Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: Impact on generic drug substitution
Nitika Kaushal, Sachin Kumar Singh, Monica Gulati, Yogyata Vaidya, Munish Kaushik
DOI: 10.7324/JAPS.2016.60430Pages: 206-222

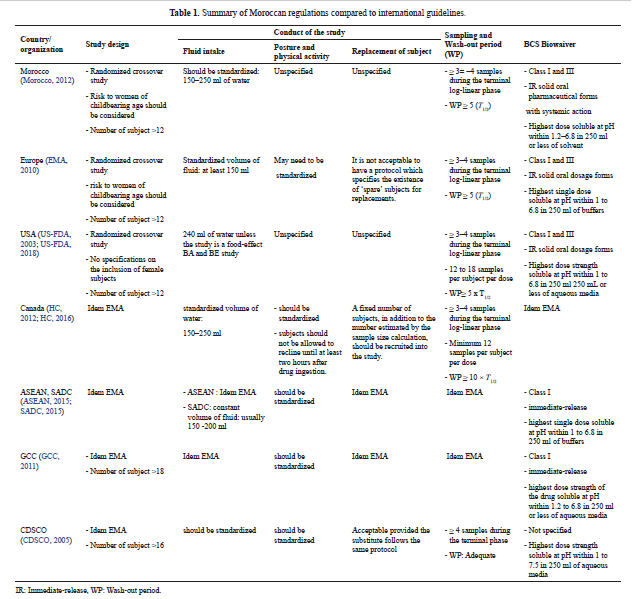
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035