INTRODUCTION
Dengue is a viral disease, most prominently found in tropical and subtropical areas. Aedes aegypti, an urban breeding mosquito, spreads dengue among people. Approximately half of the world’s population is now living in places where dengue transmission is endemic, and close to 125 nations are affected by the disease every year. According to reports around the world, every year, around 500,000 people are affected by severe dengue and around 20,000 people die of dengue. Further, it is predicted that there will be an increase in the incidence and prevalence of dengue infection, mainly because of uncontrolled climate change, travel, socioeconomic factors, trading, and viral resistance [1].
Clinical manifestation of dengue fever ranges from mild flu to coagulopathy, increased vascular permeability, and plasma leakage that results in dengue shock syndrome. The severe form of dengue causes dengue hemorrhagic fever, which frequently results in dengue-associated mortality [2].
Dengue fever is reported annually throughout the world and, globally, half a million patients are hospitalized due to thrombocytopenia and microvascular leakage, every year. Thrombocytopenia is characterized by low platelet count. Platelets protect the vascular integrity by protecting endothelial damage. Thrombocytopenia increases vascular permeability, leading to microvascular leakage, which in turn causes fluid and protein extravasation, interstitial edema, and organ failure. Microvascular leakage and increased systemic vascular permeability are the most serious complications of dengue infection. Other complications include coagulopathy and hypovolemia. Vascular leakage and hypovolemia increase the severity of the condition. These complications are outcomes of inflammatory response due to sepsis. Apart from these, the patients also suffer from hepatic damage and high fever, which need to be addressed to stop further deterioration of the patient’s condition [3].
The lack of adequate treatment adds to the severity of dengue fever [4]. The clinical signs of dengue fever are usually treated with fluid balance, electrolyte supplementation, and blood clotting factors. Sometimes, anti-D immunoglobulin therapy is given for severe thrombocytopenia. Dengvaxia, the first dengue vaccine, was approved for clinical use in 2015 [5]. However, this may not provide complete protection against all serotypes of dengue infection.
In tropical and subtropical areas, most people rely on a holistic approach of treatment for dengue fever, in addition to allopathic treatment. Herbal remedies have long been used for therapeutic purposes, since the dawn of human civilization. Due to its safety and cost-effectiveness, there is a resurgence of interest in herbal medicine among the general population. Some of the herbs, such as Andrographis paniculata, Alternanthera philoxeroides, Carica papaya L., Cladosiphon okamuranus, and Momordica charantia are known to have the ability to fight dengue fever [4]. The juice of C. papaya leaves, for example, is commonly used to improve platelet count in dengue cases in many Asian nations [6,7].
The aim of this study was to evaluate the effect of HPH-031508PP on complications associated with dengue such as thrombocytopenia, microvascular permeability, hepatic damage, and pyrexia using animal models. Further, platelet-increasing and white blood cell–increasing properties and membrane-stabilizing properties of C. papaya leaf extract were also analyzed.
MATERIALS AND METHODS
Preparation of HPH-031508PP
HPH-031508PP is a blend of aqueous extracts of Tinospora cordifolia L. stem, hydroalcoholic extract of Phyllanthus maderaspatensis L. whole plant, and C. papaya L. leaves, and ethyl acetate extract of Piper nigram fruit, which are blended in the ratio of 9, 24, 66, and 1, respectively. Phytochemical parameters and analytical markers for the standardization of all plant extracts were analyzed, and pre-formulation studies were performed for powder blends. The phytochemicals or analytical markers present in the herbal ingredients of HPH-031508PP were identified and quantified using various methods: glycosides present in C. papaya L. were analyzed using gravimetry; rutin was analyzed using high-performance liquid chromatography (HPLC); flavonoids present in P. maderaspatensis L. were analyzed using ultraviolet (UV) spectroscopy; bitters present in T. cordifolia L. were analyzed using gravimetry; and piperine in Piper nigrum was analyzed using by HPLC.
The markers identified in C. papaya L., P. maderaspatensis L., T. cordifolia L and P. nigrum are glycosides, rutin, flavonoids, and piperine, respectively.
Fingerprinting and identification of analytical markers present in HPH-031508PP
Gravimetric analysis of glycosides
HPH-031508PP was accurately weighed to 5 g, and 50 ml of 90% methanol was added, mixed, and placed in a water bath and heated under a reflux condenser for 30 minutes. The mixture was allowed to cool to room temperature, and then the extract was decanted. After this, the same procedure was repeated twice. The methanolic extracts were combined, filtered, collected, and concentrated to 50–60 ml using a water bath. Then, it was transferred into a separating funnel and shaken vigorously for a few minutes till the two layers got separated. The aqueous layer was concentrated to less than 5 ml using a water bath. To this, 25 ml of cool acetone was added and mixed, and the acetone layer was decanted [8,9].
Fingerprinting using thin-layer chromatography
Thin-layer chromatography (TLC) was done as explained in Pyka et al.’s study [10]. During the procedure, 10 μl of the sample and 5 μl of the reference standard were used for spotting. The concentration of the standard rutin was 1 mg/ml, and the sample was 100 mg/ml. The solutions were added to 12-mm plates. Further, the plates were allowed to dry and were visualized under UV 254 nm and 366 nm using a UV cabinet (Fig. 1).
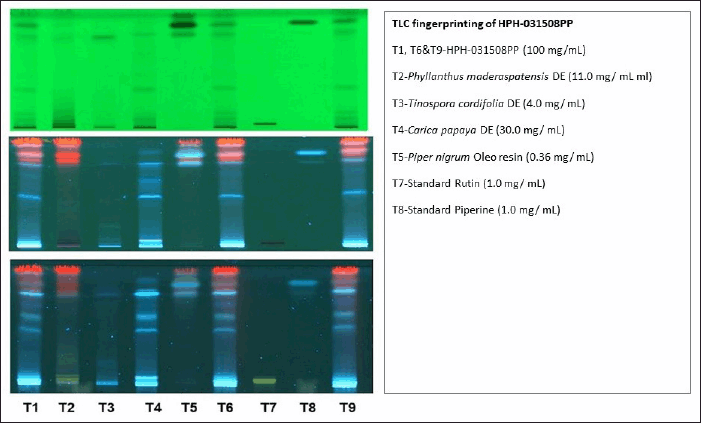 | Figure 1. TLC fingerprint of HPH-031508PP at 254 and 366 nm after spraying with vanillin sulfuric acid. [Click here to view] |
Identification of analytical marker (estimation of rutin and piperine by HPLC)
HPLC condition: Column: Ascentis Express C18 (100 × 4.6) mm/particle size: 2.7 µ; wavelength: 354 nm; flow rate-: 1 ml/min; volume of injection: 10 μl; mobile phase Solvent-A: 0.1% trifluoroacetic acid (TFA) in purified water, Solvent-B: 0.1% TFA in Acetonitrile: water (70:30) (Gradient); run time 33 minutes; approximate retention time about 12 minutes; column temperature 40°C; and diluent: 70% ethanol. The concentration of standard rutin and piperine solutions used for analysis was 0.005 mg/ml and HPH-031508PP was 5 mg/ml.
Estimation of total flavonoids by UV spectrophotometer
The colorimetric method was used to determine total flavonoids using a Shimadzu UV-1700 spectrophotometer, and rutin was taken as a standard. Flavonoids form a yellow complex upon reaction with aluminum chloride. Methanolic extracts of the samples were treated with aluminum chloride reagent, and the absorbance of the resulting complex was measured at 410 nm against a reagent blank.
Stability studies were performed under controlled conditions of 30°C, 65% relative humidity and 40°C, 75% relative humidity to evaluate potential degradation of analytical markers. The results confirmed that glycosides, total flavonoids, rutin, and piperine remained stable throughout the study period.
Experimental animals
Male Wistar rats were used in this study. The rats were housed in a standard environment of light and temperature and were provided a standard diet and water ad libitum. All experiments in these animals were carried out as per the standard ethical guidelines of the Committee for the Purpose of Control and Supervision of Experiments, Government of India. The study protocol (164/16, 2016) was approved by the Institutional Animal Care and Ethics Committee of Himalaya Wellness Company (Bengaluru, India).
Effect of HPH-031508PP on thrombocytopenia
The platelet augmentation activity of HPH-031508PP was evaluated in male Wistar rats by inducing thrombocytopenia with cyclophosphamide, as described in Venkataraman et al.’s study [11]. Thirty-two Wistar rats (body weight 200–220 g) were divided into four groups of eight each: Groups 1 and 2 received water at a dose of 10 ml/kg b.wt. p.o. and served as normal control and positive control, respectively. Groups 3 and 4 received HPH-031508PP suspended in demineralized water, at doses of 250 and 500 mg/kg b.wt. p.o., respectively, for 2 consecutive weeks. Except for normal control, cyclophosphamide was administered to all the animals at a dose of 25 mg/kg b.wt./day, subcutaneously, consecutively for the first 3 days after 1 hour of treatment with HPH-031508PP to induce thrombocytopenia. On days 1, 4, 7, 11, and 14, blood was collected through retro-orbital sinus under isoflurane anesthesia from all the animals, and the blood samples were subjected to hematologic evaluation. On day 14, after 2 hours of treatment, blood clotting time was estimated. All the animals were euthanized using excess isoflurane and bone marrow smear was collected and subjected for the estimation of megakaryocytes.
Effect of HPH-031508PP on lipopolysaccharide-induced microvascular permeability
Microvascular leakage was evaluated by estimating Evans blue concentration, a dye that binds to proteins such as albumin. The amount of extravasation of Evans blue into the surrounding tissues helps understand the microvascular leakage, and it was quantified spectrophotometrically [12].
Thirty-two male Wistar rats (body weight 220–250 g) were divided into four groups of eight each. These animals were acclimatized for 7 days, and then they were treated with HPH-031508PP for the following 7 days. Groups 1 and 2 received water at a dose of 10 ml/kg b.wt. p.o. and served as normal control and positive control (pathological control), respectively. Groups 3 and 4 received HPH-031508PP at doses of 250 and 500 mg/kg b.wt. p.o., respectively. On day 7, after 1 hour of receiving HPH-031508PP, lipopolysaccharide (LPS) was injected at a dose of 5 mg/kg b.wt. to all the groups (except normal control) through the intraperitoneal route. After 5 hours of LPS injection, the animals were anesthetized using urethane (1.25 g/kg b.wt. i.p.), and jugular vein cannulation was performed. Evans blue dye (100 mg/kg) was administered through the jugular vein. The animals were then euthanized by giving an excess of isoflurane anesthesia after 30 minutes of Evans blue infusion. The liver, lungs, and kidneys were weighed, and 200 mg of each organ sample was transferred to a separate tube containing 1 ml of acetone. Tissues were minced properly and were kept overnight to extract Evans blue color. After extraction, the samples were centrifuged, 200 µl of supernatant was taken, and color was measured at 620 nm spectrophotometrically. Evans blue standard curve was used to calculate the concentration of Evans blue present in the tissues [13,14].
Effect of HPH-031508PP on Brewer’s yeast-induced pyrexia
Pyrexia was induced as described in Bhat et al.’s study [15]. Thirty male Wistar rats (body weight 225–240 g) were divided into five groups of six each. Group 1 served as the normal control. Group 2 served as a pyretic control. Groups 3 and 4 were treated with HPH-031508PP at doses of 250 and 500 mg/kg b.wt. p.o., respectively. Group 5 was treated with the reference standard Paracetamol at a dose of 120 mg/kg b.wt. p.o. The basal rectal temperature was recorded using a digital thermometer. All the animals, except the normal control, were administered 20% (w/v) of Brewer’s yeast at a dose of 20 ml/kg in 0.9% saline, s.c., to induce pyrexia, and non-pyrogenic saline was administered to the animals in the normal control group. Eighteen hours after Brewer’s yeast administration, the animals were treated with HPH-031508PP. After 30 minutes of the treatment, the rectal temperature of all animals in all groups was recorded at hour 0, 1, 2, 3, 4, and 6.
Effect of HPH-031508PP on CCl4-induced hepatic damage
The hepatoprotective activity of HPH-031508PP was evaluated using the CCl4-induced hepatic damage method, as described in Anturlikar et al.’s study [16]. Twenty-four male Wistar rats (body weight 230–250 g) were divided into three groups of eight each. Group 1 received water at a dose of 10 ml/kg and served as control. Group 2 received water at 10 ml/kg and CCl4 at a dose of 0.5 ml/kg b.wt. i.p., on day 14, and served as positive control (pathological control). Group 3 received HPH-031508PP at a dose of 500 mg/kg (for 2 consecutive weeks) + CCl4 at a dose of 0.5 ml/kg b.wt. i.p., on day 14. All the rats, except Group 1 rats, were administered with CCl4 at a dose of 0.5 ml/kg b.wt. i.p., which was suspended in olive oil (1:9 v/v), on day 14. Blood was collected from all the animals under isoflurane anesthesia from the retro-orbital plexus, 36 hours after CCl4 administration. Serum was separated and subjected to the estimation of serum glutamate pyruvic transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), and alkaline phosphatase (ALP) using auto analyzer kits from Erba Mannheim (Transasia Bio-Medicals Ltd, Mumbai, India).
Expression of thrombopoietin in HepG2 cells through quantitative polymerase chain reaction
This analysis was carried out using human liver cell line (HepG2 cells; 2 × 105 cells/well). The investigational test product, HPH-031508PP, was incubated with cells for 24 hours at a standard temperature of 37°C with 5% CO2. After 24-hour incubation, the media was discarded, and adherent cells were subjected to total RNA isolation. Total RNA was extracted from HepG2 cells using an RNA isolation kit (Krishgen Biosystems, Mumbai, India). Total RNA and oligo(dT) were used for the first-strand cDNA synthesis through reverse transcriptase. The polymerase chain reaction amplification was carried out in a reaction volume of 20 µl containing 2 µl of cDNA, reverse and forward primers, and 10 µl of SYBR Green Supermix (Bio-Rad Laboratories Inc., USA). The expression levels were normalized to that of glyceraldehyde-3-phosphate dehydrogenase RNA expression [17].
Acute oral toxicity study
Acute oral toxicity of HPH-031508PP was conducted in female Wistar rats. OECD Guideline No. 423 (the acute toxic class method) was followed for evaluation. A limit test was conducted on six female rats at a dose of 2,000 mg/kg body weight [18].
Statistical analysis
The values are expressed as mean ± SEM. The results were analyzed statistically using one-way ANOVA followed by post-hoc Dunnett’s multiple comparison test using GraphPad Prism 6.07 (GraphPad Software Inc, San Diego, CA). A p value < 0.05 was considered statistically significant.
RESULTS
Thrombocytopenia
HPH-031508PP was evaluated for its platelet augmentation activity in rats with cyclophosphamide-induced thrombocytopenia. Cyclophosphamide administration significantly decreased the platelet count on days 4 and 7 (p < 0.001) compared with normal control. HPH-031508PP was given at doses of 250 and 500 mg/kg b.wt. Results revealed that on day 11 after receiving HPH-031508PP, there was an increase in platelet count with a 500 mg/kg dose (p < 0.001). On day 14, both doses (250 mg/kg, p < 0.01; 500 mg/kg, p < 0.05) resulted in an increase in platelet count compared with that in positive control animals (Fig. 3).
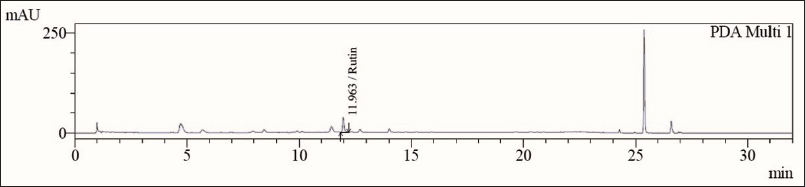 | Figure 2. HPLC chromatogram of rutin, an analytical marker of HPH-031508PP. [Click here to view] |
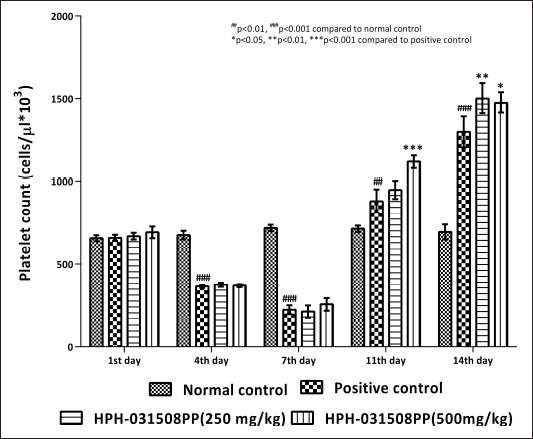 | Figure 3. Effect of HPH-031508PP on platelet count on different days of treatment. [Click here to view] |
Furthermore, cyclophosphamide administration caused a significant (p < 0.01) increase in blood clotting time and a decrease in megakaryocyte number in animals in positive control (pathological control) compared with that in normal control animals. After 14 days of treatment with HPH-031508PP, there was a dose-dependent decrease in blood clotting time significantly (250 mg/kg, p < 0.05 and 500 mg/kg, p < 0.01) compared with that in the positive control group (Fig. 4).In addition, a significant and dose-dependent increase in megakaryocyte count was observed (250 mg/kg, p < 0.05 and 500 mg/kg, p < 0.001) (Figs. 5 and 6).
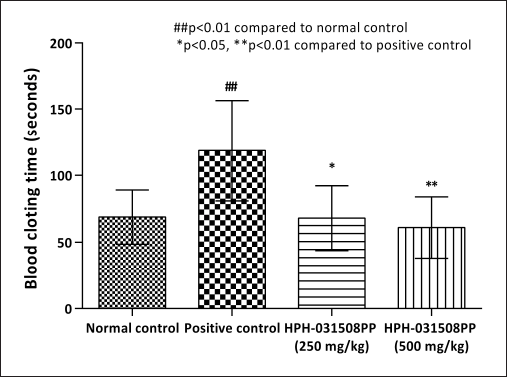 | Figure 4. Effect of HPH-031508PP on clotting time after 14 days of treatment. [Click here to view] |
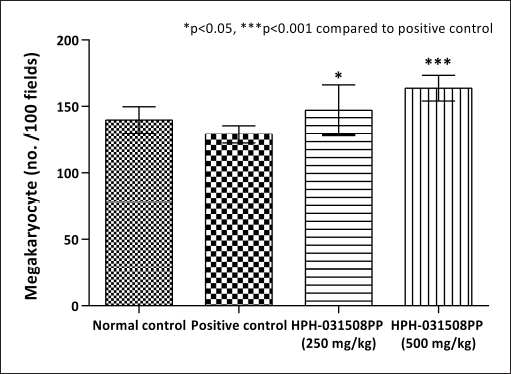 | Figure 5. Effect of HPH-031508PP on megakaryocytes after 14 days of treatment. [Click here to view] |
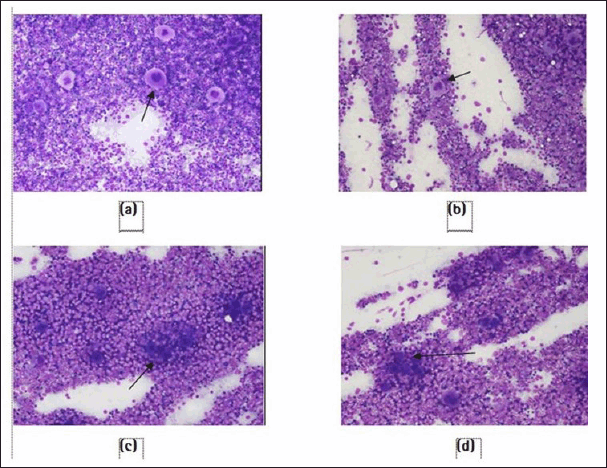 | Figure 6. Photomicrograph showing an increase in the number of megakaryocytes in HPH-031508PP-treated groups compared with the positive control group: (a) normal control, (b) positive control, (c) HPH-031508PP at 250 mg/kg, and (d) HPH-031508PP at 500 mg/kg. [Click here to view] |
Microvascular permeability
In the liver tissues, there was a significant (p < 0.001) increase in the concentration of Evans blue in the positive control group compared with that in the normal control group, which indicates a significant increase in microvascular leakage in the LPS-induced sepsis model. However, vascular leakage significantly decreased after treatment with HPH-031508PP in both groups that received 250 mg/kg (p < 0.05) and 500 mg/kg (p < 0.01) (Table 1).
Table 1. Microvascular leakage in the organs with the help of Evans blue dye.
| Group | Liver(ng/200 mg sample) | Lungs(ng/200 mg sample) | Kidneys(ng/200 mg sample) |
|---|---|---|---|
| Normal control | 532 ± 148 | 835 ± 109 | 1,470 ± 176 |
| Positive control | 1,846 ± 236### | 2,127 ± 191### | 2,700 ± 222## |
| HPH-031508PP (250 mg/kg) | 1,153 ± 134* | 1,435 ± 99** | 1,790 ± 165** |
| HPH-031508PP (500 mg/kg) | 994 ± 81** | 1,377 ± 42** | 1,549 ± 106*** |
‘Values are expressed as mean ± SEM; ##p < 0.01, ###p < 0.001 compared to normal control; *p < 0.05, **p < 0.01, ***p < 0.001 compared to positive control.
In the lungs, there was a significant (p < 0.001) increase in Evans blue concentration in the positive control group compared with that in the normal control group. It was found that treatment with HPH-031508PP significantly (p < 0.01) decreased the Evans blue concentration (Table 1).
In the kidneys, there was a significant (p < 0.01) increase in Evans blue concentration in the positive control group compared with that in the normal control group. However, there was a significant decrease in Evans blue concentration in HPH-031508PP-treated groups (with both doses; 250 mg/kg, p < 0.01 and 500 mg/kg, p < 0.001) (Table 1).
Antipyretic activity
Animals in the positive control group that were administered Brewer’s yeast showed a significant increase (p < 0.05) in rectal temperature, from hours 1 to 6, compared with animals in the normal control group. Treatment with HPH-031508PP at a dose of 500 mg/kg showed a significant decrease in rectal temperature at hour 2 (p < 0.05), 3 (p < 0.001), 4 (p < 0.05), and 6 (p < 0.05) compared with that in the positive control (pyretic) group. Further, as expected, Paracetamol-treated animals showed a significant decrease (p < 0.001) in rectal temperature at hours 1, 2, 3, and 4 compared with that in positive control animals. Thus, it can be inferred that HPH-031508PP showed mild-to-moderate antipyretic activity (Fig. 7).
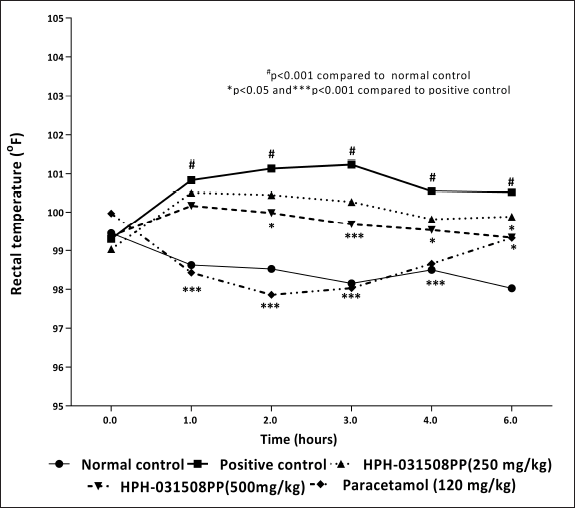 | Figure 7. Effect of HPH-031508PP on the rectal temperature of rats at different time points. [Click here to view] |
Hepatoprotective activity
To evaluate the hepatoprotective activity, a single dose of 500 mg/kg b.wt. HPH-031508PP was used. This dose was found to be effective in decreasing the hepatic enzymes such as SGPT (p < 0.05), SGOT, and ALP, the levels of which were elevated due to the administration of CCl4. These findings depicted that HPH-031508PP exhibits hepatoprotective activity at 500 mg/kg (Table 2).
Table 2. Effect of HPH-031508PP on reducing elevated hepatic enzyme levels.
| Group | SGPT (U/L) | SGOT (U/L) | ALP (U/L) |
|---|---|---|---|
| Normal control | 58.71 ± 3.6 | 119.8 ± 14.1 | 233.6 ± 11.7 |
| Positive control | 244.8 ± 64## | 488.8 ± 104.4## | 345.6 ± 28.4# |
| HPH-031508PP (500 mg/kg) | 128.1 ± 14.6* | 329.8 ± 47.4 | 282.6 ± 31.6 |
Values are expressed as mean ± SEM; #p < 0.05, ##p < 0.01 compared to normal control; *p < 0.05 compared to positive control.
Expression of thrombopoietin in HepG2 cells through quantitative polymerase chain reaction
HPH-031508PP helped in increasing the expression of the thrombopoietin gene at 50 μg/ml and 100 μg/ml, which in turn boosts platelet production (Fig. 8).
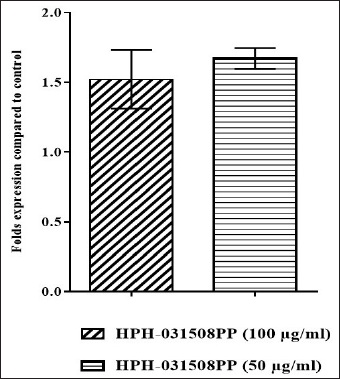 | Figure 8. Expression levels of thrombopoietin gene (after normalizing control levels to 1). [Click here to view] |
Acute oral toxicity study
The outcome of the study indicated that the minimum lethal dose of HPH-031508PP was more than 2,000 mg/kg b.wt., which classifies it under the Globally Harmonized System category 5.
DISCUSSION
According to the World Health Organization reports, the incidence of dengue fever has significantly increased globally in recent decades. Each year, between 100 and 400 million cases are reported, globally [19]. Dengue is characterized by a spectrum of complications, especially in case of severe dengue. It results in a potentially fatal condition because of plasma leakage, accumulation of fluid, difficulty breathing, bleeding, and organ damage. Severe dengue can even cause death. Hence, dengue needs a holistic treatment approach that not only addresses basic symptoms of thrombocytopenia but also severe conditions such as microvascular leakage, fever, and liver function.
Polyherbal formulations retain a high reputation in traditional medical systems and have been used as remedies for many diseases that are mainly aggravated by inflammatory responses [20]. Traditional knowledge and modern scientific research should be combined to validate the efficacy and safety profiles of such herbal formulations.
In this study, the efficacy of HPH-031508PP in the holistic treatment of complications associated with dengue infection was analyzed.
In this study, microvascular leakage was induced in rats through LPS, a well-known endotoxin and a major component of the bacterial cell wall responsible for toxic activity due to its lipid A moiety. LPS works by binding to components of blood plasma such as high-density lipoproteins or LPS-binding proteins (LBPs). The LBP–LPS complex interacts with CD14, a high-affinity receptor for LPS, expressed on monocytes/macrophages, resulting in cell activation in the blood to stimulate endothelial cells that do not express CD14, resulting in vascular endothelial barrier dysfunction and microvascular leakage. It was observed that HPH-031508PP-treated groups showed a significant reduction in microvascular leakage compared with that in the positive control group. This finding could be attributed to the phytoactive, rutin, present in HPH-031508PP.
Carica papaya has been utilized in the treatment of a variety of diseases since ancient times. Scientific research proved that extracts of C. papaya leaves, fruits, and seeds exhibit a variety of therapeutic effects [21]. Carica papaya leaf extract is proved to have larvicidal activity against the dengue virus’s vector, A. aegypti mosquito [22]. An in vitro experiment carried out by Ranasinghe et al. [23] found that C. papaya leaf extract prevents hemolysis of erythrocytes in hypotonicity and heat-induced hemolysis in both healthy and dengue-infected individuals, which proves its ability to stabilize membranes and protect blood cells from stress. This property in turn helps prevent platelet lysis in patients suffering from dengue fever. This property is attributed to the flavonoids and other phenolic compounds present in C. papaya leaves. Furthermore, these flavonoids can block a protease involved in viral assembly, according to a study that used bioinformatics approaches. Additionally, the leaf extract of C. papaya contains antioxidant and free radical-scavenging properties, according to Okoko and Ere, which can help reduce hemolysis and bleeding [24]. Carica papaya leaf extract also helps lower viral load within cells, which proves its anti-dengue property [25].
In silico studies discovered that T. cordifolia is a potent inhibitor of the NS2B-NS3, which is a viral protease, specifically a serine protease, that plays a crucial role in the replication cycle of flaviviruses such as Dengue, Zika, and West Nile viruses. It also exhibited immunomodulatory activity against human immunodeficiency virus [26,27].
Innate and acquired immunity are two arms of the immunomodulatory system. Parveen et al.’s research [28] proved that phytoactives present in T. cordifolia and P. nigrum synergistically exhibit immunomodulatory activity. Both humoral and cellular immunity improved because of this activity. In addition, P. nigrum is used traditionally in India for the treatment of many diseases characterized by a rise in body temperature [29,30].
Sharma et al. [31] reported the dose-dependent hepatoprotective activity of P. maderaspatensis in tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells, and hence, recommended that P. maderaspatensis can provide a promising therapeutic intervention against hepatic damage. Furthermore, a study by Asha et al. [32] proved significant hepatoprotective activity of P. maderaspatensis against acetaminophen-induced hepatotoxicity.
In our study, the gravimetric and HPLC analyses revealed that 12.2% (w/w) total glycosides and 0.1% (w/w) rutin are present in HPH-031508PP. These are the active constituents of the formulation and are responsible for the desired pharmacological activity. Piperine increases bioavailability of active compounds and permeability of epithelial membranes [33].
One of the major active components present in HPH-031508PP is rutin. According to Ganeshpurkar and Saluja’s study [34], the anti-platelet activity of rutin may involve the following pathways: rutin inhibits phospholipase C activation, followed by inhibition of protein kinase C activity and thromboxane A2 formation, thereby leading to inhibition of the phosphorylation of P47 and intracellular Ca2+ mobilization, finally resulting in inhibition of platelet aggregation. Rutin also dramatically slows down the growth and reproduction of S. aegypti larvae [35]. In addition, quercetin and narasin, present in C. papaya, also possess significant anti-dengue virus properties [36]. Quercetin has been reported to block NS2B-NS3 serine protease, which is required for dengue virus assembly [23]. Quercetin also inhibits ADP (adenosine diphosphate)-induced platelet aggregation [37].
Thrombopoietin is a glycoprotein hormone produced by the liver and the kidneys and regulates platelet formation. It is also known as a megakaryocyte growth and development factor because it enhances the production and differentiation of megakaryocytes. Increased thrombopoietin level can be beneficial during thrombocytopenia [38]. In our study, HPH-031508P helped in upregulating the thrombopoietin genes and the number of megakaryocytes in the bone marrow smear which may be due to the presence of C. papaya L. leaves which induces thrombopoietin secretion from mesenchymal stem cells and hematopoietic cells, thereby upregulating the thrombopoietin gene [39].
Carica papaya L. is known to improve platelet synthesis by increasing arachidonate 12-lipoxygenase and platelet-activating factor receptor gene expression. Flavonoids and other phenolic compounds present in HPH-031508P help increase platelet count while also reducing peripheral platelet breakdown due to their membrane-stabilizing activity. T. cordifolia is known to strengthen the host immune system by activating macrophages, NF-κβ translocation, and cytokine production. T. cordifolia and P. maderaspatensis are reported to possess anti-pyretic, anti-viral, and hepatoprotective activities. Piperine has substantial anti-inflammatory characteristic, which aids in the dramatic reduction of clinical signs and symptoms of dengue fever. Thus, HPH-031508P helps treat thrombocytopenia by upregulating the thrombopoietin gene and megakaryocytes, which are responsible for increasing platelet count.
The herbal actives that are known to be efficient in preventing dengue virus infection are used as anti-dengue medicines [40,41]. Many natural compounds that have shown substantial inhibitory efficacy in vitro against specific viruses and targets have, however, failed to advance to clinical trials [42].
CONCLUSION
Based on the study results, we conclude that HPH-031508P comprising potential herbs helps in treating complications such as thrombocytopenia, microvascular permeability, pyrexia, and hepatic damage associated with dengue. Hence, HPH-031508P may be effective in treating dengue and associated complications, which is a holistic treatment approach.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was funded by Himalaya Wellness Company, Bangalore, India. This research did not receive any funding from the Government or public or not-for-profit sectors.
CONFLICTS OF INTEREST
All authors are full-time employees of the Himalaya Wellness Company, Bengaluru, India. The tested herbal composition was developed by the company’s in-house Research and Development Centre, where the study was also conducted. The company provided institutional infrastructure and logistical support; however, it had no involvement in the study’s conceptualization, design, data acquisition, statistical analysis, interpretation of results, manuscript writing, or the decision to submit the work for publication. The authors affirm that the research was conducted and reported independently, in accordance with ICMJE and COPE standards for ethical publishing.
ETHICAL APPROVALS
Ethical approvals details are given in the ‘Materials and Methods’ section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zerfu B, Kassa T, Legesse M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Trop Med Health. 2023;51(1):11. CrossRef
2. Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564–81. CrossRef
3. Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Dengue Virus. 2010;338:83–98. CrossRef
4. Abd Kadir SL, Yaakob H, Zulkifli RM. Potential anti-dengue medicinal plants: a review. J Nat Med. 2013;67(4):677–89. CrossRef
5. Agnandji S. Malaria vaccine: WHO position paper–January 2016. N Engl J Med. 2011;365(20):1863–75. CrossRef
6. Patil S, Shetty S, Bhide R, Narayanan S. Evaluation of platelet augmentation activity of Carica papaya leaf aqueous extract in rats. J Pharmacogn Phytochem. 2013;1(5):57–60.
7. Subenthiran S, Choon TC, Cheong KC, Thayan R, Teck MB, Muniandy PK, et al. Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evid Based Complement Alternat Med. 2013; 2013:1–7. CrossRef
8. Jayasinghe CD, Gunasekera DS, De Silva N, Jayawardena KK, Udagama PV. Mature leaf concentrate of Sri Lankan wild type Carica papaya Linn. modulates nonfunctional and functional immune responses of rats. BMC Complement Altern Med. 2017;17:1–4. CrossRef
9. Ranasinghe P, Ranasinghe P, Abeysekera WK, Premakumara GS, Perera YS, Gurugama P, et al. In-vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacognosy Res. 2012;4(4):196–202. CrossRef
10. Pyka A. Detection progress of selected drugs in TLC. Biomed Res Int. 2014;16:1–19. CrossRef
11. Venkataraman N, Pamukuntla S, Banoth J, Boini P, Sampathi S. Platelet augmentation activity of Andrographis paniculata extract and andrographolide against cyclophosphamide induced thrombocytopenia in rats. Pharm Pharmacol Int J. 2015;2(4):00029. CrossRef
12. Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80(20):10208–17. CrossRef
13. Radu M, Chernoff J. An in-vivo assay to test blood vessel permeability. J Vis Exp. 2013;(73):1–4.
14. Adams JA, Uryash A, Lopez JR, Sackner MA. Whole body periodic acceleration improves survival and microvascular leak in a murine endotoxin model. PLoS One. 2019;14(1):1–14. CrossRef
15. Bhat AS, Tandan SK, Kumar D, Krishna V, Prakash VR: Interaction between inhibitors of inducible nitric oxide synthase and cyclooxygenase in Brewer?s yeast induced pyrexia in mice: an isobolographic study. Eur J Pharmacol. 2005;511:137–42. CrossRef
16. Anturlikar S, Rafiq M, Azeemuddin M, Viswanatha G, Jagadeesh M, Rao KS, et al. Free radical scavenging and hepatoprotective activity of HD-03/ES in experimental models. J Exp Integr Med. 2012;2:161–6. CrossRef
17. Berlian G, Tandrasasmita OM, Tjandrawinata RR. Trombinol, a bioactive fraction of Psidium guajava, stimulates thrombopoietin expression in HepG2 cells. Asian Pac J Trop Biomed. 2017;7(5):437–42. CrossRef
18. Organisation for Economic Co-operation and Development, Test no. 423: Acute oral toxicity—acute toxic class method. In: OECD Guidelines for the testing of chemicals, Section 4; Paris, France: Organisation for Economic Co-operation and Development; 2002.
19. World Health Organization, Special Programme for Research, Training in Tropical Diseases, World Health Organization. Department of Control of Neglected Tropical Diseases, World Health Organization. Epidemic, Pandemic Alert. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; 2009.
20. Bellini MF, Angeli JP, Matuo R, Terezan AP, Ribeiro LR, Mantovani MS. Antigenotoxicity of Agaricus blazei mushroom organic and aqueous extracts in chromosomal aberration and cytokinesis block micronucleus assays in CHO-k1 and HTC cells. Toxicol In Vitro. 2006;20(3):355–60. CrossRef
21. Sarala N, Paknikar SS. Papaya extract to treat dengue: a novel therapeutic option? Ann Med Health Sci Res. 2014;4(3):320–4. CrossRef
22. Kovendan K, Murugan K, Kumar AN, Vincent S, Hwang JS. Bioefficacy of larvicidal and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2012;110(2):669–78. CrossRef
23. Senthilvel P, Lavanya P, Kalavathi Murugan Kumar RS, Anitha P, Bag S, Sarveswari S, et al. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents dengue 2 viral assembly. Bioinformation 2013;9(18):889–95. CrossRef
24. Okoko T, Ere D. Reduction of hydrogen peroxide–induced erythrocyte damage by Carica papaya leaf extract. Asian Pac J Trop Biomed. 2012;2(6):449–53. CrossRef
25. Sharma N, Mishra KP, Chanda S, Bhardwaj V, Tanwar H, Ganju L, et al. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch Virol. 2019;164(4):1095–110. CrossRef
26. Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, AbuBakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med. 2012;12(1):1–9. CrossRef
27. Bency BJ, Helen PA. In silico identification of dengue inhibitors in Giloy (Tinospora cordifolia) and Papaya. J Emerg Technol Innov Res. 2018;5:506–11.
28. Parveen A, Zahiruddin S, Agarwal N, Siddiqui MA, Ansari SH, Ahmad S. Modulating effects of the synergistic combination of extracts of herbal drugs on cyclophosphamide-induced immunosuppressed mice. Saudi J Biol Sci. 2021;28(11):6178–90. CrossRef
29. Ghiware NB, Nesari TM. Antipyretic activity of Piper nigrum and Nyctanthes arbor-tristis in different dosage forms. Res J Pharm Technol. 2010;3:157–60.
30. Wadhwa S, Singhal S, Rawal S. Bioavailability enhancement by piperine: a review. Asian J Biomed Pharm Sci. 2014;4(36):1–8.
31. Sharma SK, Arogya SM, Bhaskarmurthy DH, Agarwal A, Velusami CC. Hepatoprotective activity of the Phyllanthus species on tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells. Pharmacogn Mag. 2011;7(27):229–33. CrossRef
32. Asha VV, Akhila S, Wills PJ, Subramoniam A. Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J Ethnopharmacol. 2004;92:67–70. CrossRef
33. Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Piperine—the bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf. 2017;16(1):124–40. CrossRef
34. Sheu JR, Hsiao G, Chou PH, Shen MY, Chou DS. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J Agric Food Chem. 2004;52(14):4414–8. CrossRef
35. Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J. 2017;25(2):149–64. CrossRef
36. Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, AbuBakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J. 2011;8(1):1–11. CrossRef
37. Tzeng SH, Ko WC, Ko FN, Teng CM. Inhibition of platelet aggregation by some flavonoids. Thromb Res. 1991;64(1):91–100. CrossRef
38. Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci. 2002;17(1):6–10. CrossRef
39. Aziz J, Abu Kassim NL, Abu Kasim NH, Haque N, Rahman MT. Carica papaya induces in vitro thrombopoietic cytokines secretion by mesenchymal stem cells and haematopoietic cells. BMC Complement Altern Med. 2015;15(1):1–8. CrossRef
40. Frabasile S, Koishi AC, Kuczera D, Silveira GF, Verri WA, Dos Santos CN, et al. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci Rep. 2017;7(1):1–11. CrossRef
41. Ocazionez RE, Meneses R, Torres FÁ, Stashenko E. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in-vitro. Mem Inst Oswaldo Cruz. 2010;105:304–9. CrossRef
42. Low JS, Wu KX, Chen KC, Ng MM, Chu JJ. Narasin, a novel antiviral compound that blocks dengue virus protein expression. Antivir Ther. 2011;16(8):1203–18. CrossRef