INTRODUCTION
As bones grow and changes throughout a person’s lifetime, osteoblasts and osteoclasts work together to continuously and precisely remodel the bones. The most common cause of imbalanced bone remodeling is the deregulated coupling between the major bone cells, the increment of osteoclast resorption activity over osteoblast bone formation rate, or the declination of bone turnover rate. Osteoporosis is a common bone disease in adults and is the consequence of this process (Boyle et al., 2003; Rachner et al., 2011). New research indicates that long-term use of antiosteoporotic drugs may have negative health effects on humans, despite their ability in order to boost bone strength and reduce the risk of fracture (Curtis et al., 2015). It is imperative that new, less toxic agents are developed for osteoporosis prevention and treatment. Numerous drugs have been developed to help prevent and treat this condition. In contrast to the traditional prevention focus on other trace elements such as calcium and vitamin D, the identification of multiple nutrients with bone-sparing properties has been made possible by recent preclinical strategies for prevention. It has been demonstrated that natural products, in particular, can be valuable sources of novel osteoporosis drugs (Schulman et al., 2011).
A female mammal’s placenta is a temporary organ which grows during pregnancy. The placenta acts as a blood vessel connecting the mother’s uterus to the fetus to allow oxygen and nutrients to flow to the developing baby (Kapila and Chaudhry, 2021). As a consequence, the placenta generates and enriches bioactive components such as growth factors and hormones as well as essential minerals, vitamins, and cytokines (Phuapradit et al., 2000). Placenta extract is used by a diverse range of mammals, such as porcine, bovine, and humans, and has been shown to improve the effectiveness of biological and therapeutic applications such as increasing personal health and energy (Cross, 2016), promoting hair regrowth (Kim et al., 2020), reducing and relieving pain (Park and Cho, 2017), and increasing wound healing activity (Shukla et al., 2004), along with anti-inflammatory effects (Heo et al., 2018), antimicrobial properties (King et al., 2007), and antioxidant effects (Rozanova et al., 2012). Some studies have shown that porcine placenta extract (PPE) has an enhancement effect on osteoblasts. According to a recent study, the placenta regulates ER stress and the production of reactive oxygen species in osteoblasts (Lee et al., 2016), while placental-derived adherent cells prevented bone loss and promoted bone formation by inhibiting the formation of osteoclasts and stimulating the differentiation of osteoblasts in the host (Li et al., 2011). Therefore, understanding the mechanism underlying this process would be highly valuable in evaluating the effect of PPE in an in vitro assay. The present study demonstrates that PPE can enhance human osteoblast activity, including cell proliferation differentiation and mineralization, mediated by induction of the prosurvival signaling pathway. These results suggest that a possible source of bioingredients from agricultural industry waste for osteoporosis treatment and prevention, according to these findings, is PPE.
MATERIALS AND METHODS
Cell culture
Human osteoblast hFOB 1.19 cells were purchased from the American Type Culture Collection (ATCC). Osteoblast cells were maintained to standard guidelines of good cell culture practice in a 1:1 mixture of Ham’s F12 Medium to Dulbecco’s Modified Eagle’s Medium, with 2.5 mM L-glutamine (without phenol red). G418 0.3 mg/ml was added to the base medium, and fetal bovine serum was supplemented at 10% final concentration.
Preparation of PPE
The PPE was obtained from the Faculty of Sciences, Mahidol University, Bangkok, Thailand. The porcine placenta was washed and homogenized in a phosphate buffer saline (PBS) solution. Subsequently, the sonication and centrifugation of the homogenate were conducted at 4°C for 1 hour. Eventually, the supernatant was collected and sterilely filtrated with 0.2 µm filters. The Bradford assay was performed to measure the protein concentration that the PPE lastly labeled with the exact concentration.
Cell viability assessment using MTT assay
Cell viability was determined by the MTT assay. In brief, seeded osteoblast cells were grown overnight at a density of 5×103 cells per well in a 96-well cell culture plate. PPE was then added in distinct concentrations and incubated for desired incubation times. At the end of incubation, the MTT reagent was dispensed and sequentially incubated for 4 hours. The Dimethyl sulfoxides (DMSO)-solubilized formazan crystal was used to measure the light absorbance by a microplate spectrophotometer at 570 nm. The cell viability was calculated as follows: [(PPE treated Abs570)/(control Abs570)] × 100 (%).
Alkaline phosphatase activity measurement
Osteoblast cells were grown overnight at a density of 1 × 103 cells per well in a 6-well cell culture plate. The cell culture medium was replaced with an osteogenic induction medium (0.1 µM dexamethasone, 10 mM β-glycerophosphate, and 50 µg/ml of ascorbic acid) containing PPE at the desired concentration and incubated for 3, 5, and 7 days. Treated cells were washed twice with a PBS solution before adding 50 mM Tris-HCl, pH 7.5, plus protease inhibitor cocktail and lysed using ultrasonic sonication. The extract protein was collected using high-speed centrifugation, and enzyme activity was measured by using Cobas c 111 analyzers.
Mineralization assay
Seeded osteoblast cells were grown overnight at a density of 5 × 103 cells per well in a 96-well cell culture plate. The cell culture medium was replaced with an osteogenic induction medium containing PPE at the desired concentration and incubated for 7 and 14 days. Cells were then washed twice with a PBS solution before fixing with 10% formaldehyde for 10 minutes. Subsequently, the cells were stained with Alizarin red and photographed under an inverted microscope to observe mineralization. The level of calcium deposition was quantified by elution of Alizarin red with 10% acetic acid, and the light absorbent was measured at 405 nm. Data is presented as relative to the control (the osteogenic induction medium).
RNA extraction and real-time polymerase chain reaction (PCR)
hFOB 1.19 cells were treated with an osteogenic induction medium and/or with PPE for 4 days. Nucleic acid was extracted with the Total RNA Mini Kit (Bio-Rad, CA) as per the manufacturer’s recommendation. First-strand cDNA was synthesized using the Tetro cDNA synthesis kit (Bioline, UK) and real-time PCR was performed. Primer sequences are listed in Table 1. The gene expression was analyzed as the relative quantification with the values of 2−ΔΔCT, normalized with the GAPDH expression level.
Western blot analysis
Human osteoblast cells were treated with PPE with or without inhibitors at the indicated concentration. The cells were then washed with ice-cold PBS and intracellular protein harvested with a RIPA buffer. Protein concentration was quantified by using the Bradford assay. The protein was then separated by SDS-gel electrophoresis and transferred onto PVDF membranes. After blocking with a 5% skim milk buffer, the membrane was then incubated and gently agitated with anti-pAkt, anti-Akt, anti-pERK, anti-ERK, anti-pJNK, anti-JNK, anti-β-actin, and anti-cyclin D1 (CST, MA) at 4°C overnight. After that, the membranes were washed and incubated with HRP-linked anti-rabbit antibody (CST, MA) for 1 hour at room temperature. The incubated membranes were washed twice and the expression signal was detected by using ChemiDoc™ XRS and analyzed by Image Lab (Bio-Rad, CA).
Statistical analysis
All values are presented as mean ± SEM. One-way analysis of variance was used to determine significant differences between groups, accompanied by the Tukey–Kramer test regarding appropriation. p value < 0.05 was accounted as a statistically significant difference. Statistical testing was analyzed using GraphPad Prism version 7.
 | Table 1. Primer sequences for quantitative real-time PCR. [Click here to view] |
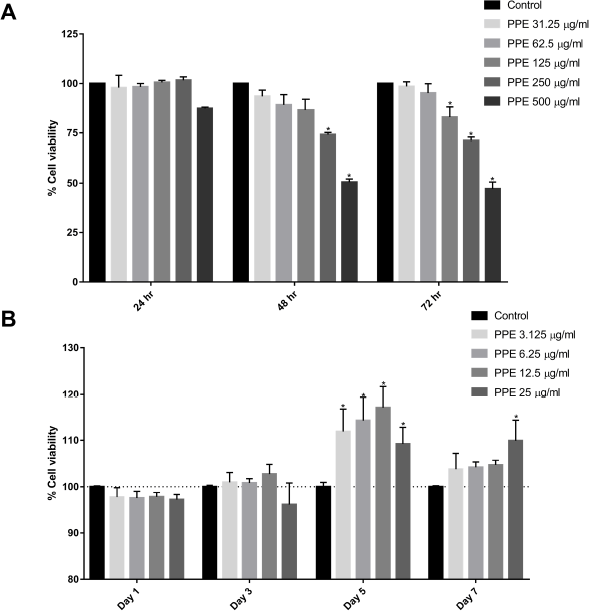 | Figure 1. PPE activated osteoblast proliferation. (A) Cytotoxicity screening of PPE on hFOB 1.1.9 cell at 0–500 µg/ml. (B) Proliferation effect of PPE at a concentration of 0–25 µg/ml on human osteoblast cells. hFOB 1.1.9 cells were seeded in a 96-well plate and incubated with the indicated concentration of PPE. Cell viability was accessed using the MTT assay. Data presented as % cell viability normalizing with control. Each value is expressed as mean ± SEM (n = 9). p value < 0.05 was included as a statistically significant difference. [Click here to view] |
 | Figure 2. PPE enhances osteogenic phenotypes of human osteoblast cells. hFOB 1.1.9 cell was treated with an osteogenic induction medium supplemented with PPE at the indicated concentration. (A) ALP enzyme activity was measured from total cell lysate at days 3, 5, and 7 and showed as an international unit enzyme. (B) Treated cells were stained with Alizarin red and photographed under an inverted microscope and subsequently eluted for quantification compartment (C). Each value is expressed as mean ± SEM (n = 6). p value < 0.05 was included as a statistically significant difference. [Click here to view] |
 | Figure 3. PPE promotes osteogenesis-related genes. hFOB 1.19 cells were seeded into a 6-well plate and subsequently incubated with either osteogenic induction medium or PPE at the indicated concentration for 4 days. The cellular mRNA was extracted and the expression of osteogenesis-related genes measured using real-time PCR. Data was calculated as relative mRNA expression to the osteogenic induction medium group. Data are shown as the mean ± SEM (n = 6). p value < 0.05 was included as a statistically significant difference. [Click here to view] |
RESULTS
PPE-induced osteoblast proliferation
To optimize the nontoxic concentration of PPE, hFOB 1.19 cells were incubated with various concentrations of PPE at 0–500 µg/ml and viability was observed for 3 days. The results show that high concentrations of PPE (over 125 µg/ml) significantly suppressed the viability of osteoblasts. Medium concentrations (62.5–125 µg/ml) slightly suppressed the viability at 48 and 72 hours, whereas low concentrations of PPE did not affect osteoblast viability (Fig. 1A). Therefore, the optimal concentrations of PPE for use in this study were 0–30 µg/ml.
The optimal concentration of PPE was further investigated for osteoblast proliferative effect. After coincubation with PPE for 7 days, 3.125–25 µg/ml of PPE significantly increased cell proliferation rate when compared with the control group (Fig. 1B). The peak of cell proliferation was detected at day 5 regarding PPE concentration, suggesting that a low concentration of PPE could induce osteoblast proliferation.
PPE enhanced osteogenic activity of osteoblast cells
Osteoblast maturation is a crucial process for bone formation, which increases alkaline phosphatase (ALP) enzyme production, matrix deposition, and mineralization. The present study investigated the context of PPE on osteoblast maturation by measuring ALP activity and mineralization. The results show that PPE significantly increased ALP enzyme activity compared with the control, with an incremental effect in a time-dependent manner (Fig. 2A). Moreover, PPE could enhance calcium deposition demonstrated by overstaining of red color when compared with the induction medium group, as shown in Figure 2B. To confirm, stained cells were eluted, and the light absorbent was measured. The results demonstrate that increasing PPE concentration significantly enhanced mineralization in human osteoblasts (Fig. 2C). From these results, it can be concluded that PPE positively enhances osteogenic activity involved with the activation of human osteoblast maturation.
PPE promotes osteogenesis-related markers
To further confirm the osteogenic differentiation of human osteoblasts, PPE-treated cells were measured for their relative expression of osteogenesis-related genes, including ALP, COL1 (collagen type 1), OCN (osteocalcin), and RUNX2 (Runt-related transcription factor 2) genes. PPE treatment markedly increased the expression levels of ALP, COL1, and RUNX2 genes; peak expression was observed at 12.5 µg/ml of PPE, whereas the expression level of the OCN gene showed an increasing trend compared with the osteogenic induction medium group (Fig. 3). Importantly, PPE promoted osteogenesis differentiation of human osteoblasts.
PPE induced phosphorylation of ERK1/2, Akt, and JNK
The activation of the MAPK, AKT, and JNK signaling pathways is well known to be associated with cellular proliferation and maturation of osteoblast cells. Therefore, the researchers investigated the effects of PPE on the induction of the signaling pathway associated with osteogenesis. The results indicate that PPE induced phosphorylation of Akt, ERK1/2, and JNK as early as 5–30 minutes after the addition of PPE to the hFOB 1.19 cell lines, respectively (Fig. 4A). Phosphorylation reached a maximum level at 5–60 minutes after exposure and then decreased. These are confirmed in Figure 4B, the addition of LY294002 (Akt inhibitor), PD98059 (ERK inhibitor), and SP600125 (JNK inhibitor) that PPE treatment condition attenuated the phosphorylation of Akt, ERK1/2, and JNK, respectively, when compared with the untreatment condition. These suggest that PPE is involved in the induction of the ERK, PI3K-AKT, and JNK signaling pathways.
 | Figure 4. PPE induced phosphorylation of Akt, ERK1/2, and JNK. (A) hFOB 1.19 cells were incubated with and without indicated PPE concentration and times. The survival-associated signaling proteins were determined by using immunoblotting. (B) Additional incubation with LY294002, PD98059, and SP600125 for 15 μM on PPE treatment attenuated signaling protein overexpression. β-actin was used as protein loading control of immunoblotting experiment. [Click here to view] |
The molecular mechanism of PPE was further investigated, targeting cell cycle regulatory protein, which is involved in cell cycle transition from the G0 to S phase. Interestingly that PPE markedly induced the expression of the cyclin D1 protein. In addition to the inhibitory effect of the signaling protein (Fig. 4B), all of them attenuated PPE-induced overexpression of cyclin D1. This indicates that the induction effect of PPE on osteoblast proliferation is likely associated with overexpression of cyclin D1 and is mediated by phosphorylation of ERK1/2, Akt, and JNK signaling.
DISCUSSION
Osteoblasts are mesenchymal cells that play a major role in osteogenesis, including synthesizing the bone matrix and coordinating the mineralization of the skeleton. This study demonstrates the effectiveness of PPE on osteogenic activity enhancement. PPE proficiently induced osteoblast proliferation and differentiation which was reflected by incrementally increased osteogenesis-related markers and mineralization.
Several bioactive compounds are components of PPE that have been extensively studied for their potential to improve skin care (Nagae et al., 2020), strengthen immune function (Lee et al., 2013), and play a role as an anti-antioxidant and improve metabolic functions (Nensat et al., 2021). Insulin-like growth factor-1 is a key regulator of fetal development found in the placenta, which is associated with the activation of type I collagen synthesis in osteosarcoma cells (Kudo et al., 1996). Active components in PPE that are entirely used in the present study are also characterized by LC-MS/MS and data analyzed against the domestic porcine Sus scrofa proteome database. The PPE in this study consists of 391 protein sequences from the entire 447 proteins. The researcher suggests the PPE in this study is mostly comprised signal-involved proteins, phosphoproteins, and disulphide-bond proteins (Padhomchai Pumbthongthae, 2020). This suggests that the signal-associated proteins may play an important function in the induction of osteoblast differentiation, such as insulin-like growth factor-1 and basic fibroblast growth factors.
With 60 µg/ml of PPE extract, osteoblast proliferation was induced without toxicity. The low concentrations of PPE exhibited bioactivities greater than higher concentrations, which may be associated with the toxicity from the high protein content of the extract. This is similar to other preparations of PPE in that 20–200 µg/ml of extract activates cell proliferation but results in higher concentrations of toxicity (Imamura et al., 2017). Moreover, the researchers also investigated the effect of PPE in various cell types including endothelial cells, fibroblasts, and keratinocytes in which PPE was found to exhibit bioactivity at low concentrations (data not shown). Therefore, 25 µg/ml of PPE was selected as the highest dose to investigate osteogenic activity on osteoblasts. As is well known, new bone formation is mainly mediated by osteoblasts which either increase the osteoblast proliferation or induce osteoblast differentiation (Ducy et al., 2000). PPE dramatically increased osteoblast proliferation on day 5 and slightly induced it on day 7. The extract likely enhances osteoblast proliferation 5 days after maturation. As shown in Figure 3, PPE markedly enhanced ALP production and mineralization after 7 days. These phenotypes indicate the proficient property of PPE on osteoblast maturation and differentiation enhancement.
The induction of osteoblast differentiation was confirmed by the upregulation of osteogenic genes that are involved in bone formation markers. Runx2 genes play an important function as transcription factors for other osteogenic proteins. Our findings demonstrate that PPE enhances the expression of Runx2, ALP, COL1, and OCN genes, indicating that PPE induced osteogenic maker through upregulation of Runx2. It has been shown that Runx2 is the central regulator of bone formation, which is influenced by many signals such as fibroblast growth factor (Choi et al., 2005; Kim et al., 1998) and bone morphogenetic protein 2 (Lee et al., 2003). Runx2-deleted mice caused osteoblast deficiency and failed to form bone (Otto et al., 1997). Moreover, the level of functional Runx2 is also responsible for bone loss in estrogen deficiency (Maruyama et al., 2007). Therefore, upregulation of Runx2 by PPE enhancement could provide a therapeutic application in bone disorders.
Activation of growth factor receptors subsequently results in phosphorylation of the MAPK pathway. It has been reported that FGF induced ERK MAP kinase phosphorylation and later increased Runx2 and was involved in the induction of osteoblast differentiation (Park et al., 2010). In addition to the PI3K-Akt signaling pathway, this signal has been implicated as a critical pathway for the differentiation of skeletal component cells, including chondrocytes and osteoblasts (Ghosh-Choudhury et al., 2002; Hidaka et al., 2001). A lack of Akt1 and Akt2 is the cause of delayed bone development (Peng et al., 2003). Interestingly, regulation of osteoblast differentiation and migration is cooperatively dependent on PI3K-Akt signaling and Runx2. The present study presented the effect of PPE on the induction of ERK and Akt phosphorylation. Moreover, PPE also upregulated the phosphorylation of JNK. This finding is in accordance with a previous study which showed that JNK is a key mediator of osteoblast differentiation and mineralization activity (Xu et al., 2017). Taking these data together, PPE regulated and enhanced the proliferation and differentiation of human osteoblasts mediated by either the ERK MAP kinase, PI3K-Akt, and JNK signaling pathways and/or subsequent upregulation of Runx2 (Graphical Abstract). In this study, we intently present the osteogenic activities of PPE in terms of basic characteristics and main molecular mechanisms in human osteoblasts. Although an in vitro setting appropriately demonstrates the bioactivities of PPE, further information is still crucial for confirmation and development. The effects of the compound on other bone cells such as osteoclasts, bone matrix conformation, and in vivo models are urgently needed to prove the exhibition of the overall function of PPE in bone remodeling.
CONCLUSION
The present study demonstrated the bioactivity of PPE on human osteoblast activity, including cell proliferation, differentiation, and mineralization. In addition, the researchers confirmed the positive benefits of cell signaling investigation. These results suggest that PPE is a potential bioactive compound from agriculture industry waste that could enhance osteoblast activity for osteogenesis. Further investigation of active bioingredients of PPE and their effectiveness in animal models is required to improve clinical applications for bone disorders.
ACKNOWLEDGMENTS
The authors thank Kumphune S. for providing essential chemicals and excellent suggestions.
FINANCIAL SUPPORT
This study has been funded by the Office of National Higher Education Science Research and Innovation Policy Council (NXPO), Thailand, through the Program Management Unit for Manpower Development and Funding for Higher-Education Institute Development, Research and Innovation (PMU-B, Grant number B05F630066), and Program Management Unit for National Competitiveness Improvement (PMU-C), CCF Energy Supplement (Thailand) Limited, and Mahidol University (Grant number C10F640021).
AUTHORS’ CONTRIBUTIONS
WS, CN, WK, and AJ originated and outlined experiments; WS conducted the experiments; WS, CN, and AJ interpreted the data; AJ, WK, TS, and RT provided laboratory materials. All authors contributed to manuscript preparation.
CONFLICTS OF INTEREST
The authors disclose that they have no conflicts of interest in this paper.
DATA AVAILABILITY
All the data generated in the study is included in the article itself.
ETHICAL APPROVAL
This study does not include any experiments on humans or animals.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature, 2003; 423:337–42. CrossRef
Choi KY, Kim HJ, Lee MH, Kwon TG, Nah HD, Furuichi T, Komori T, Nam SH, Kim YJ, Kim HJ, Ryoo HM. Runx2 regulates FGF2-induced Bmp2 expression during cranial bone development. Dev Dyn, 2005; 233:115–21. CrossRef
Cross JC. Adaptability and potential for treatment of placental functions to improve embryonic development and postnatal health. Reprod Fertil Dev, 2016; 28:75–82. CrossRef
Curtis EM, Moon RJ, Dennison EM, Harvey NC, Cooper C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin Med (Lond), 2015; 15 Suppl 6:s92–6. CrossRef
Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science, 2000; 289:1501–4. CrossRef
Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem, 2002; 277:33361–8. CrossRef
Heo JH, Heo Y, Lee HJ, Kim M, Shin HY. Topical anti-inflammatory and anti-oxidative effects of porcine placenta extracts on 2,4-dinitrochlorobenzene-induced contact dermatitis. BMC Complement Altern Med, 2018; 18:331. CrossRef
Hidaka K, Kanematsu T, Takeuchi H, Nakata M, Kikkawa U, Hirata M. Involvement of the phosphoinositide 3-kinase/protein kinase B signaling pathway in insulin/IGF-I-induced chondrogenesis of the mouse embryonal carcinoma-derived cell line ATDC5. Int J Biochem Cell Biol, 2001; 33:1094–103. CrossRef
Imamura Y, Honda Y, Masuno K, Nakamura H, Wang P-L. Effects of placental extract on cell proliferation, type i collagen production, and ALP secretion in human osteosarcoma cell line Saos-2. J Hard Tissue Biol, 2017; 26:157–60. CrossRef
Kapila V, Chaudhry K. Physiology, placenta. In StatPearls. StatPearls Publishing, Treasure Island, FL, 2021.
Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development, 1998; 125:1241–51. CrossRef
Kim MH, Kim K, Lee H, Yang WM. Human placenta induces hair regrowth in chemotherapy-induced alopecia via inhibition of apoptotic factors and proliferation of hair follicles. BMC Complement Med Ther, 2020; 20:230. CrossRef
King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JRG. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta, 2007; 28:161–9. CrossRef
Kudo Y, Iwashita M, Iguchi T, Takeda Y. The regulation of type-I collagen synthesis by insulin-like growth factor-I in human osteoblast-like SaOS-2 cells. Pflugers Arch, 1996; 433:123–8. CrossRef
Lee HY, Chae HJ, Park SY, Kim JH. Porcine placenta hydrolysates enhance osteoblast differentiation through their antioxidant activity and effects on ER stress. BMC Complement Altern Med, 2016; 16:291. CrossRef
Lee KH, Park HJ, Seo HG, Kim JH, Lim GS, Lee WY, Kim NH, Kim JH, Lee JH, Jung HS, Sung SH, Song H. Immune modulation effect of porcine placenta extracts in weaned the pig. J Anim Sci, 2013; 91:2405–13. CrossRef
Lee M-H, Kwon T-G, Park H-S, Wozney JM, Ryoo H-M. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun, 2003; 309:689–94. CrossRef
Li X, Ling W, Pennisi A, Wang Y, Khan S, Heidaran M, Pal A, Zhang X, He S, Zeitlin A, Abbot S, Faleck H, Hariri R, Shaughnessy JD Jr, van Rhee F, Nair B, Barlogie B, Epstein J, Yaccoby S. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem cells (Dayton, Ohio), 2011; 29:263–73. CrossRef
Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R, Miyazaki T, Kitaura H, Nakamura K, Fujita T, Kanatani N, Moriishi T, Yamana K, Liu W, Kawaguchi H, Nakamura K, Komori T. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn, 2007; 236:1876–90. CrossRef
Nagae M, Nagata M, Teramoto M, Yamakawa M, Matsuki T, Ohnuki K, Shimizu K. Effect of porcine placenta extract supplement on skin condition in healthy adult women: a randomized, double-blind placebo-controlled study. Nutrients, 2020; 12:1671. CrossRef
Nensat C, Songjang W, Tohtong R, Suthiphongchai T, Phimsen S, Rattanasinganchan P, Metheenukul P, Kumphune S, Jiraviriyakul A. Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC Complement Med Ther, 2021; 21:66. CrossRef
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 1997; 89:765–71. CrossRef
Padhomchai Pumbthongthae PH, Roytrakul S, Janvilisri T, Ounjai P. Analysis of protein profile of crude porcine placenta extract. In: The 50th National Graduate Research Conference. King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand, 2020.
Park KM, Cho TH. Therapeutic effect of acupuncture point injection with placental extract in knee osteoarthritis. J Integr Med, 2017; 15:135–41. CrossRef
Park O-J, Kim H-J, Woo K-M, Baek J-H, Ryoo H-M. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization*. J Biol Chem, 2010; 285:3568–74. CrossRef
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev, 2003; 17:1352–65. CrossRef
Phuapradit W, Chanrachakul B, Thuvasethakul P, Leelaphiwat S, Sassanarakkit S, Chanworachaikul S. Nutrients and hormones in heat-dried human placenta. J Med Assoc Thai, 2000; 83:690–4.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet, 2011; 377:1276–87. CrossRef
Rozanova S, Cherkashina Y, Repina S, Rozanova K, Nardid O. Protective effect of placenta extracts against nitrite-induced oxidative stress in human erythrocytes. Cell Mol Biol Lett, 2012; 17:240–8. CrossRef
Schulman RC, Weiss AJ, Mechanick JI. Nutrition, bone, and aging: an integrative physiology approach. Curr Osteoporos Rep, 2011; 9:184–95. CrossRef
Shukla VK, Rasheed MA, Kumar M, Gupta SK, Pandey SS. A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J Wound Care, 2004; 13:177–9. CrossRef
Xu R, Zhang C, Shin DY, Kim JM, Lalani S, Li N, Yang YS, Liu Y, Eiseman M, Davis RJ, Shim JH, Greenblatt MB. c-Jun N-terminal kinases (JNKs) are critical mediators of osteoblast activity in vivo. J Bone Miner Res, 2017; 32:1811–5. CrossRef