 1. Previous studies showed that EEPS contains various plant secondary metabolites, such as alkaloids, tannins, saponins, and glycosides. These natural compounds can scavenge free radical DPPH I the solution by donating hydrogen atoms [18]. The antioxidant property of flavonoids arises from their ability to scavenge free radicals, particularly through chelation [19,20], This is an important mechanism in targeting inflammatory progression [11,21]. The antioxidant activity of the extract is expressed as the percentage of scavenged free radical DPPH upon extract treatment. In this study, EEPS demonstrated antioxidant activity, and having the potency as a promising natural defense against oxidative damage provoking inflammation. EEPS showed a free radical DPPH scavenging activity IC50 (55.97 ± 0.96 µg/ml). This antioxidant activity is in line with the previous study showing that the ethanol (96%) extract of P. scutellaria leaves demonstrated antioxidant activity by effectively scavenged free radical DPPH [14]. However, the activity of EEPS is lower compared to that of ascorbic acid (IC50 19.62 ± 0.19 μg/ml) in this study, as well as a previous study (13.68 μg/ml) [22]. Oxidative stress can initiate inflammatory signaling pathways via NF-κB, resulting in the synthesis of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 [23].
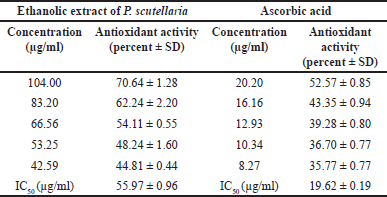 | Table 1. Free radical scavenging activity of ethanolic extract of P. scutellaria leaves and ascorbic acid assessed by DPPH assay at 517 nm (n = 3). [Click here to view] |
Anti-inflammatory activity
EEPS contains flavonoid and phenolic compounds with free radical scavenger potency as presented in Table 1. This study further investigates the anti-inflammatory activity of EEPS on Wistar rat hind paw oedema induced with carrageenan. Phenolic compounds such as flavonoids and terpenoids have been shown to inhibit the reactive oxygen species (ROS) produced upon carrageenan administration to induce inflammation [24,25]. Carrageenan can induce ROS formation, which triggers injury leading to acute inflammation [26]. The injection of carrageenan (0.1 ml, 1% w/v) into a rat hind paw leads to a steady increase in swelling that peaks 180 minutes after injection. This is then followed by a gradual decrease in oedema for the subsequent 360 minutes [27]. This pattern was also seen in this study. The increase in oedema volume was observed up to 180 minutes, then gradually decreased. As expected, Figure 1 revealed that sodium diclofenac possessed the strongest anti-inflammatory activity among the groups. Although sodium diclofenac treatment demonstrated the highest anti-inflammatory activity, the anti-inflammatory activity was not significant compared to the EEPS 400 mg/kg. The order of the anti-inflammatory activity from weakest to strongest based on the oedema volume were: sodium diclofenac (3.04 ± 1.045) < EEPS 400 (2.94 ± 0.62) < EEPS 200 (3.59 ± 0.72) < EEPS 100 (4.38 ± 0.29) < solvent group (4.09 ± 0.82). Oedema was significantly decreased in all EEPS-treated groups as well as the sodium diclofenac group compared to the oedema control group (p < 0.05).
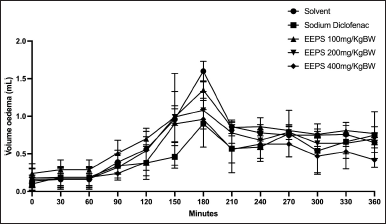 | Figure 1. The effects of ethanolic extract of P. scutellaria leaves on the volume of carrageenan-induced rat paw oedema. Data are presented as mean ± SD (n = 5). *Statistically significant (p < 0.05) difference when compared to the solvent group. EEPS, ethanolic extract of P. scutellaria leaves; SD, standard deviation. [Click here to view] |
The percentages of anti-inflammatory activity of EEPS after 360 minutes of carrageenan injection were presented in Figure 2. The anti-inflammatory activity for each treatment was as follows: solvent: 0%, sodium diclofenac: 30.57%, EEPS 100: 0%, EEPS 200: 12.09%, and EEPS 400: 28.10%. Statistical analysis showed that although there was a decrease in paw oedema in rats treated with EEPS, only EEPS at 400 mg/kg showed significant inhibition of inflammation compared to the solvent group (p < 0.05). Interestingly, there was no significant difference in inflammatory inhibition in rats treated with EEPS 400 mg/kg (28.1%) and sodium diclofenac 13.65 mg/kg (30.57%; p > 0.05), indicating that the anti-inflammatory effects of EEPS 400 mg/kg are roughly equivalent to those of sodium diclofenac 13.65 mg/kg.
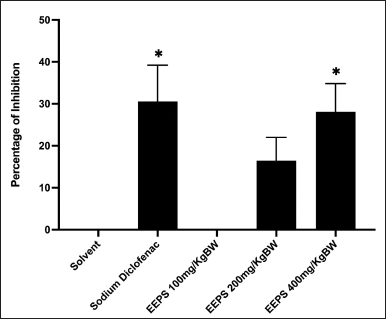 | Figure 2. The percentages of anti-inflammatory effect of ethanolic extract of P. scutellaria leaves and sodium diclofenac on carrageenan-induced paw oedema in rats (n = 5). Data are presented as mean ± SD. *Statically significant (p < 0.05) difference when compared with the solvent group. EEPS, ethanolic extract of P. scutellaria leaves; SD, standard deviation. [Click here to view] |
Cytokines level measurement
Cytokines are inflammatory modulators that play a vital role in both acute and chronic inflammation. Pro-inflammatory mediators (IL-1β, IL-6, and TNF-α) are crucial in beginning and enhancing immune responses after infection or tissue damage [28]. In this study, pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, play a crucial role in carrageenan-induced inflammation [29,30]. The results of our analysis of the amounts of TNF-α, IL-1β, and IL-6 cytokines in the untreated group, solvent group, sodium diclofenac group, and EEPS groups are displayed in (Fig. 3). TNF-α is a potent pro-inflammatory cytokine, influences immunity, inflammation, differentiation, and apoptosis [31,32]. It also significantly contributed to the pathophysiology of inflammatory-related diseases, such as Crohn’s disease, ulcerative colitis, and rheumatoid arthritis. Over the past decade, TNF-α is a promising therapeutic target for treating inflammatory diseases [33]. In this study, we found that the rats treated with sodium diclofenac, EEPS at the doses of 100, 200, and 400 mg/kg significantly decreased the level of TNF-α compared to the rats treated with solvent (Fig. 3). This indicated that EEPS exerted anti-inflammatory activity by suppressing TNF-α level. Considering the pivotal role of TNF-α in inflammation [34], EEPS served a potential source for the discovery of anti-inflammatory agents derived from natural products. TNF-α production resulted in a decrease in NO [35,36].
The effect of EEPS on IL-1β levels is presented in Figure 3B. The rats treated with sodium diclofenac and EEPS 400 mg/kg showed a significant reduction of IL-1β level compared to the solvent group (p < 0.05). Interestingly, EEPS at the lower dose (100 mg/kg) failed to reduced the level of IL-1β, but it increased the IL-1β compared to the solvent group. This might be due to the hormesis phenomenon [37]. This refers to the concept that low doses of a substance might have a beneficial or stimulatory effect, while higher doses can be suppressive. At low dose, EEPS might activate pathways that promote IL-1β production, stimulating an immune response. At higher dose (400 mg/kg), however, EEPS might overwhelm the immune system, leading to an inhibitory effect that reduces IL-1β production. Another possibility is that due to the feedback mechanism [38]. At low dose of EEPS, the extract enhances IL-1β production. However, at the higher dose, negative feedback loops could be activated, reducing IL-1β synthesis to prevent overactivation of the immune response. This suggests that EEPS was only effective as IL-1β suppressant at a higher dosage (400 mg/kg). Tissue injury can damage cell membranes, resulting in an accumulation of inflammatory cytokines. The injection of carrageenan significantly triggered the production of inflammatory cytokines, including IL-1 β. IL-1 β is an important mediator of inflammation in the early stage [39,40]. In the medium and late stage of inflammation, IL-1 β can contribute to the development of systemic inflammation and organ dysfunction [41].
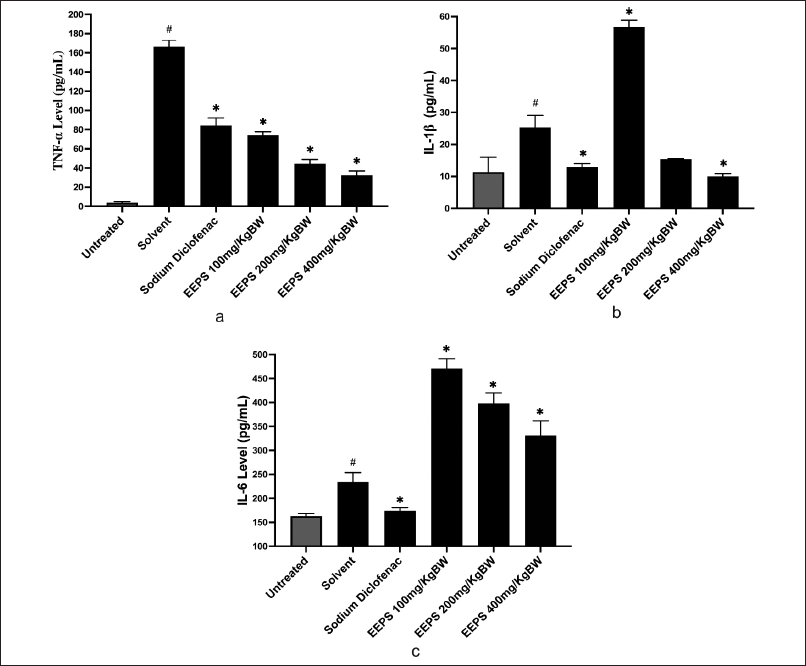 | Figure 3. The effects of ethanolic extract of P. scutellaria leaves on TNF-α, IL-1β, IL-6, and (pg/ml) levels in a rat paw oedema model. Data are presented as mean ± SD. *Statistically significant (p < 0.05) difference when compared with the solvent group. #versus the untreated group. a) TNF-α; b) IL-1β; c) IL-6. [Click here to view] |
Regarding the effect of EEPS on the production of IL-6 (Fig. 3), we could confirm that the EEPS failed to inhibit the expression of IL-6 at 100, 200, and 400 mg/kg. In contrast, EEPS tended to increase the expression of IL-6 at dose dependent manner. This might be due to the presence of various compounds in EEPS that potentially induced the expression of IL-6, such as phenylalanine that have been identified in EEPS by LC-HRMS. Besides exerted anti-inflammatory activity by targeting cytokines [42,43], phenylalanine was able to induce the expression of pro-inflammatory cytokines, including IL-6 [44]. Further mechanistic study to investigate the effect of chemical constituents of EEPS on IL-6 is required to clarify this effect. The reference drug, sodium diclofenac consistently inhibited the expression of all cytokines in this experiment.
The ability of EEPS to reduce the expression of pro-inflammatory cytokines suggests its therapeutic potential in the management of both acute and chronic inflammatory conditions [45–49]. The compounds responsible for the anti-inflammatory activity of EEPS are still unknown. Therefore, we performed LC-HRMS analysis to determine the metabolite constituents of EEPS [50].
Phytochemical analysis using LC-HRMS
Analysis of the plant metabolites found in EEPS in positive and negative ionization modes yielded 10 compounds from three updated online databases: Predicted Compositions, mzCloud Search, and ChemSpider Search. Filtering of the detected metabolite compounds on each database based on name and presence relative abundance yielded the chromatograms and metabolite compounds presented in Figure 4 and Table 2. Some of the detected compounds exert antioxidant effects by inhibiting ROS and act as anti-inflammatory agents by inhibiting various receptors or mediators in inflammation. When ROS is expressed, it activates inflammatory receptors and mediators [51,52]. Activated ROS induces the formation of IκB kinase, thereby triggering the transcription of NF-κB, leading to increased synthesis of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [53]. Of the ten major compounds detected in EEPS by LC-HRMS, two compounds have been reported to have antioxidant or anti-inflammatory effects in previous studies. These compounds are ursolic acid [54] and chlorogenic acid (Fig. 5) [55,56].
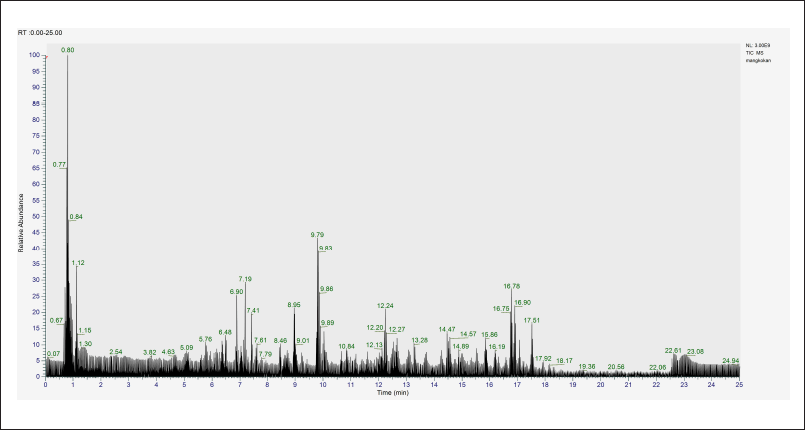 | Figure 4. Total ion chromatogram of ethanolic extract of P. scutellaria leaves analyzed using LC-HRMS with a retention time of 0.00–25.00 minutes. Only peaks with relative abundance more than 1% were selected. LC-HRMS, liquid chromatography-orbitrap high-resolution mass spectrometry. [Click here to view] |
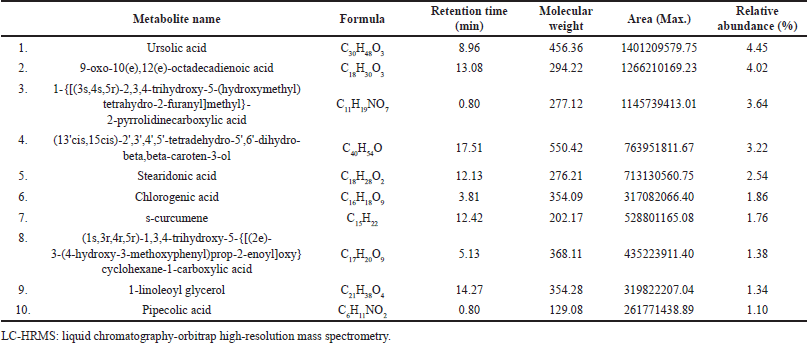 | Table 2. The phytochemical constituents of ethanolic extract of P. scutellaria leaves detected by LC-HRMS. [Click here to view] |
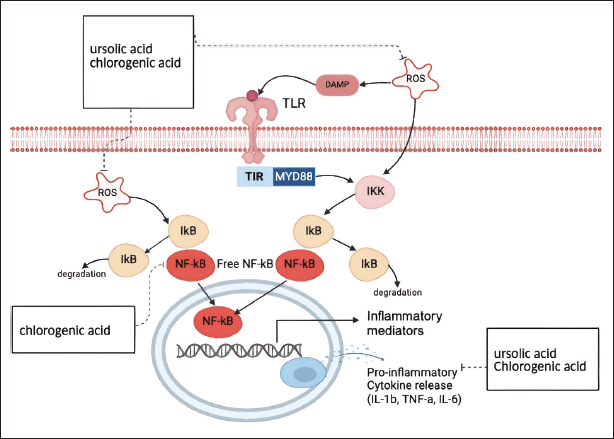 | Figure 5. Secondary metabolites identified in ethanolic extract of P. scutellaria leaves and their mechanisms of action. The identification of the secondary metabolites was done using LC-HRMS. The mechanism of action of the compound is predicted using published literatures. Created with BioRender.com. [Click here to view] |
CONCLUSION
EEPS demonstrated anti-inflammatory activity in carrageenan-induced rat hind paw oedema at doses of 400 mg/kg. The anti-inflammatory mechanism of the extract involves the inhibition of pro-inflammatory cytokines TNF-α and IL-1β. Phytochemical analysis using LC-HRMS revealed the presence of several promising compounds, including ursolic acid and chlorogenic acid. This finding highlights the need for further exploration of the active compounds responsible for the anti-inflammatory activity from P. scutellaria.
ACKNOWLEDGMENTS
The authors would like to thank the Center for Higher Education Funding (BPPT) and Indonesia Endowment Fund for Education (LPDP) for their financial support to the first author through a Doctoral Scholarship Scheme (00751/J5.2.3/BPI.06/9/2022).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The protocol of this study was approved by the Institutional Ethics Committee of LPPT, Universitas Gadjah Mada, Yogyakarta, Indonesia (approval number: 00045/04/LPPT/IX/2023).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bagad AS, Joseph JA, Bhaskaran N, Agarwal A.. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv Pharmacol Sci 2013;2013:1–7. CrossRef
2. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010;140(6):771–776. CrossRef
3. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 2007;147(2):227–235. CrossRef
4. Nathan C, Ding A. Nonresolving inflammation. Cell 2010;140(6):871–882. CrossRef
5. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140(6):805–820. CrossRef
6. Chertov O, Yang D, Howard OMZ, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev 2000;177(1):68–78. CrossRef
7. Zhou S, Huang G. The inhibitory activity of natural products to xanthine oxidase. Chem Biodivers 2023;20(5):e202300005. CrossRef
8. Nasution SLR, Awanis, Elsafarindo S. Effect of mangkokan (Polyscias scutellaria) leaf extract on blood sugar levels in alloxan-induced male white rats. Maj Kedokt Bdg. 2021;53(3):132–7. CrossRef
9. Paphassarang S, Raynaud J, Lussignol M, Becchi M. Triterpenic glycosides from Polyscias scutellaria. Phytochemistry 1989;28(5):1539–41. CrossRef
10. Esseh K, Afanyibo YG, Ahama-Esseh KY, Idoh K, Koudouvo K, Agbonon A, et al. Screening phytochimique, etude toxicologique, evaluation des activités antiplasmodiale et antiradicalaire de la tige feuillée de senna occidentalis linn (Fabaceae). Eur Sci J ESJ 2019;15(6):411. CrossRef
11. Saleem A, Saleem M, Akhtar MF. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: an ethnomedicinal plant of Moringaceae family. South Afr J Bot. 2020;128:246–56. CrossRef
12. Nur S , Mus S, Fadri A, Marwati M, Nursamsiar N, Sami FJ, et al. Determination of total phenolic and flavonoid levels of Mangkokan leaf extract (Polyscias scutellaria). J Pharm Med Sci. 2020;5.
13. Muhar AM, Velaro AJ, Prananda AT, Nugraha SE, Çamlik G, Wasnik S, et al. Polyscias scutellaria: an emerging source of natural antioxidants and anti-inflammatory compounds for health. Pharmacia. 2023;70(4):1463–70. CrossRef
14. Islam MA, Zilani MNH, Biswas P, Khan DA, Rahman MH, Nahid R, et al. Evaluation of in vitro and in silico anti-inflammatory potential of some selected medicinal plants of Bangladesh against cyclooxygenase-II enzyme. J Ethnopharmacol. 2022;285:1–14. CrossRef
15. Wulandari W, Jamarun N, Wellia DV, Emriadi E. Green precipitation method using Polyscias scutellaria extract in the synthesis of hydroxyapatite/alginate/copper (II) oxide composites as a drug carrier. J Appl Pharm Sci. 2024;14(6):147–53. CrossRef
16. Suharsanti R, Wahyuono S, Astuti P, Yuniarti N. Isolation and characterization of curcumenotone, a sesquiterpene from Curcuma aeruginosa roxb as antioxidant. Indones J Pharm. 2023;34(4):593–602. CrossRef
17. Triastuti A, Pradana DA, Setiawan ID, Fakhrudin N, Himmi SK, Widyarini S, et al. In vivo anti-inflammatory activities of Plantago major extract and fractions and analysis of their phytochemical components using a high-resolution mass spectrometry. Res Pharm Sci. 2022;17(6):665–76. CrossRef
18. Waheed I, Ahmad M, Syed NH, Ashraf R. Investigation of phytochemical and antioxidant properties of methanol extract and fractions of ballota limbata (Lamiaceae). Indian J Pharm Sci. 2014;76(3):251–6.
19. Grazul M, Budzisz E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev. 2009;253(21–22):2588–98. CrossRef
20. Kasprzak MM, Erxleben A, Ochocki J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015;5(57):45853–77. CrossRef
21. Mucha P, Skoczy?ska A, Ma?ecka M, Hikisz P, Budzisz E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 2021;26(16):4886. CrossRef
22. Al Owaisi M, Al Hadiwi N, Khan SA. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac J Trop Biomed. 2014;4(12):964–70. CrossRef
23. Osorio JS. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J Dairy Sci. 2014;97(12):7437–50. CrossRef
24. Heldin CH, Lu B, Evans R, Gutkind JS. Signals and receptors. Cold Spring Harb Perspect Biol. 2016;8(4):a005900. CrossRef
25. Prakash V. Terpenoids as source of anti-inflammatory compounds. Asian J Pharm Clin Res. 2017;10(3):68. CrossRef
26. Raker VK, Becker C, Steinbrink K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front Immunol. 2016;7:123. CrossRef
27. Purnomo Y, Tilaqza A. Analgesic and anti-inflammatory activities of Urena lobata L. leaf extracts. Indones J Pharm. 2022;33:566–74. CrossRef
28. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6(36):1–22. CrossRef
29. Lopes AH, Silva RL, Fonseca MD, Gomes FI, Maganin AG, Ribeiro LS, et al. Molecular basis of carrageenan-induced cytokines production in macrophages. Cell Commun Signal. 2020;18(1):141. CrossRef
30. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta BBA - Mol Cell Res. 2014;1843(11):2563–82. CrossRef
31. Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24(6):1297–305. CrossRef
32. Bradley J. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60. CrossRef
33. Khoury T, Ilan Y. Introducing patterns of variability for overcoming compensatory adaptation of the immune system to immunomodulatory agents: a novel method for improving clinical response to anti-TNF therapies. Front Immunol. 2019;10:2726. CrossRef
34. Jang D, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021;22(5):2719. CrossRef
35. Barale C, Russo I. Influence of cardiometabolic risk factors on platelet function. Int J Mol Sci. 2020;21(2):623. CrossRef
36. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. CrossRef
37. Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. CrossRef
38. Gabryšová L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10–secreting Th1 cells. J Exp Med. 2009;206(8):1755–67. CrossRef
39. Wen H, Ting JP, O’Neill LAJ. A role for the NLRP3 inflammasome in metabolic diseases—did warburg miss inflammation? Nat Immunol. 2012;13(4):352–7. CrossRef
40. Li H, Qian F, Liu H, Zhang Z. Elevated uric acid levels promote vascular smooth muscle cells (VSMC) proliferation via an nod-like receptor protein 3 (NLRP3)-inflammasome-dependent mechanism. Med Sci Monit. 2019;25:8457–64. CrossRef
41. Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274(1):330–53. CrossRef
42. Yi C, Liang H, Huang D, Yu H, Xue C, Gu J, et al. Phenylalanine plays important roles in regulating the capacity of intestinal immunity, antioxidants and apoptosis in largemouth bass (Micropterus salmoides). Animals. 2023;13(18):2980. CrossRef
43. Zhang Q, Chen S, Guo Y, He F, Fu J, Ren W. Phenylalanine diminishes M1 macrophage inflammation. Sci China Life Sci. 2023;66(12):2862–76. CrossRef
44. Tang Y, Yu Y, Li R, Tao Z, Zhang L, Wang X, et al. Phenylalanine promotes alveolar macrophage pyroptosis via the activation of CaSR in ARDS. Front Immunol. 2023;14:1114129. CrossRef
45. Darabi P, Khazali H, Mehrabani Natanzi M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol Endocrinol. 2020;36(7):582–7. CrossRef
46. Guazelli CFS, Fattori V, Ferraz CR, Borghi SM, Casagrande R, Baracat MM, et al. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem Biol Interact. 2021;333:109315. CrossRef
47. He X, Li W, Xie Y, Zhao Y. Long-term inhibition of dipeptidyl-peptidase 4 reduces islet infiltration and downregulates IL-1β and IL-12 in NOD mice. Int Immunopharmacol. 2020;88:106945. CrossRef
48. Stringham NT, Holmes PV, Stringham JM. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol Behav. 2019;211:112650. CrossRef
49. Taherkhani S, Suzuki K, Castell L. A Short overview of changes in inflammatory cytokines and oxidative stress in response to physical activity and antioxidant supplementation. Antioxidants. 2020;9(9):886. CrossRef
50. Windarsih A, Suratno, Warmiko HD, Indrianingsih AW, Rohman A, Ulumuddin YI. Untargeted metabolomics and proteomics approach using liquid chromatography-Orbitrap high resolution mass spectrometry to detect pork adulteration in Pangasius hypopthalmus meat. Food Chem. 2022;386:132856. CrossRef
51. Singh A, Yau YF, Leung KS, El-Nezami H, Lee JC. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants. 2020;9(8):669. CrossRef
52. Xu Y, Chen F. Antioxidant, anti-inflammatory and anti-apoptotic activities of Nesfatin-1: a review. J Inflamm Res. 2020;Volume 13:607–17. CrossRef
53. Armutcu F, Akyol S, Ustunsoy S, Turan FF. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp Ther Med. 2015;9(5):1582–8. CrossRef
54. Zhao M, Wu F, Tang Z, Yang X, Liu Y, Wang F, et al. Anti-inflammatory and antioxidant activity of ursolic acid: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1256946. CrossRef
55. La Rosa G, Sozio C, Pipicelli L, Raia M, Palmiero A, Santillo M, et al. Antioxidant, anti-inflammatory and pro-differentiative effects of chlorogenic acid on M03-13 human oligodendrocyte-like cells. Int J Mol Sci. 2023;24(23):16731. CrossRef
56. Ontawong A, Duangjai A, Vaddhanaphuti CS, Amornlerdpison D, Pengnet S, Kamkaew N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon. 2023;9(3):e13917. CrossRef