INTRODUCTION
Cancer is known as uncontrolled proliferation in the cells of body, and it eventually causes death. In 2018, Liver tumor is predicted to be the major diagnosed and the crucial cause of death, with about 841,000 novel cases and 782,000 deaths annually. In addition, there are about 2.1 million patients of breast cancer in 2018, about one in four cancer cases between women. Breast cancer is the most diagnosed cancer in the most countries and is the cause of cancer death in more than 100 countries (Bray et al., 2018). In Egypt, the National Plan for Cancer Control 2017–2020 announced the recent rates of disease which are expected to increase from 113 per 100 to 341 per 100 thousand of populations as a result of infections between males with this disease (rate of liver cancer 33.63%) and between women is 32.04% (breast cancer) and 13.54% (liver cancer). Nowadays, despite considerable efforts cancer remains a destructive killer worldwide (Hanahan and Weinberg, 2011). The familiar and efficient regimens of cancer treatment are chemotherapy and radiotherapy together with surgery; all of these treatments have many defects and drawbacks (Safarzadeh et al., 2014). So, many drugs especially cytotoxic drugs that maintain to achieve a main task in treatment of cancer is important but often they have many side effects. Moreover, the two famous types of treatment chemotherapy and radiotherapy cause a gradual resistance of infected cells against the used drugs (Pereira et al., 2012; Qi et al., 2010). So, there is a continuous persistent need to have a novel, an efficient and an inexpensive anticancer drug (Coseri, 2009). In addition, natural extracted isothiocyanates are valuable chemo-protective agents at the laboratory level treatment (Hecht, 1995; Morse et al., 1993; Stonger et al., 1991) in inhibition of many types of tumor geneses in rat such as liver, small intestine, bladder, lung mammery gland, and esophegous (Hecht, 1995; 2000). They also reduce risk for prostate cancer (Kellof et al., 1999; Zhao et al., 2001). Moreover, many literatures cited that various isothiocyanate derivatives inhibit the growth of cell depending on apoptosis mechanism which involving apoptosis that is effectively involved in the isothiocyanates anti-carcinogenesis (Doerr-O Rourkek et al., 1991; Miyoshi et al., 2004). Furthermore, various naturally occurring derivatives have been used in in vitro and animal studies to get safe and potent anticancer drugs for breast cancer treatment. Several surveys have reported that naturally occurring 3-isothiocyanato-1-propene (AITC; sulfur including organic compound) display, as a result of glucosinolate sinigrin enzymatic hydrolysis, as prospective cancer preventing agents, and display carcinogenesis promoting effects. Its biosynthsis is very high and formulations for controlled eliminate of AITC were developed recently (Abu Sayeed et al. 2018), benzyl isothiocyanate (BITC) regulation of genes in breast cancer. BITC also prevents breast cancer growth and metastasis, and urges apoptosis, and phenethyl isothiocyanate (PEITC) (2-isothiocyanatoethyl) benzene) have anti-breast cancer potentials (Abu Sayeed et al., 2017). Contrarily, active and stabilized phosphonium ylides are known in the literature with various industrial applications (Kobayashi et al., 2000; Vasishtha et al., 1989), and their different pharmacological applications are well known. Also, it is known that triphenylphosphonium ion is a target moiety for delivery of antioxidants to isolate mitochondria, as well as the mitochondria of intact cells and whole organisms. As pharmacological agents, definite phosphonium salts have verified anti-microbial activity against gram negative and positive bacteria and the parasite Trypanosoma cruzi, antiglycemic, and anti-proliferative activities in cell and animal-based systems. In addition, as anticancer agent, phosphonium salts reveal huge promise for neoplasm treatment (Millard et al., 2010). Abd-El-Maksoud et al. (2014) confirmed that the phosphanylidene-aziridinyl chromenone revealed announced in vitro antitumor activities against human MCF-7 and HepG2 carcinoma cell lines. Also, cytotoxicity of phosphanylidene-cyclobutane, oxaphosphetane, and pyridazinone was confirmed by (El-Hussieny et al., 2015; Maigali et al., 2015).
Therefore, the present investigation aims to evaluate the potential antitumor activity of some tested isocyanates, isothiocyanates, and their nitrogen and sulfur heterocyclic phosphorus derivatives. In vitro cytotoxic and growth inhibitory activities of compounds against human HepG2 hepatocellular carcinoma and MCF-7 adenocarcinoma breast cancer were screened and compared with the 5-flurouracil as reference drug.
MATERIALS AND METHODS
Synthesized compounds
The tested iso(thio)cyanate organophosphorus derivatives namely, thietanes, thiazinanes, and azetidines (Table 1, No: 6a, 6b, 7a, 7b, and 8a, 8b), respectively, were synthesized from the result of compounds 3, 4, and 5 (Li et al, 2013), (Milelli et al, 2014) with the active phosphacumulene ylides, namely (N-phenyliminovinylidene) triphenylphosphorane (1a) (Bestmann and Sandmeier, 1976), and (2-oxovinylidene)triphenylphosphorane (1b).
Complex phosphonium compounds namely phosphanylidene derivatives (9a, 9b) (10a, 10b) and (11a, 11b) were obtained, respectively, through the association of compounds 3, 4, and 5, with the stabilized phosphonium ylides, namely, methoxycarbonyl (2a), and acetyl- methylenetriphenylphosphorane (2b) (Bestmann and Kratzer, 1962; Ramirez and Dershowitz, 1957), as shown in Table 1.
The structure of the newly prepared products has been confirmed through completely evidences of analytical and spectroscopy techniques together with the X-ray crystallography of compounds 6a and 10a in Figures 1 and 2 (Hashem et al., 2018).
In vitro anticancer activity evaluation
Chemicals
MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide], Dimethylsulph-oxide (DMSO), and 5-flurouracil were gotten from German Merck company. And the other reagents and chemicals were purchased from American Sigma Aldrich (St. Louis, MO). Human liver cancer HepG2 and breast cancer MCF-7 cell lines were gotten from Company for Biological Products & Vaccine (VACSERA).
Synthesized compounds dose preparation
Synthesized compounds were prepared at doses of (0, 10, 20, 40, 80, and 160 μg/ml). In brief, each compound was prepared in the concentration of 160 μg/ ml and considered as a working solution. Nearly, 500 μl RPMI-1640 media without fetal bovine serum (FBS) was taken in five Eppendorf tubes for each compound. After that, 500 μl of compound working solution was put to the first Eppendorf and mixed well then 500 μl of this concentration was transferred to next tube by serial dilution to get the required concentration of the compounds.
Cell lines and culturing
HepG2 and MCF-7 cell lines were propagate in RPMI-1640 medium supplemented with 10% heat inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, MO), penicillin and streptomycin at 37ºC in 5% CO2 incubator. After the cells became 80%, confluent sub culturing was done. The growth medium was replaced with complete RPMI-1640 media without FBS and rinsed gently by tilting. Trypsin-ethylenediaminetetraacetic acid (EDTA) (0.025% trypsin and 0.02% EDTA) using for cells harvested at 37ºC for 5 minutes for disaggregation. The complete media (5 ml) was added to the cells by using serological pipette. After trypsinization and harvest suspension cells by centrifugation, HepG2 and MCF-7 cells were seeded at a concentration of 1 × 106 cells per well, in 100 μl complete RPMI-1640 medium in 96-well culture plates (Falcon) and were incubated in a humidified 5% CO2 incubator at 37°C for 24 hours to assure total attachment.
Cytotoxicity assay
Cytotoxicity was performed to assessment the anticancer activity of the novel compounds using MTT (3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenylte trazolium bromide). Cells suspended in 100 μl of complete medium were seeded in 96-well microplate at a cell density of 3 × 103 cells/ well and incubated at 37ºC, in 5% CO2 overnight. Cells were treated with serial concentration of test compounds and 5-Flurouracil as reference drug (0, 10, 20, 40, 80, or 160 μg/ml) for 48 hours. After incubation, 20 μl/well MTT at conc. (0.5 mg/ml) added and incubated for 4 hours at 37°C and then media discarded and replaced by 100 μl of DMSO to dissolve the formed purple formazan crystals. The optical density in each well was measured spectrophotometrically at wavelength 570 nm using ELISA reader (Mosmann, 1983) (Microplates reader, Asys Hitech, Austria). Compounds cytotoxicity was calculated as IC50, the compound concentration that inhibits the cells growth by 50% as compared with the untreated cells as follows: growth inhibition % = 100 − (OD of treated cells/OD of control cells) × 100) (Mosmann, 1983). Sigma plot software 11 was used to calculate the IC50 for tested compounds.
 | Table 1. Synthesized thietane (6a, 6b), thiazinane (7a, 7b), azetidine (8a, 8b), acid ester (9a, 10a, 11a), and amide Compounds (9b, 10b, 11b) resulted from the reaction of iso(thio)cyanate derivatives (3, 4, and 5) with the active- (1a, 1b) and stabilized-phosphonium ylides (2a, 2b). [Click here to view] |
Statistical analysis
Data are expressed as mean ± standard error (SE) for at least three independent determinations in triplicate for each experimental point. Data were analyzed using IBM SPSS Statistics 16 software. For all the measurements, one-way analysis of variance followed by Duncan’s new multiple range test (p ≤ 0.05) was used to assess the statistically significance of deference between control and treated groups.
RESULTS AND DISCUSSION
Substituted thiazole compounds take part in nature and have a varied range of biological effects. As antitumor against Ehrlich ascites carcinoma cells and five human cell lines (Ghorab and Al-Said, 2012; Leoni et al., 2016; Tung et al., 2013). Antibacterial (Li et al., 2014), antimicrobial (Bondock et al., 2010), anti-viability against FLT3-dependent human acute myeloid Leukemia cell line MV4-11 (Lin et al., 2015), anti-inflammatory (Geronikaki et al., 2007), antiviral (Barradas et al., 2011), antiproliferative (Grozav et al., 2014), and anticandidal (Altıntop et al., 2014).
 | Figure 1. X-ray crystallography of compound 6a. [Click here to view] |
 | Figure 2. X-ray crystallography of compound 10a. [Click here to view] |
The synthesized thietane (6a, 6b) and thiazinane compounds (7a, 7b) cytotoxicity was investigated using MTT assay against human HepG2 hepatocellular carcinoma and MCF-7 adenocarcinoma breast cancer in comparison with the known anticancer drug 5-flurouracil as a reference drug. The results are expressed as inhibition concentration (IC50) which is the concentration of the tested compounds that reduces the cancer cell survival to 50% compared with control (Table 1).
As shown in Table 2 and Figure 3, all thietane and thiazinane compounds showed concentration dependent increase in the growth inhibition percentage against HepG2 and MCF-7 cancer. In Table 3, the results revealed that thietane compounds 6a, 6b and thiazinane compounds 7a, 7b showed anticancer activity with IC50 value of (20, 8.9 and 12.7, 32.5 μg/ml), respectively, against HepG2 and (20, 10.3 and 20, 20 μg/ml), respectively, against MCF-7 cancer cell lines. The obtained results show that compound 6b has the most efficient growth inhibition compared with the other three compounds (6a, 7a, and 7b) in cells of HepG2 and MCF-7.
This results was concordant with, Wang et al. (2012) synthesized a series of novel multithioether derivatives by the integration of thiazoline and thiazine with dibromides and evaluated their antitumor activity. Data revealed that these compounds exhibited higher antitumor actions on A549 (human lung cancer) and Bcap-37 (human breast cancer cell). Ferreira et al., (2013) prepared thiazinediones that displayed anticancer behavior against Leukemia cells. It was noticed that various aromatic group linked to the 1,3-thiazine-2,4-diones maybe intercalate with DNA leading to DNA fragmentation and ultimate cell death. Some 1,6-diazaphenothiazines showed anticancer against melanoma C-32 and breast cancer MCF-7 (Morak-Mlodawska et al., 2014). In addition, the novel synthesized complex 2-(2,4-dihydroxyphenyl) thieno-1,3-thiazin-4-one (BChTT) be able to use to stop many types of developing cancers and it is safe to body cells and organs. These derivatives end the synthesis of DNA and suppress the cell growth stages by decreasing the activity of p38 kinase and cyclin D1 enzymes (Juszczak et al., 2016).
 | Table 2. Growth inhibition percentage of synthesized compounds (Thietane, 6a, 6b and Thiazinane 7a, 7b) against cancer cell lines. [Click here to view] |
 | Figure 3. Concentration dependent effect of Thietane (6a, 6b) and Thiazinane (7a, 7b) synthesized compounds against HepG2 and MCF-7 cancer cell lines. [Click here to view] |
 | Table 3. Median growth inhibitory concentration (IC50 μg/ml) of Thietane (6a, 6b) and Thiazinane (7a, 7b) synthesized compounds in human cancer cell lines. [Click here to view] |
Moreover, the novel azetidines (8a, 8b) were investigated in vitro for anticancer activity against human HepG2 hepatocellular carcinoma and MCF-7 adenocarcinoma breast cancer using MTT assay. The results showed that azetidines 8a and 8b induced concentration dependent increase in the percentage of cell growth inhibition with IC50 of (13.5 and 32.5 μg/ml) against HepG2 and (10 and 25.9 μg/ml) against MCF7cancer as tabulated in Tables 4 and 5. Compound 8a showed more potent activity than 8b against both types of cancer cell lines as illustrate in Figure 4.
This result was compatible with (Chimento et al., 2013) who revealed the effects of new chloro-azetidin derivatives as growth regulatory in MCF-7 and SkBr3 cancer. They concluded that these compounds showed moderate to high antitumor activity and their capability to elicit selective inhibitory effects on breast cancer might be taken into account towards novel pharmacological access in breast cancer therapy. This result was compatible with (Chimento et al., 2013) who revealed the effects of new chloro-azetidin derivatives as growth regulatory in MCF-7 and SkBr3 cancer. They concluded that these compounds showed moderate to high antitumor activity and their capability to elicit selective inhibitory effects on breast cancer might be taken into account towards novel pharmacological access in breast cancer therapy. Kabilan et al. (2013) mentioned that N-sulphonic acid substituted derivatives of azetidinone exhibited significant inhibitor activity against the proliferation of MCF-7 cancer. In addition, (Noolvi et al., 2014) showed that novel azetidine-2-one derivatives of 1H-benzimidazole showed good in vitro cytotoxic activity. Deep et al. (2016) indicated that the synthesized compound N-[2-(3-chloro-2-oxo-4-styrylazetidin-1-ylamino)-2-oxoethyl] benzamide exhibited potent anticancer effect in MCF-7.
As shown in Table 6 and Figure 5, the cytotoxicity of Iso (thio)cyanates (3, 4, and 5), and malonamic acid methyl ester synthesized compound (11a) were tested against HepG2 and MCF-7 and 5-Flu as a reference drug. A gradual increase in growth inhibition of cancer cells with tested compounds increasing concentration was seen. Compounds 3 and 4 IC50 values are (25.9, 12.3 and 40, 20), respectively, against HepG2 and MCF-7. This shows that the compound 4 is more effective than compound 3 in both two cell lines. IC50 value of compound 5 on HepG2 is equal (10) but it similar in potency to 5-Flu as an anticancer drug with an IC50 value 5.3 μg/ml versus 5 μg/ml for 5-Flu against MCF-7. It should be noted that the compound 5 appears to be the most effective one, especially in MCF-7. While, compound 11a not induced any effect on HepG2 or MCF-7 as illustrated in Table 7.
 | Table 4. Growth inhibition percentage of synthesized compounds azetidine (8a, 8b) against human cancer cell lines. [Click here to view] |
 | Table 5. Median growth inhibitory concentration (IC50 μg/ml) of azetidine synthesized compounds in human cancer cell lines. [Click here to view] |
The isothiocyanates (ITCs) cytotoxicity was documented with large number of studies and clinical reports. Particularly, they are capable on cell proliferation block, apoptosis, interfere with all essential steps of neovascularization, and inhibition of potential cancer cells metastatic (Bertl et al., 2006; Fimognari and Hrelia, 2007; Thejass and Kuttan, 2006). Moreover, Turrini et al. (2014) reported the ability of ITCs to increase the anticancer efficacy of conventional anticancer drugs. In addition, Ristic et al. (2016) show that isothiocyanates suppressed the growth of many cells lines. BITC was the most potent growth inhibitor with half-maximum IC50 values on HeLa, Fem-x and LS 174 cells. Penthala et al. (2015) reported that trans-cyanostilbene analogues which contained the 3,4,5-trimethoxyphenyl and dimethoxyphenyl moieties exhibited low micromolar growth inhibition against human cancer in the panel. Luo et al. (2017) suggested that acetyl chloride-mediated creation of PEITC derivatives proves to be convenient and provides the expected excellent products yields. Biological activity and structure association analysis revealed that the novel compound appeared the greatest anticancer activity against human cancer by elevated the intracellular ROS and depleted GSH, causing cancer cell death.
The compounds 9a and 10a were screened for in vitro anticancer activity at various concentrations. The percentage growth inhibition of HepG2 and MCF-7 cancer cells was determined by MTT assay. Table 8 and Figure 6 show the maximum inhibition percentage of 9a and 10a maximum concentrations in μg/ml with IC50 (34.9 and 22.5 μg/ml; 35.5 and 21.6 μg/ml, respectively) as in Table 9.
Previously, amide-containing heterocycles were known as a category of compounds displaying wide biological activities, which consist of natural and synthetic yields. its possibly considered building blocks for the manufacture of bioactive compounds in pharmaceutical drug design and agrochemical industry (Aggarwal et al., 2014; Chen et al., 2015; Liu et al., 2011; 2014; Yan et al., 2014; Yang et al., 2015).
 | Figure 4. Concentration dependent effect of azetidine synthesized compounds 8a, 8b against HepG2 and MCF-7 cancer cell lines. [Click here to view] |
 | Table 6. Growth inhibition percentage of Iso(thio)cyanates (3, 4, and 5), and malonamic acid methyl ester synthesized compound (11a) against cancer cell lines. [Click here to view] |
 | Figure 5. Concentration dependent effect of Iso(thio)cyanates (3, 4, and 5), and malonamic acid methyl ester synthesized compound (11a) against HepG2 and MCF-7 cancer cell lines. [Click here to view] |
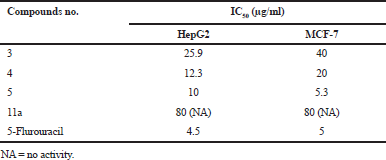 | Table 7. Median growth inhibitory concentration (IC50 μg/mL) of Iso(thio) cyanates (3, 4, and 5), and malonamic acid methyl ester synthesized compound (11a) against human cancer cell lines. [Click here to view] |
 | Table 8. Growth inhibition percentage of synthesized compounds (acetic acid methyl ester) against human cancer cell lines. [Click here to view] |
 | Table 9. Median growth inhibitory concentration (IC50 μg/ml) of (acetic acid methyl ester) synthesized compounds in human cancer cell lines. [Click here to view] |
 | Figure 6. Concentration dependent effect of (Acetic Acid Methyl Ester) synthesized compounds 9a, 10a against HepG2 and MCF-7 cancer cell lines. [Click here to view] |
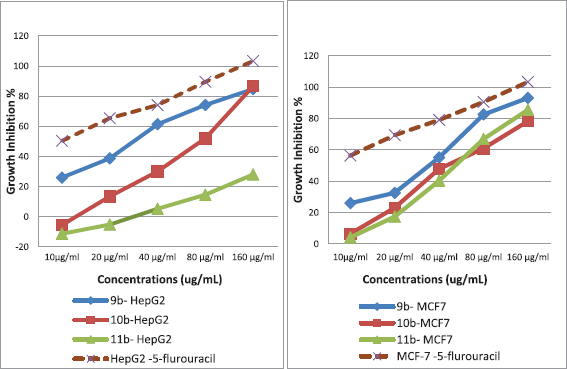
 | Table 10. Growth inhibition percentage of synthesized compounds (butyramide) against human cancer cell lines. [Click here to view] |
 | Table 11. Median growth inhibitory concentration (IC50 μg/ml) of butyramide synthesized compounds in human cancer cell lines. [Click here to view] |
 | Figure 7. Concentration dependent effect of (butyramide) synthesized compounds against HepG2 and MCF-7 cancer cell lines. [Click here to view] |
The results listed in Tables 10 and 11 revealed that the tested compound 9b induced concentration dependent increase growth inhibition against HepG2 cells with IC50 (28 μg/ml), while 10b and 11b compounds did not exert activity as show in Figure 7. However, compounds 9b and 10b exhibited cytotoxic activity against MCF-7 cancer cells with IC50 (34.2 and 42.3 μg/ml), While, 11b did not exhibited the effect.
Jiang et al. (2012) study the cytotoxicity of novel N-alkylated amino acid-derived hydroxamate, 2-[Benzyl-(2-nitro-benzenesulfonyl)-amino]-N-hydroxy-3-methyl-N-propyl-butyramide. They showed that it caused breast cancer inhibition, as well as, colony formation of MDA-MB-231 cells and suppresses breast cancer-mediated angiogenesis of vascular endothelial cells in vitro.
CONCLUSION
In the present study, the cytotoxicity of the synthesized compounds against human cancer cell lines (HepG2 and breast MCF-7) indicated that all compounds showed growth inhibitory activity on liver HepG2, and breast MCF-7 cancer cell except compounds 10b, 11a and 11b had no effect. Moreover, the thietane 6b showed the highest growth inhibitor activity against human cancer cell lines (HepG2) and breast MCF-7. While, the thiazinane 7a showed high activity against (HepG2) than MCF-7. In the case of azetidine 8a, it showed high activity against HepG2 and MCF-7, however, isocyanate 5 showed high activity against MCF-7.
REFERENCES
Abd-El-Maksoud MA, Maigali SS, Soliman FM. Chemistry of phosphonium ylides. Part 39: facile synthesis of aziridine, pyridine, pyrolotriazole chromenones and azaphosphinin chromenones as antitumor agents. J Heterocyclic Chem, 2014; 51:1830–7. CrossRef
Abu Sayeed Md, Bracci M, Ciarapica V, Malavolta M, Provinciali M, Pieragostini E, Gaetani S, Monaco F, Lucarini G, Rapisarda V, Di Primio R, Santarelli L. Allyl isothiocyanate exhibits no anticancer activity in MDA-MB-231 breast cancer cells. Int J Mol Sci, 2018; 19:145–57.
Abu Sayeed Md, Bracci M, Lazzarini R, Tomasetti M, Amati M, Lucarini G, Di Primio R, Santarelli L. Use of potential dietary phytochemicals to target miRNA: promising option for breast cancer prevention and treatment? J. Funct Foods, 2017; 28:177–93.
Aggarwal N, Kumar R, Srivastava C, Dureja P, Khurana JM. Synthesis, biological activities and SAR studies of novel 1-ethyl-7-methyl-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid based diacyl and sulfonyl acyl hydrazines. Pest Manag Sci, 2014; 70:1071–82. CrossRef
Altıntop MD, Özdemir A, Turan-Zitouni G, Ilgın S, Atlı Ö, Demirci F, Kaplancıklı ZA. Synthesis and in vitro evaluation of new nitro-substituted thiazolyl hydrazone derivatives as anticandidal and anticancer agents. Molecules, 2014; 19:14809–20. CrossRef
Barradas JS, Errea MI, D’Accorso NB, Sepulveda CS, Damonte EB. Imidazo [2,1-b]thiazole carbohydrate derivatives: synthesis and antiviral activity against Junin virus, agent of Argentine hemorrhagic fever. Eur J Med Chem, 2011; 46:259–64. CrossRef
Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther, 2006; 5:575–85. CrossRef
Bestmann HJ, Kratzer O. Reaktionen mit phosphin-alkylenen, III 1) uber die wittig-reaktion mit tricyclohexylphosphin-alkylenen. Chem Ber, 1962; 95:1894–901. 385.
Bestmann HJ, Sandmeier D. Simple synthesis of ketenylidenetriphenylphosp-horane and its thioanalogs. Angew Chem Int Ed, 1975; 14:634. C A: 1976; 84:5070s.
Bondock S, Fadaly W, Metwally MA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur J Med Chem, 2010; 45:3692–701. CrossRef
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68:394–424.
Chen K, Liu Q, Ni JP, Zhu HJ, Li YF, Wang Q. Synthesis, insecticidal activities and structure-activity relationship studies of novel anthranilic diamides containing pyridylpyrazole-4-carboxamide. Pest Manag Sci, 2015; 71:1503–12. CrossRef
Chimento A, Sala M, Gomez-Monterrey IM, Musella S, Bertamino A. Biological activity of 3-chloro-azetidin-2-one derivatives having interesting antiproliferative activity on human breast cancer cell lines. Bioorganic Med Chem Lett, 2013; 23:6401–5. CrossRef
Coseri S. Natural products and their analogues as efficient anticancer drugs. Mini Rev Med Chem, 2009; 9:560–71. CrossRef
Deep A, Kumar P, Narasimhan B, Lim SM, Ramasamy K, Mishra RK, Mani V. 2-Azetidinone derivatives: synthesis, antimicrobial, anticancer evaluation and qsar studies. Acta Pol Pharm, 2016; 73(1):65–78.
Doerr-ORourkek K, Trushin N, Hecht SS, Stoner GD. Effect of phenethyl isothiocyanate on the metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by cultured rat lung tissue. Carcinogenesis, 1991; 12:1029–34. CrossRef
El-Hussieny M, Abd-El-Maksoud MA, Maigali SS, Soliman FM, Soliman AM. Chemistry of phosphorus ylides. Part 42: reaction of dipyridyl ethamedione with phosphorus reagents-cytotoxic activity of phosphanylidene-cyclobutane, oxaphosphetane, and pyridazinone. Phosphorus Sulfur Silicon Relat Elem, 2015; 190:1845–56. CrossRef
Ferreira M, Assunção LS, Filippin-Monteiro FB, Creczynski-Pasa TB, Sa MM. Synthesis of 1, 3-thiazine-2,4-diones with potential anticancer activity. Eur J Med Chem, 2013; 70:411–8. CrossRef
Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res, 2007; 635:90–104. Toxins, 2015; 7:550–2.
Geronikaki A, Hadjipavlou-Litina D, Zablotskaya A, Segal I. Organosilicon-containing thiazole derivatives as potential lipoxygenase inhibitors and anti-inflammatory agents. Bioinorg Chem Appl, 2007; 1–8. doi:10.1155/2007/92145 CrossRef
Ghorab MM, Al-Said MS. Antitumor activity of novel pyridine, thiophene and thiazole derivatives. Arc Pharm Res, 2012; 35:965–73. CrossRef
Grozav A, Găină LI, Pileczki V, Crisan O, Silaghi-Dumitrescu L, Therrien B, Zaharia V, Berindan-Neagoe I. The synthesis and antiproliferative activities of new arylidene-hydrazinyl-thiazole derivatives. Int J Mol Sci, 2014; 15:22059–72. CrossRef
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell, 2011; 144(5):646–74. CrossRef
Hashem AI, El-Hussieny M, Abd-El-Maksoud MA, Maigali SS, Mansour Sh T, Soliman FM. Synthesis of phosphoranylidene thietane, azetidine and thiazinane derivatives as potent chemo preventative agents. Phosphorus Sulfur Silicon Relat Elem, 2018; 193(1):1–9; doi:10.1080/10426507.2017.1370467.
Hecht SS. Chemoprevention by isothiocyanates. J Cell Biochem Suppl, 1995; 22:195–209. CrossRef
Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev, 2000; 32:395–411. CrossRef
Jiang J, Thyagarajan-Sahu A, Krchňák V, Jedinak A, Sandusky GE. NAHA, a novel hydroxamic acid-derivative, inhibits growth and angiogenesis of breast cancer in vitro and in vivo. PLoS One, 2012; 7(3):e34283. CrossRef
Juszczak M, Walczak K, Matysiak J, Lemieszek MK, Langner E, KarpiÅ„ska MM, Pożarowski P, Niewiadomy A, Rzeski W. New derivative of 2-(2,4-dihydroxyphenyl)thieno-1,3-thiazin-4-one (BChTT) elicits antiproliferative effect via p38-mediated cell cycle arrest in cancer cells. Bioorg Med Chem, 2016; 24:1356–61. CrossRef
Kabilan C, Shankar D, Alagarsamy V, Asiya Parvin A. Synthesis and antiproliferative activity of novel thiazolidine and azetidinone derivatives. Res J Pharma Biol Chem Sci, 2013; 4(2):1770–80.
Kellof GJ, Growell JA, Steele VE, Ann NY. Progress in cancer chemoprevention. Acad Sci, 1999; 889:1–13. CrossRef
Kobayashi M, Sanda F, Endo T. Application of phosphonium ylides to latent catalysts. 2. Kinetic study on the thermal latency of the phosphonium ylides in the polyaddition of bisphenol A diglycidyl ether with bisphenol A. Macromolecules, 2000, 33:5384–7. CrossRef
Leoni A, Locatelli A, Morigi R, Rambaldi M. Novel thiazole derivatives: a patent review (2008–2014. Part 2). Expert Opin Ther Pat, 2016; 26(2):149–73; doi: 10.1517/13543776.2016.
Li JR, Li DD, Wang RR, Sun J, Dong JJ, Du QR, Fang F, Zhang WM, Zhu HL. Design and synthesis of thiazole derivatives as potent FabH inhibitors with antibacterial activity. Eur J Med Chem, 2014; 75:438–47. CrossRef
Li Z-Y, Ma H-Z, Han C, Xi H-T, Meng Q, Chen X, Sun X-Q. Synthesis of isothiocyanates by reaction of amines with phenyl chlorothionoformate via one-pot or two-step process. Synthesis, 2013; 45:1667–74. CrossRef
Lin XD, Yang HW, Ma S, Li WW, Zhang CH, Wang WJ, Xiang R, Li LL, Yang SY. Discovery of 6-phenylimidazo[2,1-b]thiazole derivatives as a new type of FLT3 inhibitors. Bioorg Med Chem Lett, 2015; 25:4534–8. CrossRef
Liu XH, Tan CX, Weng JQ. Phase transfer-catalyzed, one-pot synthesis of some novel N-pyrimidinyl-N1-picotinyl thiourea derivatives. Phosphorus Sulfur Silicon Relat Elem, 2011; 186:552–7. CrossRef
Liu XH, Weng JQ, Wang BL, Li YH, Tan CX, Li ZM. Microwave-assisted synthesis of novel fluorinated 1,2,4-triazole derivatives, and study of their biological activity. Res Chem Intermed, 2014; 40:2605–612. CrossRef
Luo B, Wang J, Xiaobing L, Wenhua L, Jing Y, Yumin H, Peng H, Shijun W. Simple approach to isothiocyanates: a class of potent anticancer agents. Molecules, 2017; 22:773. CrossRef
Maigali SS, El-Hussieny M, Soliman F M. Chemistry of phosphorus ylides. Part 37. The reaction of phosphonium ylides with indoles and naphthofuranes. Synthesis of phosphanylidenes, pyrans, cyclobutenes, and pyridazine as antitumor agents. J Heterocycl Chem, 2015; 52:15–23. CrossRef
Millard M, Pathania D, Shabaik Y, Tahen L, Jinxia D, Neamati N. Preclinical evaluation of novel triphenylphosphonium salts with broad-spectrum activity. PLoS One, 2010; 5:1.
Milelli A, Fimognari C, Ticchi N, Neviani P, Minarini A, Tumiatti V. Isothiocyanate synthetic analogs: biological activities, structure-activity relationships and synthetic strategies. Mini Rev Med Chem, 2014; 14: 963–77.
Miyoshi N, Talcabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis, 2004; 25:567–75. CrossRef
Morak-MÅ‚odawska B, Pluta K, Latocha M, Jelen M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1,6-diazaphenothiazines. Med Chem Res, 2016; 25:2425–33.
Morse MA, Zu H, Galati AJ, Schmidt CJ, Stoner GD. Dose-related inhibition by dietary phenethyl isothiocyanate of esophageal tumorigenesis and DNA methylation induced by N-nitrosomethylbenzylamine in rats. Cancer Lett, 1993; 72:103–10. CrossRef
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immuno Methods, 1983; 65:55–63. CrossRef
Noolvi M, Agrawal S, Patel H, Badiger A, Gaba M, Zambre A. Synthesis, antimicrobial and cytotoxic activity of novel azetidine-2-one derivatives of 1H-benzimidazole. Arab J Chem, 2014; 7(2):219–26.
Penthala NR, Zong H, Ketkar A, Madadi NR, Janganati V, Eoff RL, Guzman ML, Crooks PA. Synthesis, anticancer activity and molecular docking studies on a series of heterocyclic trans-cyanocombretastatin analogues as antitubulin agents. Eur J Med Chem, 2015; 92:212–20; doi:10.1016/j.ejmech. 2014.12.050
Pereira DM, Valentao P, Orreia-da-Silva G, Teixeira N, Andrade PBP. Plant secondary metabolites in cancer chemotherapy: where are we current. Pharm Biotech, 2012; 13:632–50. CrossRef
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N, Li XK, Tang W. Chinese herbal medicines as adjuvant treatment during chemo-or radio-therapy for cancer. Bio Sci Trends, 2010; 4(6):297–307.
Ramirez F, Dershowitz SJ. Phosphinemethylenes-II-triphenylphosphineacyl methenes. Org Chem, 1957; 22:41–5. CrossRef
Ristic AK, Stanojkovic T, Srdic-Rajic T, Dilber S, Djordjevic B, Stankovic I, Juranic Z. In vitro assessment of antiproliferative action selectivity of dietary isithicyanates for tumor versus normal humal cells. Vojnosanit Pregl, 2016; 73(7):636–42. UDC: 615.322::616-006-084.
Safarzadeh E, Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull, 2014; 4: 421–7.
Stonger GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, Wagner SA. Inhabitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethyl amine in carcinogenesis in the rat esophagus. Cancer Res, 1991; 51:2063–8.
Thejass P, Kuttan G. Antimetastatic activity of sulforaphane. Life Sci, 2006; 78:3043–50. CrossRef
Tung TT, Oanh DTK, Dung PTP, Hue VTM, Park SH, Han BW, Kim Y, Hong JT, Han SB, Nam NH. New benothiazole/thiazole-containing hydroxamic acids as potent histone deacetylase inhibitors and antitumor agents. Med Chem, 2013; 9:1051–7. CrossRef
Turrini E, Ferruzzi L, Fimognari C. Natural compounds to overcome cancer chemoresistance: Toxicological and clinical issues. Expert Opin Drug Metab Toxicol, 2014; 10:1677–90. CrossRef
Vasishtha R, Saini S, Nigam SK, Srivastava AKJ. Applications of ylides in the polymerization of vinyl monomers macromolecules. Sci Rev Macromolecules Chem Phys, 1989; 29:39–53.
Wang W, Zhao B, Xu C, Wu W. Synthesis and antitumor activity of the thiazoline and thiazine multithioether. Int J Organic Chem, 2012; 2:117–20.
Yan SL, Yang MY, Sun ZH, Min LJ, Tan CX, Weng JQ, Wu HK, Liu XH. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett Drug Des Discov, 2014; 11:940–3. CrossRef
Yang MY, Zhao W, Sun ZH, Tan CX, Weng JQ, Liu XH. Synthesis and biological activity of acylthiourea derivatives contain 1,2,3-thiadiazole and 1,3,4-thiadiazole. Lett Drug Des Discov, 2015; 12:314–8. CrossRef
Zhao B, Seow A, Lee EJD, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase-M1,-T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev, 2001; 10:1063–7.