INTRODUCTION
Estrogen is a hormone that has a selective function in female reproduction, as well as physiological functions in almost all tissues in the body [1]. Estrogen deficiency can occur in women who experience menopause or have an oophorectomy [2]. Treatment of estrogen deficiency can be done with the aromatase enzyme, estrogen receptor antagonists, aryl hydrocarbon receptors, phytoestrogens, or hormone replacement therapy (HRT) [3,4].
In recent years, HRT has apparently caused side effects in patients, namely, the appearance of spots and itching on the skin, pain in the breasts, and weight gain [2,5]. Several studies also state that HRT can also increase the risk of cancer [6–9], coronary heart disease [10–12], stroke [13], and osteoporosis and endometriosis [1,14–16]. Therefore, treatment of estrogen deficiency is recommended through alternative therapy, such as the use of phytoestrogens [2,17,18].
The characteristic feature of phytoestrogens is the presence of phenolic rings, which are a prerequisite for binding to estrogen receptors [18]. Phytoestrogens are divided into four groups, namely, chalcones, flavonoids (flavones, flavonols, flavanones, and isoflavonoids), lignans, and stilbenoids [19]. Phytoestrogens can bind to estrogen receptors, so they can carry out the same function as estrogen in the body [20]. Apart from that, this compound is also reported to have estrogenic and antiestrogenic effects, which depend on the body’s estrogen levels [21]. Phytoestrogens can be found in vegetables, fruits, and grains [22,23].
In previous research, we succeeded in identifying five compounds from the ethyl acetate and ethanol fractions of R mucronate fruit flour. The five compounds are aromadendrin, cianidanol (flavonoid group), zearalenone, ethylestrenol, and pinoresinol. Ethylestrenol has similarities to the molecular structure of 17β estradiol and ethynyl estradiol in mammals, which act as estrogen receptors α and β. In this research, studies were carried out on the estrogenic potential, druglikeness, molecular docking, and pathway analysis of these five compounds. The molecular structures of the test and control compounds (ethynyl estradiol and 17β estradiol). It is hoped that this research can become a reference in exploring the potential of R mucronata fruit flour as an alternative therapy to replace the hormone estrogen.
MATERIAL AND METHODS
Estrogenic activity study
Estrogenic activity was analyzed for potency using Way2drug Pass online (https://www.way2drug.com/passonline/). Tracing the Pa value (probability to be active) is used to describe the potential of a compound being tested. If the Pa value is more than 0.7, it means that the compound is predicted to have high estrogenic potential by computing and laboratory tests. If the Pa value is more than 0.3 but less than 0.7, it means that the compound has computationally estrogenic capabilities but has not been proven in laboratory tests or has little potential. The control compounds used were ethynyl estradiol and 17β estradiol, which were used as comparisons because they have the potential to act as estrogen agonists [24]. The molecular structures of the test and control compounds (ethynyl estradiol and 17β estradiol) are presented in Figure 1.
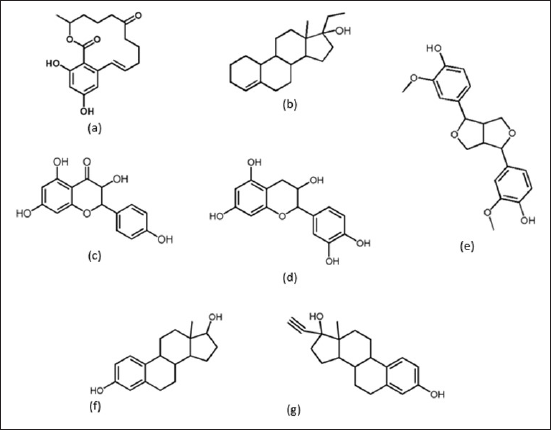 | Figure 1. Molecular structure of test and control compounds: (a) zearalenone, (b) ethylestrenol, (c) aromadendrin, (d) cianidanol, (e) pinoresinol, (f) 17β estradiol/control-1 dan, and (g) ethynil estradiol/control-2. [Click here to view] |
Druglikeness analysis
The compounds were analyzed with SwissAdme software to determine their ability as medicinal compounds. Compounds were selected based on the rule of five (RO5) requirements [25]. Next, the binding strength with the estrogen protein as a receptor was analyzed to obtain the best binding model with the lowest binding affinity.
Molecular docking study
Receptor determination
The material used for molecular docking studies is a protein with the code NF-?B.
Receptor preparation
The material used for the molecular docking study was the NF-?B protein (pdb id: 4IDV), which was downloaded from https://www.rcsb.org/structure/4IDV. The 3D structure of the NF-?B protein binding the ligand 4-{3-[2-amino-5-(2-methoxyethoxy) pyrimidin-4-yl]-1H-indol-5-yl}-2-methylbut-3-yn -2-ol (13V) is presented in Figure 2. This research uses a computer with Intel® CoreTM i7 processor specifications, 8550U @ 1.80GHz 1.99GHz, 8.00 GB RAM. Meanwhile, the software used includes HyperChem 8, Chimera 1.10.1, Autodock 4.2, and Discovery studio® 3.1. (Accelerys, San Diego, USA).
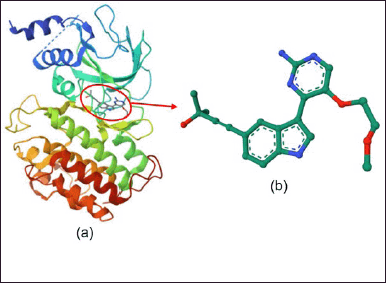 | Figure 2. Structure 3D (a) protein NF-?B and (b) ligan standard (13V). [Click here to view] |
Ligand preparation
The 3D structure of the ligand was taken from the PubChem website. The ligand file is downloaded and then saved. Ligand files in the sdf format were then converted into the pdb format using PyMol version 2.4.1. The file is then converted to the pdbqt format using AV.
Docking receptor ligan
NF-?B protein preparation begins by selecting the active form of the protein that binds to the original ligand (13V). Next, the NF-?B protein and native ligand were separated using the Chimera 1.10.1 program to provide space (pockets/cavities) so that the pocket shape and pocket coordinates were known as docking material.
Validation of the molecular docking method
Validation of the molecular docking method was carried out by docking the native ligand back to the target protein, which had the native ligand removed using the Autodock 4.2 program. The method is said to be valid if the root mean square deviation (RMSD) value obtained is ≤3 Å so that the test compound can be docked with the target protein [26]. An RMSD value ≤3 Å indicates that the native position of the ligand is not much different before and after redocking.
Docking data analysis
The docking analysis stage was carried out using the Discovery studio® 4.5 visualizer program. Data analysis is carried out based on the binding energy and interactions formed between the ligand and the receptor protein. The smaller the binding energy, the more stable the bond between the receptor protein and the ligand. Apart from that, docking data analysis was also carried out by looking at the type, number, and distance of bonds formed between the ligand and the NF-?B protein.
RESULT AND DISCUSSION
Estrogenic activity study
Based on studies of estrogenic activity using Way2drug Pass online, it is known that the five compounds tested have a Pa value (probability to be active) ≥0.3. Pa values above 0.3 indicate that these five compounds have the potential to have estrogenic activity. The estrogenic activity of cianidanol, pinoresinol, and aromadendrin was predicted via estrogen beta receptor agonist and estrogen agonist. The estrogenic activity of zearalenone is via estrogen agonist, while ethylestrenol is via estrogen agonist, estradiol 17β dehydrogenase stimulant, and estrone sulfotransferase simultaneously [27–29]. The Pa values of the five compounds compared with estradiol and ethynylestradiol (as control) are presented in Figure 3.
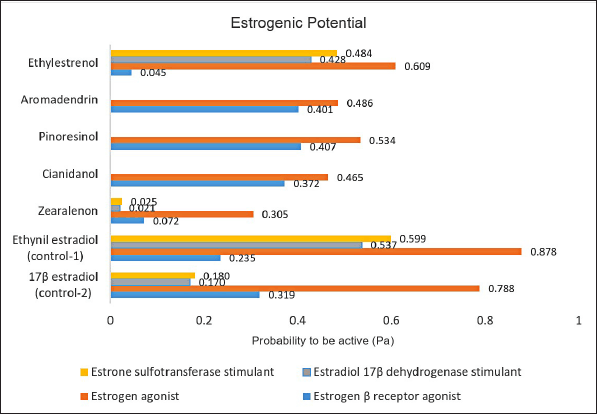 | Figure 3. Probability to be active (Pa) values for test and control compounds. [Click here to view] |
Druglikeness analysis
Based on a druglikeness study using the SwissAdme program, it is known that four test compounds (ethylestrenol, aromadendrin, pinoresinol, and zearalenone) have the potential as drug compounds for oral administration because they meet the provisions of the RO5. Cianidanol cannot be given orally because it has a number of hydrogen bond donors of 5. Lipinski et al. [25] explain that the drug compound can be given orally if it has a molecular weight (<500Da) and high lipophilicity (LogP < 5), hydrogen bond donors are less than 5, hydrogen bond acceptors are less than 10, and molar refractivity is between 40 and 130. Data from the druglikeness analysis of the five test and control compounds are presented in Table 1.
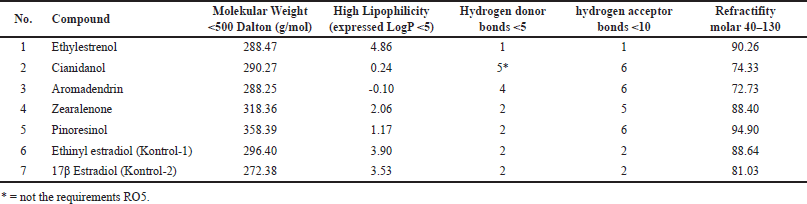 | Table 1. Data from druglikeness analysis of test and control compounds. [Click here to view] |
Molecular docking study
Validation of the molecular docking method through standard ligand redocking (13V) obtained an RMSD value of 0.51 Å and a binding energy of –5.56 kcal/mol. This value indicates that the standard ligand conformation before and after readdition is not much different. A comparison of the conformations of the standard 13V ligand before and after redocking is presented in Figure 4.
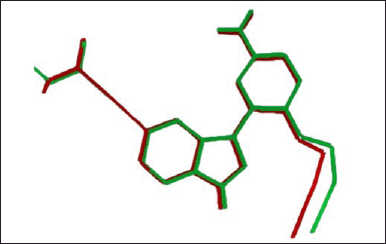 | Figure 4. Comparison of the conformations of the standard 13V ligand before (red) and after (green) redocking. [Click here to view] |
The conformations and grid boxes resulting from the redocking of standard 13V ligands were then used as a reference for molecular docking studies of the compounds: zearalenone, ethylestrenol, aromadendrin, cianidanol, and pinoresinol. These compounds are compounds contained in the ethyl acetate and ethanol fractions of mangrove fruit based on the results of our previous research. Ethynil estradiol and estradiol were used as controls. Data on the molecular formula, molecular weight, PubChem CID, and canonical smiles for each compound are presented in Table 2. Meanwhile, the 3D molecular structure resulting from geometric optimization of the seven compounds is presented in Figure 5.
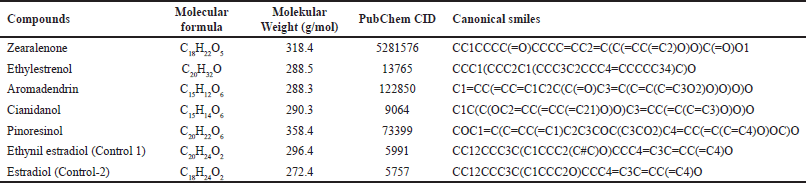 | Table 2. Molecular formula, molecular weight, PubChem CID, and canonical smiles of test and control compounds. [Click here to view] |
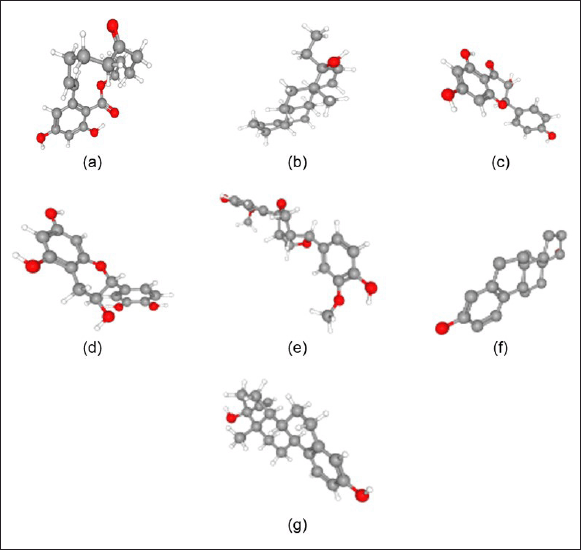 | Figure 5. 3D molecular structures of (a) zearalenone, (b) ethylestrenol, (c) aromadendrin, (d) cianidanol, (e) pinoresinol, (f) 17β estradiol/control-1, and (g) ethynil estradiol/control-2. [Click here to view] |
The results of molecular docking analysis show that the zeralenone compound forms hydrogen bonds with the amino acid residues Glu353, Leu387, and Arg394 in the receptor protein. The distance of the hydrogen bonds formed is 2.55, 2.33, and 2.98 Å. In addition to hydrogen bonds, zearalenone also forms alkyl and pi-alkyl bonds with the amino acid residues Met388, Leu346, Leu391, Ala350, Leu384, Trp383, and Leu525. The van der Waals interaction between zearalenone and the receptor protein occurs at the amino acid residues Leu428, Ile424, Gly521, Met421, Met343, Val533, Thr347, and Leu349 (Fig. 6).
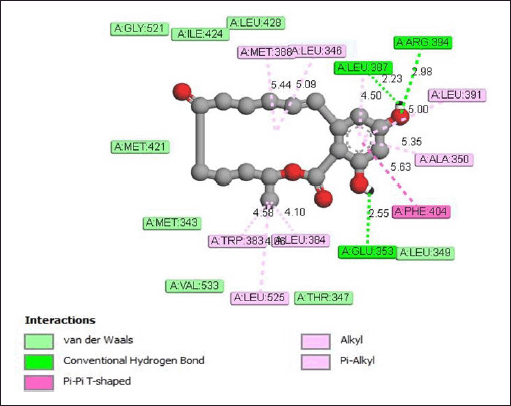 | Figure 6. Interaction of zearalenone with estrogen receptor protein. [Click here to view] |
The interaction of ethylestrenol with the estrogen receptor protein (Fig. 7) occurs through the formation of alkyl and Pi-Alkyl bonds with the amino acid residues Leu346 (4.47 Å), Leu 384 (4.38 Å), Leu428 (5.42 Å), Met388 (4.72 Å), Leu391 ( 4.53 Å), Ala350 (5.15 Å), Leu349 (4.60 Å), Phe404 (5.41 Å), and Leu387 (4.98 Å). In addition to alkyl and pi-alkyl bonds, ethylestrenol also produces van der Waals interactions with the amino acid residues Gly521, His524, Glu353, Met343, Met421, Arg394, and Ile424.
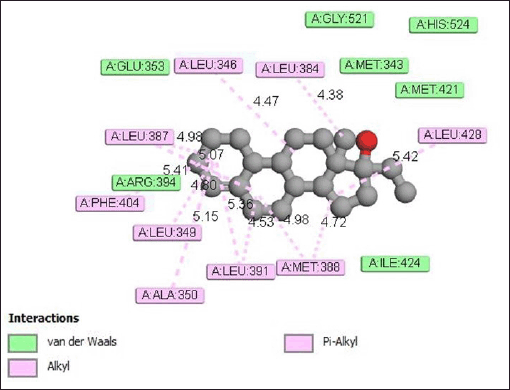 | Figure 7. Interaction of ethylestrenol with estrogen receptor protein. [Click here to view] |
Figure 8 shows the interaction between aromadendrin and the receptor protein through the formation of hydrogen bonds with the amino acid residues Glu353 (2.36 Å) and Gly521 (2.49 Å). In addition, pi-alkyl bonds are also formed with amino acid residues Leu387 (5.34 Å), Leu346 (5.42 Å), Leu391 (5.13 Å), and Ile424 (5.41 A). Meanwhile, van der Waals interactions occur with the amino acid residues Trp383, Ala360, Leu384, Leu525, Leu349, His524, Arg394, and Met421.
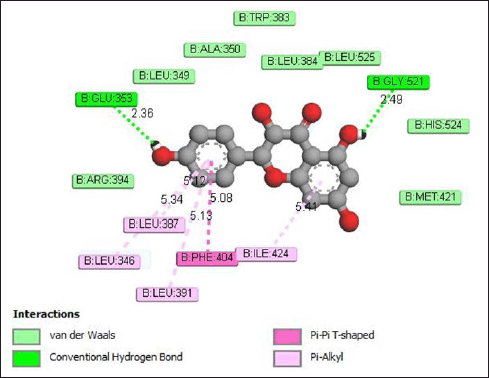 | Figure 8. Interaction aromadendrin with estrogen receptor protein. [Click here to view] |
The interaction of cianidanol with the estrogen receptor protein (Fig. 9) occurs through the formation of hydrogen bonds to the amino acid residues Gly521 and Glu353. The hydrogen bond distances formed are 2.43 and 3.00 Å. The pi-alkyl bond between cianidanol and the estrogen receptor protein occurs at amino acid residues Leu346 (5.37 Å), Leu391 (5.21 Å), and Leu387 (5.04 Å).
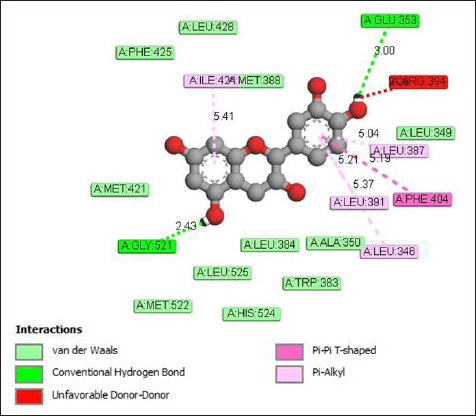 | Figure 9. Interaction of estrogen receptor protein with the cianidanol compound. [Click here to view] |
The interaction of pinoresinol with the estrogen receptor protein (Fig. 10) occurs through the formation of hydrogen bonds with the Arg394 amino acid residue with a bond distance of 2.80 Å. Apart from that, carbon–hydrogen bonds are also formed with amino acid residues Trp393 (3.39 Å), Pro324 (5.40 Å), and Pro325 (3.51 Å), as well as alkyl and pi-alkyl bonds to Phe445 (5.30 Å) and Pro324 (4.24 Å). Meanwhile, van der Waals interactions occur on the amino acid residues His356, Glu353, Glu323, Ile326, Met357, Leu387, Ile386, Lys449, and Gly390.
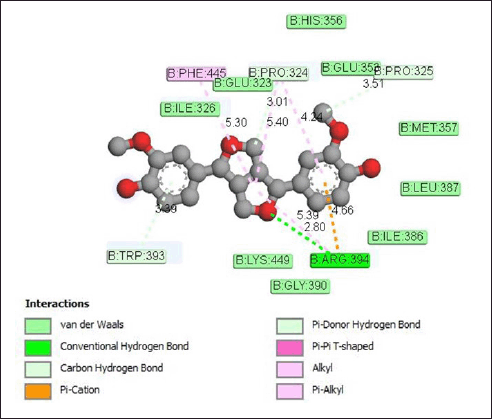 | Figure 10. Interaction pinoresinol with estrogen receptor protein. [Click here to view] |
Based on the results of molecular docking studies, it is known that the compounds, zearalenone, ethylestrenol, aromadendrin, cianidanol, and pinoresinol, form alkyl bonds with several amino acid residues found in the estrogen receptor protein. The formation of hydrophobic or fat-soluble alkyl bonds plays a role in the drug–receptor interaction mechanism. Of the five test compounds, zearalenone produces a greater number of hydrogen bonds than the other four compounds, namely, three hydrogen bonds. The more bonds produced, the better the interactions that occur between a molecule and the target protein. Apart from that, hydrogen bonds are also known to be the dominant interaction in studying the activity of a medicinal compound [30,31]. The strength of hydrogen bonds varies greatly and generally occurs in groups of electron-rich heteroatoms and electron-deficient hydrogen atoms [32,33]. Most hydrogen bonds have a binding energy of 16 to 60 kJ/mol or about 10 times less than covalent bonds.
The strong interaction between zearalenone and the estrogen receptor protein can also be seen from the large binding affinity produced (Fig. 11). The binding affinity value between zearalenone and the estrogen receptor protein is –9.23 kcal/mol. This value is higher than the control compounds (17β estradiol and ethynyl estradiol), which have binding affinity values of 8.63 and 8.83 kcal/mol, respectively. Thus, the interaction produced by zearalenone with the estrogen receptor protein is better than the control compound.
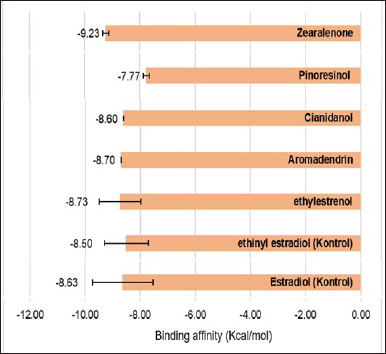 | Figure 11. Binding affinity of estrogen receptor protein with test and control compounds. [Click here to view] |
Pathway analysis
The results of the molecular docking analysis showed that the interaction produced by zearalenone with the estrogen receptor protein was better when compared to control compounds (ethinyl estradiol and 17β estradiol), as well as four other test compounds (pinoresinol, cianidanol, aromadendrin, and ethylestrenol). Therefore, the study continued with stitch analysis to predict the interaction mechanism between zearalenone and receptor proteins. The results of the stitch analysis are presented in Figure 12.
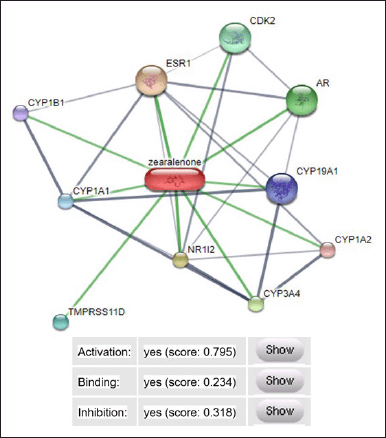 | Figure 12. Mechanism of interaction of zearalenone with estrogen receptor protein. ESR1 = estrogen reseptor, CYP1A1 = cytochrome P450 family 1 subfamily A1, CYP1B1 = cytochrome P450 family 1 subfamily B1, TMPRSS11D = transmembrane protease serine 11D, NR112 = nuclear receptor subfamily 1, AR = androgen receptor, CDK2 = cyclin dependent kinase 2. [Click here to view] |
The prediction results for zearalenone interactions through stitch analysis obtained an activation score of 0.795, a binding strength of 0.234, and an inhibition of 0.318. The strongest interaction is shown by the thick and short green line, which indicates that zearalenone has estrogenic potential through the activation of the estrogen receptor (ESR1), nuclear receptor subfamily 1 (NR112), and androgen receptor (AR). Another interaction that occurs is through activation of the zearalenone compound with CDK2, CYP1A1, CYP1B1, TMPRSS11D, CYP3A4, CYP1A2, and CYP19A1.
CONCLUSION
Zearalenone, pinoresinol, aromadendrin, and ethylestrenol from the ethyl acetate and ethanol fractions of R. mucronata fruit flour are known to have potential estrogenic activity. The strongest interaction with the estrogen receptor protein was shown by zearalenone with a binding affinity of –9.23 Kcal/mol and the formation of hydrogen bonds to the amino acid residues Glu353, Leu387, and Arg394. The estrogenic potential of zearalenone is through activation of the estrogen receptor (ESR1), nuclear receptor subfamily 1 (NR112), and AR.
ACKNOWLEDGMENTS
We want to acknowledge Brawijaya University, Indonesia, for providing research facilities and Postgraduate School Universitas Airlangga for support by Mandatory Grant with Reference Number 1809/B/UN3.SPS/PT.01.03/2024. This study was funded by the Ministry of Education, Culture, Research, and Technology, Government of Indonesia (Contract No. 009/SP2H/PPKM/LL7/2022).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
AVAILABILITY OF DATA AND MATERIALS
The corresponding author can provide access to the data used to support the findings of this research upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109–46. CrossRef
2. Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220–31.
3. Barenberg BJ, Pickett SD. Menopausal hormone therapy BT. In: Shoupe D, editor. Handbook of gynecology. Cham, Switzerland: Springer International Publishing; 2023. pp. 639–47. CrossRef
4. Fait T. Menopause hormone therapy: latest developments and clinical practice. Drugs Context. 2019;8:1–9.
5. David PS, Sobel T, Sahni S, Mehta J, Kling JM. Menopausal hormone therapy in older women: examining the current balance of evidence. Drugs Aging [Internet]. 2023;40(8):675–83. CrossRef
6. Landrum LM, Zuna RE, Walker JL. Endometrial hyperplasia, estrogen therapy, and the prevention of endometrial cancer [Internet]. In: DiSaia PJ, Creasman WT, editors. Clinical gynecologic oncology. 9th ed. Amsterdam, The Netherlands: Elsevier Inc.; 2018. pp. 105–20.e6. CrossRef
7. Eliyahu E, Katz MG, Vincek A, Freage-Kahn L, Ravvin S, Tal S, et al. Effects of hormone replacement therapy on women’s lung health and disease. Pulm Ther [Internet]. 2023;9(4):461–77. CrossRef
8. Curran M, Wolde T, Vazquez A, Mihulka O, Moore J, Rojas KE. Best practices for hormonal contraception and menopause therapy in women at increased risk for breast cancer. Curr Breast Cancer Rep [Internet]. 2024;16:342–50. CrossRef
9. Loizzi V, Dellino M, Cerbone M, Arezzo F, Chiariello G, Lepera A, et al. Hormone replacement therapy in BRCA mutation carriers: how shall we do no harm? Hormones [Internet]. 2023;22(1):19–23. CrossRef
10. Gu Y, Han F, Xue M, Wang M, Huang Y. The benefits and risks of menopause hormone therapy for the cardiovascular system in postmenopausal women: a systematic review and meta-analysis. BMC Womens Health [Internet]. 2024;24(1):60. CrossRef
11. Mikkola TS, Tuomikoski P, Lyytinen H, Korhonen P, Hoti F, Vattulainen P, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–94.
12. Rouhana S, Glen Pyle W. Menopause and the bridge to cardiovascular disease BT. In: Kirshenbaum L, Rabinovich-Nikitin I, editors. Biology of women’s heart health. Cham, Switzerland: Springer International Publishing; 2023. pp. 145–64. CrossRef
13. Chang WC, Wang JH, Ding DC. Menopausal hormone therapy with conjugated equine estrogen is associated with a higher risk of hemorrhagic stroke than therapy with estradiol: a retrospective population-based cohort study. Maturitas [Internet]. 2022;165(707):72–7. CrossRef
14. Hashemzadeh M, Romo R, Arreguin JM, Movahed MR. The effects of estrogen and hormone replacement therapy on cardiovascular systems. Future Cardiol. 2021;17(2):347–53.
15. Lee SR, Cho MK, Cho YJ, Chun S, Hong SH, Hwang KR, et al. The 2020 menopausal hormone therapy guidelines. J Menopausal Med. 2020;26(2):69.
16. Rani J, Swati S, Meeta M, Singh SH, Tanvir T, Madan A. Postmenopausal osteoporosis: menopause hormone therapy and selective estrogen receptor modulators. Indian J Orthop [Internet]. 2023;57(s1):105–14. CrossRef
17. Rietjens IMCM, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol. 2017;174(11):1263–80.
18. Wyse J, Latif S, Gurusinghe S, McCormick J, Weston LA, Stephen CP. Phytoestrogens: a review of their impacts on reproductive physiology and other effects upon grazing livestock. Animals. 2022;12(19):1–17.
19. Nikoli? MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife. 2017;6:1–33.
20. Risjani Y, Darmawan A, Putri Renitasari D, Lorma Ayuknita A, Rahma F, Effendi S, et al. Histopathological aberration and 17-β-estradiol imbalance in green mussel Perna viridis population cultured in Java Sea, Indonesia. Egypt J Aquat Res [Internet]. 2022;49:197–203. CrossRef
21. Yu Z, Jiao Y, Zhao Y, Gu W. Level of Estrogen in females—the different impacts at different life stages. J Pers Med. 2022;12(12):1995.
22. Bacciottini L, Falchetti A, Pampaloni B, Bartolini E, Carossino AM, Brandi ML. Phytoestrogens: food or drug? Clin Cases Miner Bone Metab. 2007;4(2):123–30.
23. Dutta S, Mahalanobish S, Sil PC. Chapter 8?Phytoestrogens as novel therapeutic molecules against breast cancer. In: Brahmachari G, editor. Natural product drug discovery [Internet]. Amsterdam, The Netherlands: Elsevier; 2021. pp. 197–229. Available from: https://www.sciencedirect.com/science/article/pii/B9780128212776000088
24. Kar P, Goyal AK, Das AP, Sen A. Antioxidant and pharmaceutical potential of Clerodendrum L.: an overview. Int J Green Pharm. 2014;8(4):210–6.
25. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev [Internet]. 2012;64(SUPPL):4–17. CrossRef
26. Jain AN, Nicholls A. Recommendations for evaluation of computational methods. J Comput Aided Mol Des. 2008;22(3–4):133–9.
27. Ernawati, Adam MA, Widiastuti IM, Insivitawati E, Nikmatullah M, Riyadi PH, et al. Exploring the anti-menopausal potential of Rhizophora mucronata lam. ethanol extract: a comprehensive study on estrogen receptor β agonist activity. Ilmu Kelaut Indones J Mar Sci. 2024;29(3):414–24.
28. Adam M, Talbia H, Ariyanti D, Kristianto S, Chairunnisa N, Aprilia M, et al. Microplastics contamination in environment and marine animals at Kodek Bay, Lombok, Indonesia. Water Air Soil Pollut [Internet]. 2024;235(789):1–16. CrossRef
29. Zhu J. Effector CD4+ T lymphocytes. Ref Modul Biomed Sci. 2014;4:1–12. CrossRef
30. Agu PC, Afiukwa CA, Orji OU, Ezeh EM, Ofoke IH, Ogbu CO, et al. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci Rep [Internet]. 2023;13(1):1–18. CrossRef
31. Rauf A, Al-Awthan YS, Bahattab O, Shah ZA, Rashid U, Bawazeer S, et al. Potent urease inhibition and in silico docking study of four secondary metabolites isolated from Heterophragma adenophyllum seem. South African J Bot [Internet]. 2021;142:201–5. CrossRef
32. Ernawati E, Adam MA, Widiastuti IM, Insivitawati E. Physical and chemical characterization of African catfish smoked sausage with different liquid smoke concentrations and immersion durations. E3S Web Conf. 2021;322:04001.
33. Adam MA, Khumaidi A, Ramli R, Widiastuti IM, Ernawati E, Insivitasti E, et al. Detoxification mechanisms in oxidative stress and reactive oxygen species (ROS) in gills of gambusia fish (Gambusia affinis) exposed to Cadmium. E3S Web Conf. 2021;322:01025