INTRODUCTION
The human digestive system primarily comprises the digestive tract, including the mouth, stomach, small intestine, large intestine, and other auxiliary organs such as the pancreas [1]. Among these, the stomach, pancreas, and small intestine are the most crucial digestive organs. These organs are responsible for digestion, nutrient absorption, excretion of metabolite products, serving as barriers to prevent pathogenic bacteria and toxins, and maintaining immune system balance [2,3]. Damage to these organs can lead to various diseases and even a fatal condition. To support digestive health and optimize its functions, functional foods may be consumed as a preventive measure.
Functional foods are defined as foods that offer potential and beneficial effects on health beyond their basic nutritional value [4]. These diets contain biologically active compounds that, in certain amounts, are effective and nontoxic [5]. Sea cucumbers have been used as a traditional food source with many health advantages for a long time. Sea cucumbers are a food source that is very rich in nutrients and bioactive compounds, making them a promising candidate for functional foods, in addition to other purposes, such as pharmaceuticals and cosmetics [6].
The sea cucumber Holothuria atra (black sea cucumber) is abundant in Indonesian waters. This species has shown many promising bioactivities, such as its ethanolic extract exhibited cytotoxicity against cancer cell lines [7]. Its anticancer activity is associated with saponins, including Holothurin A, Holothurin A5, Echinoside A, Echinocide B, and 24-dehydroechinoside A [8,9]. In addition to its anticancer properties, H. atra also demonstrated strong α-glucosidase inhibitory activity [10], immunostimulant effects [11], and hepatoprotective properties [12]. Furthermore, the fatty acid-rich extract of H. atra showed anti-hyperuricemic activity in rat models [13].
Considering the numerous potential of H. atra as a source of functional food, medicines, and cosmetic ingredients, it is essential to conduct toxicity studies of H. atra to ensure its safety. The acute toxicity of H. atra extract in mice was evaluated by Hashim et al. [14] and Hanafi et al. [15]. As part of our research on the bioprospecting of Indonesian tropical sea cucumbers, our previous findings showed that H. atra ethanolic extract was safe on the liver and kidneys of rats at doses up to 100-mg/kg BW[16]. Therefore, we continue our research on the subchronic toxicity of H. atra ethanolic extract on the digestion organs of the stomach, pancreas, and small intestine for 90 days.
MATERIALS AND METHODS
Preparation of ethanolic extract
Fresh sea cucumber of H. atra (local name: oler, cera hitam) was collected from the Halmahera Islands, North Maluku Province, Indonesia. Viscera-free samples were stored in an iced cool box and immediately were transported to the Biotechnology Laboratory of the Research Centre for Marine and Fisheries Product Processing and Biotechnology, Jakarta. All samples were preserved at −20°C for further research. Sample identification was conducted in the Research Center for Oceanography, Indonesian Institute of Science, Jakarta. Samples were macerated using 96% ethanol for 12 hours (three times). Ethanol was evaporated using a vacuum rotary evaporator (Buchi, Switzerland) until a concentrated extract was obtained. The extract was further dried with a freeze dryer (Christ, Germany) at a temperature ±43°C.
Experimental animals
In vivo study
The in vivo study protocol was conducted according to the Experimentation Ethics Committee on Animal Use of the Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia. The experimental animals were male Sprague-Dawley rats (Rattus norvegicus) obtained from The National Agency of Drug and Food Control of the Republic of Indonesia (BPOM RI). The in vivo study was carried out at the Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia.
Animal acclimatization
After 2 weeks of acclimatization, 18 rats weighing between 220 and 250 g were fed PureitTM ad libitum along with a standard rodent pellet diet. Before the commencement of the experiment, pre-treatment was carried out by administering antihelmintic drugs (Univerm Total®, dose 10-mg/kg BW), antibiotics (Azythromicin®, dose 10-mg/kg BW), and anti-protozoa (Flagyl®, dose 10-mg/kg BW). The pre-treatment was conducted for internal parasite examination using the McMaster egg per gram feces method [17]. The examination was conducted before and after the pre-treatment to ensure that no parasites were involved in the main experiment. Then, the rats were rested for 2 weeks before the treatment.
Subchronic toxicity study
To determine the doses of sea cucumber ethanolic extract to be administered, the animal’s body weight was measured prior to treatment. Rats were divided into six groups and each group consisted of three rats. These groups were the control group, which was given 1 ml of distilled water (group A), group B (dose 25-mg/kg body weight; BW), group C (dose 50-mg/kg BW), group D (dose 100-mg/kg BW), group E (200-mg/kg BW), and group F (dose 400-mg/kg BW). The extract was dissolved in distilled water. Each group of rats was given 1 ml of extract daily for 90 days using oral gavage. This subchronic toxicity test was guided by OECD-408 [18]. After 90 days, the rats were euthanized and necropsied to take the stomach, pancreas, and small intestine samples. Euthanasia was performed with 10% ketamine (dose 100-mg/kg BW) and 2% xylazine (10-mg/kg BW) by intraperitoneal injection. Organ samples were fixed in a 10% buffered neutral formalin (Sigma-Aldrich) solution and left to fix for 1 week in preparation for histopathological examination.
Histopathological analysis
Histopathological analysis was conducted according to Cheville [19]. The stomach, pancreas, and small intestine samples were cut with a thickness of 0.5 cm × 1 cm × 1 cm. Then, thin pieces of the organs were placed in a tissue cassette and put into an automatic tissue processor (Leica) overnight to be dehydrated with ethanol. The tissue was then clarified with xylol and infiltrated with liquid paraffin at 56°C for 30 minutes. The next stage was embedding the organs in liquid paraffin. Paraffin blocks were cut using a microtome (Thermo) with a thickness of 3–5 µm. A thin piece of tissue was floated on the water at a temperature of approximately 45°C for a few moments so that the paraffin stretched, and then, the piece of tissue was placed and flattened on a glass object and stored in an oven at 58°C overnight. The preparations were stained with hematoxylin–eosin (HE) staining.
Histopathological preparations of the stomach, pancreas, and small intestine were observed using a light microscope (Leica, Germany) and data were collected from micrographic photographs. Quantitative data collected were the number of erosion lesions in the mucosal layer of the gastric fundus and gastric gland cells, including parietal cells, chief cells, and mucus neck cells. The observed pancreatic organ was the number of acinar cells, including normal cells, degeneration, and necrosis as well as inflammatory cells (ICs) and the percentage of congestion in the pancreas. The observed parts of the duodenum small intestine were the height and width of the villi, the number of ICs, and crypt cells. The surface width of the villi was calculated using the following formula [20]:
Data analysis
The histopathological analysis calculation was carried out by using ImageJ software. The observations were statistically analyzed using the one-way ANOVA (SPSS 16.0 software), followed by Duncan’s multiple range test to determine significant differences at the 5% probability level.
RESULTS AND DISCUSSION
Gastric histopathology
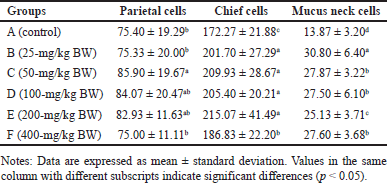
Anatomically, the stomach consists of the fundus, corpus, and pylorus, while histologically, it consists of the mucosa, gastric glands, and tunica muscularis. The gastric gland cells observed in the gastric mucosa include parietal cells, chief cells, and mucus neck cells. The average number of these cells after being fed with H. atra ethanolic extract is presented in Table 1. Microphotography of parietal cells (PCs), chief cells (CCs), and mucus neck cells in the rat stomachs fed with the extract is presented in figures 1 and 2.
 | Table 1. Average number of gastric gland cells of R. norvegicus rats fed with H. atra ethanolic extract for 90 days. [Click here to view] |
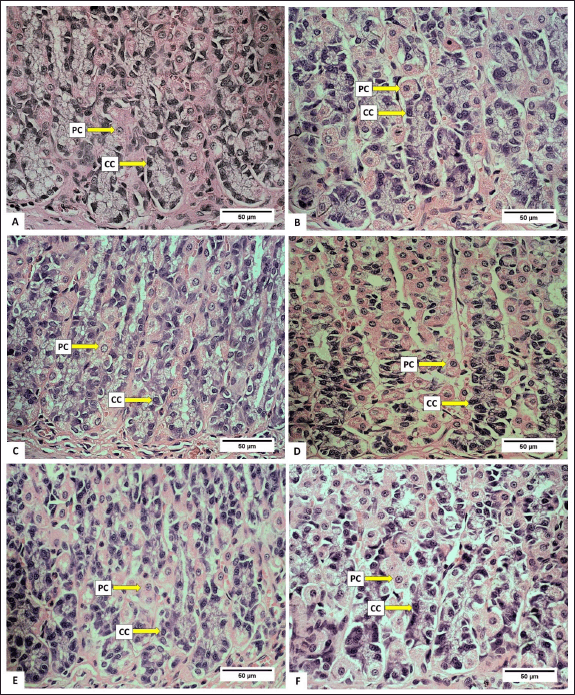 | Figure 1. Photomicrograph of parietal cells (PCs) and chief cells (CCs) in gastric R. norvegicus rats fed with H. atra ethanolic extract; (Staining: H&E, magnification: 40×, bar: 50 µm). Photomicrograph showed an overall increase in parietal cells and chief cells. A (distilled water as control), B (25-mg/kg BW, C (50-mg/kg BW), D (100-mg/kg BW), E (200-mg/kg BW), and F (400-mg/kg BW). [Click here to view] |
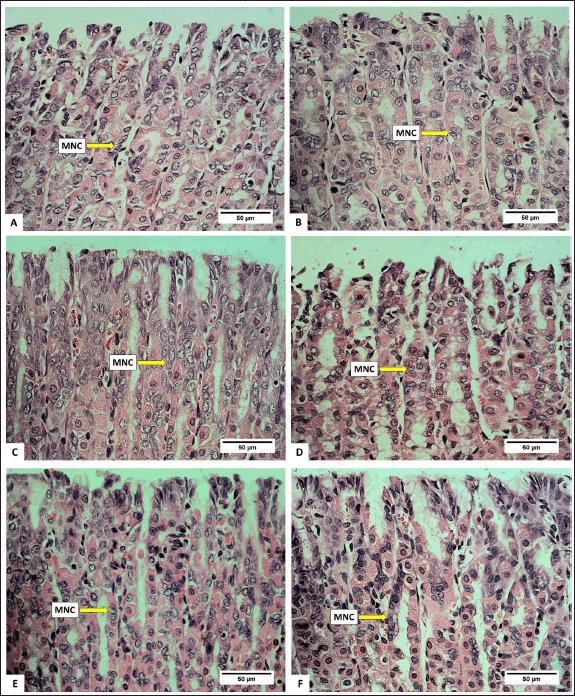 | Figure 2. Photomicrograph of mucus neck cell in gastric R. norvegicus rats fed with H. atra ethanolic extract; (Staining: H&E, magnification: 40×, bar: 50 µm). Photomicrograph showed an overall increase in parietal cells and chief cells. A (distilled water as control), B (25-mg/kg BW), C (50-mg/kg BW), D (100-mg/kg BW), E (200-mg/kg BW), and F (400-mg/kg BW). [Click here to view] |
The number of parietal cells tended to increase after exposed by the extract. The highest number of parietal cells was observed in a dose of 50-mg/kg BW (p < 0.05) (Table 1). The increase in the number of parietal cells indicated that the ethanolic extract of H. atra can be digested by the stomach. Parietal cells are located in the proximal region of mucous glands. It has a function to secrete hydrochloric acid (HCl) in the form of hydrogen and chloride ions to activate digestive enzymes [21]. The concentration of HCl in the stomach is very high, and in this case, HCl functions to denature proteins and increases the exposure of the protein’s peptide bonds to further digestive processes by enzymes [22]. Thus, an increase in the number of parietal cells enhances gastric acid secretion and maintains an acidic environment in the stomach [23].
The number of chief cells exposed by the extract was higher than the control group (p < 0.05). However, at a concentration of 400-mg/kg BW, we suspected that the extract was toxic since the number of chief cells appeared to decrease. Chief cell activity is related to parietal cell activity. Hydrochloric acid from parietal cells can induce pepsinogen secretion by chief cells [24]. According to Reece et al. [22], pepsinogen is converted into an active proteolytic enzyme, called pepsin, after interacting with HCl. Chief cells have a greater number than parietal cells in normal stomach conditions [25]. Based on these observations, the exposure of the extract positively affected the function of gastric gland cells by supporting the growth of parietal and chief cells.
Observation of the number of mucus neck cells showed that, in general, exposure of the extract increased the number of mucus neck cells (Table 1). Group B (25-mg/kg BW) had the highest number, and meanwhile, the number of mucus neck cells in group A (control) was the lowest compared to other groups (p < 0.05). Increased digestive activity due to the increase in the number of parietal and chief cells accompanied by the addition of mucus neck cells serves to maintain the balance and function of parietal cells. Mucus neck cells are mucus-producing cells whose function is to protect the stomach from acid, pepsin, pathogens, and ingesta substances by maintaining a more alkaline state [26]. According to Kang et al. [27], an increase in the number of mucus neck cells can be caused by an increase in the protective function of mucus against the stomach from acid, an injury due to bacterial infection or the host’s immune response, and an increase in progenitor cell proliferation, which supports the conversion of mucus neck cells into parietal cells. The exposure of the extract can provide a cytoprotective effect by stimulating the growth and healing of gastric mucosal wounds [28]. Wound healing is related to the high fatty acid content in sea cucumbers [29].
The feed of the extract at doses of 25-mg/kg BW and 50-mg/kg BW appeared to increase intact mucosa surfaces and reduce erosion/desquamation on the gastric mucosa (Table 2). The histopathological analysis of the gastric mucosa exposed by the extract at various doses is presented in figure 3. The surface of the gastric epithelium is sensitive and prone to detachment. Rough textures, such as food ones, can cause the epithelium to detach from the mucosa. However, the detachment of mature, outer epithelial cells has no significant impact. According to Chauhan [30], erosion is defined as the loss of the epithelial surface part. Erosive lesions can occur due to the death or detachment of epithelial cells at a rate that exceeds their regeneration. Erosion in the stomach of adult rats is commonly observed [31]. Erosive lesions are typically not dangerous unless accompanied by bleeding. Epithelial cell loss can be caused by luminal factors, e.g., stomach acid, chemical induction, and disease, as well as physical or mechanical factors [32].
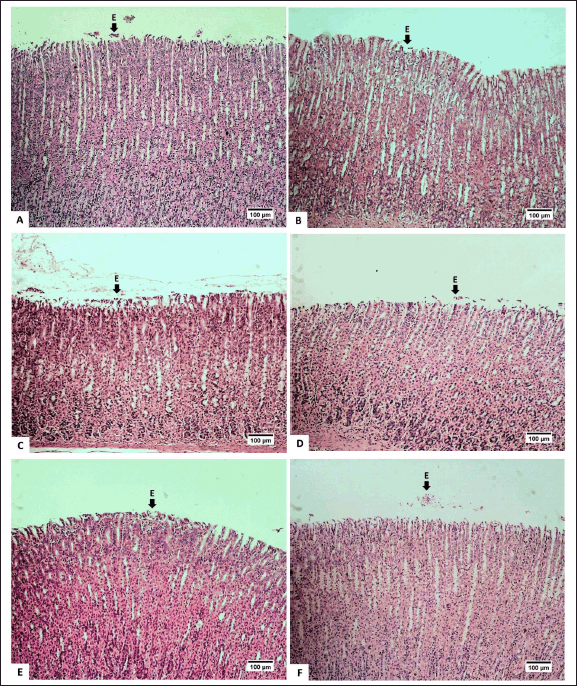 | Figure 3. Photomicrograph of gastric mucosa of R. norvegicus rats fed with H. atra ethanolic extract; (Staining: H&E, magnification: 10×, bar: 100 µm). The desquamation/erosion lesion (E) was indicated by the arrow. A (distilled water as control), B (25-mg/kg BW), C (50-mg/kg BW), D (100-mg/kg BW), E (200-mg/kg BW), and F (400-mg/kg BW). [Click here to view] |
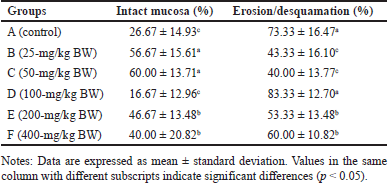 | Table 2. Percentage of desquamation or erosion on the gastric mucosa of R. norvegicus rats fed with H. atra ethanolic extract for 90 days. [Click here to view] |
Pancreatic histopathology
Normally, the number of acinar cells is greater than the number of degenerated cells and necrotic cells. Photomicrographs of normal acinar cells, degenerated acinar cells, and acinar cell necrosis are presented in figure 5. Meanwhile, a photomicrograph of the rat pancreas showing IC and congestion is presented in figure 6. Overall, the number of normal acinar cells showed no difference (p > 0.05) compared with degenerated and necrotic acinar cells (Table 3). This indicated that the pancreas was still functioning well to produce digestive enzymes. Antonucci et al. [33] stated that stress, nutritional deficiencies, and pathogen infection can influence the number of acinar cells. Damage to acinar cells can occur through degeneration or necrosis. Group D had the lowest number of acinar cell degeneration (p < 0.05). Cell degeneration is a reversible cell alteration characterized by the presence of vacuoles in the cytoplasm [34]. Cell degeneration can take the form of hydropic degeneration, fatty degeneration, and autophagocytosis. Cell degeneration can be caused by acute damage and autophagocytosis (hydropic degeneration). Acute damage leads to water accumulation in the endoplasmic reticulum, mitochondria, or vacuoles. In contrast, sublethal damage often results in lipid accumulation in the cytoplasm (lipid degeneration) or autophagocytosis of damaged cellular organelles. Acinar cell degeneration is also frequently found in aged rats [35]. According to Saad et al. [36], H. atra exhibited an antiapoptotic effect, likely relatable to its bioactive components from the lysophospholipid and polysaccharide groups.
 | Figure 6. Photomicrograph of the height of the villi and crypts of the small intestine of R. norvegicus rats fed with H. atra ethanolic extract; (Staining: HE, magnification: 10×, bar 100 µm). High villi and the number of crypts increased in groups B, C, and D, while the surface area of the villi increased in all groups. [Click here to view] |
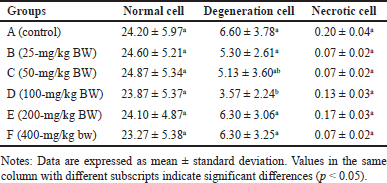 | Table 3. Number of pancreatic acinar cells in R. norvegicus rats fed with H. atra ethanolic extract for 90 days. [Click here to view] |
The number of necrotic acinar cells was not significantly different (p > 0.05) between the control and treated groups of animals (Table 3). This showed that the extract did not significantly cause cell damage. Necrosis, a form of cell death, begins with changes in the cell nucleus such as pyknosis (nucleus shrinkage and condensation), karyorrhexis (nucleus fragmentation), and karyolysis (nucleus disappearance). The cytoplasm and cell membrane become irregular, and the nucleus undergoes shrinkage in cases of lethal cell damage [37].
The number of IC was lower in all treated groups compared to the control (p < 0.05), with groups D and E exhibiting the least number of IC (Table 4). This suggested that the extract effectively inhibited the growth of IC. The relatively low number of IC is likely attributed to the anti-inflammatory effect of H. atra extract [38]. Inflammation is the body’s response to cell injury that occurs in vascularized tissues in multicellular organisms. The primary goal of the inflammatory response is to eliminate the cause of the injury and facilitate healing [39].
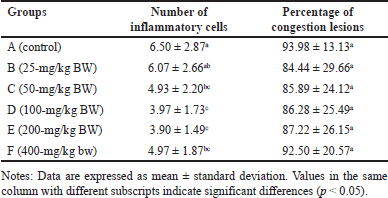 | Table 4. Number of inflammatory cells and percentage of pancreatic congestion lesions of R. norvegicus rats fed with ethanolic extract of H. atra for 90 days. [Click here to view] |
The percentage of lesion congestion in the rat pancreas revealed no significant difference between the control and treatment groups (p > 0.05). The overall percentage of congestion incidence was high, ranging from 84.44% ± 29.66% to 93.98% ± 13.13% (Table 4). The congestion in this experiment was thought to be caused by the euthanasia agents. According to Reilly [40], euthanasia drugs, such as the ketamine/xylazine combination, can induce congestion. This drug combination can lead to progressive bradycardia, which may result in heart failure [41]. High doses of ketamine can depress the cardiovascular system, causing congestion [42]. Congestion, also referred to as passive hyperemia, is a hemodynamic disorder [39] and passive engorgement of blood vessels due to decreased outflow and increased inflow of blood. Passive congestion can be either acute or chronic. Acute passive congestion can occur as a response to acute heart failure, after euthanasia, in organs affected by the smooth muscle relaxing effects of barbiturate euthanasia anesthesia drugs, and in euthanasia, which can cause dilatation of blood vessels [37]. The histopathological features of the pancreas are presented in figures 4 and 5.
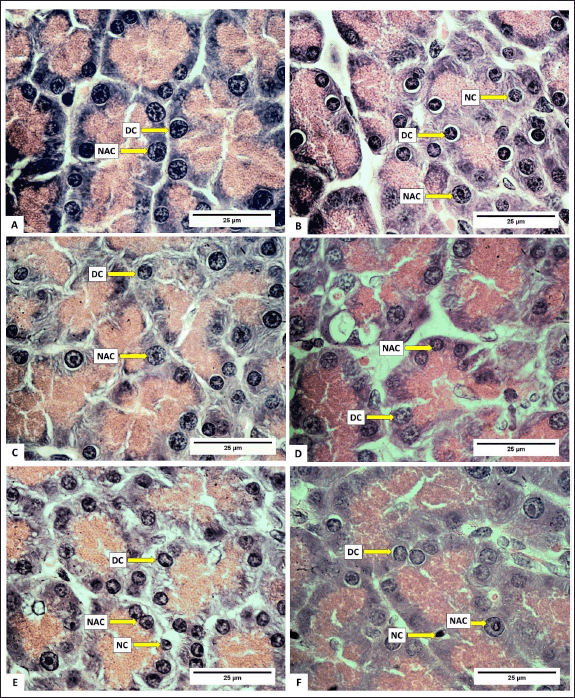 | Figure 4. Photomicrograph of normal acinar cells, degenerated acinar cells, and acinar cells necrosis in the pancreas of R. norvegicus rats fed with H. atra ethanolic extract; (Staining: H&E, magnification: 100×, bar: 25 µm). Overall, the number of normal acinar cells was greater than degenerate and necrotic acinar cells. A (distilled water as control), B (25-mg/kg BW), C (50-mg/kg BW), D (100-mg/kg BW), E (200-mg/kg BW), and F (400-mg/kg BW). [Click here to view] |
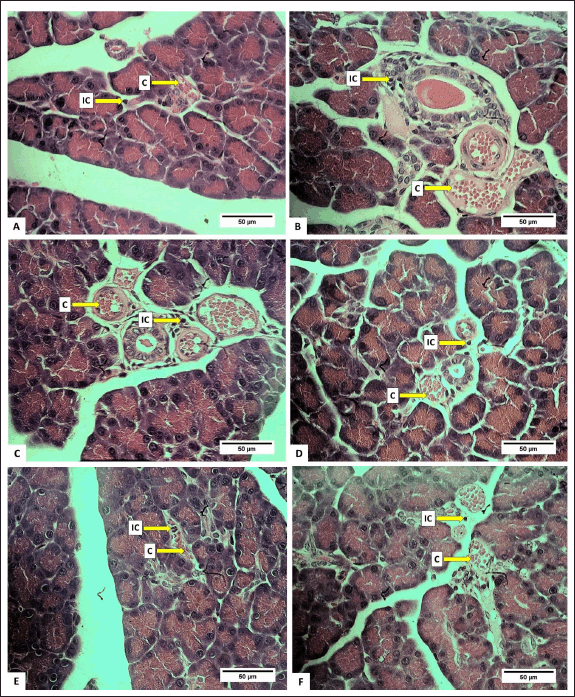 | Figure 5. Photomicrograph of R. norvegicus rats pancreas fed with H. atra ethanolic extract showing inflammatory cells and congestion (C); (Staining: HE, magnification: 40×, bar: 50 µm). A (distilled water as control), B (25-mg/kg BW), C (50-mg/kg BW), D (100-mg/kg BW), E (200-mg/kg BW), and F (400-mg/kg BW). [Click here to view] |
Small intestine histopathology
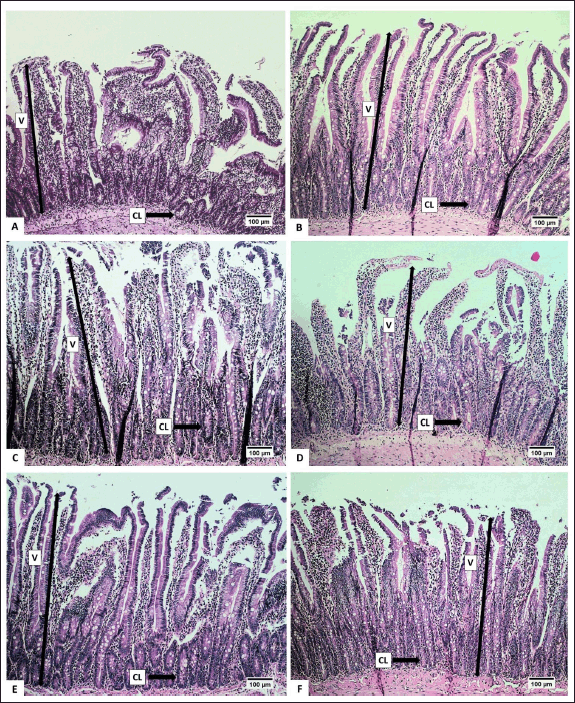
The height and area of the villi, an increase in the number of crypts (CL), and a reduction in the number of IC were observed to assess the effect of the extract on the digestive organs of rats. Histopathological analysis of the small intestine, encompassing the height and surface area of villi, the number of CL, and submucosal IC are presented in Table 5. Histopathological analysis of the villi (V), CL, and IC of the small intestine of R. norvegicus fed with the extract is presented in figures 6 and 7.
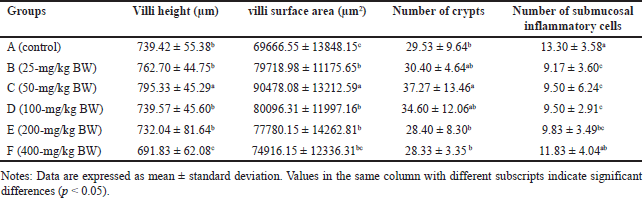 | Table 5. Average villi height, villous surface area, number of crypts, and number of submucosal inflammatory cells in the small intestine of R. norvegicus rats fed with H. atra ethanolic extract for 90 days. [Click here to view] |
The height and surface area of the villi in group C (50-mg/kg BW) exhibited the highest values (p < 0.05), as did the number of CLs in the small intestine of R. norvegicus. The highest increase in height and area of villi in group C accompanied by a small number of submucosal ICs was thought to have the best effect on the growth of small intestinal villi. Therefore, the extract may enhance nutrient absorption by the small intestine so that it can maintain healthy digestive conditions.
According to Buchanan [43], villi height is an indicator of healthy digestion. Conversely, a reduction in villi height is a response to poor health conditions. An increase in villi height enhances the absorption area, thereby improving the performance of enzymatic digestion in the small intestine and nutrient transport through the surface of the villi [44]. An increase in the height of small intestinal villi can be influenced by an increase in feed consumption due to an increase in absorption capacity. Additionally, the height of the villi also depends on the rate of enterocyte proliferation, which is also influenced by hormones [45].
In addition to increasing the height and surface area of the villi, the extract of 50-mg/kg BW also significantly increased the number of small intestinal CLs. The number of CLs is related to the height of the villi. Under normal conditions, Lieberkuhn’s CL functions as a “nursery” site because the CL has stem cells that are highly active in mitosis so that the epithelial cells lining the villi of the small intestine are shed and replaced at high speed. This mechanism causes new cells to be continuously produced in the CL and then migrate to the villi to replace old cells at the tips of the villi [46]. According to Clevers [47], the kinetics of cell replacement begin with cell growth in the CL, proliferation, and differentiation, and end with cell release into the lumen. Crypts play a crucial role in intestinal defense from abrasive and corrosive conditions and protect stem cells from bacterial attack by the presence of Paneth cells, which produce bactericidal compounds, namely, lysozyme and defensin [48].
The number of submucosal ICs showed a significant decrease in groups B, C, and D (p < 0.05). It was most likely related to the content of certain bioactive compounds in the extract. Several types of sea cucumbers are reported to have anti-inflammatory properties [49,50]. Lymphocytes, plasma cells, macrophages, eosinophils, monocytes, granulocytes, and mast cells are types of IC that can be found in the submucosa of the digestive tract [51]. The histopathological analysis of the height of the villi and CL of the small intestine is presented in figures 6 and 7.
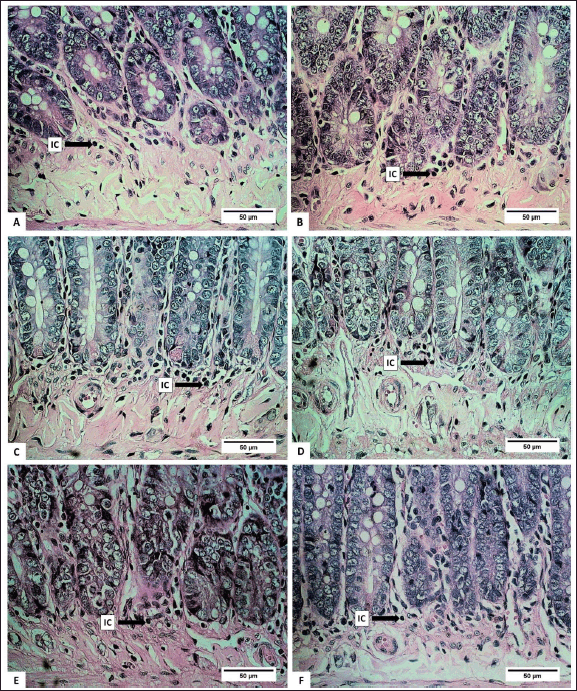 | Figure 7. Photomicrograph of inflammatory cells of small intestinal submucosa in R. norvegicus rats fed with H. atra ethanolic extract. Overall, total submucosal inflammatory cells decreased due to the exposure of the extract (Staining: HE, magnification: 40×, bar 50 µm). [Click here to view] |
In the small intestine, macromolecules undergo enzymatic hydrolysis. The majority of nutrients are absorbed into the bloodstream by the event [22]. The duodenum, jejunum, and ileum are the three sections that make up a rat’s small intestine [52]. Most absorption takes place in the jejunum and duodenum. The surface of the small intestine forms folds containing villi to expand the absorption area. The height of the villi varies depending on the region and type of animal and is related to the level of absorption in the small intestine [53]. The small intestinal mucus contains short tubular structures that curve inward as the protective side of stem cells known as CL [46]. Goblet, inflammatory, and enteroendocrine cells can also be found throughout the digestive tract [54].
The data shown in this study are in line with other studies regarding the positive effects of H. atra extracts. As shown by Esmat et al. [55] and Dakrory et al. [12], H. atra extract plays an important role as a hepatoprotector. In another in vivo study, Nursid et al. [11] showed that H.atra extract can even stimulate immunity while suppressing IC. At a dose of 50-mg/kg BW, H. atra extract had a positive effect on rat liver. In this case, the positive influence of H. atra ethanolic extract in the digestive system, including its role in reducing IC in the digestive system, can be observed from our study. The findings presented in this paper can increase opportunities for utilizing H. atra extracts for human health.
CONCLUSION
The exposure of H. atra ethanolic extract indicated beneficial effects on the digestive tract organs, including the stomach, pancreas, and small intestine. Oral feed of the extract, at a dose of 50-mg/kg BW, increased gastric gland cells, reduced damage to pancreatic acinar cells, increased the height and surface area of villi, and increased small intestinal CL. At the same dose, the extract also showed an effect on reducing IC in the pancreas and small intestinal submucosa, as well as maintaining the surface of the gastric mucosa by reducing lesions or desquamation. Based on this research, the H. atra ethanolic extract has the potential to be used for digestive tract health at the right dose.
ACKNOWLEDGMENT
The authors would like to thank the laboratory technicians: Mr. Soleh and Mr. Kasnadi from the Laboratory Animal Management Unit, Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The funding of this research was provided by the Research Center for Marine and Fisheries Product Processing and Biotechnology, Ministry of Marine Affairs and Fisheries, Republic of Indonesia number: SP DIPA-032.12.2.403835/2018.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The animal experimental procedures were approved by the Experimentation Ethics Committee on Animal Use of the Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia, with approval number 18-2016 IPB.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sensoy I. A review on the food digestion in the digestive tract and the used in vitro models. Curr Res Food Sci. 2021;4:308–19. Available from: https://www.sciencedirect.com/science/article/pii/S2665927121000307
2. Zimmermann C, Wagner A. Impact of food-derived bioactive compounds on intestinal immunity. Biomolecules. 2021;11(12):1901. Available from: https://www.mdpi.com/2218-273X/11/12/1901
3. Xu T, Sun R, Zhang Y, Zhang C, Wang Y, Wang Z, et al. Recent research and application prospect of functional oligosaccharides on intestinal disease treatment. Molecules [Internet]. 2022;27(21):7622. Available from: https://www.mdpi.com/1420-3049/27/21/7622
4. Essa M, Bishir M, Bhat A, Chidambaram S, Al-Balushi B, Hamdan H, et al. Functional foods and their impact on health. J Food Sci Technol [Internet]. 2023;60(3):820–34. doi: https://doi.org/10.1007/s13197-021-05193-3
5. Yan T, Babji A, Lim S, Sarbini S. A systematic review of edible swiftlet’s nest (ESN): nutritional bioactive compounds, health benefits as functional food, and recent development as bioactive ESN glycopeptide hydrolysate. Trends Food Sci Technol [Internet]. 2021;115:117–32. Available from: https://www.sciencedirect.com/science/article/pii/S0924224421004088
6. Hossain A, Dave D, Shahidi F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar Drugs [Internet]. 2022;20(8):521. Available from: https://www.mdpi.com/1660-3397/20/8/521
7. Nursid M, Marraskuranto E, Chasanah E. Cytotoxicity and apoptosis induction of sea cucumber Holothuria atra extracts. Pharmacognosy Res. 2019;11(1):41–6.
8. Grauso L, Yegdaneh A, Sharifi M, Mangoni A, Zolfaghari B, Lanzotti V. Molecular networking-based analysis of cytotoxic saponins from sea cucumber Holothuria atra. Mar Drugs [Internet]. 2019;17(2):86. Available from: https://www.mdpi.com/1660-3397/17/2/86
9. Shahinozzaman M, Ishii T, Takano R, Halim M, Hossain M, Tawata S. Cytotoxic desulfated saponin from Holothuria atra predicted to have high binding affinity to the oncogenic kinase PAK1: a combined in vitro and in silico study. Sci Pharm [Internet]. 2018;86(3):32. Available from: https://www.mdpi.com/2218-0532/86/3/32
10. Puspitasari Y, Tuenter E, Foubert K, Herawati H, Hariati A, Aulanni’am A, et al. Saponin and fatty acid profiling of the sea cucumber Holothuria atra, α-glucosidase inhibitory activity and the identification of a novel triterpene glycoside. Nutrients [Internet]. 2023;15(4):1033. Available from: https://www.mdpi.com/2072-6643/15/4/1033
11. Nursid M, Patantis G, Dewi A, Achmad M, Sembodo P, Estuningsih S. Immunnostimulatory activity of Holothuria atra sea cucumber. Pharmacia [Internet]. 2021;68:121–7. Available from: https://doi.org/10.3897/pharmacia.68.e58820
12. Dakrory A, Fahmy S, Soliman A, Mohamed A, Amer S. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced hepatorenal diseases in rats. Biomed Res Int [Internet]. 2015;2015(1):563652. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1155/2015/563652
13. Ikhsan I, Idroes R, Azharuddin A, Nasution R, Yusnaini R, Iqhrammullah M. Fatty acid-rich extract from Holothuria atra for hyperuricemia via expressions modulation of GLUT9a and GLUT9b in rat model. Molecules [Internet]. 2023;28(10):3981. Available from: https://www.mdpi.com/1420-3049/28/10/3981
14. Hashim R, Azizan N, Zamli Z, Zulkipli F, Mazlan N, Althunibat O. Toxicity effects of water extracts of Holothuria atra jaeger in mice. Asian Pac J Trop Biomed. 2014;4(8):614–7.
15. Hanafi P, Sutrisno A, Murniasih T, Harijono, Putra M, Untari F. Acute oral toxicity assessment of the ethanol extract of Holothuria atra in mice. Asian J Pharm Clin Res. 2019;13:150–3.
16. Nursid M, Dewi A, Patantis G, Marraskuranto E, Habibullah U, Subagja M, et al. Subchronic toxicity of Holothuria atra ethanol extract for 90 days in histopathology of the liver and kidney of rats. Farmacia. 2023;71(2):322–8.
17. Roepstorff A, Nansen P. Epidemiology, diagnosis and control of helminth parasites of swine. Rome, Italy: Food and Agriculture Organization of the United Nations; 1998. p 161.
18. OECD. OECD guideline for the testing of chemicals; test no. 408: repeated dose 90-day oral toxicity study in rodents [Internet]. (OECD guidelines for the testing of chemicals, Section 4). Paris, France: OECD; 2018 [cited 2024 Aug 1]. Available from: https://www.oecd-ilibrary.org/environment/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en
19. Cheville NF. Introduction to veterinary pathology. 3rd ed. Hoboken, NJ: Wiley-Blackwell; 2006. 1–384 pp.
20. Iji P, Saki A, Tivey D. Body and intestinal growth of broiler chicks on a commercial starter diet. 1. Intestinal weight and mucosal development. Br Poult Sci [Internet]. 2001;42(4):505–13. doi: https://doi.org/10.1080/00071660120073151
21. Gartner L, Hiatt J. Color textbook of histology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2007. p 396.
22. Reece J, Urry L, Cain M, Wasserman S, Minorsky P, Jackson R. Campbell biology. 9th ed. Boston, MA: Pearson Learning Solutions; 2011. p 1464.
23. Xu RJ, Cranwell PD, editors. The neonatal pig: gastrointestinal physiology and nutrition. England: Nottingham University Press; 2003. p 1–360.
24. Engelking L. Review of veterinary physiology. Jackson, WY: Teton NewMedia; 2002. pp 1–657.
25. Parker G, Papenfuss T. Chapter 10?Immune system. In: Parker GA, Picut CA, editors. Atlas of histology of the juvenile rat [Internet]. Boston, MA: Academic Press; 2016. pp. 293–347. Available from: https://www.sciencedirect.com/science/article/pii/B9780128026823000100
26. Ermund A, Schütte A, Johansson M, Gustafsson J, Hansson G. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol-Gastrointest Liver Physiol [Internet]. 2013;305(5):G341–7. doi: https://doi.org/10.1152/ajpgi.00046.2013
27. Kang W, Rathinavelu S, Samuelson L, Merchant J. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Investig. 2005;85(5):702–15.
28. Fahmy S, Amer M, Al-Killidar M. Ameliorative effect of the sea cucumber Holothuria arenicola extract against gastric ulcer in rats. J Basic Appl Zool [Internet]. 2015;72:16–25. Available from: https://www.sciencedirect.com/science/article/pii/S2090989615000223
29. Subramaniam B, Amuthan A, D’almeida P, Arunkumar H. Efficacy of gamat extract in wound healing in albino wistar rats. Int J Pharm Sci Rev Res [Internet]. 2013;20(1):142–5. Available from: https://api.semanticscholar.org/CorpusID:46960424
30. Chauhan R. Illustrated veterinary pathology: general and systematic pathology. 2nd ed. Lucknow, India: International Book Distributing Co.; 2008.
31. Jones T, Popp J, Mohr U. Digestive system [Internet]. Berlin, Germany: Springer Berlin Heidelberg; 1997. (Monographs on pathology of laboratory animals). Available from: https://books.google.co.id/books?id=Fy1tAAAAMAAJ
32. Toljamo K. Gastric erosions?clinical significance and pathology?: a long-term follow-up study [Internet]. Oulu, Finland: University of Oulu; 2012. Available from: https://api.semanticscholar.org/CorpusID:43797210
33. Antonucci L, Fagman J, Kim J, Todoric J, Gukovsky I, Mackey M, et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A [Internet]. 2015;112(45):E6166–74. Available from: https://www.pnas.org/doi/abs/10.1073/pnas.1519384112
34. Shirahata S, Hamasaki T, Teruya K. Advanced research on the health benefit of reduced water. Trends Food Sci Technol [Internet]. 2012;23(2):124–31. Available from: https://www.sciencedirect.com/science/article/pii/S0924224411002408
35. Suttie A, Masson R, Schutten M. Chapter 10?Exocrine pancreas. In: Suttie AW, editor. Boorman’s pathology of the rat [Internet]. 2nd ed. Boston, MA: Academic Press; 2018. pp. 107–22. Available from: https://www.sciencedirect.com/science/article/pii/B9780123914484000101
36. Saad D, Baiomy A, Mansour A. Antiseptic effect of sea cucumber (Holothuria atra) against multi-organ failure induced by sepsis: molecular and histopathological study. Exp Ther Med. 2016;12(1):222–30.
37. Munday JS. Pathologic basis of veterinary disease [Internet]. In: Zachary JF, editor. Veterinary dermatology. 6th ed. St. Louis, MO: Elsevier; 2017. Vol. 28, p 258. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/vde.12434
38. Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs [Internet]. 2011;9(10):1761–805. Available from: https://www.mdpi.com/1660-3397/9/10/1761
39. Damjanov I. Pathology secrets. Maryland Heights, MO: Mosby/Elsevier; 2009. p 509.
40. Reilly J. Euthanasia of animals used for scientific purposes. Adelaide, Australia: ANZCCART; 2001. p 106.
41. Zolhavarieh S, Nourian A, Sadeghi-Nasab A. A new method for on-farm euthanasia with animal welfare considerations. Iran J Vet Surg [Internet]. 2011;6(1–2):55–64. Available from: https://www.ivsajournals.com/article_3134.html
42. Duke-Novakovski T, de Vries M, Seymour C. BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Chorley, UK: British Small Animal Veterinary Association; 2016. pp 1–464.
43. Buchanan N. Diet formulation and manufacturing technique interactions affect pellet quality and broiler growth [dissertation]. Morgantown, WV: West Virginia University; 2008.
44. Tufarelli V, Desantis S, Zizza S, Laudadio V. Performance, gut morphology and carcass characteristics of fattening rabbits as affected by particle size of pelleted diets. Arch Anim Nutr [Internet]. 2010;64(5):373–82. doi: https://doi.org/10.1080/1745039X.2010.496945
45. Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A. The mouse in biomedical research: normative biology, husbandry, and models. 2nd ed. In: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A, editors. San Diego, CA: Academic Press; 2006. Vol. 3.
46. Sherwood L. Human physiology: from cells to systems. 6th ed. Melbourne, Australia: Cengage Learning Australia; 2016.
47. Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell [Internet]. 2013;154(2):274–84. Available from: https://www.sciencedirect.com/science/article/pii/S0092867413008386
48. Clevers H, Bevins C. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol [Internet]. 2013;75:289–311. Available from: https://www.annualreviews.org/content/journals/10.1146/annurev-physiol-030212-183744
49. Olivera-Castillo L, Grant G, Kantún-Moreno N, Acevedo-Fernández J, Puc-Sosa M, Montero J, et al. Sea cucumber (Isostichopus badionotus) body-wall preparations exert anti-inflammatory activity in vivo. PharmaNutrition [Internet]. 2018;6(2):74–80. Available from: https://www.sciencedirect.com/science/article/pii/S2213434418300264
50. Wargasetia T, Ratnawati H, Widodo N, Widyananda M. Antioxidant and anti-inflammatory activity of sea cucumber (Holothuria scabra) active compounds against KEAP1 and iNOS protein. Bioinform Biol Insights [Internet]. 2023;17:11779322221149612. Available from: https://doi.org/10.1177/11779322221149613
51. Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat M, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol [Internet]. 2014;7(8):4557–76. Available from: www.ijcep.com/
52. Sharp P, Villano J. The laboratory rat. 2nd ed. Boca Raton, FL: CRC Press; 2012.
53. Eurell J, Frappier B. Dellmann’s textbook of veterinary histology. 6th ed. Eurell JA, Frappier BL, editors. Ames, IA: Blackwell Publishing Professional; 2006.
54. Treuting P, Dintzis S, Frevert C, Liggitt D, Montine K. Comparative anatomy and histology: a mouse and human atlas (Expert consult). 1st ed. London, UK: Academic Press; 2012.
55. Esmat AY, Said MM, Soliman AA, El-Masry KSH, Badiea EA. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition [Internet]. 2013;29(1):258–67. Available from: https://www.sciencedirect.com/science/article/pii/S089990071200264X