INTRODUCTION
Infectious diseases remain one of the leading causes of morbidity and mortality. The majority of infections are contracted in a public place [1,2]. Antibiotic resistance has developed as an unprecedented global health threat, driven by its swift and widespread transmission [3]. Antibiotic-resistant pathogens have developed into a major concern in recent years, affecting both community and healthcare-associated infections (HAIs) [4]. The global spread of antibiotic-resistant bacteria reflects an increasing prevalence and highlights the urgent need for effective sterilization products to curb the growth and transmission of pathogens [5]. The prevalence of diseases caused by pathogenic filamentous fungi has risen substantially, yet these conditions remain underestimated [6–8]. Fungal contamination in indoor environments can lead to allergic reactions [9], and inhalation of fungal spores, fragments, or metabolites can contribute to respiratory issues, such as asthma, rhinitis, and bronchitis [10]. Molds can exacerbate asthma, cause skin irritation, and headaches, and trigger allergic responses [11,12]. Immunocompromised individuals are particularly at risk, with significant evidence linking mold exposure to various respiratory diseases [6,9]. Several studies highlight the critical role of microbial contamination on surfaces and devices in facilitating pathogen transmission. The efficiency of pathogen spread is contingent upon multiple factors, including the resilience of microorganisms to persist on dry surfaces and the frequency with which contaminated surfaces or devices contact both patients and healthcare personnel [13].
Disinfecting contaminated surfaces effectively lowers the overall infection level while also preventing the dissemination of bacteria to other surfaces [4]. Disinfectants are extensively recognized for eliminating microorganisms from the surfaces of objects and transmission media [14]. They are highly effective in controlling infectious diseases by preventing or eliminating the growth of pathogenic microorganisms within transmission modes [15,16]. They are crucial for maintaining ecological health and safety, with significant applications across various sectors, including healthcare, water treatment and distribution, food processing, agriculture, and other industries [17]. However, the emergence of disinfectant resistance poses a significant threat to public health and safety, as well as to the optimal use of resources, due to the decreased effectiveness of disinfectants [18].
Bacterial resistance to disinfectants plays a critical role in the management of HAIs [19,20]. Microorganisms may display intrinsic resistance to disinfectants, frequently attributed to the low permeability of their cellular structures. Prolonged exposure to disinfectants can further exacerbate microbial resistance through genetic mutations or the acquisition of resistance-conferring genetic elements [13]. Several studies reported that benzalkonium chloride (BC) which is a quaternary ammonium compound class of chemical disinfectants, is resistant against various microorganisms, such as Staphylococcus aureus [21], Escherichia coli [22], Pseudomonas aeruginosa [23], Acinetobacter baumannii [24], Enterobacter sp. [25], Listeria monocytogenes [22], Aspergillus ochraceus [26], and Aspergillus fumigatus [27]. Regular exposure to commonly used chemical disinfectants increases the risk of chronic obstructive pulmonary disease, asthma, and eye irritation [28,29]. Additionally, there is growing evidence that such exposure may lead to infertility and negatively affect brain development in children [30]. Health workers and individuals who are regularly exposed to these disinfectants are particularly vulnerable, as continuous contact with these chemicals may contribute to long-term health complications [28]. Moreover, it can have detrimental effects on human health, including skin irritation, pruritus, headaches, and dizziness [31]. Residual chemicals left on surfaces often contain hazardous compounds that are implicated in a range of health issues such as cancer, respiratory disorders, skin irritation, central nervous system dysfunction, and oxidative stress, all of which contribute to a broad spectrum of adverse human health outcomes [28]. Additionally, rainfall facilitates the runoff of disinfectants, leading to contamination of aquatic systems, soil, and atmospheric components. Both direct and indirect discharges of sewage effluents finally enter freshwater bodies such as lakes and rivers, presenting substantial threats to aquatic ecosystems and biodiversity by disrupting ecological stability and endangering the health of wildlife [28,30].
Being an ideal disinfectant, a natural plant-derived disinfectant should possess potent antimicrobial activity and be non-toxic, non-corrosive, cost-effective, user-friendly, and safe for most surfaces. Additionally, it should be safe for skin contact and inhalation and environmentally sustainable [32,33]. Syzygium cumini is a large evergreen tree from the Myrtaceae family, known for its wide range of medicinal properties [34,35]. The leaf extract of S. cumini has been demonstrated to have significant antibacterial [36,37], antifungal [37,38], and antiviral [39] activities due to the presence of rich bioactive compounds, including tannins, saponins, terpenoids, flavonoids, and phenols, such as sitosterol, betulinic acid, crategolic acid, quercetin, myricetin, and kaempferol [40–42]. Tannins are capable of inhibiting bacterial growth or killing bacteria by binding to bacterial protein cells. This interaction causes protein denaturation, damaging the cell wall and causing lysis [43]. Terpenoids also inhibit bacterial growth [44], while saponins compromise the integrity of cell membranes [45]. Flavonoids form complex bonds with extracellular proteins in the bacterial cell wall, weakening its structure and ultimately causing cell wall breakdown and lysis [43]. Furthermore, S. cumini leaf extract is reported to be non-toxic in various cell lines [37,46].
In the present work, environmental bacterial and fungal organisms from different floor surfaces of Sathyabama Dental College and Hospital premises were isolated and identified, and the effectiveness of S. cumini leaf extract-derived disinfectant was evaluated toward the antibacterial and antifungal activity in comparison to routinely used chemical disinfectant. Furthermore, the antibiotic and antifungal susceptibility tests against environmental isolates were also determined. This study is the first to report on the antibacterial and antifungal efficacy of S. cumini leaf extract against isolates from the environmental floor.
MATERIALS AND METHODS
Isolation of environmental organisms from different surfaces of hospital and college premises
Samples were collected by wiping with a sterile moistened swab from various areas of Sathyabama Dental College and General Hospital (SDCGH) floor surfaces, including the patient waiting area, hospital reception, lecture hall, hospital laboratory, staircase, department of microbiology, auditorium, office, college mess, library, and corridor. The study was approved by the Institutional Biosafety and Ethical Committee (Ref: 331/IRB-IBSEC/SIST Dated 18th October 2023). The collected swabs were inoculated onto the sterile Nutrient agar (NA) plates and Sabouraud dextrose agar (SDA), respectively. The plates were subsequently incubated at 37°C for 24 hours to facilitate bacterial isolation and at ambient temperature for 72 hours to enable fungal isolation. In order to obtain a pure isolate, individual colonies were picked from each plate and sub-cultured on respective media and then incubated at respective temperatures and days as mentioned earlier [47,48].
Identification of the environmental isolates
Identification of the environmental bacterial isolates was carried out by standard microbiological procedures, such as colony morphological analysis, Gram staining, plated on differential as well as on selective media, and biochemical tests [47]. The isolates were identified based on Bergey’s Manual of Systematic Bacteriology and further confirmed by Vitek MS (Biomerieux) [4]. Environmental fungal isolates were identified by colony morphological analysis, Lactophenol cotton blue (LPCB) staining, and confirmed by Vitek MS (Biomerieux) [4,49].
Preparation of extract
Fresh S. cumini leaves were collected and thoroughly washed with distilled water, dried, and then ground into fine powder. About 15 g of powder was added to 150 ml of sterile distilled water. Extraction was carried out using the hot percolation method at 60°C for 2 hours. Then, the extract was filtered through Whatman filter paper and then dried overnight in an oven at 40°C–45°C. It was ground into fine powder and stored for further use [37]. Extraction yield % was calculated as the weight of the solvent-free extract/dry weight of the sample ×100 [50].
Chemical disinfectants
The ingredients present in CD-1 are BC solution, water, lauryl alcohol ethoxylate, sodium bicarbonate, cocamidopropyl betaine, perfume, tetrasodium Ethylenediaminetetraacetic acid (EDTA), denatonium benzoate, and CI 47005. CD-2 contains BC solution, water, lauryl alcohol ethoxylate, lauramine oxide, sodium bicarbonate, disodium EDTA, isopropyl alcohol, cocamidopropyl betaine, perfume, and CI 47005. CD-3 contains BC, water, non-ionic surfactant, perfume, preservative, and colorant.
Preparation of the inoculum
The bacterial isolates were inoculated on NA medium and incubated at 37°C for 24 hours. After incubation, 2–3 colonies were taken from the plate and diluted with NB. The turbidity was standardized to the 0.5 McFarland standard to reach a cell density of 108 cells/ml. Fungal cultures were inoculated on to SDA medium and incubated at 28°C for 72 hours. Fungal isolates were adjusted to the spore density of 106 spores/ml using a spectrophotometer [51].
Antimicrobial activity of S. cumini leaf extract and routinely used chemical disinfectant against the bacterial and fungal environmental isolates
The antimicrobial activity of the S. cumini extract was compared with three routinely used chemical disinfectants, which was determined by the agar well diffusion method. The lawn culture was made with the isolated organisms onto the Muller–Hinton agar [47]. Chemical disinfectants were diluted in sterile distilled water based on the instructions provided by the manufacturers. Hundred microliters of S. cumini extract (10%), three chemical disinfectants, and distilled water were loaded into the respective wells using a sterile micropipette. The plates were then incubated at 37°C for 24 hours for bacterial cultures and 3 days at room temperature for fungal cultures. Following incubation, the diameter of the inhibition zone was determined. The experiment was conducted in triplicate.
Antibiotic susceptibility test against the bacterial environmental isolates
Antibiotic susceptibility test against the bacterial isolates was done on Muller–Hinton agar by disc diffusion method according to Clinical and Laboratory Standards Institute guidelines [52]. Antibiotics used for Gram-positive isolates were Cephalothin (CEP30), Vancomycin (VA30), Novobiocin (NV30, Erythromycin (E15), Oxytetracycline (O30), Amikacin (AK10), Amoxicillin (AMX10), and Bacitracin (B10). Antibiotics used for Gram-negative isolates were Ciprofloxacin (CIP10), Co-Trimazine (CM25), Kanamycin (K30), Nitrofurantoin (NIT300), Streptomycin (S10), Tetracycline (TE30), Amikacin (AK10), and Carbenicillin (CB100). Lawn cultures of bacterial isolates were inoculated onto Muller Hinton agar (MHA). Respective antibiotics were then placed on the media and incubated at 37°C for 24 hours. The zone of inhibition was measured after incubation. The experiment was conducted in triplicate.
Antifungal susceptibility test against the fungal environmental isolates
Antifungal susceptibility tests against the fungal isolates were also done using the disk diffusion method according to CLSI M51-A [53]. Antimycotic drugs used were Nystatin (NS), Amphotericin B (AP), Clotrimazole (CC), Fluconazole (FLC), Itraconazole, and Ketoconazole (KT). Lawn cultures of fungal isolates were prepared on MHA. Antimycotic drugs were then placed on it and incubated at room temperature for 2–3 days, respectively. Subsequent to incubation, the diameter of the zone of inhibition was determined. The experiment was conducted in triplicate.
Statistical analysis
One-way ANOVA and Tukey’s HSD post hoc test were used to analyze the significance level of data (IBM SPSS version 25.0). A p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Isolation and identification of the environmental isolates
Totally, 61 environmental organisms were isolated from SDCGH floor surfaces, of which 41 were bacterial isolates and 20 were fungal isolates, as shown in (Fig. 1). The isolated bacteria were characterized by colony morphology, gram staining, and biochemical tests and identified using Bergey’s Manual of Systematic Bacteriology and further confirmed by Vitek MS. The identified bacteria were Staphylococcus sp., Bacillus sp., Providencia rettgeri, Micrococcus sp., P. aeruginosa, and Enterobacter hormaechei (Table 1). Among the Staphylococcus sp., six of them were S. aureus. This is the first study reported on P. rettgeri isolated from environmental floors. Fungal isolates were identified by colony morphology, LPCB staining and further confirmed by Vitek MS. The identified fungi were Mucor sp., Alternaria sp., Fusarium sp., Penicillium sp., Aspergillus flavus, and Aspergillus niger (Fig. 2 and Table 2). Palmer and Onifade [48] revealed that S. aureus, Str. pyogenes, E. coli, P. aeruginosa, Klebsiella pneumoniae, Bacillus cereus, A. niger, A. fumigatus, and Candida albicans were isolated from hospital environmental surfaces. Similarly, A. flavus, A. niger, A. ustus, A. fumigatus, Rhizopus stolonifera, Scopulariopsis sp., Trichoderma sp., Mucor racemosus, Mucor hiemalis, and Wallemia sp. were isolated from carpet and floor dust samples of various indoor environments [54]. Staphylococcus sp., Streptococcus sp., Bacillus sp., Aspergillus sp., Mucor sp., Fusarium sp., Penicillium sp., Candida sp., Rhizopus sp., and Verticillium sp. were isolated from the hospital indoor environment [55]. The dominant percentage of bacterial and fungal isolates is shown in (Fig. 3). In the case of bacterial isolates, Bacillus sp. was found predominantly, followed by Staphylococcus sp., P. aeruginosa, E. hormaechei, P. rettgeri, and Micrococcus sp., respectively. In the case of fungal isolates, A. niger was the most common fungi, followed by Penicillium sp., Fusarium sp., A. flavus, Mucor sp., and Alternaria sp. According to Viegas et al. [56], the most prevalent bacteria isolated from the indoor environment were Micrococcus sp., followed by Staphylococcus sp. and Neisseria sp., and in the case of fungal isolates, Chrysosporium sp. were prevalently found, followed by Penicillium sp. and Chrysonilia sp.
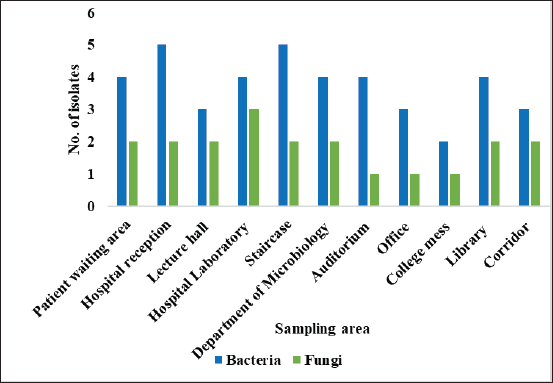 | Figure 1. Number of bacteria and fungi isolated from various floors. [Click here to view] |
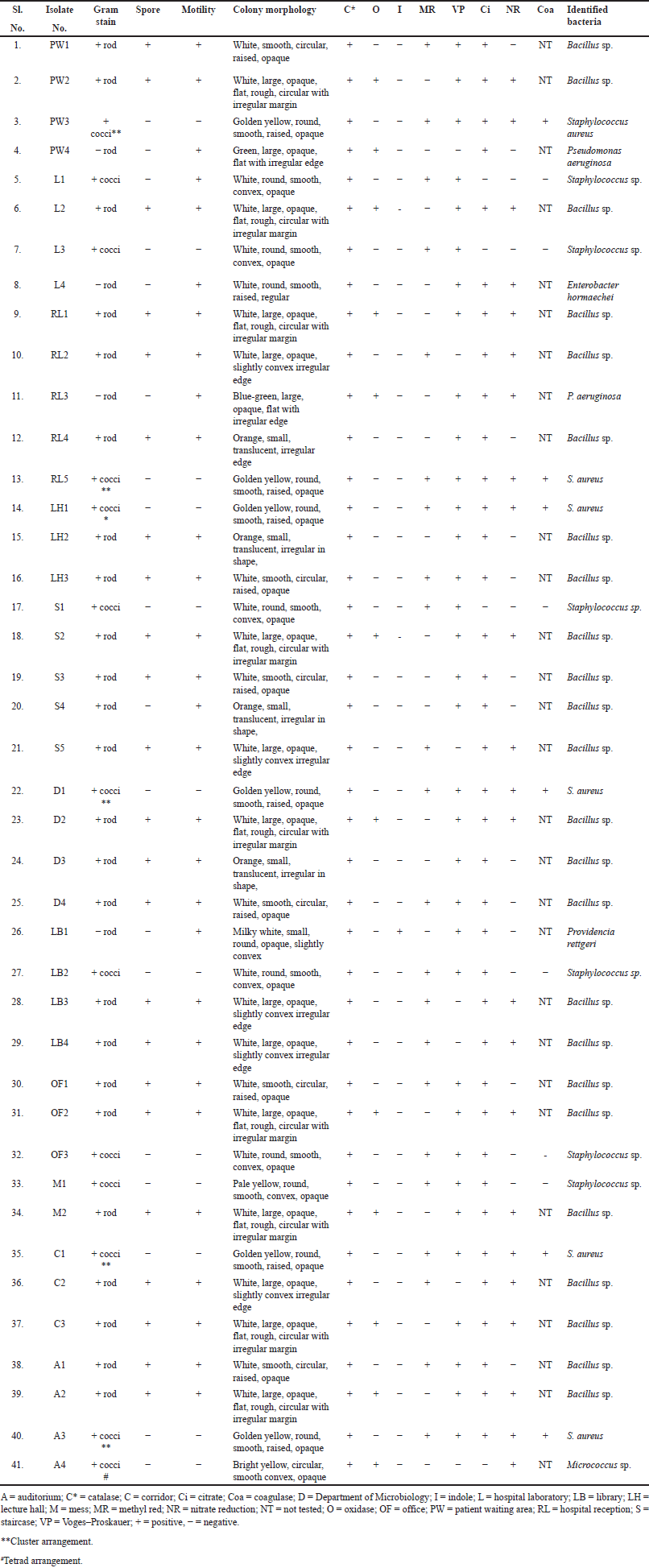 | Table 1. Morphological and biochemical tests of bacterial environmental floor isolates. [Click here to view] |
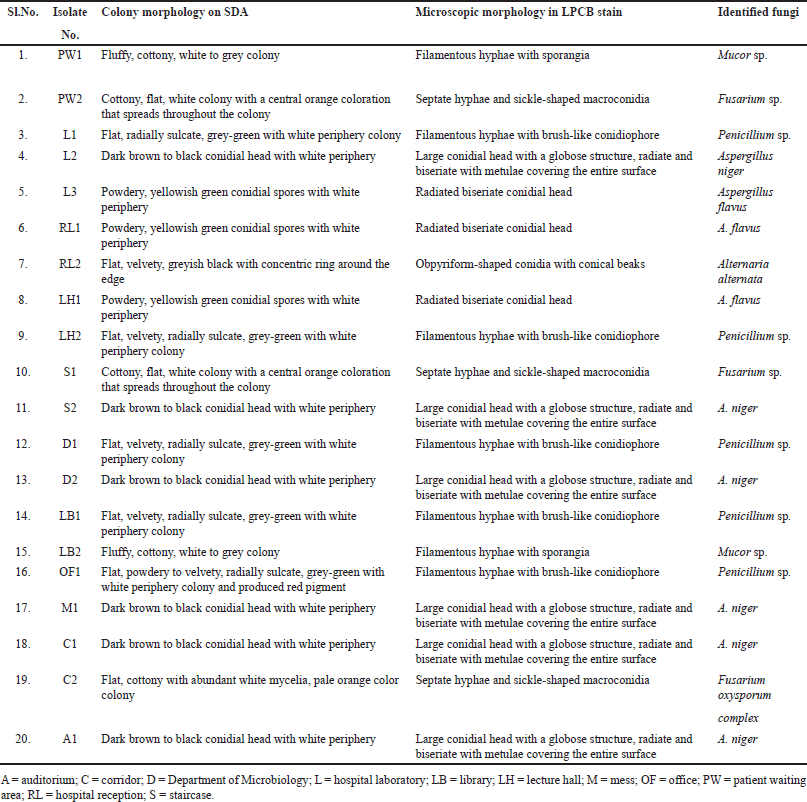 | Table 2. Colony morphology and microscopic characteristics of fungal environmental floor isolates. [Click here to view] |
 | Figure 2. Fungal floor isolates on SDA. [Click here to view] |
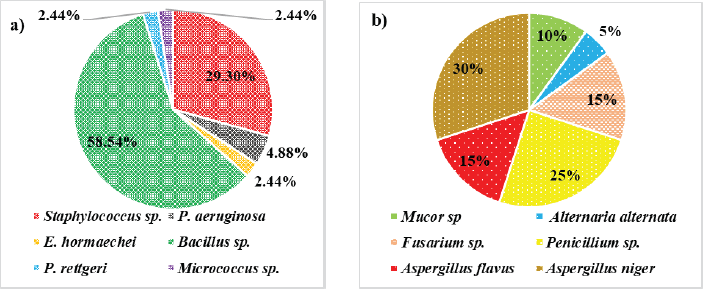 | Figure 3. Dominant percentage of a) bacterial isolates and b) fungal isolates. [Click here to view] |
Extraction yield
Syzygium cumini leaves were extracted using distilled water as a solvent. The obtained extract was brown in color and crystalline in nature after drying. The extraction yield % of S. cumini leaves was calculated using the formula mentioned above, and 9.5% of yield was obtained.
Effect of S. cumini leaf extract and routinely used chemical disinfectant against the bacterial and fungal environmental isolates
The antibacterial activity of S. cumini extract and three routinely used chemical disinfectants, such as CD1, CD2, and CD3 were determined against the organisms isolated from SDCGH floor surfaces by the agar well diffusion method (Table 3). Syzygium cumini extract showed good activity against all the bacterial environmental floor isolates, such as Staphylococcus sp., Bacillus sp., Micrococcus sp., P. aeruginosa, E. hormaechei, and P. rettgeri (Fig. 4). Syzygium cumini extract produced a significant zone of inhibition against Staphylococcus sp. in the range of (18.07–25.3 mm), Bacillus sp. (12.8–20.5 mm), Micrococcus sp. (28.87 mm), P. aeruginosa (14.13–18.3 mm), E. hormaechei (20.33 mm), and P. rettgeri (21.83 mm). The significant antibacterial activity of Syzygium cumini extract (SCE) may be due to the presence of various secondary metabolites such as tannins, terpenoids, saponins, and flavonoids [40].
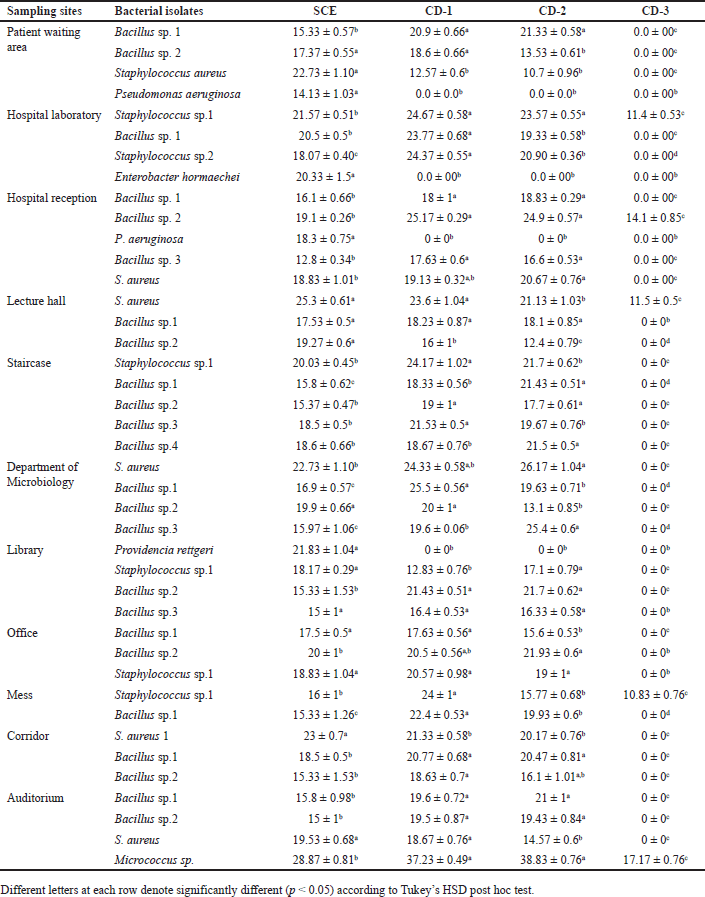 | Table 3. Antibacterial activity comparison of SCE and chemical disinfectants against the bacterial environmental floor isolates. [Click here to view] |
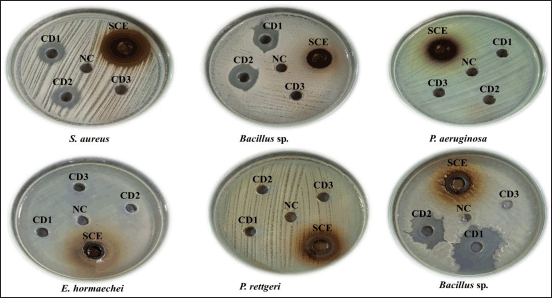 | Figure 4. Zone of inhibition of SCE and chemical disinfectants against the bacterial environmental floor isolates. [Click here to view] |
BC, the active ingredient of the routinely used chemical disinfectant, was used in the current investigation. In the case of chemical disinfectant, CD1 showed good activity against bacterial isolates, followed by CD2 and CD3. In this study, there was a significant difference (p < 0.05 according to Tukey’s HSD post hoc test) between the SCE and chemical disinfectants on antibacterial activity against the isolates. CD1 showed antimicrobial activity against bacterial isolates, such as Staphylococcus sp., Bacillus sp., and Micrococcus sp., but no activity was shown against P. aeruginosa, P. rettgeri, and E. hormaechei. Similarly, CD2 showed antimicrobial activity against most of the bacterial isolates, such as Staphylococcus sp., Bacillus sp., and Micrococcus sp.; however, it showed no activity against P. aeruginosa, P. rettgeri, and E. hormaechei. CD3 was found to have the least activity toward the bacterial isolates. It showed antimicrobial activity to some of the Staphylococcus sp. and Bacillus sp. only; however, no activity was shown against P. aeruginosa, P. rettgeri, and E. hormaechei. Interestingly, this study showed that S. cumini extract was potent against P. aeruginosa, E. hormaechei, and P. rettgeri. However, all the routinely used chemical disinfectants whose active ingredient was BC showed no activity against P. aeruginosa, E. hormaechei and P. rettgeri. Our results were consistent with the previous studies reported that BC does not show activity against P. aeruginosa [23,57,58] and P. rettgeri [59,60]. It also does not show activity against Enterobacter sp. isolated from the hospital environment [25].
Microbial innate resistance to disinfectants might be typically associated with the impermeability of their cell membranes, which prevents disinfectant agents from penetrating and affecting the cells [19]. This inherent defense mechanism renders certain microorganisms less susceptible to the action of disinfectants. However, continuous or prolonged exposure to disinfectants can lead to an increase in resistance [61]. This enhancement of resistance occurs through two main mechanisms: genetic mutations within the microorganism’s genome, which may lead to adaptations/alterations that enable survival in the presence of disinfectants, and the acquisition of resistance genes from other microorganisms, often via horizontal gene transfer. These processes contribute to the development and spread of disinfectant resistance, which makes it more challenging to control the microorganisms [13].
The antifungal activity of S. cumini extract and three routinely used chemical disinfectants, such as CD1, CD2, and CD3, were also determined against the environmental fungal isolates (Table 4). Syzygium cumini extract showed significant activity against all the environmental fungal isolates, such as Mucor sp., Penicillium sp., Fusarium oxysporum, Alternaria alternata, A. niger, and A. flavus (Fig. 5). Syzygium cumini extract exhibited a significant zone of inhibition against Mucor sp. in the range of (23–26.33 mm), Penicillium sp. (15–19.8 mm), F. oxysporum (11.5–19.77 mm), A. alternata (20.83 mm), A. niger (13–14.47 mm), and A. flavus (12.17–12.83 mm). Similar to that of the antibacterial activity, the marked antifungal activity of SCE could be likely due to the presence of secondary metabolites, such as tannins, terpenoids, saponins, and flavonoids. These phytochemicals are also known to possess antifungal properties, with tannins directly affecting fungi [62]. Their antifungal action is attributed to their lipophilicity, hydroxyl groups, and ability to bind proteins and adhesins, disrupting membranes, inactivating enzymes, and binding metal ions, leading to toxic effects on fungal cells [63].
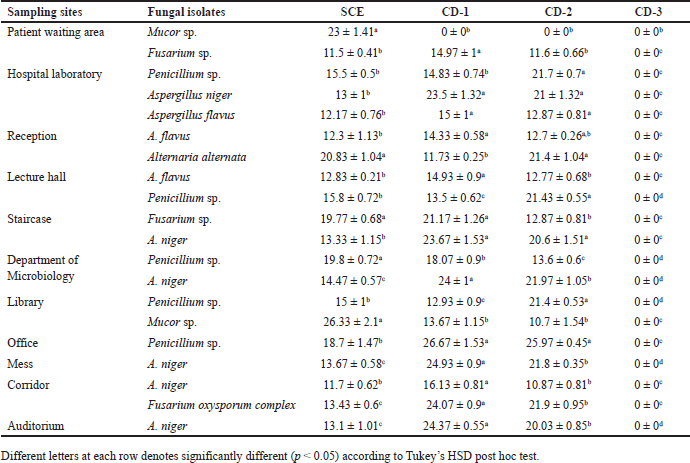 | Table 4. Antifungal activity comparison of SCE and chemical disinfectants against the fungal environmental floor isolates. [Click here to view] |
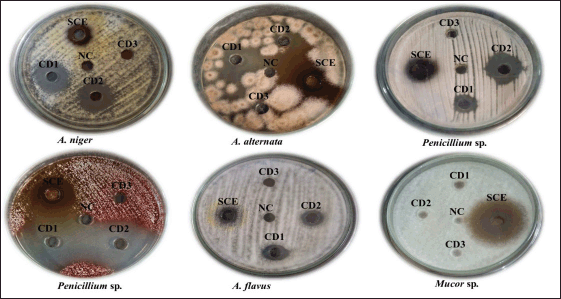 | Figure 5. Zone of inhibition of SCE and chemical disinfectants against the fungal environmental floor isolates. [Click here to view] |
Among the routinely used chemical disinfectants whose active ingredient was BC, CD1 showed good activity against most of the fungal isolates, followed by CD2. This study showed a significant difference (p < 0.05 according to Tukey’s HSD post hoc test) between the SCE and chemical disinfectants on antifungal activity against the isolates. CD1 showed antifungal activity against all the fungal isolates except one Mucor sp., which was isolated from the patient waiting area. Similarly, CD2 showed antifungal activity against all fungal isolates except that one Mucor sp., which was isolated from the patient waiting area. However, CD3 does not show activity in all the fungal isolates. Stupar et al. [64] revealed that BC showed antifungal activity against A. niger, Bipolaris spicifera, Penicillium sp., A. ochraceus, Epicoccum nigrum, and Trichoderma viride, which were isolated from cultural heritage objects. BC showed antifungal activity against Aspergillus sp. and Fusarium sp. [65]. It is noteworthy that this is the first study to report on the resistance of Mucor sp. isolated from environmental floors to BC.
Antibiotic drug susceptibility
Antibiotic drug susceptibility test was done by the disc diffusion method against the environmental Gram-positive and Gram-negative bacterial isolates. Results were interpreted according to the CLSI guidelines [52]. In the case of Gram-positive isolates, it showed resistant of 32.4% (12/37) to B, 27% (10/37) to AMX, 24.3% (9/37) to E, 21.6% (8/37) to CEP and NV, 10.8% (4/37) to O, and it showed 100% (35/35) sensitive to AK and V (Fig. 6). Among the Gram-positive isolates, Staphylococcus sp. showed resistant of 58.33% (7/12) to AMX, 50% (6/12) to E, 41.67% (5/12) to NV, and 100% (12/12) sensitive to B, CEP, O, V, and AK. Bacillus sp. showed 52% (12/24) resistant to B, 33.33% (8/24) to CEP, 16.67% (4/24) to O, 12.5% (3/24) to AMX, E, and NV, and exhibited 100% 9 (24/24) sensitive to V and AK. Micrococcus sp. showed 100% (1/1) sensitivity to all the tested antibiotics. These findings were consistent with the previous study by Mohammed et al. [66] who reported that Staphylococcus sp. isolated from hospital environment showed 67% resistant to E, and 21% to V, and Bacillus sp. showed 8% resistant to E and sensitive to V. B. cereus strains isolated from fresh vegetables were 99% resistant to amoxicillin/clavulanic acid combination and 4.5% to E [67]. Micrococcus sp. showed 100% (1/1) sensitivity to all the tested antibiotics. This is the first study to report the antibiotic susceptibility pattern of Micrococcus sp. isolated from environmental floors.
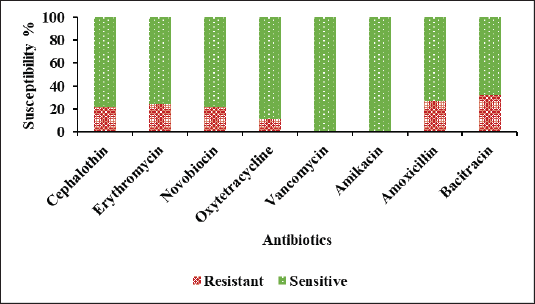 | Figure 6. Percentage of antibiotic susceptibility against the Gram-positive environmental isolates. [Click here to view] |
In the case of Gram-negative isolates, it showed resistant of 100% (4/4) to NIT, 50% (2/4) to K, 50% (2/4) to TE, 25% (1/4) to S and CM, and it showed 100% sensitive to CIP, AK and CB. (Fig. 7). Among the Gram-negative isolates, P. aeruginosa showed resistant of 100% (2/2) to NIT and K, 50% (1/2) to CM and TE, and 100% (2/2) sensitive to CIP, S, AK and CB. Eyo et al. [68] reported that environmental isolates of P. aeruginosa showed resistant to CB, CIP, and AK. Low resistant to AK and CIP against P. aeruginosa isolated from the hospital environmental isolates [69]. Pseudomonas aeruginosa isolated from residential sewage was resistant to NIT and CIP [70]. Morita et al. [71] reported that P. aeruginosa was resistant to TE. Enterobacter hormaechei showed resistant of 100% (1/1) to NIT and showed 100% (1/1) sensitive to CIP, K, CM, TE, S, AK, and CB. The result was in accordance with the previous finding [72]. Providencia rettgeri showed resistant of 100% (1/1) to NIT and TE and showed 100% (1/1) sensitive to CIP, K, S, CM, AK, and CB which was supported by the previous study [73].
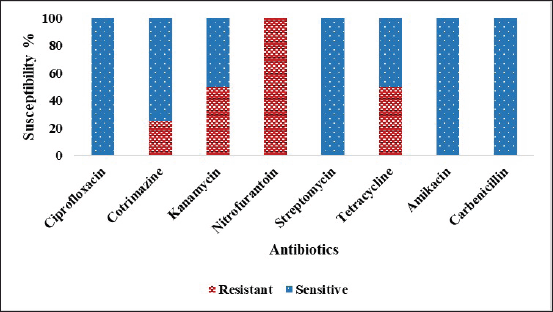 | Figure 7. Percentage of antibiotic susceptibility against the Gram-negative environmental isolates. [Click here to view] |
Antifungal drug susceptibility
Antifungal drug susceptibility test was investigated by the disc diffusion method against the environmental fungal isolates. The results were interpreted based on CLSI guidelines [74]. They showed resistant of 100% (20/20) to FLC, 30% (6/20) to Itraconazole (ITR), 15% (3/20) to AMP, and 10% (1/20) to CC (Fig. 8). However, all the fungal isolates were 100% (20/20) sensitive to NS and KT. The results are in accordance with the previous study [75]. Among the fungal isolates, Mucor sp. showed 100% (2/2) resistant to FLC and 50% (1/2) to AMP and ITR and showed 100% (2/2) sensitive to NS, CC, and KT. Alternaria alternata showed resistant of 100% (1/1) to FLC, ITR, and CC and showed 100% (1/1) sensitive to NS, AP, and KT. Penicillium sp. showed resistant of 100% (5/5) to FLC and 20% (1/5) to ITR and produced 100% (5/5) sensitive to NS, AP, CC, and KT. Fusarium oxysporum showed 100% resistant to FLC, 33.33% (1/3) to ITR and CC, and 100% (3/3) sensitive to NS, AP, and KT. Moreover, it showed 66.66% (2/3) sensitive to ITR and CC. Aspergillus niger showed 100% (8/8) resistant to FLC, 16.67% (1/6) to AMP and ITR, and 100% sensitive to NS, CC, and KT. Aspergillus flavus showed 100% resistant to FLC, 33.33% (1/3) to AMP and ITR, and 100% sensitive to NS, CC, and KT. Consistent with our findings, the environmental fungal isolates such as Aspergillus sp. and Mucor sp. were highly sensitive to AMP but had low susceptibility against Fusarium sp. [76] However, Aspergillus sp. Mucor sp. and Fusarium sp. had low susceptibility toward ITR. Penicillium sp. and Alternaria sp. were highly sensitive.to AMP and ITR. Kaur et al. [77] revealed that A. flavus and A. niger isolated from hospital environments as well as from community environments have low MIC for ITR and high MIC for AMP. Aspergillus niger, A. flavus, Penicillium sp. And Mucor sp. isolated from poultry environments were sensitive to NS but resistant to FLC [78].
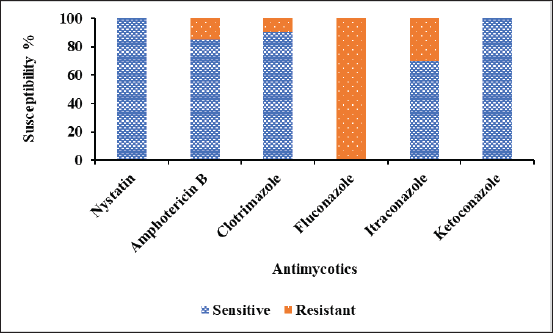 | Figure 8. Percentage of antifungal susceptibility against the fungal environmental isolates. [Click here to view] |
CONCLUSION
Syzygium cumini leaf extract showed significant antimicrobial activity against all the bacterial and fungal environmental floor isolates. Syzygium cumini extract showed a significant antimicrobial activity against the chemical disinfectant-resistant and antibiotic-resistant environmental isolates, supporting the potential use of S. cumini extract as an alternative plant-derived floor disinfectant that is eco-friendly and cost-effective. Identification of disinfectant-resistant genes should be done from the disinfectant-resistant isolates for future studies.
AUTHORS’ CONTRIBUTION
All the authors made significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICS STATEMENT
Ethical approvals details are given in the ‘Materials and Methods’ Section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Antabe R, Ziegler BR. Diseases, emerging and infectious. In: Kobayashi A, editor. International encyclopedia of human geography. 2nd ed. Amsterdam, Netherlands: Elsevier; 2020. pp. 389–91. CrossRef
2. Matini E, Shayeghi F, Vaghar M E, Nematian J, Hosseini SS, Mojri N, et al. A survey of public restrooms microbial contamination in Tehran city, capital of Iran, during 2019. J Family Med Prim Care. 2020;9(6):3131–5. CrossRef
3. Qiao M, Ying GG, Singer AC, Zhu YG. Review of antibiotic resistance in China and its environment. Environ Int. 2018;110:160–72. CrossRef
4. Rutala WA, Weber DJ, Barbee SL, Gergen MF, Sobsey MD, Samsa GP, et al. Evaluation of antibiotic-resistant bacteria in home kitchens and bathrooms: is there a link between home disinfectant use and antibiotic resistance? Am J Infect Control. 2023;51(11):A158–63. CrossRef
5. Bragg RR, Meyburgh CM, Lee JY, Coetzee M. Potential treatment options in a post-antibiotic era. Adv Exp Med Biol. 2018;1052:51–61. CrossRef
6. Paterson RRM, Lima N. Filamentous fungal human pathogens from food emphasising Aspergillus, Fusarium and Mucor. Microorganisms 2017;5(3):44. CrossRef
7. Al-Shaarani AAQA, Pecoraro L. A review of pathogenic airborne fungi and bacteria: unveiling occurrence, sources, and profound human health implication. Front Microbiol. 2024;15:1428415. CrossRef
8. Hattab Z, Ben Lasfar N, Abid M, Bellazreg F, Fathallah A, Hachfi W, et al. Alternaria alternata infection causing rhinosinusitis and orbital involvement in an immunocompetent patient. New Microbes New Infect. 2019;32:100561. CrossRef
9. Caillaud D, Leynaert B, Keirsbulck M, Nadif R. Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev. 2018;27(148):170137. CrossRef
10. Crook B, Burton NC. Indoor moulds, sick building syndrome and building related illness. Fungal Biol Rev. 2010;24(3–4):106–13. CrossRef
11. Jabber AS, Abbas FN, Abid IN, Qasim MT, Radhi LY, Hamim SS. Indoor air study of fungi contamination at internal departments for students in Thi-Qar Government. EJPMR. 2016;3(6):7–12. Available from: https://api.semanticscholar.org/CorpusID:251389186
12. Ozoaduche CL, Idemudia IB. Identification of Fungi isolated from bathrooms in female students’ Hostel, University of Benin, Benin City. AJHSE. 2021;2:25–35. CrossRef
13. Gomes IB, Malheiro J, Mergulhao F, Maillard J-Y, Simoes M. Comparison of the efficacy of natural-based and synthetic biocides to disinfect silicone and stainless steel surfaces. Pathog Dis. 2016;74(4):1–7. CrossRef
14. Maillard JY, Pascoe M. Disinfectants and antiseptics: mechanisms of action and resistance. Nat Rev Microbiol. 2024;22:4–17. CrossRef
15. Rozman U, Pušnik M, Kmetec S, Duh D, Šostar Turk S. Reduced susceptibility and increased resistance of bacteria against disinfectants: a systematic review. Microorganisms 2021;9(12):2550. CrossRef
16. Chaturvedi P, Shukla P, Giri BS, Chowdhary P, Chandra R, Gupta P, et al. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: a review on emerging contaminants. Environ Res. 2021;194:110664. CrossRef
17. Tong C, Hu H, Chen G, Li Z, Li A, Zhang J. Disinfectant resistance in bacteria: mechanisms, spread, and resolution strategies. Environ Res. 2021;195:110897. CrossRef
18. Mc Carlie S, Boucher CE, Bragg RR. Molecular basis of bacterial disinfectant resistance [published correction appears in Drug Resist Updat. 65:100867. CrossRef
19. Abreu AC, Tavares RR, Borges A, Mergulhão F, Simões M. Current and emergent strategies for disinfection of hospital environments. J Antimicrob Chemother. 2013;68(12):2718–32. CrossRef
20. Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect. 1999;43:S57–68. CrossRef
21. He GX, Landry M, Chen H, Thorpe C, Walsh D, Varela MF, et al. Detection of benzalkonium chloride resistance in community environmental isolates of staphylococci. J Med Microbiol. 2014;63(5):735–41. CrossRef
22. Pereira BMP, Tagkopoulos I. Benzalkonium chloride: uses, regulatory status, and microbial resistance. Appl Environ Microbiol. 2019;85(13):e00377-19. CrossRef
23. El-Banna T, Abd El-Aziz A, Sonbol F, El-Ekhnawy E. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Mol Biol Rep. 2019;46:3437–43. CrossRef
24. Maillard JY. Impact of benzalkonium chloride, benzethonium chloride and chloroxylenol on bacterial antimicrobial resistance. J Appl Microbiol. 2022;133(6):3322–46. CrossRef
25. Boutarfi Z, Rebiahi S-A, Morghad T, Pulido RP, Burgos MJG, Mahdi F, et al. Biocide tolerance and antibiotic resistance of Enterobacter spp. isolated from an Algerian hospital environment. J Glob Antimicrob Resist. 2019;18:291–7. CrossRef
26. Gupta AK, Ahmad I, Summerbell RC. Fungicidal activities of commonly used disinfectants and antifungal pharmaceutical spray preparations against clinical strains of Aspergillus and Candida species. Med Mycol. 2002;40(2):201–8. CrossRef
27. Terleckyj B, Axler DA. Efficacy of disinfectants against fungi isolated from skin and nail infections. J Am Podiatr Med Assoc. 1993;83(7):386–93. CrossRef
28. Bhat SA, Sher F, Kumar R, Karahmet E, Haq SAU, Zafar A, et al. Environmental and health impacts of spraying COVID-19 disinfectants with associated challenges. Environ Sci Pollut Res Int. 2022;29:85648–57. CrossRef
29. Dewey HM, Jones JM, Keating MR, Budhathoki-Uprety J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem Health Saf. 2022;29(1):27–38. CrossRef
30. Dhama K, Patel SK, Kumar R, Masand R, Rana J, Yatoo MI, et al. The role of disinfectants and sanitizers during COVID-19 pandemic: advantages and deleterious effects on humans and the environment. Environ Sci Pollut Res. 2021;28:34211–28. CrossRef
31. Hashemi F, Hoepner L, Hamidinejad FS, Haluza D, Afrashteh S, Abbasi A, et al. A comprehensive health effects assessment of the use of sanitizers and disinfectants during COVID-19 pandemic: a global survey. Environ Sci Pollut Res. 2023;30:72368–88. CrossRef
32. William A. Rutala WA, Weber DJ and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centre for Disease Control and Prevention; 2024[cited 2024 Dec 17]. Available from: https://www.cdc.gov/infection-control/media/pdfs/Guideline-Disinfection-H.pdf
33. Mandavgane SA, Rambhal AK, Mude NK. Development of cow urine based disinfectant. Natural Product Radiance. 2005;4(5):410–5. Available from: http://nopr.niscpr.res.in/handle/123456789/8129
34. Imran M, Imran M, Khan S. Antibacterial activity of Syzygium cumini leaf extracts against multidrug resistant pathogenic bacteria. J App Pharm Sci. 2017;7(3):168–74. CrossRef
35. Chhikara N, Kaur R, Jaglan S, Sharma P, Gat Y, Panghal A. Bioactive compounds and pharmacological and food applications of Syzygium cumini - a review. Food Funct. 2018;9(12):6096–115. CrossRef
36. Elfadil AG, Karamallah AA, Abualhassan AM, Hamid AA, Sabahelkhier MK. Antimicrobial activities of Syzygium cumini leave extracts against selected microorganisms. NJMBS. 2015;4:1–8. Available from: https://api.semanticscholar.org/CorpusID:87668526
37. Devi HJ, Gnanasekaran P, Devi YA, Siva D, Jayashankar J. Investigation of the antimicrobial efficacy and cytotoxicity of a natural disinfectant Syzygium cumini (L.) skeels leaf extract on vero cell lines. J Appl Pharm Sci. 2025;15(1):125–32. CrossRef
38. dos Santos KK, Matias EF, Tintino SR, Souza CE, Braga MF, Guedes GM, et al. Cytotoxic, trypanocidal, and antifungal activities of Eugenia jambolana L. J Med Food. 2012;15(1):66–70. CrossRef
39. Diningrat DS, Sari AN, Harashap NS, Kusdianti. In silico study of the toxicity and antiviral activity prediction of Jablang (Syzygium cumini) leaves essential oil as ACE2 Inhibitor. PharmacologyOnLine. 2021;3:1334–51. Available from: https://pharmacologyonline.silae.it/files/archives/2021/vol3/PhOL_2021_3_A146_Diningrat.pdf
40. Devi HJ, Gnanasekaran P, Devi YA. Selection of effective plant extract as a disinfecting agent using hot and cold-water extraction. Eco. Env Cons. 2022;28(4):1874–81. CrossRef
41. Qamar M, Akhtar S, Ismail T, Wahid M, Abbas MW, Mubarak MS, et al. Phytochemical profile, biological properties, and food applications of the medicinal plant Syzygium cumini. Foods 2022;11(3):378. CrossRef
42. Rizvi MK, Rabail R, Munir S, Inam-Ur-Raheem M, Qayyum MMN, Kieliszek M, et al. Astounding health benefits of jamun (Syzygium cumini) toward metabolic syndrome. Molecules 2022;27(21):7184. CrossRef
43. Wirahmi N, Masrijal CDP, Amri F, Ikhsan, Triyansyah MI. Formulation and antibacterial activity of natural disinfectant combination of Psidium guajava and Piper betle leaf infusion against Staphylococcus aureus. Proceedings of the 2nd International Conference on Contemporary Science and Clinical Pharmacy 2021 (ICCSCP 2021). Dordrecht, Netherlands: Atlantis Press; 2021. pp 91–7. CrossRef
44. Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, et al. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res. 2017;196:44–68. CrossRef
45. Li J, Monje-Galvan V. In vitro and in silico studies of antimicrobial saponins: a review. Processes 2023;11(10):2856. CrossRef
46. Pereira JV, Freires IA, Castilho AR, da Cunha MG, Alves HDS, Rosalen PL. Antifungal potential of Sideroxylon obtusifolium and Syzygium and their mode of action against Candida albicans. Pharm Biol. 2016;54(10):2312–19. CrossRef
47. Gahongayire S, Aliero AA, Kato CD, Namatovu A. Prevalence and detection of qac genes from disinfectant-resistant Staphylococcus aureus isolated from salon tools in Ishaka Town, Bushenyi District of Uganda. Can J Infect Dis Med Microbiol. 2020;2020:1–11. CrossRef
48. Palmer OG, Onifade AK. Microorganisms isolated from hospital environmental surfaces in Akure Metropolis, Ondo State, Nigeria. J Adv Microbiol. 2019;15(3):1–8. CrossRef
49. Chand S, Saha K, Singh PK, Sri S, Malik N. Determination of minimum inhibitory concentration (MIC) of routinely used disinfectants against microflora isolated from clean rooms. Int J Curr Microbiol App Sci. 2016;5(1):334–41. CrossRef
50. Suryani AE, Nisa K, Handayani S, Darsih C, Wuryastuty H, Yanuartono Y. The UHPLC-HRMS profiling, in vitro antioxidant and pancreatic lipase inhibitory activities of Peronema canescens leaves extract and fractions from Indonesia J Appl Pharm Sci. 2025;15(05):75–89. CrossRef
51. Nayaka S, Chakraborty B, Bhat MP, Nagaraja SK, Airodagi D, Swamy PS, et al. Biosynthesis, characterization, and in vitro assessment on cytotoxicity of actinomycete-synthesized silver nanoparticles on Allium cepa root tip cells. Beni-Suef Univ J Basic Appl Sci. 2020;9:51. CrossRef
52. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100; 2023.
53. Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of nondermatophyte filamentous fungi; approved guideline. Wayne, PA: CLSI document M51-A; 2010.
54. Buhari A-SM, Kamaldeen A-S, Hassan Ummu KM. Isolation of fungal flora in carpet and floor dust samples as an indicator of indoor air quality (IAQ): a case study of a nigerian institution. Int J Sci Res. 2012;2(8):1–7. Available from: https://www.ijsrp.org/research-paper-0812/ijsrp-p0804.pdf
55. Bhatia L, Vishwakarma R. Hospital indoor airborne microflora in private and government owned hospital in Sagar City, India. WJMS. 2010;5(3):65–70.
56. Viegas C, Dias M, Monteiro A, Faria T, Lage J, Carolino E, et al. Bioburden in sleeping environments from Portuguese dwellings. Environ Pollut. 2021;273:116417. CrossRef
57. Loughlin MF, Jones MV, Lambert PA. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J Antimicrob Chemother. 2002;49(4):631–9. CrossRef
58. Sakagami Y, Yokoyama H, Nishimura H, Ose Y, Tashima T. Mechanism of resistance to benzalkonium chloride by Pseudomonas aeruginosa. Appl Environ Microbiol. 1989;55(8):2036–40. CrossRef
59. El Moug T, Rogers DT, Furr JR, El-Falaha BMA, Russell AD. Antiseptic-induced changes in the cell surface of a chlorhexidine-sensitive and a chlorhexidine-resistant strain of Providencia stuartii. J Antimicrob Chemother. 1986;16:685–9. CrossRef
60. Stickler DJ, Thomas B, Chawla JC. Antiseptic and antibiotic resistance in Gram-negative bacteria causing Urinary tract infection in spinal cord injured patients. Spinal Cord. 1981;19:50–8. CrossRef
61. Russell AD, Suller MTE, Maillard JY. Do antiseptics and disinfectants select for antibiotic resistance? J Med Microbiol. 1999;48(7):613–5. CrossRef
62. Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS, et al. Natural products- antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11(7):621–38. CrossRef
63. Kursa W, Jamio?kowska A, Wyrostek J, Kowalski R. Antifungal effect of plant extracts on the growth of the cereal pathogen Fusarium spp.—an in vitro study. Agronomy 2022;12(12):3204. CrossRef
64. Stupar, Grbi? ML, Džami? A, Unkovi? N, Risti? M, Jeliki? A, et al. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S Afr J Bot. 2014;93:118–24. CrossRef
65. Day S, Lalitha P, Haug S, Fothergill AW, Cevallos V, Vijayakumar R, et al. Activity of antibiotics against Fusarium and Aspergillus. Br J Ophthalmol. 2009;93(1):116–9. CrossRef
66. Mohammed WE, Hassan HE, Yousif MA. Antibiotic sensitivity pattern of bacteria isolated from the environment of intensive care unit of Wad Medani emergency hospital, Gezira state, Sudan. Int J Curr Pharm Res. 2020;12(5):82–5. CrossRef
67. Fiedler G, Schneider C, Igbinosa EO, Kabisch J, Brinks E, Becker B, et al. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019;19:250. CrossRef
68. Eyo AA, Ibeneme EO, Thumamo BD, Asuquo AE. Antibiotic resistance profiles of clinical and environmental isolates of Pseudomonas aeruginosa in Calabar, Nigeria. J Pharm Biol Sci. 2015;10(4):09–15. Available from: https://www.iosrjournals.org/iosr-jpbs/papers/Vol10-issue4/Version-1/B010410915.pdf
69. Karami P, Mohajeri P, Mashouf RY, Karami M, Yaghoobi MH, Dastan D, et al. Molecular characterization of clinical and environmental Pseudomonas aeruginosa isolated in a burn center. Saudi J Biol Sci. 2019;26(7):1731–6. CrossRef
70. Adesoji AT, Onuh JP, Palang IP, Liadi AM, Musa S. Prevalence of multi-drug-resistant Pseudomonas aeruginosa isolated from selected residential sewages in Dutsin-Ma, Katsina State, Nigeria. J Public Health Afr. 2023;14(2):5. CrossRef
71. Morita Y, Tomida J, Kawamura Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol. 2014;4:422. CrossRef
72. O’Hara CM, Steigerwalt AG, Hill BC, Farmer JJ, Fanning GR, Brenner DJ. Enterobacter hormaechei, a new species of the family Enterobacteriaceae formerly known as enteric group 75. J Clin Microbiol. 1989;27(9):2046–9. CrossRef
73. Malviya M, Kale-Prathan P, Coyle M, Giuliano C, Johnson LB. Clinical and drug resistance characteristics of provindencia infections. Microorganisms 2024;12(10):2085. CrossRef
74. Clinical and Laboratory Standards Institute. Performance standards for antifungal disk diffusion susceptibility testing of nondermatophyte filamentous fungi; informational supplement; CLSI M51-S1. 2010.
75. Farian E, Wojcik-Fatla A. Diversity and drug resistance of filamentous fungi isolated from the fresh Raspberries. Indian J Microbiol. 2022;62:146–51. CrossRef
76. Badiee P, Ghadimi-Moghadam A, Bayatmanesh H, Soltani J, Salimi-Khorashad AR, Ghasemi F, et al. Environmental surveillance of fungi and susceptibility to antifungal agents in tertiary care hospitals. Microbiol Spectr. 2024;12(1):e02270-23. CrossRef
77. Kaur M, Singla N, Aggarwal D, Kundu R, Gulati N, Kumar MB, et al. J. Antifungal susceptibility profile of clinical and environmental isolates of Aspergillus species from a tertiary care center in North India. Cureus 2024;16(2):e54586. CrossRef
78. Mgbeahuruike AC, Aliyoo K, Karlsson M, Chah KF. Identification, antifungal susceptibility and phylogenetic comparison of fungi in poultry environment in Nigeria. Int J Poult Sci. 2020;19(12):548–56. Available from: https://scialert.net/abstract/?doi=ijps.2020.548.556