INTRODUCTION
In December 2019, the world was shaken by the emergence of the novel SARS-CoV-2 virus, leading to a rapid decline and great havoc in global health, economy, and society. This situation escalated into a full-blown pandemic, officially declared by the World Health Organization (WHO) on March 11th, 2020 [1,2]. The pandemic accumulated a total of 700 million reported cases alongside a death toll of 7 million deaths as of 2024. High morbidity and mortality rates were a consequence of this infectious disease; the United States of America was the hardest hit country with a record-breaking prevalence of approximately 112 million reported cases and 1.2 million deaths [3]. Additionally, the UAE suffered through around 1 million officially reported cases of coronavirus 2019 (COVID-19) and 2,000 subsequent deaths in the COVID-19 period (2020–2024) [4].
The recent prevalence of cases has been detected in integrated sentinel surveillance within the global influenza surveillance and response system, as reported in the WHO epidemiological update, which showed that the positivity of SARS-CoV-2 PCR decreased from 8.2% in the first week of the reporting period to 5.8% in the last week from 19 August to 15 September 2024 [5].
The primary mode of transmission of COVID-19 is through respiratory droplets and close contact, with most patients experiencing mild to moderate symptoms such as fever, dry cough, sore throat, loss of smell and taste, or head and body aches [6]. However, severe cases may lead to acute respiratory distress syndrome and multiorgan failure, particularly in older adults and patients with underlying health conditions such as cardiovascular diseases, hypertension, and diabetes [7].
The principal mechanism through which the coronavirus enters body cells involves an interaction between the virus’s spike (S) protein and the human ACE-2. The S protein permits viral attachment and entry into human cells by binding to the angiotensin-converting enzyme 2 receptor, which is found predominantly in the cells of the respiratory tract, lungs, heart, kidneys, and intestines [8]. Subsequently, these organs are more susceptible to virus infection and damage [9].
A study conducted amongst hypertensive Chinese patients has reported that the ABO blood group is associated with ACE activity and ACE inhibitor-induced cough [10,11].
The ABO blood group system represents the fundamental categorization of blood types based on specific antigenic markers present on the surface of erythrocytes. Hence, the presence of these antigens, namely, A and B, or their respective absence, define the ABO blood groups—A, B, AB, and O. In contrast, the Rh blood group system focuses on the presence of the Rh factor (or D antigen) on the surface of red blood cells [12]. Individuals possessing this antigen are classified as Rh-positive (Rh+), while those lacking it are Rh-negative (Rh-). Blood group antigens (A, B, and D) are genetically encoded and may be a predisposing factor for some diseases but a protective factor against others. Studies have shown that the ABO blood group is associated with rheumatologic diseases, cancer, and certain viral infections such as Norwalk virus and Hepatitis B [13,14].
Clinical studies examine the relationship between SARS-CoV-2 and blood groups [15]. Initial findings suggest that certain blood types may be associated with different levels of susceptibility to COVID-19 infection or the severity of the disease. However, the results of these studies are often inconsistent, highlighting the need for further research to clarify these associations. Understanding the impact of blood group variations across different populations is particularly important, as the distribution of ABO and Rh blood groups can differ significantly between communities.
This study aimed to investigate the distribution of ABO and Rh blood groups among patients who have recovered from COVID-19, alongside an analysis of their clinical characteristics. By exploring these relationships, we hope to contribute to a more nuanced understanding of how blood group phenotypes may influence COVID-19 outcomes, which could have implications for patient management and public health strategies.
MATERIALS AND METHODS
Participants and study design
This retrospective study was conducted at RAK Medical and Health Sciences University (RAKMHSU), UAE, with 217 participants, including students, staff, and faculty members. Purposive sampling was used to choose participants, focusing on those who gave informed consent and had a positive COVID-19 test result. This strategy aimed to ensure accessibility and facilitate participant selection.
Ethical considerations
The project received ethical clearance (Ethical Approval No.: RAKMHSU-REC-153-2020/21-F-M). The study’s goal, methods, and participants’ rights were explained to participants, guaranteeing their consent and confidentiality. All methods followed the relevant guidelines and regulations or Declaration of Helsinki.
Data collection
A self-administered questionnaire was employed to gather demographic and health data. The questionnaire was validated as follows: the researchers clearly outlined the purpose of the questionnaire, including the specific constructs or variables it aimed to measure, which guided the entire validation process. They gathered feedback from subject matter experts to ensure that the items comprehensively covered the domain, reviewing each question’s clarity, relevance, and appropriateness. A preliminary questionnaire test was conducted with a small, representative sample of the target population, helping to identify any ambiguities or misunderstandings in the questions. Cronbach’s alpha was calculated to evaluate internal consistency, and a higher alpha (typically above 0.70) indicated good internal consistency. Additionally, the questionnaire was administered to the same group of respondents at two different points to measure stability, with a high correlation between the two sets of responses indicating good reliability.
Based on feedback and data from the pilot testing, necessary revisions were made to the questionnaire, involving rewording questions, removing poorly performing items, or adding new ones to enhance clarity and relevance.
The questionnaire included closed-ended questions covering various aspects such as gender, age, smoking and alcohol habits, infection history, symptom severity, blood type, medical conditions, contact history, and vaccination status.
Data entry and analysis
Data collected from the questionnaires were coded and meticulously entered into Microsoft Excel and subsequently analyzed using IBM SPSS Statistics for Windows, version 29. 4.5.1. (IBM Corp., Armonk, NY). Analysis of Variance, or ANOVA, was used to compare the COVID-19 case averages among the various blood types (A, B, O, and AB). The alternative hypothesis suggested that at least one blood type had a different average. In contrast, the null hypothesis claimed that the average number of instances was the same for all blood groups. The F-statistic evaluated the ratio of variability between groups to variability within groups, which an ANOVA produced. Chi-square test: tested if specific blood types were prone to infection by examining the relationship between blood group and COVID-19 infection status. Using logistic regression, the probability of COVID-19 infection across blood types was evaluated. Odds ratios and 95% confidence intervals were produced, showing the likelihood of disease for each blood type and the accuracy of these estimations. Correlation analysis assessed the links between different variables. For statistical significance, a threshold of p < 0.05 was established. Cronbach’s Alpha was used to gauge the internal consistency of the survey questions. Values above 0.7 were considered satisfactory, showing that the items accurately measured the same underlying concept. The statistical significance threshold was chosen at p < 0.05 to provide strong and accurate findings.
RESULTS
Table 1 shows a correlation between blood types and the severity of COVID-19. The analysis revealed a statistically significant difference in COVID-19 severity between different blood groups (F = 4.271 and p = 0.015). With a p-value below the generally recognized cutoff of 0.05, its significance indicates a statistically significant difference in COVID-19 severity between people of different blood types.
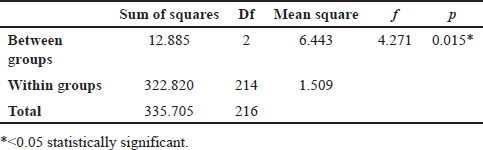 | Table 1. Association between blood groups and COVID-19 severity. [Click here to view] |
Table 2 reveals a significant correlation between the severity of COVID-19 and various blood groups. Remarkably, our findings imply that some blood types have a positive relationship with the Rh factor, emphasizing the possible influence of Rh positivity on COVID-19 outcomes. A weak positive correlation was observed between COVID-19 severity and blood group categories (r = 0.125) but was not statistically significant. Also, we noted that the COVID-19 severity and Rh factor correlation were negligible (r = 0.004) and statistically nonsignificant (p = 0.952). These findings suggest that the blood group alone may not strongly influence COVID-19 severity but might interact with other factors contributing to disease outcomes.
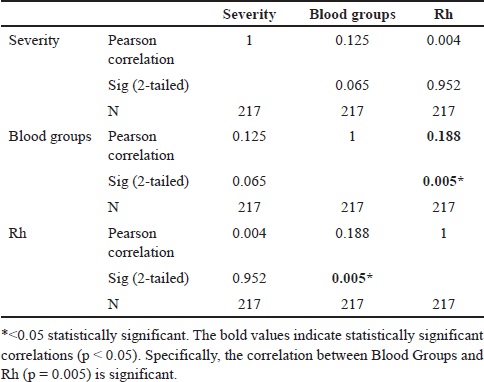 | Table 2. Correlation between the COVID-19 severity and blood group categories. [Click here to view] |
Table 3 displays the results of logistic regression comparing ABO/Rh groups to AB blood groups for COVID-19 patients. According to the analysis, those with blood group AB are significantly less likely to develop COVID-19 than those with blood group A, based on an odds ratio of less than 1. In comparison to blood type A, our study indicates that blood group AB is significantly linked to a decreased risk of COVID-19 infection overall. However, blood types O and B do not correlate substantially with COVID-19 infection at the standard threshold.
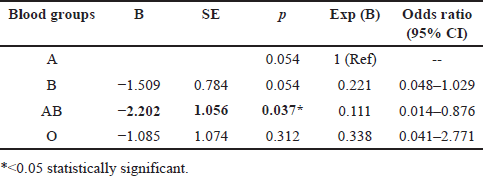 | Table 3. Logistic regression analysis of ABO blood groups and Rh factor for COVID-19 patients. [Click here to view] |
These graphs illustrate our sample’s demographic makeup. According to the first chart, 76 (35%) participants were men and 141 (65%) were women. The second graph shows that 20 (9%) participants were smokers, while the remaining 197 (91%) were non-smokers. The third graph shows that seven respondents (3%) said that they consumed alcohol, whereas 210 respondents (97%) said that they did not (Figs . 1-3).
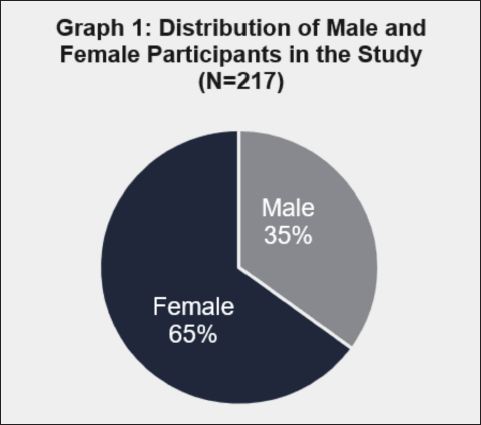 | Figure 1. Gender-wise distribution of study participants (n = 217). [Click here to view] |
 | Figure 2. Smoking history of the study patients. [Click here to view] |
 | Figure 3. Alcoholic history of the study patients. [Click here to view] |
This bar graph shows how the number of cases varies among different blood groups for males and females. Interestingly, regardless of gender, blood group AB has the fewest cases, while blood group O has the highest. This indicates a consistent trend: blood group O is the most common among reported cases, regardless of whether the patient is male or female (Fig. 4).
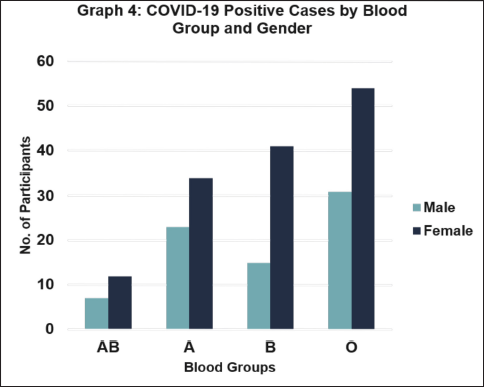 | Figure 4. Categorization of COVID-19 positive cases according to blood group and gender. [Click here to view] |
Graph 5 depicts COVID-19 cases by infection severity and gender. Regardless of gender, most people only exhibit mild and moderate symptoms, with a small percentage displaying severe symptoms. This suggests that mild and moderate cases are more prevalent in our research population (Fig. 5).
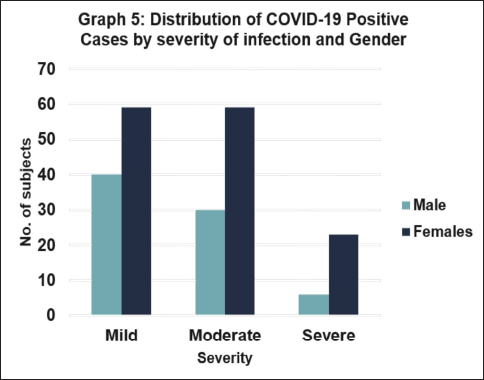 | Figure 5. Distribution of COVID-19 positive cases by severity of infection and gender. [Click here to view] |
Graph 6 depicts the severity of COVID-19 across age groups, which confirms previous findings. The majority of participants, regardless of age, have mild symptoms. Those under the age of 20 are more likely to have mild infections, while moderate and severe cases are present but less common, focusing on the prevalence of mild cases in our study’s younger cohort (Fig. 6).
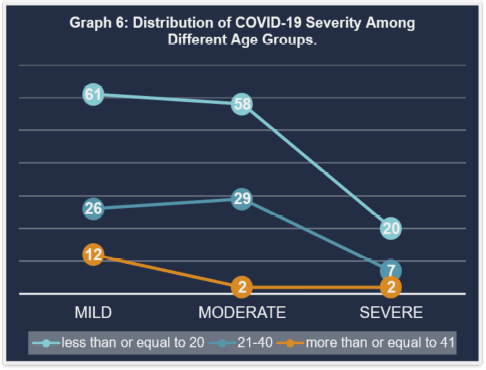 | Figure 6. Distribution of COVID-19 severity among different age groups. [Click here to view] |
Graph 7 compares COVID-19 cases and infection frequencies, confirming blood type O’s greater susceptibility. A small percentage of the 217 individuals had more than one infection; the majority only had one. This suggests acquired immunity, in which the chance of a recurrent infection may be decreased following an original infection (Fig. 7).
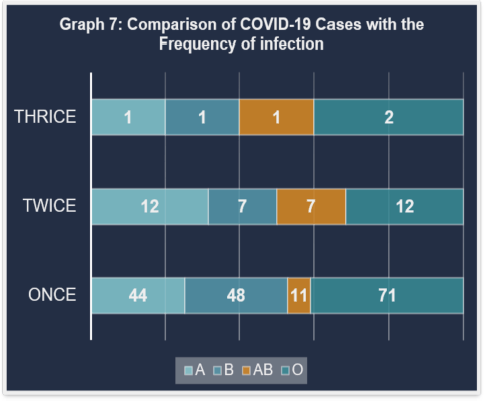 | Figure 7. Comparison of COVID-19 cases with the frequency of infection. [Click here to view] |
Among the 217 patients, 201 (92.63%) were Rh-positive (Rh+), while 16 (7.37%) were Rh-negative (Rh−). The association between ABO blood groups, Rh factor, and COVID-19 severity was statistically significant (?p = 0.021). This analysis of patient clinical characteristics and ABO blood types have found a substantial correlation between the Rh factor and COVID-19 severity. This indicates that Rh positivity and negativity distribution varies significantly among patients with different blood groups concerning COVID-19 severity (Table 4).
 | Table 4. Association between clinical characteristics of patients and ABO blood groups and severity of COVID-19. [Click here to view] |
DISCUSSION
Numerous studies discuss the correlation between blood types and COVID-19, where certain blood groups exhibit protective qualities, while others succumb to dire outcomes. With 217 participants, the results showed that 64.97% of the cases were in females, indicating a greater frequency in women. This result is inconsistent with research by Stokes [16], showing that the vulnerability to COVID-19 varies little in males and females. They further pointed out that men are more prone to suffer from serious consequences. Additionally, Jin et al. [17] discovered that while the frequency of COVID-19 is the same in men and women, males, regardless of age, are more likely to have severe consequences, including death. To support that, a study conducted by Ad’hiah et al. [18] showed males to be infected at a higher proportion. A survey by Pradhan and Olsson [19] reports greater severity and mortality from COVID-19 in males. This disparity is likely due to several factors, including females’ stronger immune response, the influence of sex hormones, and genetic differences linked to the X chromosome. In addition, females’ higher stress endurance may contribute to their greater resilience against COVID-19.
The findings showed a significant correlation between blood type and age. More specifically, COVID-19 was more common in individuals under 20 years old (139 instances) than in people over 41 (just 16 cases). However, the fact that the study mainly involved university students should be taken into consideration. These results confirm those of Malmgren et al. [20], who found that a significant proportion of COVID-19 infections in Washington State occurred in the age ranges of 0–19 and 20–39. These younger groups do not have a substantial risk of hospitalization or death, even though they may be more likely to get the virus and transmit it. On the other hand, Li et al.’s [21] study shows a much-increased mortality risk among seniors but a very low frequency in younger people, which runs opposite to our findings.
The ABO blood group system may further function as a susceptibility COVID-19 biomarker. Among the blood groups, blood type O exhibited a heightened vulnerability in contrast to other blood types, while blood type AB demonstrated a decreased susceptibility. These outcomes diverged from the research by Esref et al. [22] and the study done by Almadhi et al. [23], both of which contra-indicated that blood type A was more prone to susceptibility. In contrast, blood type O showed a lesser likelihood. The discrepancy in the results may be credited to the diverse range of ethnic groups in the study, whereas other studies concentrated solely on a particular ethnicity. However, similarities were found with Almadhi et al. [23], in which individuals with blood type AB were at a lower risk of COVID-19. Furthermore, a Turkish study conducted by Nalbant et al. [24] in 2021, comprising a sample size of 220 individuals, also coincided with the findings in which individuals of blood group O, or the younger demographic, were both highly susceptible. In the UAE, a study conducted by Hafez et al. [25] of 303 patients found blood group B to have a lower risk for mortality than blood type O.
The results suggest that patients with Rh-positive blood types may be more susceptible to COVID-19. Latz et al. [26] and Esref et al. [22] found that COVID-19 patients with Rh-positive blood type B recover faster than other blood types. Although COVID-19 severity is lower in Rh-ve, A, and AB blood types, higher infection rates tend to be observed. Notably, Zietz et al.’s [27] research highlights Rh negativity’s protective properties. These three studies are consistent with the results that were found.
Our study had a few limitations. First, the small sample size, particularly for certain subgroups such as Rh-negative individuals and patients with blood group AB, may limit the generalizability of the findings. Second, the study did not account for other potential confounding variables such as age, sex, pre-existing comorbidities, and socioeconomic factors. In addition, our study is cross-sectional; it primarily makes associations rather than investigating causations. A longitudinal study would be required to determine whether blood groups and Rh factors directly influence COVID-19 susceptibility or severity. Furthermore, the study was conducted in a single center and may not be suitable for other populations or regions.
CONCLUSION
In summary, this study reveals important correlations between ABO blood types, Rh factors, and the severity of COVID-19, offering fresh perspectives on the potential roles that blood type and Rh antigen status, may have in disease outcomes. According to the results, people with blood group O might be more susceptible to COVID-19 than people with blood groups A, B, or AB, who seem to have a less severe range of symptoms. Furthermore, it was discovered that erythrocytes with the Rh antigen were associated with a higher risk of developing severe COVID-19.
These findings highlight the significance of including blood type and Rh status into risk assessment models and therapeutic approaches to better address patient vulnerabilities. Healthcare systems may improve patient management and outcomes by customizing interventions according to these criteria. However, bigger cohort studies and thorough investigations are necessary to validate these findings and explore the molecular mechanisms at play.
LIST OF ABBREVIATIONS
ACE2, Angiotensin-converting enzyme 2; COVID-19, Coronavirus Disease 2019; RAKMHSU; Ras Al Khaimah Medical and Health Sciences University; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; S Protein, Spike Protein; SPSS, Statistical Package for Social Sciences; Rh +ve, Rhesus Factor Positive; Rh –ve, Rhesus Factor Negative; UAE, United Arab Emirates; WHO, World Health Organization.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the ethical clearance Committee, Ras Al Khaimah Medical and Health Sciences University, Ras Al Khaimah, United Arab Emirates (Approval No.: RAKMHSU-REC-153-2020/21-F-M). The study’s goal, methods, and participants’ rights were explained to participants, guaranteeing their consent and confidentiality. All methods followed the relevant guidelines and regulations or Declaration of Helsinki.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(03):105924. CrossRef
2. Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–63. CrossRef
3. Worldometer. Countries where Coronavirus has spread?Worldometer [Internet]. www.worldometers.info. 2024 April 13. [cited 2024 June 15]. Available from: https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/
4. UAE Coronavirus (COVID-19) updates [Internet]. Ncema.gov.ae. 2022 April 27. [cited 2024 June 15]. Available from: https://covid19.ncema.gov.ae/en
5. World Health Organization. COVID-19 epidemiological update?6 November 2024. 173 ed. Geneva, Switzerland: WHO [Internet]; 2024 Nov 6 [cited 2024 Nov 17]. Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-173
6. Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toe. Science. 2020;368(6489):356–60. CrossRef
7. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–25. CrossRef
8. Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867–77. CrossRef
9. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. CrossRef
10. Luo JQ, He FZ, Luo ZY, Wen JG, Wang LY, Sun NL, et al. Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenet Genom. 2014;24(6):306–13. CrossRef
11. Gasso P, Ritter MA, Mas S, Lafuente A. Influence of ABO genotype and phenotype on angiotensin-converting enzyme plasma activity. J Renin Angiotensin Aldosterone Syst. 2014;15(4):580–4. CrossRef
12. Zhang Y, Garner R, Salehi S, La Rocca M, Duncan D. Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: a literature review of 23 studies. Ann Hematol. 2021;100(5):1123–32. CrossRef
13. Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9(5):548–53. CrossRef
14. Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, et al. ABO blood group, hepatitis B viral infection, and risk of pancreatic cancer. Int J Cancer. 2012;131(2):461–8. CrossRef
15. Wang H, Zhang J, Jia L, Ai J, Yu Y, Wang M, et al. ABO blood group influence COVID-19 infection: a meta-analysis. J Infect Dev Ctries. 2021;15(12):1801–7. CrossRef
16. Stokes EK. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mort Weekly Rep [Internet]. 2020;69(24):759–65. CrossRef
17. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. CrossRef
18. Ad’hiah AH, Abdullah MH, Alsudani MY, Shnawa RMS, Al-Sa’ady AJR, Allami RH, et al. Association between ABO blood groups and susceptibility to COVID-19: profile of age and gender in Iraqi patients. Egypt J Med Hum Genet. 2020;21(1):76. CrossRef
19. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ. 2020;11:53. CrossRef
20. Malmgren J, Guo B, Kaplan HG. Continued proportional age shift of confirmed positive COVID-19 incidence over time to children and young adults: Washington State March—August 2020. PLoS One. 2021;16(3):e0243042. CrossRef
21. Li H, Wang S, Zhong F, Bao W, Li Y, Liu L, et al. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med. 2020;7:190. CrossRef
22. Esref AR, Solmaz I, Akkoc H, Donmezdil S, Karahan Z, Safak KA, et al. Association between the Rh blood group and the Covid-19 susceptibility. Int J Hematol Oncol. 2020;30(2):81–6. CrossRef
23. Almadhi MA, Abdulrahman A, Alawadhi A, Rabaan AA, Atkin S, AlQahtani M. The effect of ABO blood group and antibody class on the risk of COVID-19 infection and severity of clinical outcomes. Sci Rep. 2021;11(1):5745. CrossRef
24. Nalbant A, Ayd?n A, Yaylac? S, Kaya T, Wermeulen CL, Cinemre H. Association of ABO blood group and age with COVID-19 positive test. Rev Assoc Méd Brasil. 2021;67(suppl 1):46–50. CrossRef
25. Hafez W, Ahmed S, Abbas N, Ahmed K, Kamran S, Arya A, et al. ABO blood group in relation to COVID-19 susceptibility and clinical outcomes: a retrospective observational study in the United Arab Emirates. Life [Internet]. 2022;12(8):1157. CrossRef
26. Latz CA, DeCarlo C, Boitano L, Png CYM, Patell R, Conrad MF, et al. Blood type and outcomes in patients with COVID-19. Ann Hematol [Internet]. 2020;99(9):2113–8. CrossRef
27. Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun [Internet]. 2020;11(1):5761. CrossRef