INTRODUCTION
The key characteristics of osteoarthritis (OA) include the degeneration of articular cartilage, remodeling of subchondral bone, and inflammation of the synovial membrane. OA is a persistent, degenerative joint disorder [1]. It is the predominant form of arthritis and a significant factor in pain, disability, and diminished quality of life among the aging worldwide population [2]. In 2019, around 528 million individuals globally were affected by OA [3,4]. The prevalence in Asia is 31% for women and 23% for men over 24 years and 61% for women, and 53% for men aged 40–75. In Europe, the prevalence is 14% for women and 12% for men over 22 years, and 29% for women and 16% for men over 55 years [5,6]. In Indonesia, OA prevalence is 5% among individuals under 40, 30% among those aged 40 to 60, and 65% among those over 60. Meanwhile, according to gender, OA affects 5% of men and 12.7% of women [7,8].
An imbalance in the anabolic and catabolic factors produced by chondrocytes leads to OA, a degenerative joint disease [9]. This imbalance triggers the production of cytokines and inflammatory mediators, resulting in the release of nitric oxide (NO), which induces chondrocyte apoptosis and degradation of the extracellular matrix (ECM) [10]. The progressive degeneration of articular cartilage is a distinctive feature [11,12]. Primary OA is defined as joint degeneration without an identifiable cause. Concurrently, secondary OA arises from abnormal articular cartilage, as observed in rheumatoid arthritis (RA), or from abnormal force concentration inside the joint, as seen in post-traumatic instances [13].
Joint pain, stiffness, and functional limitations are the defining characteristics of OA. Pharmacological management of OA generally includes diclofenac sodium, a member of the Nonsteroidal Anti-Inflammatory Drug (NSAID) class. Diclofenac sodium suppresses prostaglandin synthesis by obstructing the cyclooxygenase (COX) enzyme, which catalyzes the conversion of 2-arachidonic acid (AA) to prostaglandin H2 in prostanoid biosynthesis [14]. Reports indicate that 30% of patients who administered diclofenac sodium orally for an extended duration encountered adverse effects, including renal impairment, increased liver enzymes, and gastric ulcers [15]. The topical dosage form of diclofenac sodium possesses a disadvantage [16,17]. Notwithstanding a lipophilic partition coefficient of 13.4 (log p = 1.13), diclofenac sodium could not efficiently extract its constituent from the vehicle. Moreover, the topical administration of diclofenac sodium solely mitigates symptomatic knee pain; it does not reverse cartilage degeneration [18,19]. Patients with OA will experience disability if cartilage degradation is not fully rectified.
Bone tissue engineering has significantly advanced as a therapeutic strategy for addressing bone and cartilage defects by restoring and maintaining native tissue functionality. Among the various approaches, scaffolds composed of ceramics or polymers have been widely explored for their ability to support tissue regeneration [20,21]. Diclofenac sodium has been recognized for its potential to be locally and sustainably delivered through scaffolds composed of chitosan, gelatin, and chondroitin sulfate, which are also applicable for cartilage repair. However, systemic oral administration of diclofenac sodium has been associated with severe gastrointestinal and cardiovascular risks, highlighting the need for safer, localized delivery systems [22]. Scaffolds for cartilage regeneration must replicate the morphology, structure, and function of native cartilage. Within these porous structures, neighboring cells migrate, adhere, proliferate, and differentiate to form new cartilage tissue [23]. The release of diclofenac sodium from such scaffolds may reduce acute inflammatory responses by decreasing neutrophil and macrophage infiltration at the injury site, thereby alleviating pain and swelling [24]. This study presents a novel scaffold formulation and systematic physicochemical characterization of diclofenac-loaded chitosan–gelatin–chondroitin sulfate, designed to improve mechanical performance and support controlled local release of anti-inflammatory agents, potentially relevant for cartilage repair applications.
In a previous study, a scaffold composed of chitosan, gelatin, and chondroitin sulfate in a 50:25:25 ratio was employed, resulting in a compressive strength of 10.58 MPa and a high cell viability rate of 102.75%. These findings indicate that the scaffold possesses favorable mechanical properties and excellent biocompatibility, demonstrating its non-toxic nature to cells [25]. Nonetheless, this composition ratio presents a disadvantage, as the scaffold will disintegrate within about two days. A cross-linking agent is required to improve the scaffold’s properties and facilitate the progressive release of diclofenac sodium. Upon the addition of a cross-linking agent to the scaffold, it can attach to the polymer’s amino groups, resulting in the formation of α-helical connections, which causes the initially linear polymer threads to become thicker and intertwined [23,26]. The pore diameter may consequently diminish. Consequently, the scaffold degradation rate would decrease while the volume of liquid influx would increase somewhat [27,28].
A scaffold composed of chitosan, gelatin, and chondroitin sulfate, incorporating polyethylene glycol (PEG) 400 as a plasticizer and diclofenac sodium as the active pharmaceutical ingredient, was formulated using glutaraldehyde (GA) as a cross-linking agent. GA maintains bone-bonding strength by forming stable cross-links between BHA and gelatin. Previous studies have demonstrated that GA, among various cross-linking agents tested, results in superior mechanical strength. The concentration range of GA used in this study was carefully selected based on prior research [29], which indicated that concentrations up to 2.5% are non-toxic. The most effective concentrations for cross-linking activity were reported to be 0.5% and 1.0% [30]. Accordingly, the scaffold was formulated with varying concentrations of GA: 0%, 0.25%, 0.50%, 1.00%, and 2.50%. This formulation was designed to assess the effect of different GA concentrations on key scaffold properties, including mechanical strength, pore diameter, porosity percentage, and degradation behavior under physiological conditions. These investigations provide a scientific basis for evaluating the efficiency of cross-linking while ensuring the biocompatibility and functional performance of the scaffold.
MATERIALS and METHODS
Materials
This study utilized shrimp chitosan (CV. Multiguna, Indonesia), gelatin (Cartino, Thailand), chondroitin sulfate, GA (Sigma-Aldrich, USA), pro-analysis NaOH solution (Merck, Germany), diclofenac sodium (Kalbe, Indonesia), pro-analysis acetic acid (Mallinckrodt, UK), PEG-400, phosphate buffer saline pH 7.4, ethanol 96%, and distilled water (Interlab, Indonesia).
Methods
Preparation of chitosan–gelatin–chondroitin sulfate-diclofenac scaffold composite
The scaffold was prepared using diclofenac sodium, chondroitin sulfate, gelatin, and chitosan. The composition ratio of the three biopolymers followed a 50:25:25 proportion of chitosan:gelatin:chondroitin sulfate, which corresponds to 2.5 g of chitosan, 1.25 g of gelatin, and 1.25 g of chondroitin sulfate. This ratio was determined based on previous optimization studies showing favorable properties regarding pore size distribution and interconnectivity. Moreover, incorporating chondroitin sulfate contributed to an increase in compressive strength, reaching 10.58 MPa and a high cell viability of 102.75%. These results indicate that the scaffold exhibits excellent mechanical integrity while remaining non-toxic to cells [24]. Specifically, 2.5 g of chitosan was solubilized in 100 ml of a 2% acetic acid solution and agitated until homogeneous with a magnetic stirrer; 50 ml of warm distilled water (40°C–50°C) was utilized to dissolve 1.25 g of gelatin. The chitosan solution was subsequently included, and the mixture was stirred. The chitosan–gelatin solution was thereafter stirred while 1.25 g of chondroitin sulfate powder was incrementally introduced. A 1% NaOH solution neutralized the composition upon achieving a homogeneous mixture. A 1% sodium diclofenac solution in PEG 400 was incorporated post-neutralization and stirred until fully dissolved and homogeneous. GA was then introduced as a cross-linking agent at varying concentrations of 0.25%, 0.5%, 1%, and 2.5% (v/v) by mixing it directly into the homogeneous solution. Cross-linking was conducted at room temperature (25°C) for 24 hours. After cross-linking, the scaffolds were thoroughly washed with distilled water three times to remove unreacted GA, minimizing potential cytotoxicity. Finally, the scaffolds were subjected to freeze-drying (lyophilization) to obtain the final porous structure in dry form. Table 1 illustrates the formulation of the chitosan–gelatin–chondroitin sulfate-sodium diclofenac scaffold implant with the inclusion of GA.
Table 1. Formulation of chitosan–gelatin–chondroitin sulfate-diclofenac sodium scaffolds preparations with the addition of GA.
| Formulation | Chitosan (gram) | Gelatin(gram) | Chondroitin sulfate (gram) | Diclofenac sodium(gram) | GA(%v/v) |
|---|---|---|---|---|---|
| Control | 2.5 | 1.25 | 1.25 | 0.05 | 0 |
| I | 2.5 | 1.25 | 1.25 | 0.05 | 0.25 |
| II | 2.5 | 1.25 | 1.25 | 0.05 | 0.50 |
| III | 2.5 | 1.25 | 1.25 | 0.05 | 1.00 |
| IV | 2.5 | 1.25 | 1.25 | 0.05 | 2.50 |
Characterization evaluation
Organoleptic tests, pore diameter evaluations utilizing a scanning electron microscope (SEM) (Inspect S-50, FEI, Japan), compressive strength evaluations with Autograph 2.1 (Autograph, Indonesia), and degradation analyses employing PBS solution were conducted to characterize the chitosan–gelatin–chondroitin sulfate scaffold composite incorporating the cross-linking agent GA.
Organoleptic evaluation
The organoleptic evaluation of chitosan–gelatin–chondroitin sulfate-diclofenac sodium scaffolds was conducted to assess their color and physical properties at varying GA concentrations (0%, 0.25%, 0.50%, 1.00%, and 2.50%). Three unbiased evaluators performed the assessments in a regulated laboratory environment, and the results were recorded as qualitative descriptions for subsequent comparison.
Pore diameter
The width of the pore, measured in micrometers (μm), is referred to as the pore diameter. A scaffold must possess an appropriate pore diameter size. Pore size influences gas exchange, nutrient transport, chondrocyte migration, and nutrient infiltration into the scaffold. A scaffold specimen measuring 5 mm in width and 3 mm in height was prepared for testing. A sputter was employed to deposit a 3-minute gold coating onto the scaffold. Upon placement of the coated sample in the sample chamber, it was subjected to an electron beam at a magnification of 1000 and a voltage of 5 kV. The detector would identify the beam post-reflection. A picture may result from an order of micron expansion or the dimensions of the scaffold sample’s pores. This test was conducted using SEM [31].
Porosity test
Porosity is a percentage ranging from 0% to 100% that indicates the volume of voids inside the scaffold relative to its total free space volume. The fluid transfer technique is employed to conduct the porosity test. Ethanol was selected due to its efficient absorption in the scaffold without inducing contraction or swelling. The scaffold’s dry weight (m1) was measured in its desiccated state. The mass of the ethanol and the container was subsequently recorded as m2. The scaffold was thereafter placed in a container containing ethanol (m3). Subsequently, it is immersed for 48 hours in 96% v/v ethanol. The scaffold was dismantled after 48 hours, and the weights of the ethanol and container were recorded (m4) [31]. The following formula can be employed to ascertain porosity:
Compressive test
Compressive strength testing aimed to evaluate whether the scaffold could withstand mechanical forces exerted by surrounding tissues. Scaffold samples, 5 mm in diameter and 3 mm in height, were tested in a dry state at room temperature using an Autograph universal testing machine. The compression test was conducted at a constant speed of 5 mm/minutes. A compressive load was applied to the scaffold during the test until structural deformation was observed. The diameter and height of the scaffold were recorded prior to testing to ensure accuracy. The compressive strength value was calculated based on the maximum force (in Newtons) applied before deformation occurred, and the result was automatically displayed upon test completion [31].
Degradation test
The degradation test was carried out by immersing the scaffold in a pH 7.4 phosphate-buffered saline (PBS) solution at 37°C in an incubator. A cubic device measuring 1 × 1 × 1 cm with four interconnected holes was used. The scaffold was prepared and dried using a freeze-dryer prior to immersion. The scaffold was immersed for a total of 14 days, and the mass change was measured at specific time intervals: 1 day, 2 days, 3 days, 5 days, 7 days, 10 days, 12 days, and 14 days [31].
RESULTS AND DISCUSSION
This study focused on a scaffold utilizing sodium diclofenac as the active component, composed of chitosan, gelatin, chondroitin sulfate, and PEG-400. The scaffold exhibits non-toxic, biodegradable characteristics and material compatibility, which are essential prerequisites for further development in cartilage repair applications for OA. GA was employed as a cross-linking agent to enhance structural stability. The maximum GA concentration was limited to 2.5%, which has been reported to cause no cytotoxic effects—such as cell damage or death—in human fibroblast cell lines (WI-38) [29]. This finding is consistent with the study by Budiatin et al. [23], which demonstrated that scaffolds containing up to 2.5% GA maintained ≥60% cell viability in MTT assays, thereby classifying them as non-toxic.
The cytotoxicity of GA is also known to be time-dependent. Sun et al. [29] reported that prolonged exposure to GA (24 hours) significantly increased its toxicity, lowering the 50% toxic concentration (TC50) from 4.83 mM to 2.09 mM. However, short-term exposure with appropriate post-treatment—such as extensive washing—can maintain GA levels within biologically acceptable limits. Accordingly, in this study, the cross-linking process was conducted with limited GA exposure (24 hours), followed by three cycles of washing with distilled water to remove unreacted GA, in line with procedures recommended by previous studies [29,30]. This strategy was implemented to minimize residual GA content and reduce the risk of cytotoxicity. Furthermore, PEG-400 was incorporated as a plasticizer to improve the scaffold’s elasticity and flexibility by increasing intermolecular spacing, thereby enhancing its resemblance to the mechanical properties of native cartilage tissue [32].
Organoleptic evaluation
Dry samples (after freeze-drying) underwent organoleptic evaluations through visual inspection. An organoleptic study of the color of each chitosan–gelatin–chondroitin sulfate-PEG 400-diclofenac sodium scaffold implant sample indicated a color variation, as illustrated in Figure 1, where the scaffold transitions from white to brown with increasing GA concentration.
 | Figure 1. Differences in the color of implant scaffold preparations with the addition of different GA concentrations. (a) Control, (b) GA 0.25, (c) 0.50, (d) 1.00, (e) 2.50%. [Click here to view] |
GA serves as a cross-linking agent in the construction of this scaffold. GA is essential for enhancing the stability and porosity of the scaffold by facilitating cross-linking among the amine groups of chitosan and gelatin, as well as between chitosan and chondroitin sulfate [26]. This study illustrates that the scaffold’s color alters with increasing GA content. The observed color changes indicated that the chemical reaction between GA and chitosan facilitated the formation of chromophores [33,34]. A study by Budiatin et al. [23] showed that the chitosan–gelatin–diclofenac scaffold underwent a color change upon adding GA. The color change is a consequence of a diazotization reaction between GA and the scaffold material. An intensified brownish hue is produced with an increase in GA concentration due to the formation of more cross-linking bonds between the carbonyl group (-C=O) in GA and the amine group (-NH2) from gelatin and chitosan, along with the S-group from chondroitin sulfate [35].
Pore diameter
Figure 2 illustrates that SEM analysis was employed to conduct morphological observations and ascertain the scaffold’s pore width. Based on the results of the obtained SEM observations, data were subsequently created to analyze the observed pore diameters. Table 2 illustrates that increased GA content reduces the scaffold’s pore diameter. The study’s results revealed that all scaffold samples, save for the highest concentration of 2.5%, possess pore diameters above 100 μm.
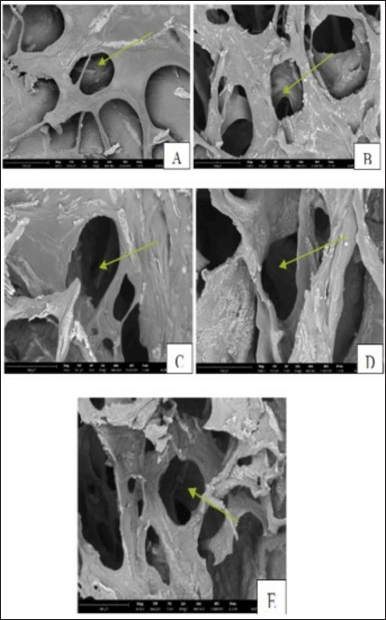 | Figure 2. The appearance of the pore diameter of scaffolds with the addition of different GA concentrations using SEM with a magnification of 600–1000x. (A) Control; (B) GA 0.25%; (C) 0.50%; (D) 1.00%; and (E) 2.50%. [Click here to view] |
Table 2. The average pore diameter values chitosan–gelatin–chondroitin sulfate-diclofenac sodium scaffolds preparations with the addition of different GA concentration.
| GA concentration | Pore diameter | p-value | |
|---|---|---|---|
| Min-Max (μm) | Mean ± SD (μm) | ||
| Control | 200–241 | 216.80 ± 21.27 | |
| GA 0.25% | 180–215 | 195.03 ±18.60 | 0.767 |
| GA 0.50% | 128–171 | 153.93 ± 22.23* | 0.043 |
| GA 1.00% | 110–112 | 111.62 ± 0.97* | 0.002 |
| GA 2.50% | 29–102 | 65.57 ± 36.05* | 0.000 |
Data are expressed as the mean ± SD of 3 replicates and analyzed by one-way ANOVA. *p-value<0.05 compared to the control group.
The presence of GA influences the dimensions of the pores that develop in the scaffold. The resultant cross-linking will form an α-helix bond, causing the initially linear polymer strands to tighten and coil around each other, enhancing the scaffold’s density [36,37]. The scaffold necessitates pore dimensions ranging from 100 to 200 μm [38,39]. Narrow pore widths may impede nutrition transfer and metabolic waste removal and restrict cellular mobility. This may lead to scaffold necrosis. Conversely, if the hole size is enormous, cells would detach more easily from the scaffold, leading to poor differentiation and proliferation processes [40–42]. The results of this work align with the prior research conducted by Zadeh and Zamanian [43], which showed that elevating the concentration of cross-linking GA may reduce the scaffold’s average pore size. Similarly, Samirah et al. [30] reported that higher concentrations of GA result in smaller pore diameters, indicating that GA significantly influences the structural characteristics of bioscrew pores.
Porosity presentation
Table 3 presents the average porosity of the chitosan–gelatin–chondroitin sulfate-diclofenac scaffold at varying doses of the GA cross-linking agent. As the concentration of GA rose, the porosity percentages of the scaffold markedly increased. Nonetheless, according to these findings, two scaffold samples—one with a GA concentration of 0.5% and another with 1%—exhibit porosity values within the requisite 75%–90% range for scaffolds.
Table 3. The porosity average of chitosan–gelatin–chondroitin sulfate-diclofenac sodium scaffolds containing different concentrations of GA.
| GA Concentration | Porosity (%) | p-value |
|---|---|---|
| Control | 64.53 ± 1.09 | |
| GA 0.25% | 74.65 ± 0.29* | 0.002 |
| GA 0.50% | 77.79 ± 1.49* | 0.002 |
| GA 1.00% | 82.53 ± 0.96* | 0.000 |
| GA 2.50% | 94.33 ± 2.08* | 0.000 |
Data are expressed as the mean ± SD of 3 replicates and analyzed by one-way ANOVA. *p-value<0.05 compared to the control group.
Incorporating GA facilitates the proliferation and differentiation of chondrocyte cells, enhances cell migration and vascularization, and increases the scaffold’s porosity to the requisite range of 75%–90% porosity percentage values [44]. The configuration of this hole is essential for enhancing bioapplicability, accelerating bone repair, and providing an increased surface area for gas and nutrient exchange [38,39]. Similar findings were seen in the work by Azami et al. [45], who reported analogous results, indicating that the porosity of the GEL-HA scaffold with GA was 85.1% more than that of the GEL-HA scaffold devoid of GA, which measured 84.6%. This study further illustrates that GA cross-linking enhances the mechanical strength and compressive resistance of the scaffold [23]. The cross-linking between the scaffold’s polymers is the reason for this. The study’s results met the criteria for a potential scaffold compressive strength (0.01–3 MPa) based on the mechanical properties of the target cartilage tissue [46,47].
Compressive strength
Table 4 presents the outcomes of experiments assessing the compressive strength of scaffolds composed of chitosan, gelatin, chondroitin sulfate, and diclofenac sodium, which incorporate differing quantities of GA. The results indicated that increasing the GA concentration significantly enhances the compressive strength of the scaffold. Scaffolds containing 0.5% GA exhibited superior compressive strength compared to scaffolds with different GA concentrations. Conversely, scaffolds lacking GA had reduced compressive strength.
Table 4. The compressive strength average of chitosan–gelatin–chondroitin sulfate-diclofenac sodium scaffolds containing different concentrations of GA.
| GA Concentration | Average ± SD (MPa) | p-value |
|---|---|---|
| Control | 0.069 ± 0.006 | |
| GA 0.25% | 0.097 ± 0.009 | 0.995 |
| GA 0.50% | 0.156 ± 0.007 | 0.769 |
| GA 1.00% | 0.368 ± 0.115* | 0.017 |
| GA 2.50% | 1.463 ± 0.168* | 0.000 |
Data are expressed as the mean ± SD of 3 replicates and analyzed by one-way ANOVA. *p-value<0.05 compared to the control group.
Degradation test
The degradation test in this study was conducted by immersing the scaffold in phosphate-buffered saline (PBS) solution at pH 7.4. While enzymatic degradation more closely replicates the physiological breakdown of cartilage tissue, PBS-only models are routinely used for preliminary scaffold assessment, as supported by recent literature. Yue et al. [48] evaluated the degradation behavior of gelatin methacrylate and PLA-silk-based scaffolds in PBS at pH 7.4 over a 30-day period. Despite the absence of enzymatic agents, the scaffolds showed progressive weight loss over time. These findings reinforced the reliability of PBS as a suitable medium for simulating early-stage scaffold degradation and drug release kinetics in physiological-like conditions.
Table 5 presents the results of the degradation test conducted in this study. The results show that increasing the GA concentration might reduce the weight loss % of the chitosan–gelatin–chondroitin sulfate-diclofenac scaffold. Figure 3 presents a bar chart illustrating the scaffold’s weight loss tendency over 14 days across different GA concentrations. The proportion of weight decrease was greater for scaffolds without GA than for those containing GA. All groups had a consistent increase in degradation over time.
Table 5. Degradation percentage average of chitosan–gelatin–chondroitin sulfate-diclofenac scaffolds containing different concentrations of GA after 14 days.
| Day (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GA Concentration | 1 | 2 | 3 | 5 | 7 | 10 | 12 | 14 | p-value | |
| Control | 23.81 ± 0.43 | 32.64 ± 0.87 | 38.82 ± 0.28 | 45.85 ± 0.33 | 51.73 ± 0.72 | 59.74 ± 0.78 | 67.13 ± 0.63 | 89.02 ± 7.15 | <0.001* | |
| GA 0.25% | 22.98 ± 0.14 | 30.98 ± 0.32 | 37.74 ± 0.75 | 45.07 ± 0.69 | 50.29 ± 0.52 | 58.78 ± 0.13 | 65.44 ± 0.72 | 80.54 ± 3.31 | <0.001* | |
| GA 0.50% | 22.06 ± 0.44 | 28.78 ± 1.15 | 36.09 ± 0.16 | 41.50 ± 0.82 | 49.51 ± 0.17 | 58.38 ± 0.21 | 64.49 ± 0.06 | 73.16 ± 2.06 | <0.001* | |
| GA 1.00% | 20.89 ± 0.60 | 26.25 ± 0.59 | 35.13 ± 0.56 | 40.54 ± 0.32 | 48.54 ± 0.32 | 56.23 ± 0.35 | 62.89 ± 1.29 | 70.38 ± 0.30 | <0.001* | |
| GA 2.50% | 19.50 ± 0.58 | 25.00 ± 0.68 | 33.75 ± 0.20 | 39.46 ± 0.55 | 46.77 ± 0.51 | 54.33 ± 1.23 | 60.89 ± 0.07 | 68.05 ± 0.30 | <0.001* | |
Data are expressed as the mean ± SD of 3 replicates and analyzed by repeated measure ANOVA.
*p-value<0.05 compared to the control group.
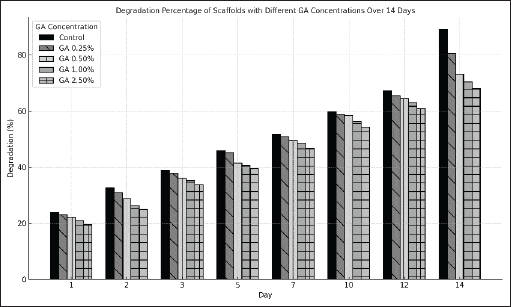 | Figure 3. Degradation percentage of scaffolds with different GA concentrations over 14 days. [Click here to view] |
GA enhances the durability of scaffolds, ensuring their stability even after a fortnight. This study corroborates other research indicating that GA can inhibit degradation by augmenting resistance to the scaffold [30,49,50]. Research conducted by Samirah et al. [30] demonstrated that GA stabilizes gelatin, which is susceptible to deterioration, with an optimal concentration ranging from 0.1% to 1%. Pinto et al. [51] indicated that GA concentrations beyond 1% may lead to cross-linking saturation, thus diminishing its benefits. The principal cause is the formation of cross-linking bonds between the carbonyl group (-C=O) in GA and the carboxyl group (COOH) from chondroitin sulfate, along with the amine group (-NH2) from gelatin and chitosan [52]. Increased cross-linking bonds are established, necessitating the rupture of additional bonds prior to the polymer’s degradation in the liquid at elevated GA concentrations. As the concentration of GA increases, the quantity of degraded particles will diminish due to a reduced fluid passage through the scaffold [53]. Furthermore, incorporating GA as a cross-linking agent in the scaffold formulation facilitates sustained release of diclofenac sodium, which may minimize potential interference with cartilage repair and provide a platform for further investigation in regenerative applications.
This study successfully characterized scaffolds incorporating various concentrations of GA as a cross-linking agent. However, several limitations should be acknowledged. First, the visual assessment primarily focused on color changes, which limits the generalizability of the findings and should be interpreted cautiously. Second, the degradation testing conditions did not fully replicate the physiological environment and thus may not accurately reflect in vivo performance. Moreover, the study did not include in vivo testing to evaluate the scaffold’s functional efficacy. Another limitation lies in the absence of cytotoxicity and in vitro cell compatibility assessments, which are essential for determining the scaffold’s biological safety, particularly considering the potential cytotoxic effects of residual GA. Furthermore, the drug release profile of sodium diclofenac, including cumulative release, burst release, and sustained release behavior, was not evaluated, despite its critical importance for understanding the scaffold’s therapeutic potential and anti-inflammatory performance. Biological and anti-inflammatory validations were likewise not included.
Further research is required to assess the scaffold’s effects on cell proliferation, viability, and differentiation, as well as to conduct quantitative drug release studies, GA cytotoxicity testing, and investigations into chondrocyte behavior and cytokine response assays. These efforts are crucial for comprehensively determining the scaffold’s safety and efficacy for biomedical applications. Despite these limitations, the findings suggest that scaffolds composed of chitosan, gelatin, chondroitin sulfate, and PEG-400, cross-linked with GA, exhibit enhanced mechanical strength, structural stability, and physicochemical properties, supporting their potential for further development in cartilage-mimicking scaffolds and localized drug delivery systems.
CONCLUSION
This study highlights the potential of GA-cross-linked chitosan–gelatin–chondroitin sulfate scaffolds as a platform for further development in OA-related cartilage repair, based on their physicochemical properties and drug-release behavior. The findings indicate that GA content significantly influences the optimization of scaffold properties. With additional GA, the resultant scaffold exhibits a more pronounced color, reduced pore diameter, increased porosity percentage, enhanced compressive strength, and diminished degradation percentage. The optimal concentration of GA as a cross-linking agent to improve the characteristics of diclofenac sodium scaffold implants made from chitosan, gelatin, and chondroitin sulfate is 0.5%.
This study did not include biocompatibility testing or in vitro assays, representing key limitations. Future studies should investigate cytotoxicity, drug release profiles, and cellular responses to comprehensively evaluate the scaffold’s safety and functionality. While this research provides critical insights into scaffold design and characterization, subsequent investigations should focus on in vivo validation to assess biocompatibility and therapeutic efficacy in relevant animal models. Exploring additional therapeutic agents and their synergistic interactions with scaffold-based delivery systems is recommended to enhance the scaffold’s applicability in various tissue engineering contexts. Interdisciplinary strategies combining biomaterials development, controlled drug delivery, and clinical translation represent a promising direction for advancing cartilage repair approaches and improving OA treatment outcomes.
AUTHORS CONTRIBUTION
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by Universitas Airlangga under Penelitian Dasar Unggulan Universitas Airlangga through research scheme No. 943/UN3.FF/PT.01.03/2024.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY STATEMENT
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. He Y, Jiang W, Wang W. Global burden of osteoarthritis in adults aged 30 to 44 years, 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Musculoskelet Disord. 2024;25(1):1–12. CrossRef
2. Welhaven HD, Welfley AH, June RK. Osteoarthritis year in review 2024: molecular biomarkers of osteoarthritis. Osteoarthr Cartil. 2024;33(1):67–87. CrossRef
3. Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil. 2020;28(3):242–8. CrossRef
4. Quicke JG, Conaghan PG, Corp N, Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthr Cartil. 2022;30(2):196–206. CrossRef
5. Coaccioli S, Sarzi-Puttini P, Zis P, Rinonapoli G, Varrassi G. Osteoarthritis: new insight on its pathophysiology. J Clin Med. 2022;11(20):1–12. CrossRef
6. Nicoliche T, Maldonado DC, Faber J, Da Silva MCP. Evaluation of the articular cartilage in the knees of rats with induced arthritis treated with curcumin. PLoS One. 2020;15(3):1–23. CrossRef
7. Hsu H, Siwiec RM. Knee osteoarhritis. Treasure Island, FL: StatPearls. 2023.
8. Sulaiman SZS, Tan WM, Radzi R, Shafie INF, Ajat M, Mansor R, et al. Comparison of bone and articular cartilage changes in osteoarthritis: a micro-computed tomography and histological study of surgically and chemically induced osteoarthritic rabbit models. J Orthop Surg Res. 2021;16(1):1–13. CrossRef
9. Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediat Inflamm. 2020;2020:1–19 CrossRef
10. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59. CrossRef
11. Budiatin AS, Khotib J, Samirah S, Ardianto C, Gani MA, Putri BRKHP, et al. Acceleration of bone fracture healing through the use of bovine hydroxyapatite or calcium lactate oral and implant bovine hydroxyapatite – gelatin on bone defect animal model. Polymers. 2022;14:1–17. CrossRef
12. Siddik M, Haryadi RD. The risk factors effect of knee osteoarthritis towards postural lateral sway. Indian J Forensic Med Toxicol. 2020;14(2):1787–92. CrossRef
13. Oliviero F, Ramonda R. Cartilage-derived biomarkers in osteoarthritis. Indian J Med Res. 2021;153:413–5. CrossRef
14. Katzung BG. Basic & clinical pharmacology. 14th ed. Katzung BG, editor. Mc Graw Hill Education. New York, NY: McGraw Hill Education; 2018. CrossRef
15. Burchum JR, Rosenthal LD (eds). Lehne’s pharmacology for nursing care. 10th ed. Amsterdam, Netherlands: Elsevier; 2019. CrossRef
16. Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Vol. 75, Drugs. Berlin: Springer International Publishing; 2015. Pp. 859–77. CrossRef
17. Zhao S, Jian Y, Wang Y, Xu Y, Liu W, Shao X, et al. Research of diclofenac sodium-loaded gelatin scaffold with anti-inflammatory activity for promoting in vivo cartilage regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023;37(1):91–100. CrossRef
18. Bariguian F, Marina R, Hagen FM. Topical diclofenac, an efficacious treatment for osteoarthritis: a narrative review. Rheumatol Ther. 2019;7(2):217–236. CrossRef
19. Reich KM, Viitanen P, Apu EH, Tangl S, Ashammakhi N. The effect of diclofenac sodium-loaded plga rods on bone healing and inflammation: a histological and histomorphometric study in the femur of rats. Micromachines. 2020;11(12):1–20. CrossRef
20. Hesaraki S, Nazarian H, Pourbaghi-Masouleh M, Borhan S. Comparative study of mesenchymal stem cells osteogenic differentiation on low-temperature biomineralized nanocrystalline carbonated hydroxyapatite and sintered hydroxyapatite. J Biomed Mater Res−Part B Appl Biomater. 2014;102(1):108–18. CrossRef
21. Sobczak-Kupiec A, Drabczyk A, Florkiewicz W, Glab M, Kudlacik-Kramarczyk S, Slota D, et al. Review of the applications of biomedical compositions containing hydroxyapatite and collagen modified by bioactive components. Materials (Basel). 2021;14(9):1–51. CrossRef
22. Tieppo Francio V, Davani S, Towery C, Brown TL. Oral versus topical diclofenac sodium in the treatment of osteoarthritis. J Pain Palliat Care Pharmacother. 2017;31(2):113–20. CrossRef
23. Budiatin AS, Su’aida N, Lamakluang AI, Rahma SS, Zulkarnain BS, Isadiartuti D. Effect of glutaraldehyde concentration variation on diclofenac sodium scaffolds as cross-linking agent. Res J Pharm Technol. 2022;15(11):4974–80. CrossRef
24. Sidney LE, Heathman TRJ, Britchford ER, Abed A, Rahman C V, Buttery LDK. Investigation of localized delivery of diclofenac sodium from poly(D,L-lactic acid-co-glycolic acid)/poly(ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng—Part A. 2015;21(1–2):362–73. CrossRef
25. Anggani OF. Karakterisasi freeze drying scaffold kitosan rajungan-gelatin sapi dan kondroitin sulfat. Surabaya, Indonesia: Faculty of Fisheries and Marine Universitas Airlangga; 2020.
26. Putri TS, Rianti D, Rachmadi P, Yuliati A. Effect of glutaraldehyde on the characteristics of chitosan–gelatin–β-tricalcium phosphate composite scaffolds. Mater Lett. 2021;304:130672. CrossRef
27. Budiatin AS, Pramesti MP, Nurfinti WO, Pratama YA, Ratri DMN, Ardianto C. Effect of diclofenac sodium on the cartilage-regeneration potential of the chitosan-gelatin-chondroitin sulfate scaffold. Iraqi J Vet Sci. 2025;39(2):243–51. CrossRef
28. Vassallo V, Tsianaka A, Alessio N, Grübel J, Cammarota M, Tovar GEM, et al. Evaluation of novel biomaterials for cartilage regeneration based on gelatin methacryloyl interpenetrated with extractive chondroitin sulfate or unsulfated biotechnological chondroitin. J Biomed Mater Res - Part A. 2022;110(6):1210–23. CrossRef
29. Sun HW, Feigal RJ, Messer HH. Cytotoxicity of glutaraldehyde and formaldehyde in relation to time of exposure and concentration. Pediatr Dent. 1990;12(5):303–7.
30. Samirah S, Sumarno S, Fauziyah AN, Afrida DI, Zaini OS, Aprilia DC, et al. Effect of glutaraldehyde on the bovine hydroxyapatite-gelatin-alendronate bioscrew and its fabrication. J Pharm Pharmacogn Res. 2025;13(1):46–57. CrossRef
31. Suyatno A, Nurfinti WO, Kusuma CPA, Pratama YA, Ardianto C, Samirah S, et al. Effectiveness of bilayer scaffold containing chitosan/gelatin/diclofenac and bovine hydroxyapatite on cartilage/subchondral regeneration in rabbit joint defect models. Adv Pharmacol Pharm Sci. 2024;2024:1–18. CrossRef
32. Kotturi H, Abuabed A, Zafar H, Sawyer E, Pallipparambil B, Jamadagni H, et al. Evaluation of polyethylene glycol diacrylate-polycaprolactone scaffolds for tissue engineering applications. J Funct Biomater. 2017;8(3):39. CrossRef
33. Martínez-Mejía G, Vázquez-Torres NA, Castell-Rodríguez A, del Río JM, Corea M, Jiménez-Juárez R. Synthesis of new chitosan-glutaraldehyde scaffolds for tissue engineering using Schiff reactions. Colloids Surfaces A Physicochem Eng Asp. 2019;579:123658. CrossRef
34. Qasim SSB, Nogueria LP, Fawzy AS, Daood U. The effect of cross-linking efficiency of drug-loaded novel freeze gelated chitosan templates for periodontal tissue regeneration. AAPS PharmSciTech. 2020;21(5):1–9. CrossRef
35. Pratama YA, Marhaeny HD, Deapsari F, Budiatin AS, Rahmadi M, Miatmoko A, et al. Development of hydroxyapatite as a bone implant biomaterial for triggering osteogenesis [In press]. Eur J Dent. 2025. CrossRef
36. Ahmed S, Annu, Ali A, Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int J Biol Macromol. 2018;116:849–62. CrossRef
37. Sharma S, Swetha KL, Roy A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int J Biol Macromol. 2019;132:97–108. CrossRef
38. Li J, Zhi W, Xu T, Shi F, Duan K, Wang J, et al. Ectopic osteogenesis and angiogenesis regulated by porous architecture of hydroxyapatite scaffolds with similar interconnecting structure in vivo. Regen Biomater. 2016;3(5):285–97. CrossRef
39. Liu HW, Su WT, Liu CY, Huang CC. Highly organized porous gelatin-based scaffold by microfluidic 3D-foaming technology and dynamic culture for cartilage tissue engineering. Int J Mol Sci. 2022;23(15). CrossRef
40. Anghelescu VM, Neculae I, Dinca O, Vladan C, Socoliuc C, Cioplea M, et al. Inflammatory-driven angiogenesis in bone augmentation with bovine hydroxyapatite, B-tricalcium phosphate, and bioglasses: a comparative study. J Immunol Res. 2018;2018:1–8. CrossRef
41. Wirawan F, Cheng CL, Kao WC, Lee DJ, Chang JS. Cellulosic ethanol production performance with SSF and SHF processes using immobilized Zymomonas mobilis. Appl Energy. 2012;100:19–26. CrossRef
42. Yoshikawa M, Tsuji N, Shimomura Y, Hayashi H, Ohgushi H. Osteogenesis depending on geometry of porous hydroxyapatite scaffolds. Calcif Tissue Int. 2008;83(2):139–45. CrossRef
43. Banafati Zadeh F, Zamanian A. Glutaraldehyde: introducing optimum condition for cross-linking the chitosan/gelatin scaffolds for bone tissue engineering. Int J Eng Trans A Basics. 2022;35(10):1967–80. CrossRef
44. Nokoorani YD, Shamloo A, Bahadoran M, Moravvej H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci Rep. 2021;11(1):16164. CrossRef
45. Azami M, Tavakol S, Samadikuchaksaraei A, Hashjin MS, Baheiraei N, Kamali M, et al. A porous hydroxyapatite/gelatin nanocomposite scaffold for bone tissue repair: In vitro and in vivo evaluation. J Biomater Sci Polym Ed. 2012;23(18):2353–68. CrossRef
46. Lee CY, Nedunchezian S, Lin SY, Su YF, Wu CW, Wu SC, et al. Bilayer osteochondral graft in rabbit xenogeneic transplantation model comprising sintered 3D-printed bioceramic and human adipose-derived stem cells laden biohydrogel. J Biol Eng. 2023;17(1):74. CrossRef
47. Vishwanath V, Pramanik K, Biswas A. Optimization and evaluation of silk fibroin-chitosan freeze-dried porous scaffolds for cartilage tissue engineering application. J Biomater Sci Polym Ed. 2016;27(7):657–74. CrossRef
48. Yue Y, Xu P, Lei Z, Li K, Xu J, Wen J, et al. Preparation and characterization of a novel drug-loaded Bi-layer scaffold for cartilage regeneration. RSC Adv. 2022;12(16):9524–33. CrossRef
49. Shajahan Haima J, Narayanan Nair S, Juliet S, Ravindran Nisha A, Nair B, Nair Dhanushkrishna B. Synthesis and characterisation of glutaraldehyde cross-linked κ-carrageenan-gelatin hydrogel. ~ 459 ~ J Pharmacogn Phytochem. 2021;10(1):459–63.
50. Sharma C, Dinda AK, Potdar PD, Chou CF, Mishra NC. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater Sci Eng C. 2016;64:416–27. CrossRef
51. Pinto R V., Gomes PS, Fernandes MH, Costa MEV, Almeida MM. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater Sci Eng C. 2020;109:110557. CrossRef
52. Arianita A, Cahyaningtyas, Amalia B, Pudjiastuti W, Melanie S, Fauzia V, et al. Effect of glutaraldehyde to the mechanical properties of chitosan/nanocellulose. J Phys Conf Ser. 2019;1317(1):1–9. CrossRef
53. Hikmawati D, Maulida HN, Putra AP, Budiatin AS, Syahrom A. Synthesis and characterization of nanohydroxyapatite-gelatin composite with streptomycin as antituberculosis injectable bone substitute. 2019;2019:1–8. CrossRef