INTRODUCTION
Cancer is a significant societal, public health, and economic challenge in the twenty-first century, responsible for approximately one in six deaths (16.8%) and one in four fatalities from non-communicable diseases (NCDs) (22.8%) worldwide. The disease constitutes 30.3% of global premature fatalities from NCDs among individuals aged 30–69 years, ranking as one of the three leading causes of death in 177 out of 183 nations. Oral cancer is the predominant malignancy in India, with a 10.4% rise in incidence and a 9.3% overall mortality rate across both genders. It ranks as the sixth most prevalent kind of cancer worldwide [1]. Surgical resection remains a crucial element of cancer therapy; however, a significant challenge is the elevated risk of cancer recurrence in numerous patients, often occurring within a few timeframe [2].
Recent research has demonstrated that medicinal plants may act on several targets within signal transduction pathways, potentially modulating gene expression, cell cycle progression, cellular proliferation, and/or apoptosis to delay the onset of certain diseases [3,4] and cancer.
Anacardium occidentale (Anacardiaceae) can be found throughout the world’s tropical climates. The true fruit of A. occidentale grows on the edge of a pseudo-fruit known as the “cashew,” and the chestnut generates cashew nut shell liquid (CNSL). CNSL is a natural source of meta-alkyl phenols. These consist of combinations of saturated and unsaturated side chains [5,6]. Cardanol, a principal constituent of CNSL, is recognized for its diverse applications, including in polymer industries, paints and varnishes, laminating resins, rubber compounding resins, epoxy resins, and as a chemical intermediary [7–9]. Cardanol was initially studied in 1987 for its anticancer efficacy against Sarcoma 180 ascites. It was extracted from the sarcotesta of Ginkgo biloba L. and evaluated via the total packed cell volume technique in mice, exhibiting significant efficacy at a dosage of 40 mg/kg [10].
Moreover, the 1,4-disubstituted triazole moiety has been associated with diverse biological activities, including the inhibition of the Bel-7402 hepatoma carcinoma cell line [11], efficacy against MCF-7, HeLa, and HEK293 cells [12], inhibition of the HDAC6 pancreatic cancer cell line [13], and cytotoxicity towards the A-549 human lung squamous carcinoma cell line [14]. Cardanol, a phenolic compound derived from the cashew tree (Anacardiaceae), has been associated with several biological effects, including antiproliferative, antibacterial, and antioxidant activities [15,16]. The specific molecular mechanism of action remains unclear.
Network pharmacology, an innovative methodology in drug discovery, offers a promising way of addressing the complexities of cancer. Conventional methods often inadequately address the intricate networks that drive cancer proliferation. Network pharmacology, integrating computational biology and network analysis, facilitates the precise targeting of dysregulated signaling pathways in cancer. This method seeks to identify optimal therapeutic targets and combinations for cancer growth inhibition by simulating drug-target interactions inside cellular networks. It has the capacity to surmount drug resistance and mitigate harmful effects by targeting the disease at the systemic level through synergistic interactions. Computational models and algorithms are essential in optimizing potential drug combinations, facilitating the advancement of effective multi-target cancer therapy [17,18]. Consequently, this study employed in silico network pharmacology to identify the principal gene targets and cellular signaling pathways influenced by Cardanol in Oral cancer, thereby establishing a foundation for future therapeutic developments.
METHODOLOGY
Drug?likeness (DL) prediction of cardanol
The DL of Cardanol was assessed using Lipinski’s Rule of Five (RO5) to ascertain its suitability as an oral medication for humans. A multitude of parameters was examined. The SMILES format of cardanol, C=CCC=CCC=CCCCCCCCC1=CC(=CC=C1)O, was submitted to the SwissADME server (http://www.swissadme.ch), an online resource for assessing parameters including absorption, distribution, metabolism, excretion, oral bioavailability, and DL [19].
Target proteins of cardanol and potential targets in oral cancer
The “Swiss Target Prediction database (http://www.swisstargetprediction.ch/)” [20] was employed to ascertain the probable targets of Cardanol by predicting the related genes. The DisGeNet database was utilized to identify genes linked to oral cancer, which were subsequently compared to those associated with Cardanol. The overlapping genes were identified and chosen for more examination.
Gene ontology (GO) and pathway enrichment
The Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/, ver. 6.8) and the ShinyGO database (http://bioinformatics.sdstate.edu/) were utilized for GO and KEGG pathway enrichment analyses, respectively. DAVID serves as a tool for annotating and understanding gene lists, while ShinyGO is specifically designed for GO and pathway enrichment analysis. KEGG is an extensive database that provides graphical representations of biological pathways [21–24]. The GO database offers substantial functional genomics data, including definitions and classifications of gene functions [25]. Clinical and pathological data were utilized to meticulously identify pathways linked to oral cancer. Bubble charts and histograms were generated for data visualization and interpretation utilizing the Bioinformatics Cloud Platform (http://www.bioinformatics.com.cn/), an online resource focused on data processing and presentation [26].
Protein-protein interaction (PPI) analysis
PPI is essential to biological processes (BPs) for understanding the complex systems functioning within living cells [27]. A cluster of target genes was analyzed using the Search Tool for the Retrieval of Interacting Genes (STRING) database (http://string-db.org/; version 11.5) to delineate the PPI network. The analysis was restricted to “Homo sapiens” to guarantee high-quality data, with a confidence threshold exceeding 0.9. The resultant PPI network was constructed using Cytoscape (https://cytoscape.org/; version 3.10.1), a widely utilized bioinformatics application for data visualization and integration [28]. The MCODE plugin of Cytoscape was utilized to identify clusters or strongly interconnected areas within the PPI network. The cytoHubba plugin (https://apps.cytoscape.org/apps/cytohubba; version 0.1) was employed to identify central proteins, or Hub Genes, within the network.
Molecular docking assessment between hub genes and cardanol
Molecular docking was employed to examine the mechanisms and interactions between the putative proteins (hub genes) and Cardanol. This analysis aimed to ascertain the interactions between the hub targets and Cardanol. CB-Dock (http://cao.labshare.cn/cb-dock) was employed for molecular docking, which autonomously identifies active sites within a protein, computes their centers and dimensions, and modifies the grid box size according to the query ligands [29]. The target protein crystal structures were sourced from the Protein Data Bank (http://www.rcsb.org), while Cardanol’s three-dimensional structure was acquired from the PubChem chemical database (https://pubchem.ncbi.nlm.nih.gov/). The protein and ligand structures were subsequently imported into CB-Dock for docking analysis, enabling researchers to examine the binding activity of the proteins with Cardanol.
Gene expression levels and overall survival analysis of hub genes
This study utilized the University of Alabama at Birmingham CANcer data analysis (UALCAN) database (https://ualcan.path.uab.edu/analysis.html) to validate the differential expression of hub genes in oral cancer and normal oral tissues. UALCAN is an interactive online platform for analyzing cancer OMICS data, developed with PERL-CGI, JavaScript, and CSS to guarantee superior graphics quality. It provides instruments for identifying biomarkers and validating prospective genes. It facilitates the generation of graphs and plots for expression profiles and patient survival data across diverse gene types, in addition to evaluating epigenetic regulation through promoter methylation and pan-cancer gene expression research. Moreover, UALCAN facilitated the examination of these genes according to pathological stages, offering essential insights into their expression patterns across various disease stages [30]. Patients with oral cancer were categorized into two groups according to the expression levels of the hub genes: low and high. A Kaplan–Meier survival plot was generated to compare the survival rates of the two groups. Moreover, hazard ratios accompanied by 95% confidence intervals and log-rank p-values were computed for additional statistical significance assessment.
RESULTS
Molecular properties of cardanol
Our research indicates that Cardanol adheres to Lipinski’s RO5. Lipinski’s RO5 characterizes a drug-like compound as possessing a molecular weight under 500 g/mol, a polar surface area of 140 Ų or less, and a calculated octanol/water partition coefficient (XLogP3) below 5. These three elements are essential for optimal drug-ligand interactions. The molecular features of cardanol conform to the RO5 requirements, indicating it possesses advantageous drug-like qualities.
Target identification and analysis
We queried the DisGeNet database for targets associated with oral cancer utilizing the keyword “Lip and Oral Cavity Carcinoma.” This screening found 734 targets linked to oral cancer. Additionally, the Swiss Target Prediction database was utilized to identify Cardanol targets, resulting in the discovery of 100 targets. A comparison of the Cardanol targets and the targets linked to oral cancer identified a common collection of 23 genes in both datasets (Table 1).
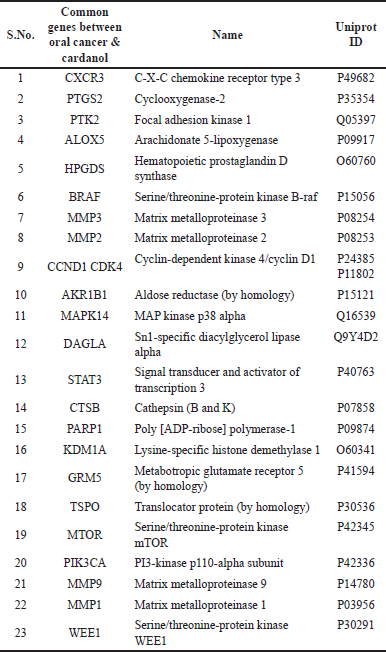 | Table 1. Number of 23 common genes present between oral cancer and cardanol with their details. [Click here to view] |
Development of PPI network and determination of key targets
The STRING Database was utilized to examine protein interactions among the specified targets. The PPI network was subsequently reanalyzed to identify modules utilizing MCODE in Cytoscape 3.10.2. The network of 23 common genes exhibited a p-value of less than 1.0e-16, signifying that these proteins interact more frequently than would be anticipated from a randomly chosen set of proteins from the genome with comparable size and degree distributions. Figure 2 illustrates that the proteins function as a biological unit. Utilizing Cytoscape’s MCODE clustering plugin, we identified clusters with a degree cut-off of 2, a node score cut-off of 0.2, a K-core of 2, and a maximum depth of 100. These were identified as linked to the negative regulation of the G1/S transition in the mitotic cell cycle, the positive control of cellular proliferation, the positive control of apoptosis, the positive regulation of cell migration, and the positive regulation of the PI3K signaling cascade (Fig. 1).
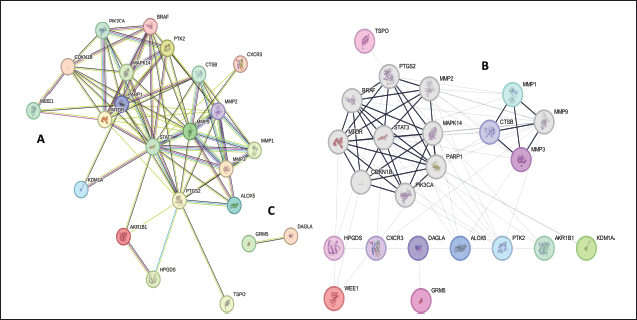 | Figure 1. PPI network of 23 common genes: (A) cluster of 23 common genes, (B) cluster 2 consisting of 21 genes, (C) cluster 2 consisting of 2 genes. MCODE clustering plugin from Cytoscape was used to identify the clusters where the max depth = 100, the degree cutoff = 2, the node score cutoff = 02, and the K-core = 2 were used as statistical parameters present in the Cytoscape. [Click here to view] |
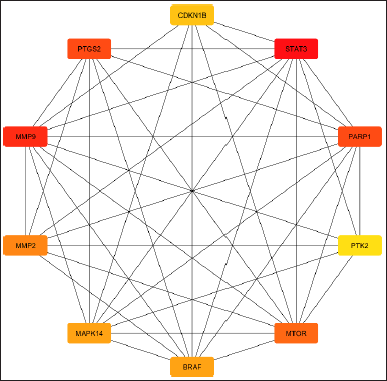 | Figure 2. Figure showing the top 10 hub genes from common 23 genes calculated by Cytohubba plugin in Cytoscape. [Click here to view] |
Top hub genes analysis
The study determined the top 10 hub genes by applying a variety of algorithms and in that degree, the algorithm was considered. These genes are as follows: BRAF, CDKN1B, STAT3, MMP9, PTGS2, PARP1, MTOR, MMP2, MAPK14, and PTK2. STAT3 was the most notably activated gene among these hub genes (Table 2, Fig. 2).
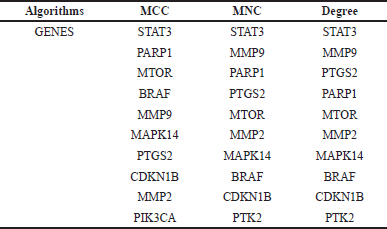 | Table 2. Various algorithms are used to determine the top 10 hub genes based on CytoHubba plugin in Cytoscape 3.10.2 software. [Click here to view] |
GO enrichment analysis
The GO enrichment analysis of the 23 hub genes identified around 98 GO terms. The research identified that these targets are associated with angiogenesis, collagen degradation, protein phosphorylation, negative control of apoptosis, and phosphorylation within BPs. The cytosol, nucleus, extracellular space, and mitochondria were incorporated into the cellular component (CC) analysis. The targets predominantly engage in serine/threonine protein kinase activity, ATP-binding, tyrosine-protein kinase activity, and enzyme binding (Fig. 3).
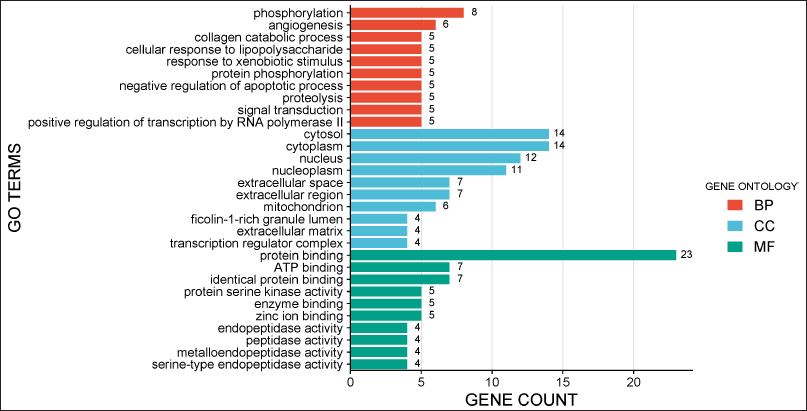 | Figure 3. GO enrichment analysis was obtained by taking 10 BPs, 10 molecular functions (MFs), and 10 CCs from DAVID database. The data are presented using SRplot tool considering a maximum number of genes involved in each process as a primary parameter. [Click here to view] |
KEGG enrichment analysis
The KEGG pathway analysis revealed 84 pathways. The top 27 paths from this group were selected for further investigation. The top 10 hub genes were demonstrated to be closely connected with a number of pathways, including the pathways in cancer, TNF and FoxO, proteoglycans in cancer, and the PI3K-Akt signaling pathway (Fig. 4). The KEGG pathway analysis identified 84 pathways. The foremost 27 pathways from this cohort were chosen for additional examination. The 10 principal hub genes were shown to be intricately associated with several pathways, including pathways in cancer, TNF and FoxO, proteoglycans in cancer, and the PI3K-Akt signaling pathway (Fig. 4). The genes MTOR, PIK3CA, CCDN1B, MMP9, STAT3, and PTGS2 were consistently implicated in the top 10 pathways. The genes exhibited a robust correlation with the PI3K-Akt signaling pathway, which governs various cellular processes associated with cancer formation, including protein synthesis, metabolic pathways, cell survival, proliferation, and gene expression [21,22,31]. The findings shown in Table 3 indicate that Cardanol may be effective in treating oral cancer, with the PI3K-Akt signaling pathway identified as a principal target.
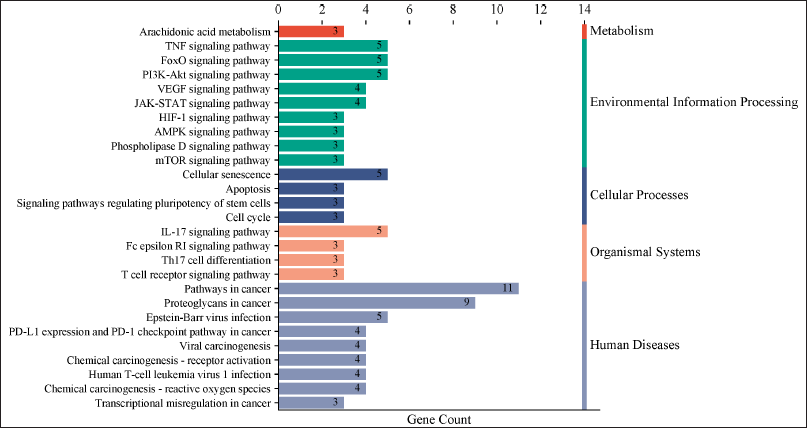 | Figure 4. An enrichment graph showing the top 27 signaling pathways linked to oral cancer. Gene count is represented by the X-axis, while various pathways that are classified into distinct BPs are represented by the Y-axis. [Click here to view] |
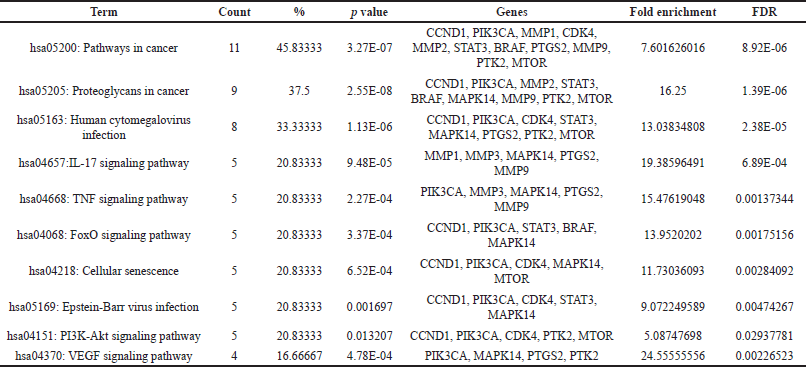 | Table 3. Top 10 pathways chosen based on the highest number of genes involved, including all significant details. [Click here to view] |
Confirmation of hub target by molecular docking
Ten hub genes underwent molecular docking studies to assess the validity of drug-target interactions. The docking capability of cardanol with STAT3, MMP9, CCDN1B, PIK3CA, PTGS2, MMP2, MAPK14, BRAF, PTK2, and MTOR was evaluated via CB-DOCK. A reduced energy value signifies an increased likelihood of interaction, as the ligand adopts a more stable shape following receptor contact. This study’s findings indicate that Cardanol exhibits significant binding affinity to the core targets, with binding energies between Cardanol and all core target proteins falling below −5.0. Table 4 presents the binding energies, whereas Figure 5 illustrates docking schematic representations that demonstrate the interaction between the target proteins and Cardanol.
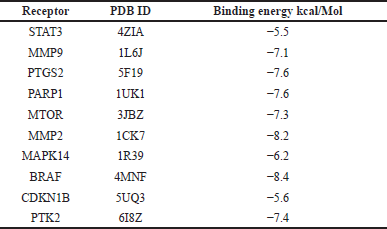 | Table 4. Molecular docking scores of cardanol and 10 hub target proteins determined by CBDock database are presented with details. [Click here to view] |
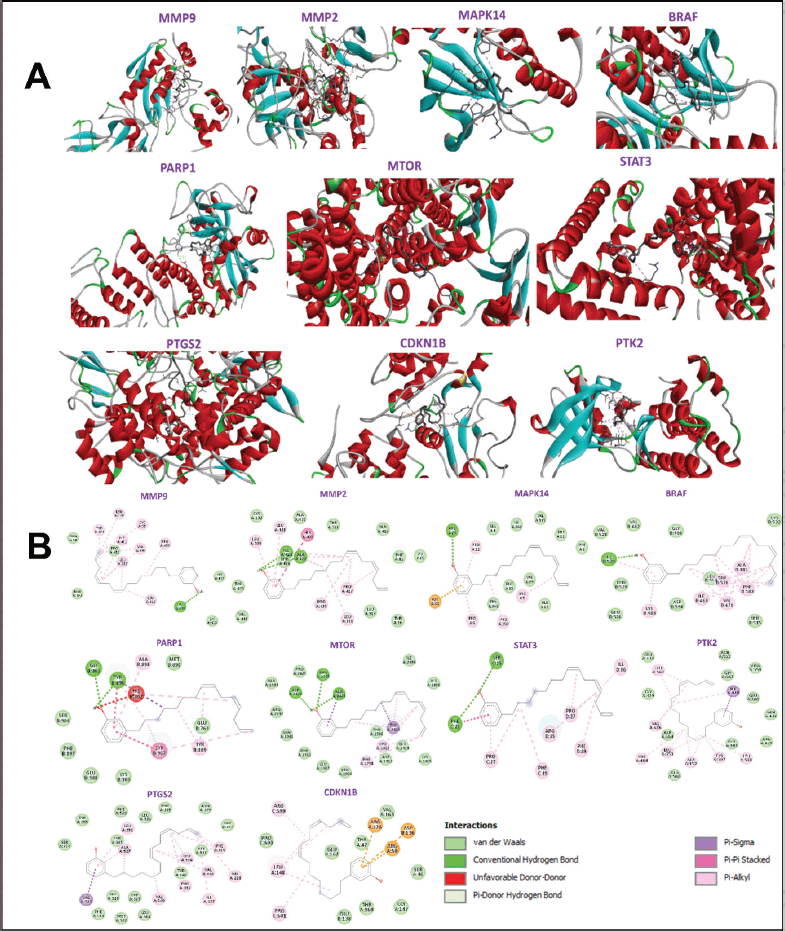 | Figure 5. Sketch and matching diagrams of molecular docking of cardanol and top 10 hub genes [target proteins]. (A) 3D presentation of interaction between cardanol and hub genes MMP9, MMP2, MAPK14, BRAF, PARP1, MTOR, STAT3, PTGS2, CDKN1B, and PTK2, (B) 2D presentation of interaction showing presence of various components like various amino acids and intermolecular bonds between cardanol and hub genes MMP9, MMP2, MAPK14, BRAF, PARP1, MTOR, STAT3, PTGS2, CDKN1B, and PTK2. [Click here to view] |
mRNA expression levels of hub genes
We examined the relationship between the pathological stages of oral cancer and the mRNA expression levels of the hub genes. The data indicated that PTGS2 and MMP9 were overexpressed in stages two and three. MAPK14 is present in stages 1 and 2, PARP1 in stages 2 and 4, MTOR in stages 1 and 3, and mp2 in stage 1. PTK2 expression was increased at every stage of the ovarian cancer process (Fig. 6). The results indicate that the expression levels of these genes may contribute to the progression of oral cancer.
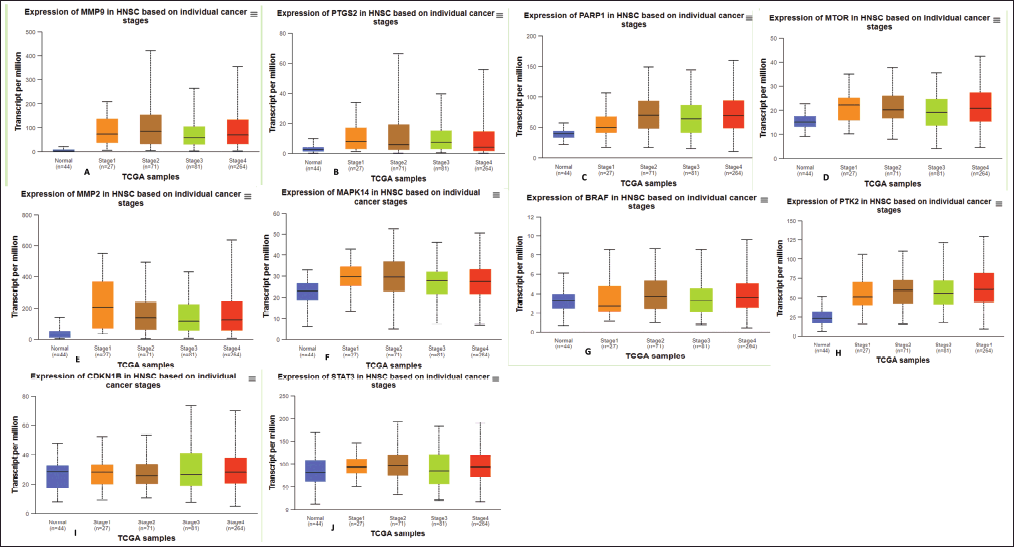 | Figure 6. The mRNA expression levels of hub genes in The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. Figure depicts mRNA expression levels of top 10 hub genes with statistically significant values in normal as well as stages of oral cancer (Box-Plot). (A) MMP9, (B) PTGS2, (C) PARP1, (D) MTOR, (E) MMP2, (F) MAPK14, (G) BRAF, (H) PTK2, (I) CDKN1B, and (J) STAT3. The UALCAN database was used for the presentation of this data. [Click here to view] |
Survival analysis of the hub genes
The ten hub genes—STAT3, MMP9, CCDN1B, PIK3CA, PTGS2, MMP2, MAPK14, BRAF, PTK2, and MTOR—underwent survival analysis. Approximately 495 patients with oral carcinoma were examined utilizing data from the TCGA database. All hub genes demonstrated a significant association (p < 0.05, Fig. 7) with poor prognosis, as indicated by the data.
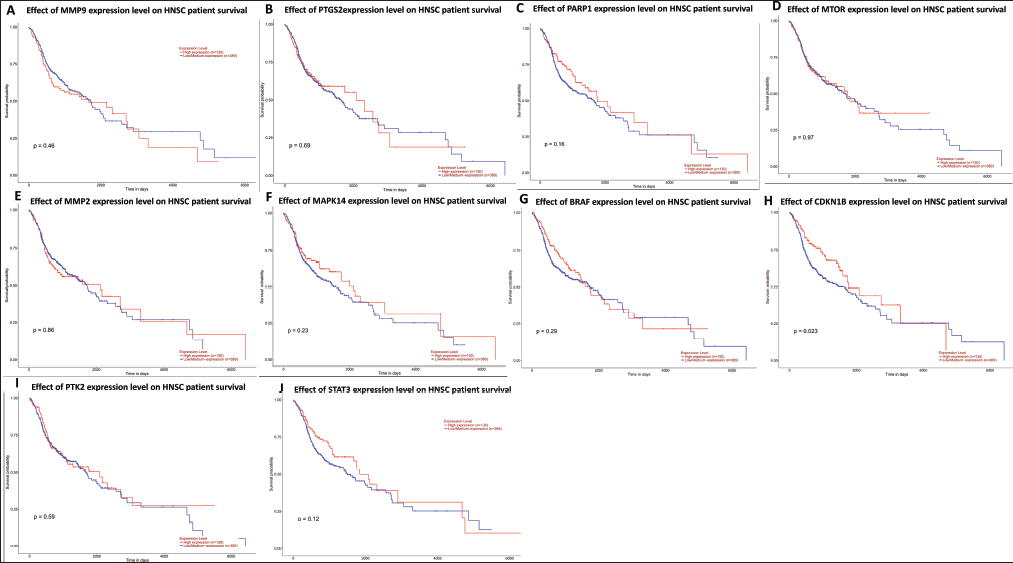 | Figure 7. Survival analyses of patients with oral cancer based on the expression of the 10 hub genes. (A) MMP9, (B) PTGS2, (C) PARP1, (D) MTOR, (E) MMP2, (F) MAPK14, (G) BRAF, (H) CDKN1B, (I) PTK2, and (J) STAT3. The UALCAN database was used for the presentation of this data. https://ualcan.path.uab.edu/cgi-bin/TCGA-survival1.pl?genenam=STAT3&ctype=HNSC). [Click here to view] |
DISCUSSION
Despite ongoing research and advancements in therapy, clinical outcomes and overall survival rates for HNSCC have not improved in recent decades, with a bleak 5-year survival rate of under 50% documented [32,33]. Due to the suboptimal clinical outcomes and elevated toxicity associated with existing head and neck cancer treatments, researchers are investigating innovative pharmaceuticals with reduced toxicity profiles. The objective is to enhance therapy results while reducing adverse drug effects. Recent years have witnessed a heightened interest in the exploration of complementary and alternative medicine as a legitimate option for cancer treatment.
This work employed an in-silico methodology to evaluate the anticancer properties of Cardanol. This investigation identified a set of 23 genes common to myricetin and oral cancer. These genes govern numerous BPs, encompassing the chemical carcinogenesis-ROS pathway, the PI3K-Akt signaling cascade, the Ras signaling pathway, cancer-related pathways, and microRNAs associated with cancer. AKT1, EGFR, and MET were recognized as pivotal genes within the top 10 hub genes. EGFR, MET, MMP2, AKT1, and MMP9 were consistently associated with all ten identified pathways. The EGFR signaling pathway, including AKT1, seems to be a pivotal mechanism for myricetin’s potential efficacy in oral cancer treatment. The mRNA expression levels of AKT1, MMP2, PARP1, MET, and CA9 were elevated in oral cancer tissues. Immunohistochemistry revealed a modest elevation in the protein expression of PARP1, CDK1, and MET in oral cancer tissues relative to normal oral tissues. The binding energies and schematic representations of AKT1 [−6.6] and MET [−7.6], derived from molecular docking data, indicated a substantial correlation and the activity of myricetin. This indicates that myricetin may engage in the PI3K-Akt pathway by influencing the AKT1 gene.
Teerasripreecha et al. [15], reported that cardanol and cardol present in propolis had significant in vitro antiproliferative and cytotoxic effects against the human colon cancer cell line SW620, resulting in necrosis. Additionally, Cardanol and Cardol have no detrimental effects in vitro on the normal Hs27 cell line. This is important since numerous current cancer therapies have harmful side effects when applied to healthy cells. N-ethyl-N-(2-methoxybenzyl)amine derivatives inhibit acetylcholinesterase (AChE) and demonstrate minimal cytotoxicity at concentrations up to 100 μM in HT-29 human colon cell lines [34].
Notwithstanding their various potential therapeutic uses, medicinal herbs may possess mutagenic, co-carcinogenic, and/or carcinogenic characteristics [35]. Foods abundant in antioxidants may aid in the prevention or treatment of diseases such as diabetes, arteriosclerosis, and cancer [36]. The issues primarily arise from errors or mutations in the metabolic process and are categorized as mutagenic compounds due to their capacity to compromise DNA integrity and elevate cancer risk. Consequently, damage to genetic material can be detected utilizing genotoxicity biomarkers, such as the micronucleus test and the comet assay [37].
Cardanol suppressed the proliferation of BT-474 cells in a time- and dose-dependent fashion, corroborating previous findings. The CEE of propolis from various regions in Korea reduced angiogenesis, including tube formation of human umbilical vein endothelial cells (HUVECs), in a dose-dependent manner (6.25–25 μg/ml). Moreover, in a dose-dependent manner (3.13–25.5 μg/ml), the CEE of propolis from the Uijeongbu and Pyoseon regions significantly inhibited HUVEC proliferation [38]. Given that Cardanol induces apoptosis. Cardanol is recognized in prior investigations as a significant antioxidant and antimutagenic compound [16, 39–41]. Cardanol and cardol elicited cytotoxicity and apoptosis in cancer cells without causing DNA damage, suggesting their potential as antiproliferative agents [15]. Neto et al. [42] conducted relevant research on the cytotoxicity of CNSL components and their derivatives in relation to oral squamous cell cancer. Cells were subjected to 25 ng/µl doses of both parental and derived compounds for durations of 24, 48, and 72 hours. Compound diluents ethanol and staurosporine (300 nM) served as positive and negative controls, respectively. The study’s findings unequivocally confirmed the cytotoxicity of CNSL and its potential application as a chemotherapeutic agent. The study focuses on a certain crucial gene, deliberately excluding a thorough investigation of the complete dataset for the time being. The authors intentionally concentrated on specific genes to simplify the analysis and maintain a reasonable scope. Although in silico methods offer robust tools for biological study, their limits highlight the need to integrate them with experimental techniques and to perpetually refine models to improve accuracy and relevance.
CONCLUSION
The research indicates that cardanol may be effective in preventing oral cancer by targeting several critical genes and pathways. Molecular docking techniques can validate positive interactions between hub genes and important targets. This methodology elucidates that in-silico analysis facilitates a comprehensive assessment across various aspects, hence enhancing our understanding of Cardanol’s mechanisms. It is essential to recognize that in-silico results are merely initial predictions that require further experimental validation to be deemed accurate and pertinent. A more prudent and equitable evaluation of the study’s results would highlight their exploratory character.
LIST OF ABBREVIATIONS
CDK, Cyclin dependent kinase; DAVID, Database for annotation, visualization, and integrated discovery; DisGeNet, Disease gene network; GA, Cardanol; GO, Gene ontology; KEGG, Kyoto encyclopaedia of genes and genome; MCC, Maximal clique centrality; MNC, Maximum neighbourhood component; OC, Oral cancer; PPI, Protein-protein interaction; ROS, Reactive oxygen species; STRING, Search tool for the retrieval of interacting genes/proteins; SwissADME, Swiss adsorption, distribution, metabolism, excretion; UALCAN, The University of ALabama at Birmingham CANcer.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was not supported by any sponsor or funder.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors, and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021 Aug;127(16):3029–30.
2. Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, et al. Surgical stress and cancer progression: the twisted tango. Mol Cancer. 2019 Sep;118(1):132.
3. Büchner FL, Bueno-de-Mesquita HB, Linseisen J, Boshuizen HC, Kiemeney LALM, Ros MM, et al. Fruits and vegetables consumption and the risk of histological subtypes of lung cancer in the European prospective investigation into cancer and nutrition (EPIC). Cancer Causes Control. 2010;21(3):357–71.
4. Soerjomataram I, Oomen D, Lemmens V, Oenema A, Benetou V, Trichopoulou A, et al. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur J Cancer. 2010;46(14):2563–80.
5. Ogunsina BS, Bamgboye AI. Pre-shelling parameters and conditions that influence the whole kernel out-turn of steam-boiled cashew nuts. J Saudi Soc Agric Sci. 2014 Jan;13(1):29–34.
6. Kubo I, Masuoka N, Ha TJ, Tsujimoto K. Antioxidant activity of anacardic acids. Food Chem. 2006;99(3):555–62.
7. Balachandran VS, Jadhav SR, Vemula PK, John G. Recent advances in cardanol chemistry in a nutshell: from a nut to nanomaterials. Chem Soc Rev. 2013;42(2):427–38.
8. Bastos FA, Tubino M. The use of the liquid from cashew nut shells as an antioxidant in biodiesel. J Braz Chem Soc. 2017;28(5):747–55.
9. Voirin C, Caillol S, Sadavarte NV, Tawade BV, Boutevin B, Wadgaonkar PP. Functionalization of cardanol: towards biobased polymers and additives. Polym Chem. 2014 Apr;5(9):3142–62.
10. Itokawa H, Totsuka N, Nakahara K, Takeya K, Lepoittevin JP, Asakawa Y. Antitumor principles from Ginkgo biloba L. Chem Pharm Bull (Tokyo). 1987;35(7):3016–20.
11. Zou Y, Zhao Q, Hu H, Hu L, Yu S, Xu M, et al. Synthesis and in vitro antitumor activities of xanthone derivatives containing 1,4-disubstituted-1,2,3-triazole moiety. Arch Pharm Res. 2012 Dec;35(12):2093–104.
12. Narsimha S, Satheesh Kumar N, Kumara Swamy B, Vasudeva Reddy N, Althaf Hussain SK, Srinivasa Rao M. Indole-2-carboxylic acid derived mono and bis 1,4-disubstituted 1,2,3-triazoles: synthesis, characterization and evaluation of anticancer, antibacterial, and DNA-cleavage activities. Bioorg Med Chem Lett. 2016;26(6):1639–44.
13. Chen Y, Lopez-Sanchez M, Savoy DN, Billadeau DD, Dow GS, Kozikowski AP. A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. J Med Chem. 2008 Jun;51(12):3437–48.
14. Bratsos I, Urankar D, Zangrando E, Genova-Kalou P, Košmrlj J, Alessio E, et al. 1-(2-Picolyl)-substituted 1,2,3-triazole as novel chelating ligand for the preparation of ruthenium complexes with potential anticancer activity. Dalton Trans. 2011 May;40(19):5188–99.
15. Teerasripreecha D, Phuwapraisirisan P, Puthong S, Kimura K, Okuyama M, Mori H, et al. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complement Altern Med. 2012 Mar;12:27.
16. Trevisan MTS, Pfundstein B, Haubner R, Würtele G, Spiegelhalder B, Bartsch H, et al. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem Toxicol. 2006 Feb;44(2):188–97.
17. Azmi AS. Adopting network pharmacology for cancer drug discovery. Curr Drug Discov Technol. 2013;10(2):95–105.
18. Tang J, Aittokallio T. Network pharmacology strategies toward multi-target anticancer therapies: from computational models to experimental design principles. Curr Pharm Des. 2014;20(1):23–36.
19. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017 Mar;7:42717.
20. Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019 Jul;47(W1):W357–3664.
21. Buva K, Kumbhar GM, Deshmukh A, Ladke VS. The assessment of the mechanism of action of lauric acid in the context of oral cancer through integrative approach combining network pharmacology and molecular docking technology. J Complement Integr Med. 2024 Mar;21(1):101–12.
22. Kumbhar GM, Jadhav AD, Kheur S, Ladke VS. Andrographolide demonstrates anti-proliferative activity in oral cancer by promoting apoptosis, the programmed cell death process. Iran J Basic Med Sci. 2024;27(10):1300–8.
23. Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021 Jan;49(D1):D545–51.
24. Ladke VS, Kumbhar GM, Joshi K, Kheur S. Systemic explanation of Glycyrrhiza glabra’s analyzed compounds and anti-cancer mechanism based on network pharmacology in oral cancer. J Oral Biosci. 2022;64(4):452–60.
25. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000 May;25(1):25–9.
26. Tang D, Chen M, Huang X, Zhang G, Zeng L, Zhang G, et al. SRplot: a free online platform for data visualization and graphing. PLoS One. 2023 Nov;18(11):e0294236. doi: https://doi.org/10.1371/JOURNAL.PONE.0294236
27. Lin JS, Lai EM. Protein–protein interactions: co-immunoprecipitation. Methods Mol Biol. 2017;1615:211–9.
28. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003 Nov;13(11):2498–504.
29. Liu Y, Grimm M, Dai W, Hou MC, Xiao ZX, Cao Y. CB-Dock: a web server for cavity detection-guided protein-ligand blind docking. Acta Pharmacol Sin. 2020 Jan;41(1):138–44.
30. Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022 Mar;25:18–27.
31. Ghafouri-Fard S, Noie Alamdari A, Noee Alamdari Y, Abak A, Hussen BM, Taheri M, et al. Role of PI3K/AKT pathway in squamous cell carcinoma with an especial focus on head and neck cancers. Cancer Cell Int. 2022 Aug;22(1):254.
32. Argiris A, Brockstein BE, Haraf DJ, Stenson KM, Mittal BB, Kies MS, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004 Mar;10(6):1956–62.
33. Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184–94.
34. Lemes LFN, De Andrade Ramos G, De Oliveira AS, Da Silva FMR, De Castro Couto G, Da Silva Boni M, et al. Cardanol-derived AChE inhibitors: towards the development of dual binding derivatives for Alzheimer’s disease. Eur J Med Chem. 2016 Jan;108:687–700.
35. Okoye FAN, Amaefule CC. Evaluation of the cytotoxicity and genotoxicity of aqueous leaf extracts of Azadirachta indica A. Juss using the Allium test. J Med Plants Res. 2012 Jun;6(22):3898–907.
36. de Sousa Leite A, Vieira Gomes DC, da Silva Oliveira GL, Correia Jardim Paz MF, Melo de Carvalho R, Oliveira Ferreira da Mata AM, et al. Net effects of cashew nuts in Saccharomyces cerevisiae front to damage induced by hydrogen peroxide. Int Arch Med. 2016;9(134):1–13.
37. Edziri H, Mastouri M, Aouni M, Anthonissen R, Verschaeve L. Investigation on the genotoxicity of extracts from Cleome amblyocarpa Barr. and Murb, an important Tunisian medicinal plant. South Afr J Bot. 2013;84:102–3.
38. Park S, Ohta T, Kumazawa S, Jun M, Ahn MR. Korean propolis suppresses angiogenesis through inhibition of tube formation and endothelial cell proliferation. Nat Prod Commun. 2014;9(4):555–60.
39. Melo Cavalcante AA, Rubensam G, Picada JN, Gomes da Silva EG, Fonseca Moreira JC, Henriques JAP. Mutagenicity, antioxidant potential, and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environ Mol Mutagen. 2003;41(5):360–9.
40. Rodrigues FHA, Feitosa JPA, Ricardo NMPS, De França FCF, Carioca JOB. Antioxidant activity of cashew nut shell liquid (CNSL) derivatives on the thermal oxidation of synthetic cis-1,4-polyisoprene. J Braz Chem Soc. 2006;17(2):265–71.
41. De Lima SG, Feitosa CM, Citó AM, Moita Neto JM, Lopes JA, Leite AS, et al. Effects of immature cashew nut-shell liquid (Anacardium occidentale) against oxidative damage in Saccharomyces cerevisiae and inhibition of acetylcholinesterase activity. Genet Mol Res. 2008;7(3):806–18.
42. Neto L, Matos N, Gonzaga W, Romeiro L, Santos M, Santos D, et al. Characterization of cytotoxic activity of compounds derived from anacardic acid, cardanol and cardol in oral squamous cell carcinoma. BMC Proc. 2014 Oct;8(Suppl 4):P30.