Comparative in vitro dissolution study of Aceclofenac Marketed Tablets in Two Different Dissolution Media by Validated Analytical Method
S.M. Ashraful Islam, Sharmi Islam, Mohammad Shahriar, Irin Dewan
Pages: 87-92

Comparative Screening analysis of Natural metabolites against a cholera toxin
R. Balajee and M.S.Dhana Rajan
DOI: 10.7324/JAPS.2013.38.S12Pages: S75-S78

Formulation and Characterization of Ketoprofen Emulgels
Ramakanth Ambala, Sateesh Kumar Vemula
DOI: 10.7324/JAPS.2015.50717Pages: 112-117

In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia
Ahmed F. Hanafy
DOI: 10.7324/JAPS.2016.60909Pages: 063-068

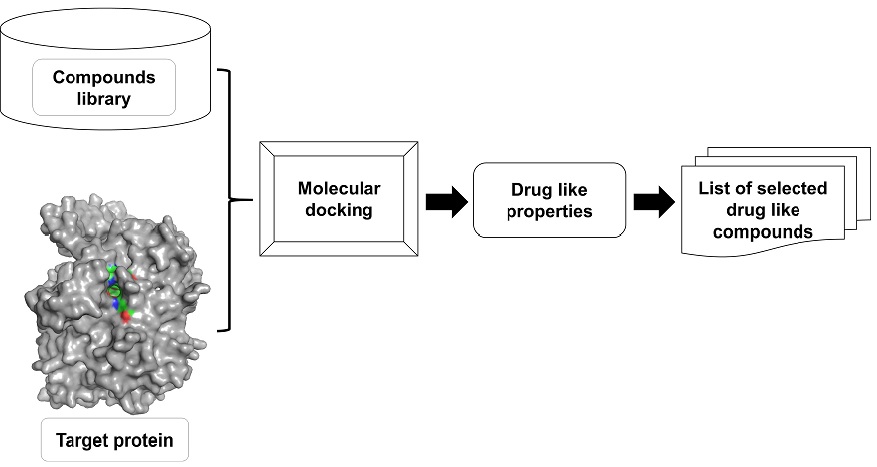
Validation of methodology for assay, pharmaceutical equivalence, and comparative dissolution profile for tablets containing amlodipine besylate
Renata Micheli Martinez, Jenifer Freitas da Silva, Larissa Regina Jorge, Rhye Lessa Ishikawa, Ana Paula Novelli, Talita Laiane Cardoso Cezar, Sandra Regina Georgetti, Marcela Maria Baracat, Rúbia Casagrande
DOI: 10.7324/JAPS.2019.91112Pages: 093-100
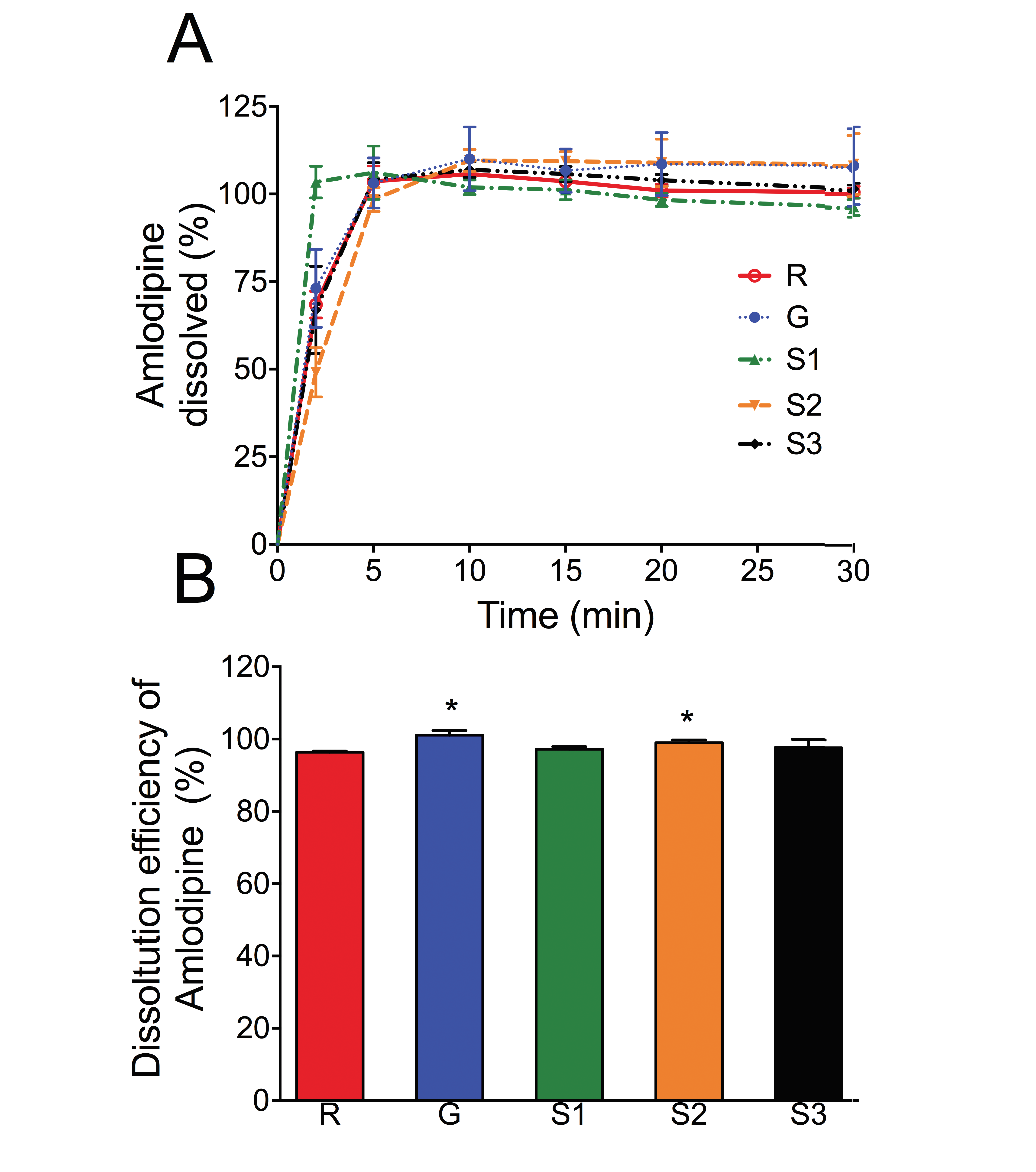
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
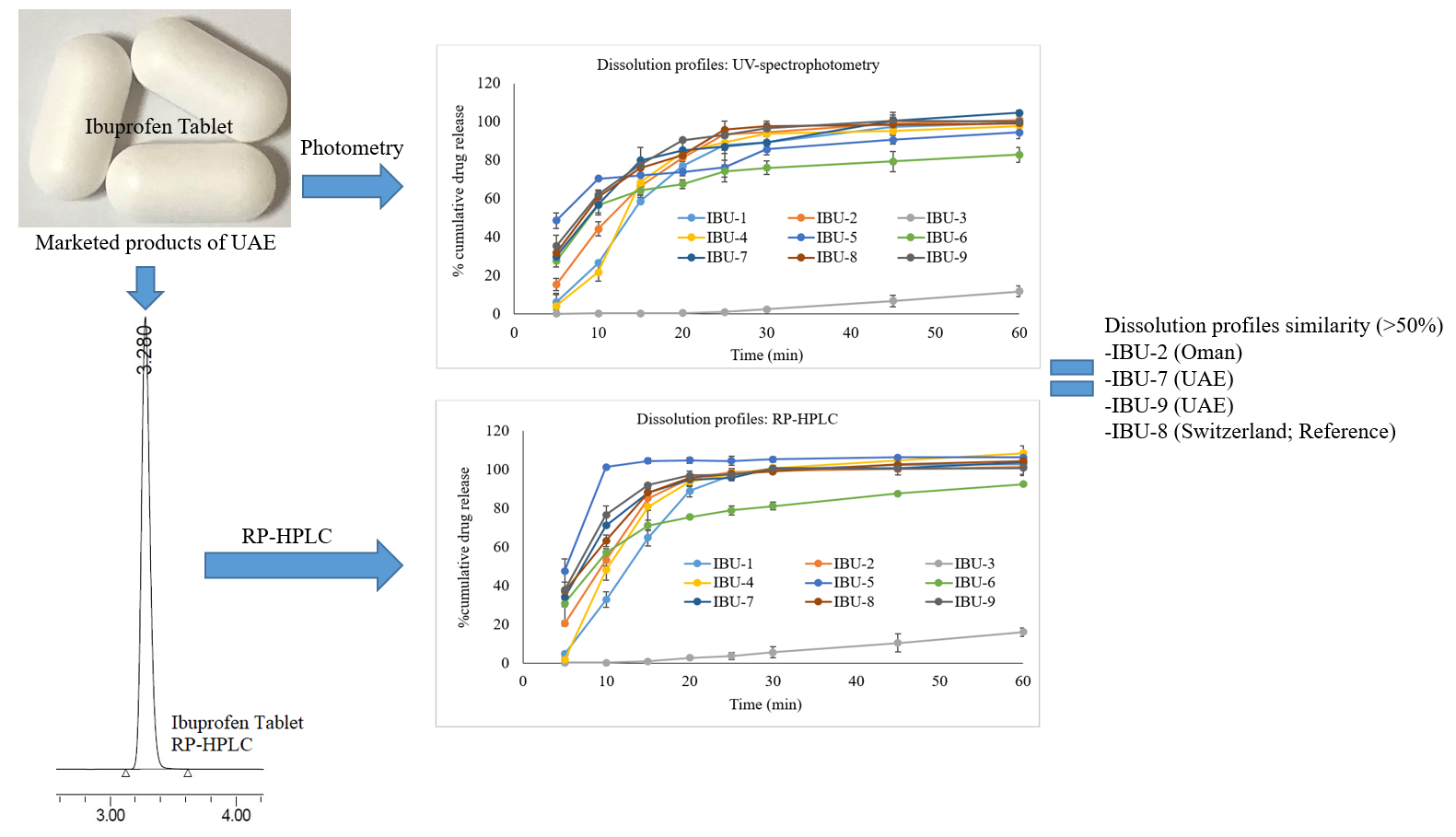
In silico screening of hispolon and its analogs: Pharmacokinetics and molecular docking studies with cyclooxygenase-2 enzyme
Mohadese Mohammadi, Mohammad Firoz Khan, Ridwan Bin Rashid, Sina Mirzaie Nokhostin, Mohammad A. Rashid
DOI: 10.7324/JAPS.2022.120509Pages: 120-128