INTRODUCTION
The generic market is a crucial medical sector and serves as an alternative to high-cost branded drugs. The market size of generic pharmaceuticals is expanding rapidly due to their cost-effective treatment goals. Before 1984, chemists were prohibited from dispensing generics once a physician had prescribed the brand name. However, these anti-substitution regulations have since been widely disregarded. Incentives are offered by governments, such as social health funds, customer reimbursement supports, and by encouraging the consumption and manufacturing of generics by cost-conscious customers. Other than boosting trade, these incentives aim at reducing healthcare costs along with improved access to quality medicines [1]. Over the past few decades, significant changes in the business context of the generic pharmaceutical industry have led to the transition of the market dynamics [2]. These changes present complex concerns such as losing market exclusivity, productivity in research and development, pricing constraints, regulatory policies, patent expiration, generic competition, and consumer perception [3]. The generic business is causing global pharmaceutical corporations that rely on proprietary chemicals to collapse. Generic versions of various other types of drugs are approved by the Abbreviated New Drug Application (ANDA) process, which is a quicker and less expensive regulatory assessment. Meanwhile, generic versions of certain specialty pharmaceuticals (complex drugs) are unavailable or do not receive approval through the standard system [4]. Such generics are called complex generics, specialty generics, super generics, hybrid drugs, value-added generics, or off-patent medications [5].
According to the United States Food and Drug Administration (US FDA), “Complex generics are products that have complex active ingredients, formulations, dosage forms, or routes of administration, or are complex drug-device combination products” [6]. Complex generics are generally nonbiologics, having complexity in manufacturing and regulatory requirements. Although biosimilars (generic versions of certain biologic drugs) face stringent regulatory requirements for approval, some of them are also categorized as a subgroup under complex generics.
The market for complex generics is expected to grow from its anticipated USD 84 billion in 2024 to USD 200 billion in 2035. The market is projected to expand at a Compound Annual Growth Rate (CAGR) of 8% from 2024 to 2035 [6]. A CAGR of 10.2% is expected for the worldwide complex generics market, valued at USD 70.0 billion in 2022 and will reach USD 184.8 billion by 2032. The market seems dynamic and diverse, with about 270 super-generic drugs produced by different companies [7]. Complex generics are affordable substitutes, especially for chronic, complex disorders, including Human immunodeficiency virus, rheumatoid arthritis, and cancer, where currently, most oncology-related complex generics are growing [6,8]. The report by a consultancy (SKYQUEST) reveals significant market growth of complex generics, projecting a value surge from USD 73.38 billion in 2022 to USD 162.02 billion in 2031, at a growth rate of 9.2% [9]. Prominent companies such as Teva (29% globally) [10], Sandoz (USD 9.7 billion sales) [11], Lupin, Apotex, Mylan (8% net sales by viatris) [12], Sun Pharmaceuticals, Accord, and Alcon dominate because of their vast product portfolios, robust research and development capacities, and substantial distribution networks. The scenario seemed more complex when Teva Pharmaceutical Industries (2023) declared it would pay USD 8.8 billion to get hold of Reata Pharmaceuticals (a specialty generic company). Furthermore, Mylan proclaimed in July 2023 that it would pay USD 16 billion to purchase the specialist generics company Viatris [13]. Over the next 10 years, the complex generics industry is expected to rise due to several factors, including upcoming regulations, lowering healthcare expenses, patent expirations, new treatment developments, and continuing technical improvements [7].
From a consumer perspective, in the context of complex generics, scarcity occurs when consumers (patients) cannot afford to buy the prescribed medication (medicine) they want at the market rate; this is known as “excess demand”[14]. The Food and Drug Administration (FDA) published reports on complex generic shortages and their hidden causes, which are still being researched. The reports emphasize that the core reason for the scarcity of certain specialty generics is related to quality issues, which also indirectly affect economic status [15]. It is important to understand the market dynamics because they affect the availability of essential drugs, profitability, overall healthcare quality, and demand and supply chain [16]. It is vital for stakeholders, including policymakers (to develop effective strategies), healthcare professionals (to make knowledgeable choices in prescribing and dispensing), and consumers (to make intelligent choices), to understand the complicated and quickly changing market dynamics to increase the supply of quality products [15,17].
While looking into the literature, there is a wide gap in knowledge, and the data were spread randomly, which creates doubts as well as a wide range of interpretations. Therefore, a comprehensive and accurate process is urgently needed to assess and combine the currently available evidence on the market dynamics of the complex generics market [18]. It can cover a wide range of topics, including the size and growth of the market, competition, pricing policies, regulatory frameworks, and the challenges and opportunities that contribute to the entry of complex generics into the market [19,20]. It may additionally focus on specific countries or product categories. The present article focuses on an extensive analysis of market dynamics from different available data sources to determine the current trends of complex generics. These data predict the future growth of these specialty medicines.
METHODS
Data collection
E Jerome McCarthy combines the fundamental pillars of marketing strategy and makes a marketing framework called the 4Ps analysis. The 4Ps provide a thorough understanding of how a product is promoted and distributed in the market by addressing all important aspects of marketing [21]. Combining several marketing components ensures that the business’s goals are met through product features, price policies, distribution methods, and promotional efforts. Because of its flexibility, the 4Ps framework can be modified to meet our needs. For example, the “Product” aspect can involve thoroughly examining product types according to dosage forms and routes of administration. In contrast, the “Price” aspect can consider the necessary investment and regulatory pricing constraints. Thus, for the market dynamic study of complex generics, a 4Ps approach is used. The 4P analysis of complex generics data was collected from 2023 to April 2024 and analyzed in the United States and India for this study. The products included in this study were selected based on the US FDA definition and those available as innovator or generic form in the US and Indian markets. Products with specialized manufacturing technologies, product-specific guidelines (PSGs), and those that classify as hybrid medicine were also included in this study. Products published in literature showing hurdles in establishing BE due to formulation, analytical, and pharmacokinetic complexity are also included in this study. The products without verified data to prove the complexity are excluded from the study. The product list was compiled using information from publications, PSG, and news and alerts from regulatory websites. Information on innovator and generic medicine prices and promotions in the US was obtained from online pharmacies such as GoodRx [22] and Medline [23]. The sources of data collection (price and promotion) from the Indian market include the Current Index of Medical Specialities book [24], 1 mg [25], and the Janaushadhi website [26]. Cross-referencing data from more than one source helps in solving discrepancies and validating the data. In case of more discrepancies, the product was considered for study based on recent regulatory updates. The availability of ongoing research determined the place of investigation. The USA was included in the study due to its importance as a centre for pharmaceutical exploration Center for Research on Complex Generics (CRCG). At the same time, Europe was left out because of its lesser use and availability of generic medications. India was added since it is a significant producer of generic drugs. Digital platforms, medical conferences, internet promotions, and pharmaceutical marketing materials were used to analyze literature-based promotional techniques. Prescription, over-the-counter (nonprescription), and specialty medicine categories were used to categorize the majority of product promotion during data analysis. This comparative study seeks to comprehend the marketing dynamics of complex generics in the USA and India by comparing and contrasting their product, prices, distribution systems, and promotional initiatives.
A systematic strength, weakness, opportunities, and challenges (SWOCs) analysis for complex generics was done by collaborating with data obtained based on a literature-based study and 4Ps analysis to offer valuable insights into the strategic positioning and market dynamics. The external and internal factors were sorted based on the 4P criteria for each aspect of SWOC to improve the business and public healthcare. Qualitative data interpretation involved categorization of relevant observations into 4Ps themes. Data triangulation was employed to enhance credibility.
Data analysis
Descriptive statistical tools were used to analyze the collected data, focusing on the number of products, types, and their promotion, and prices of complex drugs available as innovator and generic versions in both countries. Descriptive statistics like frequencies, mean, median, and percentage distribution were employed in this study. In addition, prices in relation to factors like country-specific price, Generic Drug User Fee Amendment (GUDFA) approval timing, and products coming under PSG were used for analysis. The frequencies were calculated using Jamovi software, and the figures were drawn using Microsoft Excel. No inferential statistical analysis was performed for this exploratory study.
RESULTS
4P analysis of complex generics
Market trends of complex generics
The market landscape of complex generics is dynamic and diverse, with 45 complex generic products launched by various manufacturers across both countries. A comprehensive list of 188 products was compiled from several secondary sources. These 188 products were deemed complex medicines whose generic equivalents are either now on the market or have yet to be released; in addition, those drugs whose research is ongoing to develop complex generic versions (Fig. 1). Of the 188 items, 132 complex drugs were found in the US market and 98 in the Indian market. There are 85 US-marketed products with generic versions available.
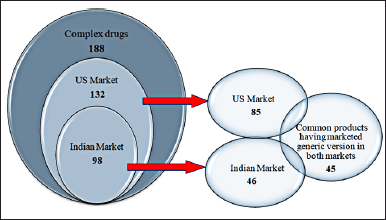 | Figure 1. Schematic representation of sorting everyday complex generic products. [Click here to view] |
In contrast, generic versions of about 50% (46 products) of drugs are available in India. Products were chosen for the 4P analysis based on drugs that have marketed generic versions in both countries (45 products). Complex generics can be administered through various routes of administration, which allows the customization of treatment plans to meet the needs of each patient at a lower cost. In addition, many technologies have also been developed, which are essential to developing, producing, and characterizing complex generics. Overall, the market landscape of complex generics is characterized by innovation, competition, and affordability, providing patients with access to high-quality and cost-effective treatment options for various disease indications.
Market share by complex generics class
Based on the rough classification by the US FDA, the super generics market was segmented into complex API, complex route of administration/formulation, complex dosage form, and complex drug-device combination products. Presently, the market is dominated by complex APIs followed by drugs with complex routes of administration/formulation (Fig. 2). Advanced formulations often offer improved efficacy or safety compared to traditional generics, but the cost of production is high; hence, the selling price will also be high for complex formulations. The expensive, complex drugs for cancer therapy come under complex API. The increase in demand for generic versions of such medications highlights the importance and unmet need for this class of value-added drugs.
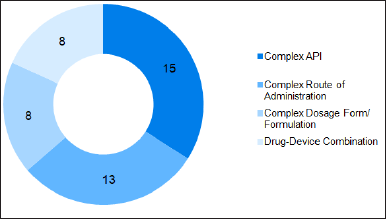 | Figure 2. Number of products based on classification of complex generics. [Click here to view] |
Market share by molecular nature
The complex generics were divided into nonbiologic, biologic, and drug-device combination products based on the nature of the molecule. In the market for value-added products, nonbiologics complex generics held the highest market share (Fig. 3). Their extensive acceptance across therapeutic fields can be due to their more straightforward developmental and analytical attributes, flexibility for dealing with different diseases, and established regulatory procedures for approval compared to the other two classes.
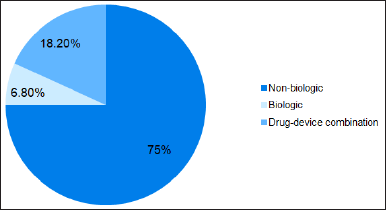 | Figure 3. Complex generics available based on the nature of molecules. [Click here to view] |
Market share by route of administrative
The complex generics market is divided into oral, topical, nasal, ophthalmic, and injectable groups based on the route of administration. The market with the largest share was complex generics that provide treatment through oral administration (Fig. 4). Easy accessibility, patient preference, and convenience of oral formulations for various therapeutic needs may be the leading causes of this hike in market share.
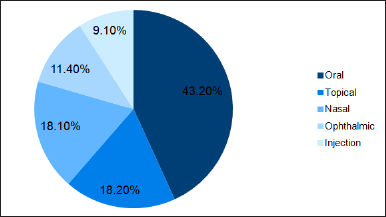 | Figure 4. Complex generics class based on route of administration. [Click here to view] |
Market share by product type
The complex generics were categorized according to the product type, including aerosols, capsules, prefilled syringes, tablets, and vials. This study shows that sales income from tablets dominates the complex generics industry, with aerosol and capsules following (Fig. 5). The increase is because tablets and capsules are widely used due to their convenience, adaptability, and ease of administration. They also have longer shelf lives than other dosage forms. Aerosol also plays a significant role as respiratory disease rates are increasing daily.
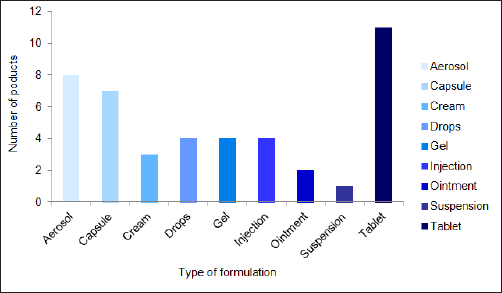 | Figure 5. Complex generics class based on dosage form or formulation. [Click here to view] |
Market share by therapeutic category
The complex generics market has been divided into several therapeutic areas based on pharmacological use. These categories include medications for autoimmunity (autocoids), neurological, gastrointestinal, respiratory, and cardiovascular disorders, and chemotherapy. Chemotherapeutics has the largest market share and is predicted to hold this position throughout the forecast (Fig. 6). From this, it is clear that complex generic medications for cancer treatment are becoming increasingly popular, further improving the public healthcare system.
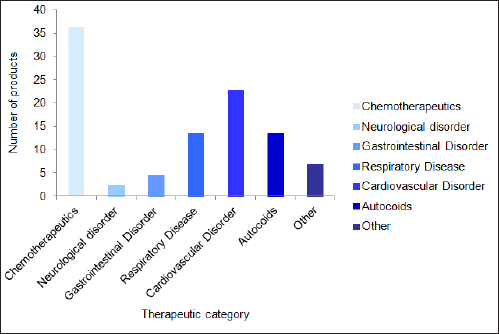 | Figure 6. Market growth of complex generics based on therapeutic use. [Click here to view] |
Market dynamics based on price
When comparing the price of innovator products and generic versions of complex drugs in the US market, there is only a 50% reduction in cost for complex generic (mean of 656$) compared to innovator drugs (mean of 1337$). Whereas in India, the price decline was more than 50% of the price of the innovator [(mean of 30.6$) and for complex generics (mean of 12.4$)]. The multivariate regression analysis (R2) shows that manufacturing challenges (p = 0.01) and regulatory challenges (p = 0.03) will be associated with generic medicine. The difference in the price decline rate is likely due to more stringent regulatory approval procedures in regulated markets like the US, which require extensive clinical study data, prolonged review time, and stringent controls. These factors increase the cost of production, which directly increases the selling price of the product. In contrast, low investment strategies are needed in the Indian market, and also, government initiatives like Jan Aushadhi significantly influence the price of the product. It can also be due to the economic status of the countries, especially developing and underdeveloped countries.
Market dynamics based on place
The major organizations and funding agencies engaged in the research and development of complex generics were identified through an analysis of secondary data from the Scopus database using keywords like “complex generic*”, “hybrid drug*”, and “specialty generic*”. The U.S. leads 41 funded projects, followed by China and India. The U.S. FDA is the top funder with 13 projects. The National Institutes of Health and the National Cancer Institute also contribute significantly. On the other hand, 37 projects were sponsored by the National Natural Science Foundation of China, which was China’s main contributor. With funding for eight and seven projects, the Department of Science and Technology and the Science and Engineering Research Board of India have also demonstrated significant commitment. The Deutsche Forschungsgemeinschaft in Germany and the National Research Foundation in South Korea are more prominent sponsors (Table 1). The prominence of funding agencies and institutes from the U.S. and India in this field highlights their crucial involvement in shaping the development of complex generics. Consequently, given their dominant positions in research financing and development, our analysis concentrated on market dynamics in the U.S. and Indian markets. To meet the varied demands of customers in all these countries, a multichannel strategy that includes retail pharmacies, internet platforms, and direct-to-hospital supplies is crucial. Developing strategic partnerships with distributors, wholesalers, and larger purchasing organizations can increase profitability and consumer reach. Furthermore, in developed countries, complex generics requiring strict storage conditions use cold-chain solutions and specialized transportation facilities.
Table 1. Top countries providing funds for complex generic-related research.
| Countries | Number of funding |
|---|---|
| United States | 41 |
| China | 37 |
| India | 15 |
| Korea | 12 |
| Germany | 11 |
| Brazil | 11 |
Market dynamics based on promotion strategies
This analysis focuses on the comparative promotion strategies used in the USA and India. The marketing techniques used for complex generics comprise 14% of nonprescription (over-the-counter) drugs, 9% of specialty drugs, and 77% of prescription drugs (Fig. 7). Prescription generics are promoted in both countries through direct-to-consumer advertising, physician targeting through engagement with sales representatives, and ongoing educational programs. Generic versions of nonprescription drugs are sold in stores and through online marketing. Specialist involvement, hospital collaboration, sponsorships, and guidance for focused patient groups promote specialty medications. This analysis demonstrates the strategies used to market complex generics in both countries. Understanding these strategies offers manufacturers significant insights to maximize their marketing endeavors in various other marketplaces. Pharmaceutical manufacturers could use continuing education courses, healthcare conferences, and online advertising strategies emphasizing clinical findings and patient satisfaction to reach medical professionals such as doctors, pharmacists, and hospital purchase managers. These unique benefits of complex generics, including affordability, improved therapeutic effects, and technology improvements, should be highlighted in practical promotion efforts to increase the supply.
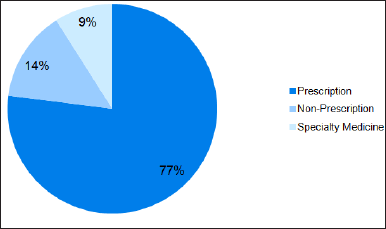 | Figure 7. Promotion strategies employed for dispensing complex generics. [Click here to view] |
Market growth rate
In this analysis, the timeframe was divided mainly into four sub-classes based on GUDFA. It comprises pre-GUDFA (2007-2011), GUDFA I (2012-2016), GUDFA II (2017-2021), and GUDFA III (2022–2027, revised as well as drafted). Depending on the number of products approved based on the product-specific guidelines, showed an increment in the number of marketed products. Even though growth was noticed, it was not exponential. It was showing slow growth. It was observed that as time passed, GDUFA was more focused on complex generics and necessary initiatives for the upbringing of this class of drugs (Fig. 8). This analysis concludes that there will be steady growth for complex generics globally within a short time.
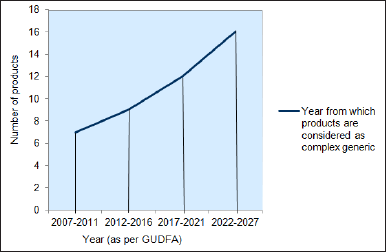 | Figure 8. Growth in market size of complex generics. [Click here to view] |
A sensitivity analysis was performed to estimate CAGR for complex generics by keeping 2024 as the base year with a market share of 84 billion USD (CAGR 8%). Using the CAGR formula, the market will shift to 195 billion USD (CAGR 8%) by 2035.
Future value = Present value × (1+CAGR)n; where n = 11.
SWOC analysis
Upon applying a SWOC analysis to the various classes of complex generics, it is apparent that although these drugs offer notable opportunities for reduced costs and increased competitiveness in the market, they also face significant challenges. Strength includes the possibility of lower costs and more accessibility when they get over development challenges. The existing technological and regulatory complexities, which restrict the number of possible alternatives to three per product, are the source of weaknesses. The opportunities include market potential and the resolution of development challenges, which may result in increased adoption and cost savings. Challenges still come from high development costs, research and development associated, regulatory obstacles, and the possibility of fewer savings than anticipated compared to noncomplex generics. The criteria above, based on existing literature, suggest a complicated landscape in which the challenges associated with introducing complex generics to the market significantly impact. A comparative SWOC analysis based on the 4Ps is given in Table 2.
Table 2. 4Ps analysis-based strategic decision of strength, weakness, opportunity, and challenges of complex generics (SWOC analysis).
| Strength | Weakness | ||||||
| Product | Place | Price | Promotion | Product | Place | Price | Promotion |
| Increase patient compliance [3] | US FDA design classifications for complex drugs so that generics can be developed quickly [31] | Reduce healthcare costs [27] | Regular updation of the Generic Drug Science and Research website [36] | All classes of complex generics have difficulty in establishing TE [34]. | Developing countries are in a tug of war about the quality and safety parameters of complex generics [44] | High regulatory approval cost [42] | PSG are getting changed from time to time, so more quality-related concerns [50] |
| Advancement of technology to improve the accessibility [27] | Companies like Aurobindo, Sun Pharma, and Lupin have introduced many complex generics into the US market [32] | Increase market competition, thus lowering specific product prices [35] | Industries can make substantial profits if they collaborate with the appropriate companies [3] | Costly comparative PD or clinical endpoint studies are needed for most complex generics nowadays [40]. | Countries use various phrases and definitions to classify complex generics [45]. | The savings from complex generics will likely be lower than those from non-complex generics [33]. | Lack of coordination between industry and academia [49] |
| Mylan merged with Pfizer's off-patent products unit to form Viatris to increase focus on complex generics [28] | 55% of the global market accounts by North America due to a large number of medical companies and research centers [33] | Increase the availability of lower-cost alternatives to higher-priced complex drugs [27]. | Mechanistic modelling and simulation grants for complex generic development were granted in GDUFA [37]. | Complex injectables show more variability in PK parameters, which causes difficulty in finding patients for crossover trials and also increases investment [41]. | Indian companies focus more on complex generics, but commercialization is less due to variations in approval requirements [32]. | The high developmental costs indirectly cause high product prices [48]. | A risk-based non-clinical approach is required for some countries that add extra burden for its faster growth [6] |
| Provide controlled drug release at a lower cost and faster rate with batch-to-batch consistency [29] | The U.S. and EMA's 2021 partnership aims to harmonize regulatory standards of complex generics [34] | Increase complex generic market share when compared to simple generics [33] | To resolve challenges through collaborative research, the USFDA established CRCG [38] | OINDPs, due to local drug delivery dynamics, face difficulties in achieving BE [42] | Low-income countries are challenging due to the low supply of chemotherapeutics [46] | High research and development investment [27,49] | Citizen petitions are filing regarding the equivalence [29] |
| Offer more therapeutic alternatives for rare disease patients [30]. | Indian companies like Dr Reddys, Lupin, Aurobindo, Glenmark and Cipla are focusing on complex generics [32] | Reduce developmental and regulatory approval costs for certain complex generic products [3] | Establish pre-ANDA meeting pathways to develop more complete submissions [39]. | Quantification and analytical challenges, as well as difficulties in establishing PE, were observed in specific classes of complex generics [43] | The hybrid pathway in the EU allows for the provision of additional (pre-) clinical data for approval [47] | Higher marketing costs due to differences in requirements of the same product approval in different countries [44] | Lack of ICH guidelines for complex generics [6] |
| Opportunity | Challenges | ||||||
| Product | Place | Price | Promotion | Product | Place | Price | Promotion |
| The market for complex generics is expected to grow from its predicted 84 billion USD (2024) to 200 billion USD (2035) [33]. | US implementing short-term negotiation in policies addressing review and approval of complex generics [52] | High profitability is expected due to timely marketing [33] | Complex generics have 3–7 years of marketing exclusivity, enabling developers to achieve high profits [56]. | Modifications in solvent and preparation techniques of microspheres impact drug load, in vitro and in vivo release [59]. | Considering the same product under different classes by regulated market confuses [3] | High developmental cost for certain drugs like DDC, nano-technological generic products [47] | Tackling anti-generic campaigns is one of the biggest challenges [29]. |
| Strength | Weakness | ||||||
| Product | Place | Price | Promotion | Product | Place | Price | Promotion |
| MDI has proven to be an extremely profitable product with tremendous growth potential [51]. | Entering developing markets might provide extra profits and substantial growth opportunities [48]. | MIFD can be used to expedite and reduce the cost of formulating generic drugs [55] | Implementation of quality by design helps in the selection of critical formulation and manufacturing variables (CFMV) [53] | Variations in inactive components, dosage form and drug load impact the adhesion and heat effects of TDS [60] | Most low-income countries approve based on the same guidelines as simple generics. [46] | High marketing cost due to extensive clinical trial reports [40] | Varying regulatory requirements across domestic and export markets, as well as patent regime-related hurdles [49,65] |
| There is no generic competition for many off-patent brand drugs. This is an opportunity to reduce costs [52] and increase accessibility [53]. | USFDA-funded University at Buffalo to develop PBPK modeling and simulation for vaginal complex products [54] | Investment in technologies to overcome complex generic challenges will increase opportunity at lower prices [27] | PBPK research, along with computational methods, is used to predict the QCP of LAI [58] | Unknown interactions will occur between the device and the patient, as well as the device and the formulation in the case of OINPS [61] | There is no particular regulatory guideline for certain products, but the number of generic manufacturers is high [3] | High technical investment is needed for clinical studies [49] | Lack of knowledge about product, process and guidelines causes severe adverse effects (Labeling defect of (NH)2SO4) [38] |
| The development of LAI is being processed, which sheds light on the faster market availability of long-back patent-expired products [42]. | Continuous revision of PSGs shows the US promote the market of quality complex generic [50] | Adaptive design can be used to save money and time by starting clinical trials with few patients [3] | The in silico model provides new insights into the development of generic versions of aerosols [58] | Complex injectables and implants show high inter-subject variability, so more subjects are needed for crossover study [62] | There are concerns over the equivalency of innovator and generic, mainly in the EU (for example, in the case of Copaxone) [5] | High marketing cost due to different regulatory approval processes (mainly DDC) [44] | Following 505b(2) and 505(j) guidelines by top companies reduce the growth of new or smaller generic companies [47] |
| A comprehensive scale-up and adjustment strategy in the study design was promoted to ensure adequate constant quality during manufacturing [3]. | 50% of complex generics of Teva (largest producer) is in the US, and 20% in the EU, highlighting its future growth [55]. | PBPK research, along with computational methods, is used to predict the QCP of LAI to reduce investment and indirectly reduce selling price [57]. | Harmonization of technical and scientific standards for complex generics presents global access to high-quality, affordable medicines [36]. | Nanotechnological products face challenges due to the intricate material design, alterations in physicochemical and manufacturing parameters and stability [63]. | USFDA guidelines suggest generic liposomes will not be approved with slight preservative, buffer, or antioxidant modifications [64,65]. | High marginal price as seen in the field of oligonucleotide drug delivery [32] | Health maintenance organizations and health funds make it more challenging for products to be on an authorized list or reimbursement [66]. |
An overview of approved complex generics available in both countries from 2007 to 2024 is included in the paper, together with information on the price of drugs, current trends in different classes of products like top market available complex generic category, top-selling drug based on molecular type, molecular nature, administration routes, dosage form/formulation, drug manufactures, and disease indications; top countries involved in the research and development; their promotion; and SWOC. Case studies of 45 authorized complex generics shed light on their growth and marketplace success. A SWOC analysis identifies vital trends, drivers, and barriers affecting the pharmaceutical industry. All the above results shed light on the market dynamics of complex generics.
DISCUSSION
The 4Ps analysis offers a simple and easy method of explaining the marketing plan to partners or investors who might need to be better versed in the complexities of the pharmaceutical industry. The 4Ps can aid in the effective planning and execution of market entry plans for complex generics, where timing and strategy are crucial. Selecting the appropriate product characteristics, pricing schemes, methods of distribution, and marketing strategies is all part of this. By adapting the marketing mix to the 4Ps analysis, manufacturers can set themselves apart from competitors with their complex generics, gaining a more significant market share and boosting their profits. For instance, Teva Pharmaceuticals is the leading company in the US market, which adopts strategic pricing policies to compete with its competitors such as Sandos, Mylan, and Endo [67]. Whereas in India, Jan Aushadi, a government initiative which spreads all over the country, provides a low-cost pricing policies which promote the growth of generics [68].
The 4Ps paradigm draws attention to crucial marketing choices that greatly influence how well a complex generic performs in the marketplace. Price and promotion are the influential factors affecting the market growth of complex generics in both countries, as of our study. Pricing competition is more prevalent in India when compared to the US, which enhances the productivity of complex generics. Digital promotion and healthcare professional awareness have more influence on the market growth of complex generics in the US than in India. In conclusion, from our study report Indian market focuses most focus on affordability and growth in the US market, on the other hand depends on professional acceptance via effective promotion activities. Increase in approval with GUDFA correlates with the descriptive trend exit between the review process, PSG revision, and findings by the US FDA. Compared to the pre GUDFA, there is approximately a 25% increment in the product count available in the market.
By examining each “P,” researchers can pinpoint the strengths, weaknesses, opportunities, and challenges associated with the present market approach. The SWOC analysis highlights the strengths and promotion strategies of different formulations like long-acting injectables (LAIs), transdermal drug delivery systems (TDSs), orally inhaled nasal drug products (OINDPs), metered dose inhalers (MDIs), as well as drug-device combination products (DDC). These insights shed light to take manufacture decisions regarding which type of product should be produced or focus on producing based on market potential.
Formulation developmental challenges and analytical weaknesses, such as changes in pharmacodynamic and pharmacokinetic (PK), critical quality attributes, and critical process parameters, significantly affect bioequivalence, therapeutic effectiveness, delay in regulatory approval, and increase in chances of regulatory revision or rejection. For example variation in particle size distribution in inhalers, viscocity of suspension, variation in BE of oral products negatively influence therapeutic outcomes and variation in batch to batch consistency. To overcome the challenges, it is important to use more advanced analytical technology like the Type IV dissolution apparatus, which provides more predictive in vitro in vivo correlation [69]. In addition, model-informed formulation development (MIDF) helps in managing formulation uncertainties by predicting real time product performance. Among these most commonly suggested MIDF for the development of complex generics is a physiologically based pharmacokinetic model (PBPK). PBPK models help in predicting ADME, BE, and drug content from patches, even based on patient populations without any extensive clinical studies [70].
By providing insights into new trends in the complex generics sector, this study enables investors to predict market dynamics changes and appropriately modify their portfolios. For example, from above, it is clear that if investors fund more for PBPK-enabled complex generic drug development, it will positively affect the market dynamics. Insurance firms can negotiate for higher reimbursement rates by knowing how complex generics are priced and the value of complex generics (highlighting how they improve therapeutic outcome) in general public health value-based pricing [71]. Companies may establish backup plans and reduce these risks early in the product lifecycle by using the SWOC analysis to identify possible strengths and risks, such as pricing pressures or regulatory hurdles. This study also helps medical professionals understand complex generics, especially regarding therapeutic equivalency. Colgan et.al. [20] study shows that several healthcare providers, pharmacists, and consumers have a negative opinion of generic medications. The broader usage of generics is probably hindered by these viewpoints [20]. Das et al. [72] study revealed the fact that few individuals had confidence in generic formulations. More than 90% of the patients in this study reported that generic medications were just as effective as name-brand ones [72]. Regulatory agencies can use these study results to develop a more robust and balanced market that benefits manufacturers and customers alike. By providing insights into new trends in the complex generics industry, this study enables investors to predict market dynamics changes and appropriately modify their portfolios. The benefits of this study for patients include more accessible access to more reasonably priced drugs. Overall, an integrated 4Ps and SWOC analysis emphasizes the need for globally harmonized guidelines, including various regulated markets, such as the European Medical Agency (EMA) and the US FDA. Lunawat and Bhat [7] study concludes that the US FDA uses both 505 (j) and 505 b(2) pathways to approve medicines, whereas the hybrid application [Article 10(3)] is used by EMA. EMA requires additional preclinical and clinical study reports for approval of complex generics. This work also highlights the need for harmonized guidelines [7]. The dual analysis studies in our paper offer a comprehensive understanding of the industry that aids in coordinating the goals of all stakeholders aiming to improve healthcare outcomes.
CONCLUSION
The market study on complex generics provides an in-depth examination of the market, emphasizing the functions of different companies in the US and India. To guarantee accuracy and dependability, this study presents an organized research approach that includes economic issues such as the price of drugs, current trends in different classes of products, and their promotional strategies from 4Ps and SWOC analysis. 4Ps analysis reveals the insightful differences of the product availability, pricing, and promotion strategies in a regulated and semi regulated country. The summary of SWOC analysis gives a high-level overview of the present and potential internal and external factors affecting the development of complex generics. Because of the complexity, generic versions of complex medicines are more challenging to analyze, replicate, and generalize. As a result, meeting the requirements for generic clearance becomes challenging. Consequently, complex generics are more expensive and put a strain on patients. Due to these challenges, there is no market competition even after the patents expire. Therefore, to address these challenges, the FDA and other regulatory authorities intend to provide several guidelines and address the scientific and regulatory challenges to improve patient affordability and market competition. This research helps manufacturers to tailor the marketing strategies of complex generics, regulators to encourage successful developments, and the outcomes of this study enhance the awareness of complex generics among healthcare providers as well as patients.
The paper concludes with great emphasis on highlighting the need to develop harmonized regulatory guidelines at a faster rate to achieve a rapid market approval, and finally highlights opportunities for future expansion. Future studies should focus on patient-centered challenges and also the global shift in policies and their impact on manufacturers. In conclusion, addressing these challenges through collaborative efforts greatly appreciated for improvising healthcare outcomes.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Rashidian A, Omidvari H, Vali Y, Sturm H, Oxman AD. Pharmaceutical policies: effects of financial incentives for prescribers. Cochrane Database Syst Rev. 2015;2015(8):CD006731. CrossRef
2. Kirking DM, Ascione FJ, Gaither CA, Welage LS. Economics and structure of the generic pharmaceutical industry. J Am Pharm Assoc. 2001;41(4):578–84. CrossRef
3. Duncan A, Viswanadh K, Baudino S, Donelan R, Lassoff P, Rhoades RA, et al. Complex generics: charting a new path [Internet]. 2016 [cited 2023 Jul 11]. Available from: https://www.iqvia.com/library/white-papers/complex-generics-charting-a-new-path
4. Demina NB, Bakhrushina EO, Anurova MN, Merkushova AG, Pomytkina MV, Rastopchina OV, et al. Complex generics as a trend of modern pharmaceutical development. Int J Appl Pharm. 2024;16:71–7.
5. Businesswire. Super generics market, 2035—ResearchAndMarkets.Com; 2024 [cited 2024 Apr 28]. Available from: https://www.businesswire.com/news/home/20240606270663/en/Super-Generics-Market-2035---ResearchAndMarkets.com
6. Roots analysis. Super generics market size & share revenue growth 2035. 2024 [cited 2024 Apr 28]. Available from: https://www.rootsanalysis.com/reports/super-generics-market/275.html
7. Lunawat S, Bhat K. Complex generic products: insight of current regulatory frameworks in US, EU and Canada and the need of harmonisation. Ther Innov Regul Sci. 2020;54(5):991–1000. CrossRef
8. FDA. Complex generics news. Center for drug evaluation and research. 2023 [cited 2024 Aug 12]. Available from: https://www.fda.gov/drugs/generic-drugs/complex-generics-news
9. Skyquestt. Specialty generics market size, growth & trends report 2031. 2024 [cited 2024 Apr 28]. Available from: https://www.skyquestt.com/report/specialty-generics-market
10. Teva blog. Complex generics: facts, figures and who they benefit. 2024 [cited 2024 Apr 20]. Available from: https://www.tevapharm.com/news-and-media/feature-stories/what-are-complex-generics/
11. Sandoz completes acquisition of Aspen’s Japanese operations, strengthening its position in world’s third largest market for generics and off-patent medicines. Sandoz [Internet]. [cited 2024 Aug 12]. Available from: https://www.sandoz.com/sandoz-completes-acquisition-aspens-japanese-operations-strengthening-its-position-worlds-third/
12. Building sustainable access to medicine. 2022 Viatris Sustainability Report [Internet]. [cited 2024 Aug 12]. Available from: https://www.viatris.com.tr//media/project/common/viatris/csrpromo/pdfs/2023_345_2023csrreport_revised.pdf
13. Specialty generics market share | Statistics report, 2032 | The Brainy Insights [Internet]. [cited 2024 Apr 8]. Available from: https://www.thebrainyinsights.com/report/specialty-generics-market-13722#summary
14. Penner SJ, Allen CM, Bralye WE, Macedo MA, Porter AD, Rothhammer HN, et al. Economics of health care. In: Penner SJ, editor. Economics and financial management for nurses and nurse leaders [Internet]. New York, NY: Springer Publishing Company; 2016. pp 1–26. Available from: https://connect.springerpub.com/content/book/978-0-8261-6002-7/part/part01/chapter/ch01
15. Skyquestt. Global specialty generics market size and forecast to 2030. 2024 [cited 2024 Apr 28]. Available from: https://www.skyquestt.com/report/specialty-generics-market
16. Frank RG, McGuire TG, Nason I. The evolution of supply and demand in markets for generic drugs. Milbank Q. 2021;99(3):828–52. CrossRef
17. Francois C, Gawlik G, Mestre-Ferrandiz J, Pana A, Perelman J, Yfantopoulos J, et al. New pricing models for generic medicines to ensure long-term sustainable competition in Europe. Front Pharmacol. 2023;14:1200641. CrossRef
18. Moosapour H, Saeidifard F, Aalaa M, Soltani A, Larijani B. The rationale behind systematic reviews in clinical medicine: a conceptual framework. J Diabetes Metab Disord. 2021;20(1):919–29. CrossRef
19. Mostafa S, Mohammad MA, Ebrahim J. Policies and practices catalyzing the use of generic medicines: a systematic search and review. Ethiop J Health Sci. 2021;31(1):167–78. CrossRef
20. Colgan S, Faasse K, Martin LR, Stephens MH, Grey A, Petrie KJ. Perceptions of generic medication in the general population, doctors and pharmacists: a systematic review. BMJ Open. 2015;5(12):e008915. CrossRef
21. Twin A. Investopedia. The 4 Ps of marketing: what they are & how to use them successfully. [cited 2024 Aug 21]. Available from: https://www.investopedia.com/terms/f/four-ps.asp
22. GoodRx. Prescription prices, coupons & pharmacy information. 2024 [cited 2024 Aug 12]. Available from: https://www.goodrx.com
23. National Library of Medicine. MedlinePlus [Internet]. Bethesda, MD: National Library of Medicine (US) [cited 2024 Aug 12]. Available from: https://medlineplus.gov/
24. CIMS. Search drug information, interactions, images, dosage & side effects. CIMS India; 2024 [cited 2024 Aug 12]. Available from: https://www.mims.com/india
25. 1 mg. 1 mg editorial policy and processes. Tata 1 mg. 2024 [cited 2024 Apr 20]. Available from: https://www.1mg.com/editorial-policy-processes
26. Janaushadhi. Pharmaceuticals & Medical Devices Bureau of India; 2024 [cited 2024 Aug 12]. Available from: https://janaushadhi.gov.in/
27. SK-Pharma. Affordable care redefined: the emerging impact of complex generics on global health. SK-Pharm blog; 2024 [cited 2024 Apr 24]. Available from: https://sk-pharma.com/media/affordable-care-redefined-the-emerging-impact-of-complex-generics-on-global-health/
28. Viatris. Our story. Viatris Homepage; 2024 [cited 2024 Apr 28]. Available from: https://www.viatris.com/en/about-us/our-story
29. Avhad PA, Chalikwar SS, Bhairav BA. A comprehensive review on complex generics. Medicine 2022;105:129–35. CrossRef
30. Perappadan BS. Generic drugs to treat four rare diseases launched. The Hindu [Internet]; 2023 [cited 2024 Jul 8]. Available from: https://www.thehindu.com/sci-tech/health/four-generic-made-in-india-drugs-to-treat-rare-diseases-offer-relief-for-patients-more-in-pipeline/article67570839.ece
31. Manual of Policies and Procedures (MAPP) 5240.10. [Internet]. [cited 2024 Jul 8]. Available from: https://www.fda.gov/media/157675/download
32. IQVIA. US generics market - evolution of Indian players. Durham, NC: IQVIA [Internet]; 2019 [cited 2024 Jul 8]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/india/us-generics-market-evolution-of-indian-players.pdf
33. Super generics market size & share revenue growth 2035 [Internet]. [cited 2024 Apr 8]. Available from: https://www.rootsanalysis.com/reports/super-generics-market/275.html
34. Quality Matters. Complex generics: are global regulators addressing the needs? | Quality Matters | U.S. Pharmacopeia Blog; 2023 [cited 2024 Apr 12]. Available from: https://qualitymatters.usp.org/complex-generics-are-global-regulators-addressing-needs
35. FDA. Reducing the hurdles for complex generic drug development. FDA; 2022 [cited 2024 Apr 9]. Available from: https://www.fda.gov/news-events/fda-voices/reducing-hurdles-complex-generic-drug-development
36. Zhang L, Lionberger RA. Generics 2030: where are we heading in 2030 for generic drug science, research, and regulation? Clin Pharmacol Ther. 2020;107(6):1293–5. CrossRef
37. Tan ML, Chandran S, Jereb R, Alam K, Bies R, Kozak D, et al. Mechanistic modeling of ophthalmic, nasal, injectable, and implant generic drug products: a workshop summary report. CPT Pharmacometrics Syst Pharmacol. 2023;12(5):631–8. CrossRef
38. Research C for DE and the center for research on complex generics. FDA [Internet]; 2024 [cited 2024 Apr 22]. Available from: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/center-research-complex-generics
39. Srivastava V, editor. Ch. 1, Peptide therapeutics: strategy and tactics for chemistry, manufacturing and controls. The Royal Society of Chemistry; 2019. pp 1–30. CrossRef
40. Outsourcing-pharma.com. Absorption systems: complex generics offer obstacles, opportunities. outsourcing-pharma.com; 2020 [cited 2024 Jul 9]. Available from: https://www.outsourcing-pharma.com/Article/2020/07/21/Complex-generic-drugs-provide-opportunities-and-obstacles
41. O’Brien MN, Jiang W Wang Y, Loffredo DM. Challenges and opportunities in the development of complex generic long-acting injectable drug products. J Control Release. 2021;336:144–58. CrossRef
42. Minghetti P, Musazzi UM, Casiraghi A, Rocco P. Old active ingredients in new medicinal products: is the regulatory path coherent with patients’ expectations? Drug Discov Today. 2020;25(8):1337–47. CrossRef
43. Kapoor V, Kaushik D. A comparative study of regulatory prospects for drug-device combination products in major pharmaceutical jurisdictions. J Generic Med Bus J Generic Med Sect. 2013;10(2):86–96. CrossRef
44. Bhatt M, Tank S, Shah J, Maheshwari D. Regulatory framework and disparities of complex generics in United States, European Union & Latin America. J Generic Med Bus J Generic Med Sect. 2023;19(3):130–40. CrossRef
45. Patil S, Kumar S, Rao DM, Rewatkar K. Current regulatory framework and challenges for the approval of complex generics in the US and the EU. Curr Indian Sci. 2024;02:e2210299X269535. CrossRef
46. Satheesh G, Sharma A, Puthean S, Ansil TPM, Jereena E, Raj Mishra S, et al. Availability, price and affordability of essential medicines for managing cardiovascular diseases and diabetes: a statewide survey in Kerala, India. Trop Med Int Health. 2020;25(12):1467–79. CrossRef
47. Klein K, Borchard G, Shah VP, Flühmann B, McNeil SE, de Vlieger JSB. A pragmatic regulatory approach for complex generics through the U.S. FDA 505(j) or 505(b)(2) approval pathways. Ann N Y Acad Sci. 2021;1502(1):5–13. CrossRef
48. Super generics industry analysis in Europe [Internet]. [cited 2024 Jul 9]. Available from: https://www.futuremarketinsights.com/reports/super-generics-industry-analysis-in-europe
49. Dutta T, Ramachandran S. Growing complex injectable portfolio in the Indian generic industries. 2023. pp 25. Available from: https://www.pharmafocusasia.com/biopharma/growing-complex-injectable-portfolio.
50. Research C for DE and upcoming product-specific guidances for generic drug product development. FDA [Internet]; 2024 [cited 2024 Jul 10]. Available from: https://www.fda.gov/drugs/guidances-drugs/upcoming-product-specific-guidances-generic-drug-product-development
51. Commissioner of the FDA. FDA Approves First Generic of a Commonly Used Albuterol Inhaler to Treat and Prevent Bronchospasm. FDA; 2020 [cited 2024 Jul 9]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-generic-commonly-used-albuterol-inhaler-treat-and-prevent-bronchospasm
52. Sari SP, Putri IDA, Nursanti B. Cost-effectiveness analysis of ceftriaxone generic and patent in children with typhoid. Int J Appl Pharm. 2018;10:76–80. CrossRef
53. Brill A. Potential savings from accelerating US approval of complex generics [Internet]. 2021 [cited 2024 Jul 9]. Available from: https://accessiblemeds.org/sites/default/files/2021-02/Potential-Savings-Complex-Generics-Feb2021.pdf
54. Simulations Plus [Internet]. Public workshop summary report on fiscal year 2021 generic drug regulatory science initiatives: data analysis and model-based bioequivalence. 2020 [cited 2024 Jul 9]. Available from: https://www.simulations-plus.com/resource/public-workshop-summary-report-on-fiscal-year-2021-generic-drug-regulatory-science-initiatives-data-analysis-and-model-based-bioequivalence/
55. Complex generics: facts, figures and who they benefit [Internet]. 2024 [cited 2024 Jul 9]. Available from: https://www.tevapharm.com/news-and-media/feature-stories/what-are-complex-generics/
56. Pillsbury D. Certara. Model-informed formulation development: insights from a case study. 2023 [cited 2024 Jul 9]. Available from: https://www.certara.com/blog/model-informed-formulation-development-insights-from-a-case-study/
57. MarketWatch [Internet]. Super generics market, 2035 - ResearchAndMarkets.com. 2024 [cited 2024 Jul 9]. Available from: https://www.marketwatch.com/press-release/super-generics-market-2035-researchandmarkets-com-91836c65
58. Lionberger RA. Innovation for generic drugs: science and research under the generic drug user fee amendments of 2012. Clin Pharmacol Ther. 2019;105(4):878–85. CrossRef
59. Andhariya, JV, Jog R, Shen J, Choi S, Wang Y, Zou Y, et al. In vitro-in vivo correlation of parenteral PLGA microspheres: effect of variable burst release. J Control Release. 2019;314:25–37. CrossRef
60. Committee for Medicinal Products for Human Use. Guideline on quality of transdermal patches. London, UK: Committee for Medicinal Products for Human Use (CHMP); 2014.
61. Lee SL, Saluja B, García-Arieta A, Santos GM, Li Y, Lu S, et al. Regulatory considerations for approval of generic inhalation drug products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285–304. CrossRef
62. Alam K. Mechanistic modeling of complex injectables: recommendations to navigate regulatory challenges. CDER | US FDA [Internet]; 2022 [cited 2024 Jul 9]. Available from: https://www.fda.gov/media/166586/download
63. Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95. CrossRef
64. Prasad V, Pooja K. Complex generics: opportunities & challenges. Int J Drug Regul Aff. 2016;4(3):1–10. CrossRef
65. Cutler D, Kirson N, Long G. Financing drug innovation in the US: current framework and emerging challenges. PharmacoEconomics. 2020;38:905–11. CrossRef
66. Barei F, Ross M. The refinement of the super generic concept: semantic challenge for product re-innovation? - GaBI J [Internet]. 2015;4(1):25–32 [cited 2024 Jul 9]. CrossRef
67. Who are the main competitors of Teva? [Internet]. [cited 2025 Jun 2]. Available from: https://synapse.patsnap.com/article/who-are-the-main-competitors-of-teva
68. TheGreySwan. Jan aushadhi and generics: is this a game changer for affordable healthcare? [Internet]. TheGreySwan; 2025 [cited 2025 Jun 2]. Available from: https://thegreyswan.substack.com/p/jan-aushadhi-and-generics-is-this
69. Medina JR, Cortes M, Romo E. Comparison of the Usp apparatus 2 and 4 for testing the in vitro release performance of ibuprofen generic suspensions. Int J Appl Pharm. 2017;9:90–5. CrossRef
70. Sowmya C, Abrar H, Prakaash K. Virtual bioequivalence in pharmaceuticals: current status and future prospects. Int J Appl Pharm. 2023;15:1–9. CrossRef
71. Ghabri S. Could or should we use cost-effectiveness thresholds in the French value-based pricing process for new drugs? PharmacoEconomics. 2024;42(8):823–7. CrossRef
72. Das M, Choudhury S, Maity S, Hazra A, Pradhan T, Pal A, et al. Generic versus branded medicines: an observational study among patients with chronic diseases attending a public hospital outpatient department. J Nat Sci Biol Med. 2017;8(1):26. CrossRef