INTRODUCTION
Messenger RNA (mRNA) vaccines and therapeutics are a revolutionary development in biomedical science, offering potential treatments for a variety of diseases. mRNA technology works by utilizing the body’s protein-making machinery for the production of particular proteins. These proteins may induce an immune response, such as in vaccines, or can replace defective proteins with therapeutic uses. mRNA vaccines are unlike traditional vaccines, which employ weakened or inactivated pathogens to stimulate the immune system because they introduce a formulated mRNA strand-coded protein of interest—like the SARS-CoV-2 virus spike protein [1]. Injected into the body, the mRNA instructs cells to create the target protein, which is identified by the immune system to elicit a protective response without introducing the virus into the body alive. mRNA’s broader use goes beyond vaccines to therapeutics in cancer, genetic disease, and metabolic disease. For example, with cancer, mRNA is programmed to code for tumor antigens to allow the immune system to identify and destroy cancer cells. With genetic disease, mRNA drugs offer a transient but reversible fix for protein replacement by delivering the correct protein blueprints without altering the genome permanently. mRNA therapeutics function by a series of critical steps. The mRNA sequence is synthesized in vitro first and then packaged within lipid nanoparticles (LNPs) for cell delivery and protection against degradation. Inside cells, ribosomes read the mRNA to produce the target therapeutic protein or antigen to trigger the desired bioresponse, e.g., immune activation or protein replacement. It opens the door for targeted, specific treatments with possible rapid development, as well as large-scale manufacturing [2,3]. Traditional treatments usually suffer from having poor target specificity, difficult manufacturing, as well as immune concerns. mRNA medications offer a scaleable, precise, temporary alternative, avoiding all these problems to create more potent, personalized drugs. This overview covers the role of mRNA-based methods in medicine today [4].
Detailed process of mRNA vaccine development and targeting mechanism
mRNA synthesis and structure
mRNA vaccine production starts with a viral protein that can elicit an immune response, like the SARS-CoV-2 spike (S) protein. After the target protein sequence has been identified, the corresponding mRNA is produced in the laboratory by a process known as in vitro transcription from DNA templates. mRNA structure consists of several crucial constituents: the 5’ Cap (usually a 7-methylguanosine cap), which stabilizes the mRNA for effective translation; the 5’ Untranslated Region, which ensures a modulation of translation efficiency; the open reading frame, coding for the viral antigen, i.e., the spike protein, to be translated into protein in host cells; the 3’ Untranslated Region, which ensures mRNA stability and protein production efficiency; and finally, the Poly(A) Tail, which extends the half-life of the mRNA by preventing degradation [5,6]. Together, these components stabilize the mRNA and are effectively translated within the host cells to cause the expression of the target antigen to elicit an immune response.
Packaging in lipid nanoparticles and delivery into cells
After synthesis, mRNA cannot be introduced into host cells due to the instability of naked mRNA. mRNA is protected against degradation and internalized into cells by encapsulating it within LNPs [7]. The components of LNPs are ionizable lipids, phospholipids, cholesterol, and polyethylene glycol (PEG). Ionizable lipids are neutral at physiological pH but become positively charged at acidic pH, for example, inside endosomes. This positive charge allows them to merge with the endosomal membrane, releasing the mRNA into the cytoplasm. Phospholipids provide structural integrity to the LNP and aid in the formation of the lipid bilayer. Cholesterol stabilizes the LNP structure and enhances its rigidity and stability. PEG, a polymer that reduces interactions with immune cells as well as proteins, prolongs the circulation of LNPs in the bloodstream and prevents rapid immune-mediated clearance [8]. Figure 1 summarizes the mechanism of mRNA vaccines, where synthetic mRNA, encapsulated within LNPs, is introduced into antigen-presenting cells (APCs). Within the cells, the mRNA is translated into antigenic proteins that are expressed at the cell surface, triggering CD8+ cytotoxic T cells as well as CD4+ helper T cells. This stimulation also triggers B cells to synthesize antibodies, creating a long-term immune memory as well as an adaptive immune response.
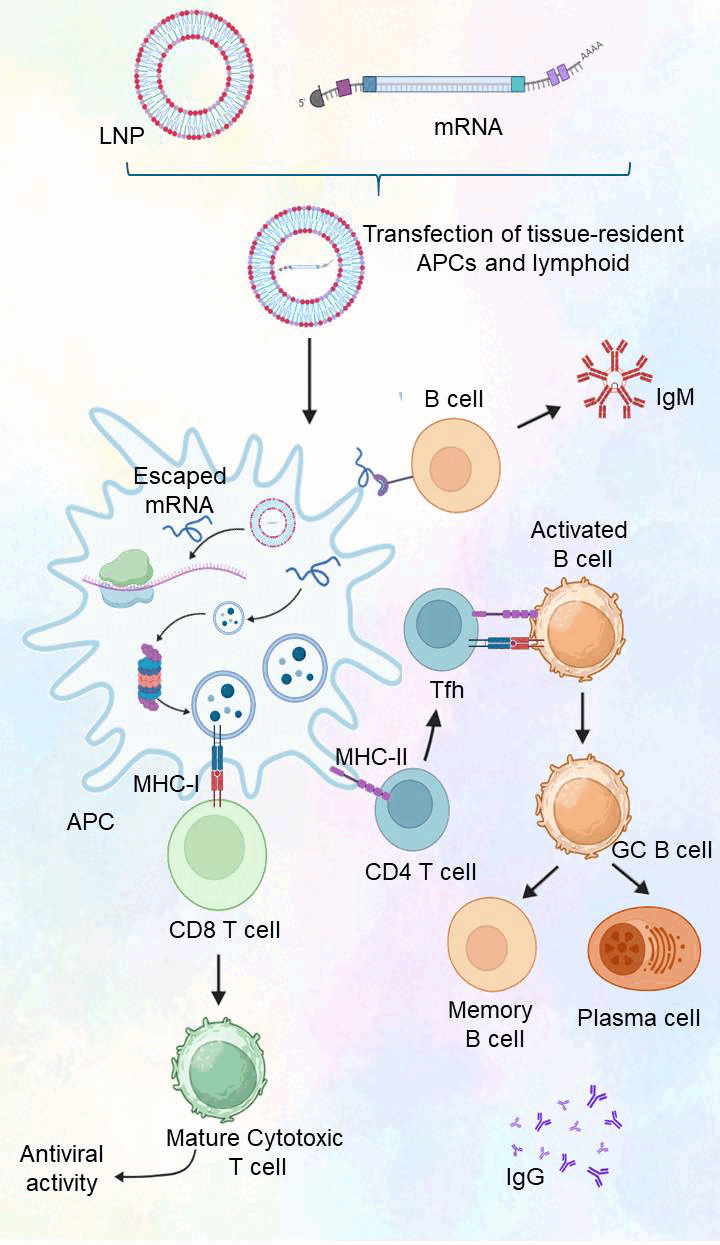 | Figure 1. Mechanism of action of mRNA-based vaccines. [Click here to view] |
When delivered, LNPs facilitate mRNA delivery to targeted cells, targeting immune cells such as dendritic cells as well as macrophages, which are responsible for antigen presentation. Such cells are primary responders to the immune response, involved in detecting foreign antigens as well as activating the adaptive immune response [9]. Upon injection, LNPs introduce mRNA into target cells, such as APCs such as dendritic cells and macrophages, which initiate the immune response. The LNPs are internalized into the cell through endocytosis, with the cell membrane surrounding the nanoparticle to form an endosome. Within the endosome, the acidic environment ionized the LNPs, which fuse with the endosome membrane—a phenomenon called endosomal escape—releasing the mRNA into the cytoplasm. This inhibits degradation within lysosomes as well as allows the mRNA to work efficiently. Within the cytoplasm, the mRNA is detected by the cell’s ribosomes, which bind to the 5’ cap to initiate translation. The ribosomes read the mRNA sequence to translate the viral protein (such as the SARS-CoV-2 spike protein) by ordering amino acids. This requires a number of significant molecules: the ribosomal subunits (40S and 60S) assemble to translate the mRNA to a polypeptide chain, and the transfer RNA molecules provide the right amino acids, as well as elongation factors, which facilitate the addition of amino acids to the chain. Following translation, the viral protein is processed within the cell as well as cleaved into peptide fragments by the action of proteasomes. Such fragments are chaperoned onto major histocompatibility complex (MHC) class I or class II molecules, contingent upon the type of APC. Within most cells, viral peptides are presented as MHC class I molecules, detected by cytotoxic CD8+ T cells, capable of directly killing virus-laden cells presenting the viral antigen. Within professional APCs, peptides are presented even on MHC class II molecules, recognized by helper CD4+ T cells, resulting in B cell activation as well as other immune cells. This facilitates antibody formation as well as amplifies cytotoxic activity [10].
When the immune system detects the viral protein through MHC molecules, it stimulates both branches of the adaptive immune response. Helper T cells stimulate B cells to secrete antibodies against the viral protein, which neutralizes the virus by binding to its outer surface, preventing infection of host cells. Activated cytotoxic T cells identify and kill virus-infected cells showing viral antigens, helping to clean the infection. A key benefit of mRNA vaccines is their ability to create long-term immunological memory. Some of the B cells and T cells are activated to become memory cells, which remain in the body for extended periods of time and respond rapidly if the virus infects again, offering extended protection. mRNA vaccine development is a complex process with multiple molecular components. From mRNA synthesis, incorporation within lipid nanoparticles, entry into cells, translation, and protein expression to immune activation, each step is designed to reliably create an immune response without the introduction of a live virus. The use of LNPs as mRNA delivery agents, combined with the adaptive response of the immune system, enables mRNA vaccines to promote immune protection that is effective as well as long-lived against a range of infectious agents.
mRNA vaccines operate through the administration of synthetic mRNA into APCs that initiate the production of pathogen-specific antigens [9]. The cells process the antigens, presenting them on the surface or secreting them, thus enabling immune cells to recognize and act, both inducing humoral and cytotoxic T-cell responses. Many studies have documented the preclinical and clinical progress of coronavirus vaccines. Although mRNA vaccines have proven effective in combating infectious diseases, several challenges persist. These include the need to improve the durability of immune responses; this may be achieved by enhancing adjuvant activity and extending protein expression to support long-term protection. Increasing the immunogenicity of these antigens by dose adjustments, delivery methods, and adjuvants is also under investigation. Reducing the adverse effects is another critical consideration because minimizing adverse reactions while maintaining efficacy is also very significant [11]. Other groups, including a company called Moderna, have shown in laboratory experiments that personalized mRNA vaccines—such as those personalized based on an individual’s immune responses—could be more potent and pose less risk. In general, mRNA vaccines have great potential as we continue to investigate and develop them to overcome existing limitations and increase their effectiveness, potency, and safety.
Developmental stages of mRNA vaccine
Pfizer-BioNTech and Moderna COVID-19 vaccines (BNT162b2 and mRNA-1273, respectively) are the first two mRNA vaccines that have been approved for human use at a large scale. The Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines were both demonstrated to have more than 90% efficacy in Phase III trials, and their effectiveness in preventing severe outcomes of COVID-19 was high in real-world settings [12,13]. There have been more than 1.5 billion doses of BNT162b2 and 900 million doses of mRNA 1273 administered globally as of 2024 helping to provide immunity to hundreds of millions [14,15]. However, the success was not universal across all candidates of mRNA vaccines. CureVac’s first-generation vaccine (CVnCoV) with only 48% effective percentage in a phase III trial was correlated with SARS-CoV-2 variants and a low protein expression [16]. The company Arcturus Therapeutics, which used a self-replicating mRNA (saRNA) platform (STARR™), has performed early-trial results with promising immunogenicity, but with limited data about efficacy on a large scale [17]. eTheRNA, which emphasized intranasal and conventional delivery routes, faced difficulties in generating potent systemic immune responses [18].
However, the idea of mRNA vaccines was in development for a few years prior to the pandemic. Work on mRNA technology for therapeutics and vaccines started early in the 1990s, and mRNA vaccines were already being tried in animal models and a few initial human trials for conditions such as rabies, influenza, and Zika [19]. The mRNA vaccine idea first arose in the early 1990s when scientists initially started investigating mRNA as a platform for the production of therapeutic proteins. The key strength of mRNA is that it can be easily and quickly designed and manufactured, hence its potential to be used as a platform for vaccines and therapeutics. Early research, though, encountered serious issues such as mRNA instability, breakdown by enzymes in the body, and inefficient cell delivery. 1990s—Early conceptualization: Early studies indicated that mRNA was able to induce protein synthesis in animal models. However, the biggest obstacle was its instability and the fact that the immune system would quickly degrade it. Early experiments showed that injection of mRNA directly was able to induce transient protein expression, but the technology was by no means practical for use in humans [19]. 2000s—Advances in mRNA Stability and Delivery: There was improvement in stabilizing the mRNA and its delivery. Chemical modifications to the mRNA structure helped reduce its immunogenicity and increase its stability [20]. Besides, advances in LNP technology as a delivery system for mRNA represented a breakthrough. LNPs protected mRNA from degradation and improved cellular uptake, making it possible to achieve efficient protein expression upon being taken into the cell.
Pre-COVID Research: Before COVID-19, mRNA vaccines were explored as candidates for infectious diseases such as rabies, Zika, and influenza [21]. Companies like Moderna and BioNTech were already in the middle of carrying out clinical trials for mRNA vaccines against diseases like rabies and cancer immunotherapies [22]. Early results indicated that mRNA vaccines were capable of inducing strong immune responses with relatively low doses. Zika and Influenza Trials: The National Institute of Allergy and Infectious Diseases in 2017 initiated clinical trials of a Zika virus vaccine using mRNA [23]. The trials demonstrated that mRNA vaccines had the ability to generate robust immune responses, although they were still in their early stages. Likewise, preclinical research of mRNA influenza vaccines showed encouraging findings against the triggering of neutralizing antibodies. Cancer immunotherapy: At the same time infectious diseases, mRNA vaccines were under investigation for cancer immunotherapy [24]. Organizations such as BioNTech produced individualized cancer vaccines that contained tumor-specific antigens, designed to activate the immune system to identify and eliminate cancer cells. COVID-19 Acceleration: The early foundation in mRNA vaccines for infectious disease and cancer established the basis for the accelerated pace of COVID-19 vaccine development. When the SARS-CoV-2 virus was sequenced, the capability of rapidly designing mRNA sequences from the viral spike protein enabled the rapid development of vaccines to result in the first mRNA vaccines licensed for broad use (Karin Bok 2021). Pre-COVID-19 mRNA vaccine development overcame many scientific challenges, but technological breakthroughs in mRNA stabilization, delivery systems, and early-stage clinical trials for conditions such as Zika and cancer laid the foundation for the unprecedented success of mRNA technology during the pandemic.
mRNA therapies have truly transformed the vaccine field, as Pfizer-BioNTech and Moderna’s COVID-19 vaccines proved the value of the platform in accelerating the development of effective vaccines for infectious diseases [25]. Beyond the prevention of infectious disease, however, mRNA technology has applications much broader. The versatility, safety profile, and capacity for rapid modification and manufacture of the platform position it as a powerful therapeutic in addressing other diseases, including cancer, genetic diseases, and tissue repair.
mRNA therapeutics offer a safer and more efficient alternative to DNA-based therapies with outstanding advantages in biomedical applications. Because mRNA acts outside the nucleus in the cytoplasm, there is no risk of genome integration and related mutations. This provides for targeted and transient protein expression, allowing for controlled biological responses. Moreover, mRNA-based therapies have the ability to induce potent immune reactions without long-term expression, minimizing probable immune complications. Their in vitro, cell-free synthesis allows for quick and large-scale manufacturing, which makes them ideal for personalized medicine and adaptation to new health issues quickly. In contrast to gene-editing tools, which create long-lasting changes, mRNA therapy offers a reversible and controlled solution, making it an appealing tool for vaccines, cancer therapies, and genetic disorder control [4,26,27].
Applications of mRNA therapeutics beyond vaccines
mRNA cancer vaccines: an innovative therapeutic approach
mRNA cancer vaccines provide an innovative solution to the treatment of cancer through the use of the immune system of the body to identify and destroy cancer cells efficiently. mRNA cancer vaccines cause a robust immune response by teaching immune cells to attack tumor-specific antigens, resulting in effective and targeted cancer destruction. One of the greatest benefits is their speed of production, whereby mRNA sequences are easily designed and produced quickly and can thus easily be adapted for various types of cancer. Compared to conventional therapy, mRNA vaccines offer targeted treatment, with only cancerous cells being influenced while keeping the healthy tissues with minimal damage. Another benefit is their affordability since their production is by simpler processes as opposed to protein-based or cell-based immunotherapy.
Another significant advantage is the reduced risk of infections, since mRNA vaccines contain no live pathogens, and there are fewer worries about viral contamination. In addition, they enable personalized therapy, whereby vaccines can be programmed to fit an individual patient’s tumor profile, with increased efficacy in treatment. Compared to certain gene therapies, mRNA vaccines are less susceptible to insertional mutagenesis, as they are not integrated into the genome, hence lowering long-term genetic alterations. Furthermore, the vaccines have fewer adverse effects and, hence, are a better alternative compared to other conventional cancer therapies such as chemotherapy and radiotherapy. With their accuracy, safety, and flexibility, mRNA cancer vaccines are a revolutionary leap forward in oncology that opens the door to more powerful and personalized treatments for cancer (Fig. 2).
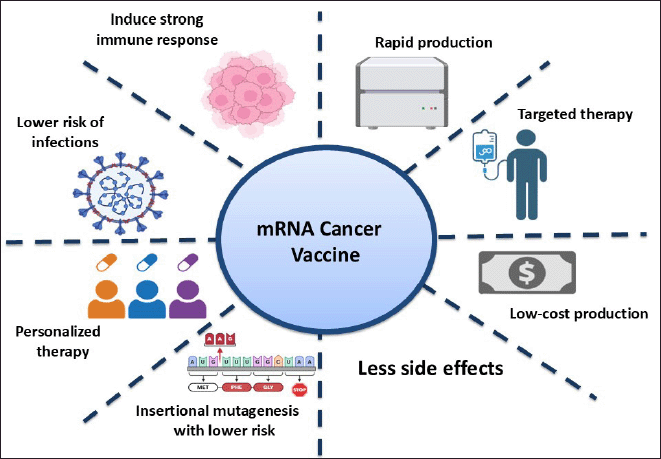 | Figure 2. Advantages of mRNA cancer vaccines. [Click here to view] |
mRNA cancer vaccines represent an innovative therapeutic approach, utilizing encoded tumor antigens—such as tumor-associated antigens (TAAs), tumor-specific antigens (TSAs), or immunomodulatory molecules—to stimulate and precisely regulate the anti-cancer immune response [28,29]. TAAs are often highly expressed in tumor cells, although it is also found in normal tissues, and may have reduced effectiveness due to T-cell tolerance [30]. On the other hand, TSAs, or neoantigens, are exclusive to cancer cells and result from somatic tumor mutations, showing greater specificity and efficacy, thereby being the most suitable targets for mRNA cancer vaccine design. Advantages of mRNA cancer vaccines include strong immune responses, rapid manufacturing, targeted treatments, low-cost manufacturing, less side effect, less risk of infection, individualized therapy, and less risk of mutagenesis. These traits render mRNA vaccines a hopeful option in precision oncology and immunotherapy.
Firms such as BioNTech and Moderna have progressed bespoke mRNA vaccines, employing tumor biopsy samples from patients to pinpoint candidate neoantigen targets using advanced sequencing and screening for immunogenicity. These are the vaccines that are tailored to a patient’s own tumor profile, and the platform based on mRNA is amenable to rapid manufacturing to enable swift conversion from biopsy to vaccine [31].
The development of mRNA cancer vaccines dates back to 1995, when Conry and colleagues conducted the initial preclinical study that utilized mRNA liposome formulations to deliver the human CEA antigen. Since that time, numerous preclinical and clinical investigations have focused on vaccines encoding TAAs via mRNA. For example, BioNTech’s BNT111, a vaccine candidate of an mRNA-lipoplex, targets four melanoma TAAs: NY-ESO-1, MAGE-A3, tyrosinase, and TPTE. Administered either independently or alongside PD-1 inhibitors, BNT111 is demonstrated as robust immune responses with respect to antigen-specific in patients with melanoma that cannot be surgically removed, leading to its Fast Track designation by the US FDA for advanced melanoma [31]. Another TAA-panel-based strategy is the generation of individualized TAA panels from a patient’s tumor tissue. In clinical trials, dendritic cells are genetically modified to express mRNA from primary tumors or particular TAAs. This approach has been tested for various cancers, such as prostate cancer (NCT01197625), acute myeloid leukemia (NCT00514189), and other cancers (NCT01334047, NCT02709616, NCT02808364, and NCT02808416) [31,32].
Neoantigens are present in most tumors and each tumor can have dozens to thousands of neoantigens. BNT122, an individualized mRNA vaccine containing up to 20 neoepitopes specific to individual patients, is being investigated for melanoma (NCT03815058) and colorectal cancer (NCT04486378). Similarly, mRNA-4157, which includes up to 34 neoantigens and is encapsulated in LNPs, is being evaluated in individuals with surgically removed solid tumors, including melanoma, bladder cancer, and non-small cell lung cancer, are being treated alone and in combination with pembrolizumab (Keytruda) (NCT03313778). Early results showed that both mRNA-4157 alone and with pembrolizumab had an acceptable safety profile and induced robust neoantigen-specific T-cell responses. Out of 13 who received the single-agent therapy, 12 remained disease-free. The efficacy of mRNA vaccines might be improved by combining them with other treatments. A Phase 1/2 clinical trial (NCT04455620) is evaluating the safety and effectiveness of BNT151, a nucleoside-modified IL-12 mRNA therapy, both as a monotherapy and in combination with other cancer-fighting agents, including adjuvants, immune checkpoint inhibitors, cytokines, or agonists, for patients with advanced solid tumors, focusing on its pharmacodynamic effects on blood and tumor samples [33].
Overall, mRNA-based cancer immunotherapy is revolutionizing oncology by providing highly targeted treatments that harness the body’s natural immune response to detect and destroy tumor cells. In contrast to traditional treatments like chemotherapy or radiation, which often harm normal tissues, mRNA vaccines selectively activate cytotoxic T cells (CD8+ T cells) to target and kill cancer cells while sparing healthy cells. For instance, the mRNA vaccines expressing TAAs like MAGE-A3 are undergoing trials for several types of cancer like melanoma, with favorable outcomes in both safety and immune stimulation [34].
Under the personalized cancer vaccine, genomic sequencing of tumor from the patient reveals neoantigens that are utilized to customize mRNA vaccines coding these particular tumor mutations. Such customized vaccines can be enhanced further by combining them with immune checkpoint inhibitors, like PD-1 inhibitors, which block tumor cells from escaping immune surveillance. Clinical trials such as those for BNT122 and mRNA-4157 have indicated that the combination of personalized mRNA vaccines with checkpoint inhibitors is promising in enhancing the anti-tumor immune response, paving the way for future breakthroughs in cancer therapy.
The role of mRNA therapeutics in replacement of protein and monogenic diseases
Monogenic diseases are genetic disorders passed down through inheritance and induced by mutations in a single gene, and over 5,000 such diseases have been identified to date. Different treatment strategies have been developed for these conditions, such as substrate-based, protein-based, mRNA-based, and DNA-based approaches. Of these, protein replacement therapy has proven to be a viable choice for treating some monogenic diseases by compensating for the lacking or faulty proteins. This approach, however, faces a number of challenges, including low delivery efficiency, difficulty in targeting proteins to the correct cellular or subcellular compartments, complex manufacturing processes, and high costs [35].
mRNA therapeutics have proven promising in the treatment of monogenic diseases, rectifying many of the issues facing protein replacement therapy. In contrast to protein therapy, mRNA drugs introduce genetic information directly to the cells to produce the normal protein necessary to replace the deficient or missing gene product. Furthermore, mRNA methods do not edit the DNA, providing a less risky, reproducible option for gene therapy, which permanently edits the genome.
Methylmalonic acidemia
An important example of mRNA-based treatment is its use in methylmalonic acidemia (MMA), a synthetic metabolic disorder due to deficiency in the methylmalonyl-CoA mutase or associated cofactor enzymes. The condition leads to the buildup of methylmalonic acid and causes extreme metabolic instability. Moderna has actively been working on an mRNA treatment for MMA, which codes for the methylmalonyl-CoA mutase (MMU) enzyme. Experiments have indicated that administration of this mRNA twice a week by intravenous injection in MMU-deficient mice lowered plasma methylmalonic acid by 60%–90%, suggesting the efficacy of this method in treating MMA in humans [36].
Acute intermittent porphyria
Acute intermittent porphyria (AIP) is another monogenic disease resulting from haploinsufficiency of the enzyme porphobilinogen deaminase (PBGD), resulting in a buildup of neurotoxic precursors such as porphobilinogen and aminolaevulinic acid. This accumulation can induce neurovisceral attacks. Experiments have shown that intravenous delivery of PBGD mRNA in LNPs increased PBGD activity in the liver by two-fold within 2 hours of intravenous delivery [37]. The increased enzyme activity lasted for 10 days, resulting in a significant decrease in the toxic precursors that cause AIP symptoms.
Fabry disease
Fabry disease is a metabolic disorder due to the deficiency of the enzyme α-galactosidase A, resulting in the accumulation of glycosphingolipids. The accumulation may cause severe organ dysfunction affecting the heart, kidneys, and nervous system. Moderna is creating an mRNA-based therapy for Fabry disease encoding α-galactosidase A. In preclinical tests, intravenous administration of α-galactosidase A mRNA contained in LNPs successfully restored the activity of the enzyme in key organs, such as the liver, spleen, heart, and kidneys, suggesting that the treatment might potentially halt or reverse the development of Fabry disease [38].
Cystic fibrosis
Cystic fibrosis (CF) is due to mutations in the CFTR gene, which lead to defective chloride transport and result in the formation of thick mucus in the lungs and digestive system. mRNA therapies seek to introduce functional CFTR mRNA into epithelial cells in the respiratory and gastrointestinal tracts, aiding in the re-establishment of normal chloride transport [39]. Translate Bio has created MRT5005, an mRNA-based treatment for CF, which yielded encouraging results in preclinical studies. In knock-out mice with cystic fibrosis transmembrane conductance regulator (CFTR), administration of CFTR mRNA resulted in the normalization of chloride secretion for up to 14 days. Moreover, a clinical trial of MRT5005 involving nebulized delivery of CFTR mRNA indicated safety and tolerability, without any severe side effects [40].
Hemophilia
Hemophilia is a genetic bleeding disorder due to mutations in factor VIII (Hemophilia A) or factor IX (Hemophilia B), resulting in impaired blood clotting. mRNA therapies are being designed to encode these clotting factors, making it possible to restore normal clotting function. Factor VIII mRNA administered in hemophilia A mouse models corrected the hemostatic defect during preclinical studies, with levels of circulating factor VIII being above therapeutic levels for more than 72 hours. In hemophilia B models also, factor IX mRNA administered in the form of LNPs induced a quick pulse of factor IX expression by 4–6 hours post-administration with longer-term effects persisting for up to 6 days [41].
Muscular dystrophy
Duchenne muscular dystrophy is caused by mutations in the dystrophin gene, leading to progressive loss of muscle tissue. mRNA therapy seeks to introduce functional dystrophin mRNA into muscle cells, allowing the synthesis of the dystrophin protein, which is essential for muscle integrity. This method is deemed safer than gene therapy because it does not cause permanent alteration of the genome while offering multiple treatment opportunities for the restoration of dystrophin function [41].
Glycogen storage disease type 1A (GSD1A)
It is a metabolic disease caused by a deficiency of the enzyme glucose-6-phosphatase (G6Pase), which is essential to maintain blood glucose levels. Treatment options currently are mainly concerned with blood sugar management by diet. mRNA-based therapies try to correct the normal functioning of the enzyme by simply introducing G6Pase mRNA into the liver. Ultragenyx Pharmaceuticals is in the process of developing mRNA-based treatments for GSD1A, which hold the promise of dramatically enhancing patients’ lives by treating the underlying enzyme deficiency [42].
mRNA therapeutics is a revolutionary method for the treatment of monogenic disorders with the potential to restore the production of normal protein without altering the genome. This renders mRNA-based therapies a reversible, transient, and safer option than gene therapy for disorders where long-term modification of DNA is not preferred. With promising results in preclinical and early-stage clinical trials, mRNA therapies are on the verge of becoming a powerful tool for treating a wide range of genetic disorders, including cystic fibrosis, hemophilia, muscular dystrophy, and metabolic disorders like methylmalonic acidemia and Fabry disease. Extensive research and clinical trials are crucial to unlocking the full potential of mRNA-based therapies for effectively managing and potentially curing these conditions.
Regenerative medicine
The versatility of mRNA therapeutics reaches regenerative medicine, where they can assist in the promotion of tissue repair and regeneration, providing new solutions to diseases and injuries that have few therapeutic alternatives. Through the encoding of growth factors, cytokines, or tissue-specific proteins, mRNA can enhance healing processes and tissue repair.
Cardiovascular disease
After a medical emergency such as a heart attack (myocardial infarction), ischemic tissues suffer tissue hypoxia, leading to cell death and impaired heart function. mRNA therapies may encode proteins such as vascular endothelial growth factor (VEGF) to induce new blood vessel formation (angiogenesis) in ischemic tissues. Animal research has demonstrated that mRNA encoding for VEGF can enhance cardiac function and induce the growth of blood vessels in ischemic tissue, providing a potential new therapy for heart failure and other cardiovascular disorders [43].
Wound healing
The mRNAs for proteins that participate in tissue repair, such as collagen or transforming growth factor-beta, can be administered directly to wounds to promote healing. This is particularly useful for the case of chronic wounds, such as diabetic ulcers, in which the body’s normal healing process does not function properly. By implementing mRNA that triggers the production of these repair proteins, the process of wound healing can be remarkably enhanced, cutting down on recovery time and avoiding complications [44].
Cartilage and bone regeneration
mRNA has been highly promising in orthopedic therapy, where it can facilitate the regeneration of bone and cartilage. In diseases like osteoarthritis, mRNA coding for cartilage-specific proteins, such as aggrecan or collagen II, can be utilized to repair degenerated cartilage. Likewise, mRNA coding for bone morphogenetic proteins can stimulate bone growth and repair in fracture or bone degenerative diseases [45].
Although research in mRNA-based regenerative medicine is only just beginning, preclinical studies have shown that it can promote tissue repair and regeneration. This innovative approach offers hope for treating a variety of degenerative diseases and injuries that currently have limited therapeutic options, including heart disease, musculoskeletal disorders, and chronic wounds [46].
Protein replacement therapy using mRNA
For diseases where protein deficiencies or malfunctions are the root cause, protein replacement therapies are often used to restore normal function. Traditional PRT presents in the form of injected synthetic proteins, which can be expensive and show limited effectiveness as a result of their quick degradation within the body or improper absorption by tissues [47]. mRNA-based PRT offers a better approach with the body’s native cells producing the protein therapeutics, reducing treatment frequency, and enhancing efficacy.
Ornithine transcarbamylase deficiency
Ornithine transcarbamylase deficiency (OTC deficiency) is a rare metabolic disease where the body is unable to efficiently clear ammonia. mRNA drugs offer a method of replacing the lacking or dysfunctional OTC enzyme with the potential to prevent the toxic build-up of ammonia. Studies have shown that mRNA therapy effectively corrects normal enzyme activity and levels in animal models of OTC deficiency [48].
Pompe disease
Pompe disease is a lysosomal storage disorder caused by a deficiency of the enzyme acid alpha-glucosidase (GAA). The treatment is now with routine enzyme replacement therapy, but mRNA therapy could be more efficient by providing genetic instructions for GAA production directly into muscle cells. This can enhance muscle function and decrease the severity of the disease [49].
Monoclonal antibodies and immunotherapeutics
mRNA-based drugs have opened new avenues for manufacturing monoclonal antibodies and immunotherapies against many diseases, including infectious diseases and cancer [50]. In 2019–2020, Moderna initiated the first human clinical trial (NCT03829384) to evaluate the safety, pharmacokinetics, and pharmacodynamics of mRNA-1944, a product of mRNA-LNP encoding a monoclonal antibody targeting the Chikungunya virus (CHKV-24). The trial had a favorable safety profile and dose-proportional elevations in the levels of serum CHKV-24 that implied potential protection against Chikungunya infection. Beyond Chikungunya, mRNA-encoded therapies have been created to protect against toxins and viral infection [42]. mRNA-LNP-encoded antibodies and antibody mimics have been tested for their prophylactic and therapeutic use against toxins like botulinum neurotoxin and Shiga toxin, and viral infections such as rabies and influenza B. Intravenous delivery of mRNA-LNP-based variable domain neutralizing agents has shown significant expression in the liver within hours and shown protective efficacy in animal models. Another innovative application of mRNA-encoded monoclonal antibodies was shown by Pardi and colleagues in their work on HIV-1. An mRNA-LNP was designed to encode the broadly neutralizing antibody VRC01 targeting HIV-1. A single dose resulted in elevated serum antibody levels and provided protection against HIV-1 infection in humanized mice [51].
mRNA-based antibody immunotherapies for cancer
mRNA technology is revolutionizing cancer immunotherapy by allowing the manufacture of therapeutic molecules including antibodies, bispecific antibodies, and chimeric antigen receptor T-cell therapies. mRNA has been used to create bispecific antibodies that target in parallel the CD3 T-cell receptor and Claudin-6 (CLDN6), a tumor-associated antigen expressed in solid tumors such as ovarian and testicular cancers. These breakthroughs indicate the promise of mRNA-based strategies in targeting cancer-specific targets. In ovarian cancer mouse models, weekly administration of these mRNA-encoded bispecific antibodies led to the complete elimination of tumors, which demonstrates a highly efficacious treatment option for solid tumors. In 2022, BioNTech launched the first human clinical trial of BNT142, an mRNA-encoded bispecific antibody against CD3 and CLDN6 in patients with advanced solid tumors expressing CLDN6 [9].
Tolerance-inducing therapies and immunosuppressives
The mRNA-based therapies are emerging as potential tools for promoting immune tolerance and treating allergies in the field of immunosuppressive treatments. Krienke and team designed a non-inflammatory mRNA vaccine intended to address experimental autoimmune encephalomyelitis, commonly used as a model for studying multiple sclerosis. This vaccine expresses myelin oligodendrocyte glycoprotein epitopes in altered mRNA, administered via non-immunostimulatory liposomes. It effectively induced tolerance by reducing antigen-specific autoreactive T cells and enhancing regulatory T cells, demonstrating its potential as a therapy for autoimmune diseases [52].
Moderna is testing the safety and pharmacokinetics of mRNA-6231, an mRNA-LNP that encodes an IL-2 mutein fusion protein, in an early-phase clinical trial. IL-2 is known to enhance effector T-cell function, but engineered versions of IL-2 are intended to preferentially enhance regulatory T cells but decrease effector T-cell activity. This specificity makes it a viable candidate for treating autoimmune and inflammatory disorders [53].
Genomic editors
mRNA-based medicines are also being utilized for genomic editing, providing the potential cause of the treatment for targeting the causative root of monogenic disorder through the correction of genetic mutations. Although viral-based gene therapies, including adeno-associated viruses, have progressed in gene therapy, they carry risks such as immune reactions and genomic integration. mRNA-based gene editing provides a safer option, typified by realizing high editing accuracy with fewer unintended consequences. A prime example is the employment of CRISPR-Cas9 administered in the form of mRNA-LNPs for curing transthyretin amyloidosis (TTR amyloidosis). In clinical tests, a single dose of LNP-encapsulated Cas9 mRNA and guide RNA was followed by a 52%–87% decrease in TTR protein levels, which demonstrates the potential for curing TTR amyloidosis through mRNA-mediated genomic editing. More recent advances in adenine base editing (a modification of the CRISPR-Cas9 system) make it enable the correction of single nucleotide mutations without causing double-stranded breaks in the DNA [54]. In an experiment targeting PCSK9, LNP-mediated delivery of adenine base editors in mice and also in macaques achieved high editing rates, leading to stable reductions in LDL cholesterol levels without inducing off-target mutations, highlighting the potential of mRNA-based genomic editing to treat metabolic and cardiovascular diseases [55].
The progress in mRNA therapeutics for monoclonal antibodies, cancer immunotherapy, immunosuppressive treatments, and genomic editing is of significant promise for the treatment of various diseases. The therapies are particularly advantageous in that they can be produced quickly, are highly specific, and present lower risks than conventional protein therapies and gene therapies. From immunoprotection against infectious diseases such as Chikungunya and Human immunodeficiency virus (HIV) to the treatment of genetic diseases by genomic editing, mRNA therapeutics hold promise to transform the field of medicine. With the ongoing development of these therapies and increasing clinical data, it is clear that mRNA technology will be at the forefront of the next generation of personalized medicine.
Broader applications: infectious disease treatment
Aside from vaccines, mRNA technology is also being investigated for the therapy of infectious diseases. In contrast to vaccines that instruct the immune system to see pathogens, mRNA infectious disease treatments present mRNA sequences encoding antimicrobial proteins or antibodies. Such treatments may provide a rapid-response capability against emerging pathogens or antibiotic-resistant infections.
mRNA-based antiviral therapies
Scientists are making mRNA-based antiviral treatments for illnesses such as HIV, influenza, and hepatitis. The treatment targets to transfer of the genetic code for the production of proteins that can directly hinder the replication of the virus or boost the immune system of the body to combat the infection [56].
mRNA for antibiotic-resistant bacteria
To address the burgeoning menace of antibiotic resistance, mRNA technology is also being investigated to generate targeted antimicrobial peptides capable of neutralizing bacteria. By customizing the mRNA to code for proteins specific to resistant bacterial species, these treatments would offer a new method for treating infections that no longer respond to traditional antibiotics [57].
The therapeutic applications of mRNA-based treatments are enormous and far-reaching beyond vaccines. Whether it is targeted cancer therapy, replacement therapy of proteins for the treatment of genetic disorders, or regeneration of tissues in degenerative diseases, mRNA can revolutionize the practice of medicine. The speed, adaptability, and scalability of mRNA therapies allow for quicker development of drugs, customized treatments, and even safer therapies. As science continues to push the limits of what mRNA can do, this technology has the potential to revolutionize not just how we treat disease today but also how we go about developing treatments for medical challenges of the future.
Advantages of mRNA vaccines
The mRNA vaccines have a number of unique benefits because of the special nature of mRNA therapy. The most important advantage is that mRNA does not become part of the host genome, reducing the risk of toxic mutations (mutagenesis). This guarantees its non-permanent effect, which is perfect for transient, controlled immune responses. Another advantage is that mRNA is extremely versatile—it can be engineered to encode virtually any protein, enabling highly targeted and specific treatments. Its quick manufacture is another big advantage, since mRNA can be produced rapidly and is thus incredibly useful in cases of emergency, such as pandemics. mRNA vaccines are most commonly packaged within LNPs. These LNPs shield the vulnerable mRNA from enzymes in the body that would degrade it, ensuring it reaches the target cells. Upon reading the genetic code inside the cells, the mRNA becomes released into the cytoplasm, where ribosomes (the protein-making machinery of the cell) read the genetic information and build the resulting protein. That protein induces an immune response, similar to how the body responds to a natural infection but without utilizing the use of a live virus or irreversible genetic changes. This makes mRNA vaccines safer, more adaptable, and quicker to be made than traditional vaccine platforms [2]. The latest developments in mRNA therapeutics have moved beyond vaccines to cancer, rare genetic disorders, cardiovascular conditions, and infectious diseases, as illustrated in Table 1.
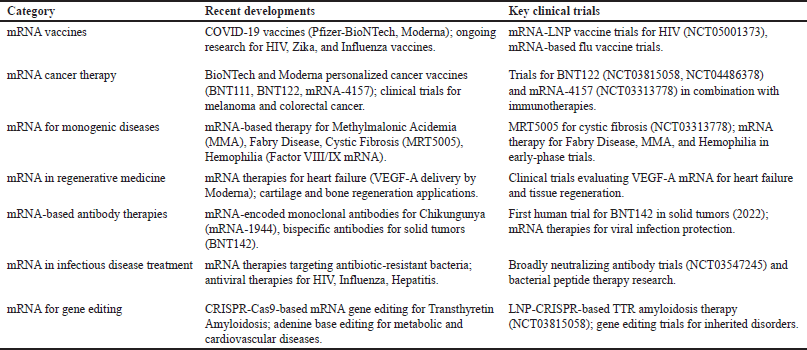 | Table 1. Recent advancements and key clinical trials in mRNA therapeutics. [Click here to view] |
Scalability and economic challenges of personalized mRNA cancer vaccines
Personalized mRNA cancer vaccines are a novel method of personalized cancer therapy, but their mass production and affordability are major challenges. In contrast to conventional vaccines, these treatments involve sequencing the tumor genome of an individual patient to define specific neoantigens, followed by bespoke mRNA synthesis and production. This highly personalized process entails sophisticated bioinformatics, specialized production, and rigorous quality control, rendering mass production costly and challenging. Also contributing to the overall expense are cold storage and targeted delivery system requirements. Cost reductions and greater efficiency, though, are coming about through advances in automated synthesis of mRNA, lipid nanoparticle delivery, and AI-based antigen selection. Economies of scale and reduced regulatory hurdles, facilitated by continued innovation in mRNA vaccine infrastructure, can expand accessibility and affordability and make personalized cancer vaccines more practical for broader clinical adoption [58,59].
Current clinical trials
Increasing numbers of clinical trials are assessing mRNA therapeutics in various therapeutic areas. A few outstanding examples include (Table 1)
Human immunodeficiency virus infection
In spite of decades of investigations, HIV is still a major worldwide health problem because of the immunogenicity of the virus, high mutation rate, and structural heterogeneity of its envelope glycoprotein (Env). Env trimers on the virus surface transition between several conformations, exposing various epitopes, making it difficult to develop broadly neutralizing antibodies (bnAbs). Neutralization of tier 2 and tier 3 viruses is crucial for protection, yet many Env immunogens only induce neutralization for tier 1 viruses, which are less challenging to neutralize [60]. Recent advances in potential HIV vaccine development have shown promising results; Preclinical studies have demonstrated that vaccinating rabbits and non-human primates with native-like trimer immunogens can induce tier 2 neutralizing antibody responses [61], which are crucial for targeting the more resistant viral strains [61]. A trial of an HIV-1 mRNA-LNP vaccine in non-human primates demonstrated that this method elicited long-lasting neutralizing antibodies for at least 41 weeks, demonstrating the long-term protection capabilities of mRNA-based HIV vaccines [62]. Trials are also underway to explore the potential for bnAbs both as therapeutics and preventives. These bnAbs target conserved sites on HIV’s membrane glycoproteins. Among these trials (NCT03547245), one was aimed at engaging germline-targeting immunogens, which have the goal of engaging the rare precursor B cells that give rise to bnAbs. The results of this trial demonstrated that the vaccine could stimulate VRC01-class responses among healthy adults, showing a clinical proof of concept for this approach [58]. However, another trial (NCT05001373) is evaluating the efficacy of mRNA-LNP-encoded antigens to generate identical bnAb responses, further clearing the path to effective HIV mRNA vaccines [52].
BioNTech’s individualized mRNA cancer therapies (BNT122)
BioNTech’s BNT122 is an individualized mRNA cancer vaccine therapy for various forms of cancer, including melanoma and colorectal cancer. This trial is on the principle of neoantigen-based immunotherapy, where the neoantigens are from a patient’s tumor. Neoantigens are mutated proteins present only in cancer cells, and not in normal tissue, such that they are perfect targets for immune-based therapy. BNT122 vaccine is engineered by sequencing an individual’s cancer to identify the neoantigens. The chosen antigens are translated into mRNA, which is introduced into the patient’s immune system through LNPs [22]. Inside the cells, the mRNA is translated into the neoantigens, which are displayed on the cell surface by MHC molecules. This stimulates CD8+ cytotoxic T cells, which recognize and destroy tumor cells presenting these neoantigens. This individualized strategy is designed to generate a strong immune response specifically against the patient’s individual tumor mutations with minimal off-target effects and improved therapeutic specificity. BNT122 is being explored as a monotherapy and combined with checkpoint inhibitors, including anti-PD-1 antibodies, to augment the total anti-tumor effect [63].
Moderna’s mRNA therapies for heart failure
Moderna’s mRNA medicine for heart failure is based on the delivery of mRNA that codes for vascular endothelial growth factor A (VEGF-A), a strong angiogenic growth factor that induces neovascularization. The therapy is intended to treat tissue injury after myocardial infarction (heart attack), which results in ischemic heart failure by causing loss of viable cardiac tissue. The treatment is systemic or local delivery of the mRNA in LNPs, which enables it to evade the immune system and travel to the target tissues within the heart. In the cell, the mRNA is translated into VEGF-A, and this promotes the formation of new blood vessels within the ischemic region [64]. This new vasculature can improve blood flow, augment oxygen delivery, and even revive function in damaged heart tissue. Preclinical models have shown that VEGF-A mRNA significantly augments cardiac repair and improves overall cardiac function in animal models of cardiac failure. The ongoing clinical trial aims to assess the safety and efficacy of this method in human patients, and results may uncover new therapeutic possibilities for treating heart disease by using mRNA-mediated tissue regeneration [64].
Translate Bio’s treatment for cystic fibrosis (mRNA-3065)
Translate Bio’s mRNA-3065 is an innovative trial for the therapy of cystic fibrosis (CF), an inherited disease as a result of mutations in the CFTR gene. CFTR is a protein used for the control of chloride ion passage across the membrane of epithelial cells, which is needed to maintain fluid balance in organs such as the lungs and pancreas. Mutations in CFTR lead to abnormal or failure to produce dysfunctional protein, resulting in abnormally thick mucus accumulation, recurrent infection, and destruction of the lungs [40]. The mRNA-3065 trial involves the delivery of mRNA coding for the normal version of the CFTR protein into CF patient cells. Encapsulated within LNPs, the mRNA is administered to the respiratory epithelium through inhalation. When the mRNA is delivered to the lung cells, it is translated into functional CFTR protein, restoring normal ion transport and thinning mucus viscosity, thus enhancing lung function and having the potential to stop disease progression. This mRNA delivery is a repeatable, but temporary, treatment, thus a safer option in comparison to gene therapy that is permanent [65,66]. The aim of the trial is to find out if this treatment is able to restore CFTR function effectively and reproducibly in patients, enhancing quality of life and disease symptoms.
These clinical trials demonstrate the applicability of mRNA therapeutics across a variety of disciplines, from cancer immunotherapy to tissue engineering and treatment of genetic disorders. Each trial demonstrates the capability of mRNA to provide customized, transient, and targeted protein expression that is relevant to the pathology of complicated diseases. With ongoing advances in delivery technologies and better formulations, mRNA therapies can be expected to transform the therapy paradigm for various diseases, with highly individualized and potent outcomes.
Challenges and opportunities in mRNA therapeutics development
The route to the development of mRNA therapeutics is more challenging than that for mRNA vaccines, especially owing to the need for greater protein production to achieve therapeutic levels [67,68]. The vaccines require minimal protein expression to stimulate the immune system, while therapeutics need to introduce much higher protein levels to target specific tissues or organs effectively. Efficient uptake in the target location therefore becomes pivotal if the intended therapeutic effect is to be delivered. The delivery itself still poses a severe bottleneck [69]. The liver can be accessed easily using intravenous injection, but this becomes more problematic when targeting solid organs. Lipid-based carrier tissue bioavailability and circulatory half-life may place constraints on effective delivery, modulating both protein expression efficiency as well as longevity [70]. The requirement for repeated dosing in chronic diseases adds to these challenges, as chronic mRNA dosing tends to induce innate immune responses that decrease therapeutic protein expression over time [71]. This problem continues even with optimized mRNA chemical modifications and sophisticated LNPs [72]. Attempts to overcome these challenges are aimed at enhancing LNP efficiency and specificity of delivery. Targeted delivery to organs other than the liver is a major area of development. Cutting-edge strategies such as enhanced tissue-specific tropism and lowered immune activation are essential for increasing the therapeutic application of mRNA. New technologies work toward increasing the stability of mRNA, prolonging therapeutic effect, and enhancing delivery system efficiency. While challenges persist, the changing dynamics of mRNA therapeutic research offer enormous promise to enhance therapies in enzyme replacement therapy, tissue repair, cancer therapy, gene editing, and more. By methodically overcoming these obstacles, mRNA therapies may transform disease treatments across the board into highly personalized, safe, and efficacious treatments [73].
Future directions
The potential of mRNA therapeutics in the future is vast and spans various areas of medicine. Further research into delivery systems, mRNA stability, and immunogenicity will broaden the scope of this technology. Beyond vaccines, mRNA therapeutics have the potential to transform cancer, genetic disease, cardiovascular disease, and other treatments. The flexibility and speed of mRNA-based therapies provide a promising future for personalized medicine and the rapid production of therapies for new diseases. Although challenges remain, the ongoing progress in the field is paving the way for a revolutionary future of healthcare.
Self-amplifying mRNA
Self-replicating mRNA is a new-generation breakthrough in mRNA technology. Unlike conventional mRNA, which requires relatively high doses to produce sufficient protein expression, saRNA also contains alphavirus sequences that enable the RNA to replicate itself once inside the host cell. This replication is achieved through RNA-dependent RNA polymerase, a viral enzyme encoded by the saRNA [74]. Therefore, saRNA must be administered in significantly reduced doses than with traditional mRNA, yet still with high protein expression. This characteristic is particularly valuable in vaccines and therapeutics, where reduced dosing could minimize the production cost, minimize side effects, and facilitate fast distribution during pandemics. For example, preclinical research on saRNA vaccines against influenza and respiratory syncytial virus has demonstrated that saRNA vaccines are capable of inducing strong immune responses using as little as a microgram of RNA saRNA vaccines against influenza and respiratory syncytial virus [75,76]. The technology is now also being investigated for cancer and other diseases when high protein yield is required to provide therapeutic benefits [77].
In vivo gene editing
The mRNA technology holds enormous potential for in vivo gene editing by the delivery of the factors needed for precise genetic repair. Specifically, mRNA has the ability to encode tools such as CRISPR-Cas9 and other nucleases that can be utilized to edit a specific gene sequence directly within the patient’s body [78]. Conventional gene therapy methods have depended on viral vectors for gene transfer, which carry safety risks, such as undesired immune reactions or insertional mutagenesis. mRNA delivery, however, is transient, non-integrative, and circumvents the long-term danger of viral vectors. By delivering mRNA that codes for CRISPR components, scientists can facilitate precise gene knockouts, insertions, or correction of mutations in targeted tissues. One of the main benefits of mRNA in gene editing is the capability to quickly change gene sequences in hard-to-transfect cells using conventional methods [79]. This would be extremely useful for single-gene mutation diseases, such as Duchenne muscular dystrophy, cystic fibrosis, and some types of inherited blindness [80]. Initial preclinical studies have demonstrated encouraging outcomes, and clinical trials are anticipated to follow as the technology develops.
Wider therapeutic applications
The applications of mRNA therapeutics are quickly broadening beyond cancer and vaccines, with research continuing to look into its application in autoimmune illnesses, metabolic diseases, and neurodegenerative diseases. mRNA can be engineered to code for proteins that regulate the immune system, and the doors open for new ways of treating diseases such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus. In autoimmune disorders, mRNA therapies may be able to instruct cells to produce tolerogenic proteins that reprogram the immune system to disregard the body’s own tissues so that the immune system will not attack itself. In the case of metabolic disorders like type 2 diabetes or lysosomal storage diseases, mRNA may be employed to code for deficient enzymes or hormones in these patients [81]. For instance, therapies using mRNA might be made to produce insulin or glucagon-like peptide-1 to modulate blood sugar levels in diabetic patients [82]. Likewise, in lysosomal storage diseases, mRNA may offer a way of generating functional enzymes to degrade cellular waste products. Finally, neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis may be helped by mRNA therapies that encode neuroprotective factors or proteins that increase synaptic function [83]. For example, mRNA may provide genes coding for proteins such as brain-derived neurotrophic factor (BDNF), which supports neuron function and survival, and is a candidate therapy for slowing or reversing neurodegeneration [84]. The changing scenario of mRNA therapeutics is driving research in diverse branches of medicine. Self-amplifying mRNA technology has the potential to lower dosing levels and increase protein expression, in vivo gene editing offers the promise of precise, transient gene correction, and broader therapeutic applications extend the reach of mRNA to treat autoimmune, metabolic, and neurodegenerative disorders. These developments have the potential to transform medicine by providing new, flexible, and potent treatments for some of the most difficult diseases.
Challenges and considerations in the clinical translation of mRNA therapeutics
Even with the promise of mRNA vaccines, various mRNA therapies have yielded disappointments during clinical trials. For example, CVnCoV for COVID-19 registered an efficacy of merely 47% in Phase 3 clinical trial, way below predictions, mainly attributed to the suboptimal design of mRNA and issues concerning delivery. On similar lines, other mRNA medicines have been axed owing to low efficacy or immunogenicity issues [85]. Therapeutic development in mRNA technologies is confronted with serious regulatory hurdles, especially outside of the vaccine space. Existing frameworks do not have well-defined criteria applicable to mRNA-based medicines, which makes the approval process challenging. Standardization, long-term safety assessment, and quality control issues further complicate things, rendering clinical translation slow and access difficult in many cases [86]. Long-term safety is a major concern for mRNA therapeutics, as their long-term impact on human health remains unclear. Some of the concerns are adverse immune responses, the risk of inducing autoimmune diseases, and off-target genetic changes. Ongoing clinical monitoring, as well as post-market surveillance, is necessary to allay these concerns and provide the long-term safety of mRNA-based therapies [87]. The scalability of mRNA therapeutics in low-income environments is threatened by high production costs, dependence on specialized infrastructure, and less access to advanced technology. Making production and distribution affordable and ensuring the development of sustainable supply chains is essential to enhancing availability in these areas. Partnering with global health organizations and investment in local production may help counteract such impediments [88]. In the field of mRNA medicines, redundancy and over-dosing are terms applied to the threats posed by chronic immune reactions and unwanted side effects that can develop when multiple injections are given or when different mRNA-based therapies are combined. They can undermine the efficacy of therapy, cause more side effects, and make it problematic to establish useful dosing schedules. It is necessary to design clinical trials cautiously with close monitoring to avoid these risks [88].
Recent developments in MicroRNA (miRNA) research
MicroRNA science has made notable progress in the past few years, especially in cancer treatment, neurodegenerative disorders, cardiovascular diseases, infectious disease treatment, regenerative medicine, and gene editing (Table 2). In cancer treatment, miRNA-targeting drugs like MRX34 and TargomiRs have been identified to address oncogenic signaling [89], while exosomal delivery of miRNAs is under investigation to increase the therapeutic effectiveness of pancreatic and breast cancers [90]. In addition, miRNA biomarkers such as miR-21, miR-155, and miR-200 are being integrated into liquid biopsy platforms for early cancer detection [91]. In neurodegenerative diseases, miRNAs such as miR-132, miR-124, and miR-155 play a key role in Alzheimer’s disease (AD) [92], while miR-7 is being studied for its neuroprotective effects in Parkinson’s disease [93]. Advances in LNPs and extracellular vesicles are further improving miRNA-based delivery systems for targeting neuronal disorders.
In cardiovascular diseases, miRNAs are being investigated for their roles in cardiac regeneration and angiogenesis [94], while circulating miRNAs like miR-1, miR-133, and miR-499 are emerging as biomarkers for early myocardial infarction detection. In infectious disease treatment, miR-122 inhibitors have shown promise in Hepatitis C Virus (HCV) therapy [95], and miRNA-based antiviral therapies for HIV and influenza are being developed to suppress viral replication [22]. Recent research on COVID-19 has also identified miRNA as potential therapeutic targets for modulating immune responses [96].
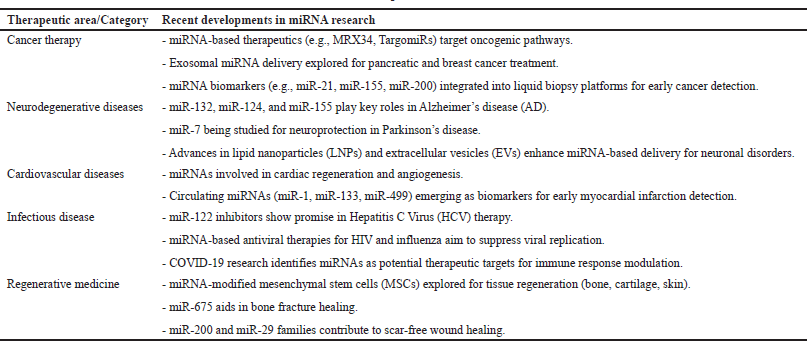 | Table 2. Recent developments in miRNA research. [Click here to view] |
In regenerative medicine, miRNA-modified mesenchymal stem cells are being explored for bone, cartilage, and skin tissue regeneration, with miR-675 showing promise in bone fracture healing and miR-200 and miR-29 families playing key roles in scar-free wound healing [97]. In addition, CRISPR-Cas9 gene editing is now integrating miRNAs to achieve tissue-specific gene modifications, with miR-CRISPR constructs being tested for tumor suppression in prostate and breast cancers. With continuous advancements in miRNA delivery systems, biomarker development, and precision medicine applications, miRNA-based therapies are rapidly evolving as a promising tool in modern medicine, holding immense potential for treating complex diseases with high specificity and minimal side effects.
CONCLUSION
The rise of mRNA therapeutics marks a transformative shift in modern medicine, extending beyond infectious disease prevention to applications in personalized cancer vaccines, gene editing, and regenerative medicine. The rapid success of mRNA-based COVID-19 vaccines has accelerated research into treatments for autoimmune and neurodegenerative disorders. While challenges such as delivery efficiency and immune modulation persist, continuous innovations are addressing these limitations. Likewise, miRNA-based therapies are becoming potent precision medicine tools, especially for neurodegenerative disease, cancer, and cardiovascular disease. Advances in delivery platforms, including lipid nanoparticles and exosomes, are enhancing therapeutic efficacy. As these technologies evolve, mRNA and miRNA therapeutics are poised to redefine treatment paradigms and revolutionize precision medicine.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors certify that they have not employed artificial intelligence (AI)-tools for writing and manuscript editing and no AI was used to edit images.
REFERENCES
1. Hossain R, Quispe C, Saikat ASM, Jain D, Habib A, Janmeda P, et al. Biosynthesis of secondary metabolites based on the regulation of MicroRNAs. Biomed Res Int. 2022;2022:9349897. CrossRef
2. Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Sadia H, et al. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021;21(1):388. CrossRef
3. Al-Kuraishy HM, Al-Gareeb AI, Butnariu M, Batiha GE. The crucial role of prolactin-lactogenic hormone in Covid-19. Mol Cell Biochem. 2022;477(5):1381–92. CrossRef
4. Shi Y, Shi M, Wang Y, You J. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduct Target Ther. 2024;9(1):322. CrossRef
5. Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106(13):5282–7. CrossRef
6. Desterro J, Bak-Gordon P, Carmo-Fonseca M. “Targeting mRNA processing as an anticancer strategy. Nat Rev Drug Discov. 2020;19(2):112–29. CrossRef
7. Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. CrossRef
8. Landesman-Milo D, Peer D. Altering the immune response with lipid-based nanoparticles. J Control Release. 2012;161(2):600–8 CrossRef
9. Stadler C, Bähr-Mahmud H, Celik L, Hebich B, Roth AS, Roth RP, et al. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat Med. 2017;23:815–7. CrossRef
10. Lemaire J, Mkannez G, Guerfali FZ, Gustin C, Attia H, Sghaier RM, et al. MicroRNA expression profile in human macrophages in response to Leishmania major infection. PLoS Negl Trop Dis. 2013;7(10):e2478. CrossRef
11. Parvin N, Joo SW, Mandal TK. Enhancing vaccine efficacy and stability: a review of the utilization of nanoparticles in mRNA vaccines. Biomolecules. 2024;14(8):1036. CrossRef
12. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. CrossRef
13. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. CrossRef
14. World Health Organization. COVID-19 vaccine tracker and landscape. Geneva, Switzerland: World Health Organization; 2023. Available from: https://www.who.int
15. CDC. COVID-19 Vaccinations in the United States. Atlanta, Georgia: CDC; 2023. Available from: https://www.cdc.gov
16. Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329–40. CrossRef
17. H? NT, Hughes SG, Ta VT, Phan LT, ?? Q, Nguy?n TV, et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat Commun. 2024;15(1):4081. CrossRef
18. REPROCELL News. eTheRNA-led international consortium starts preclinical studies of cross-strain protective COVID-19 mRNA vaccine for high risk populations. March 19, 2020. Available from: https://www.reprocell.com/news/covid-19-mrna-vaccine
19. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–8. CrossRef
20. Yamamoto A, Kormann M, Rosenecker J, Rudolph C. Current prospects for mRNA gene delivery. Eur J Pharm Biopharm. 2009;71(3):484–9. CrossRef
21. Pilkington EH, Suys EJA, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. CrossRef
22. Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–54. CrossRef
23. Wang Y, Ling L, Zhang Z, Marin-Lopez A. Current advances in Zika vaccine development. Vaccines (Basel). 2022;10(11):1816. CrossRef
24. Meisel A, Pascolo S. mRNA vaccines against infectious diseases and cancer. Heal TIMES Oncol Hematol. 2021;9(3):24–31. CrossRef
25. Noor R. Developmental status of the potential vaccines for the mitigation of the COVID-19 pandemic and a focus on the effectiveness of the Pfizer-BioNTech and Moderna mRNA vaccines. Curr Clin Microbiol Rep. 2021;8(3):178–85. CrossRef
26. Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther. 2022;7(1):166. CrossRef
27. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–30. CrossRef
28. Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–29. CrossRef
29. Besser H, Yunger S, Merhavi-Shoham E, Cohen CJ, Louzoun Y. Level of neo-epitope predecessor and mutation type determine T cell activation of MHC binding peptides. J Immunother Cancer. 2019;7(1):135. CrossRef
30. Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. CrossRef
31. He Q, Gao H, Tan D, Zhang H, Wang JZ. mRNA cancer vaccines: advances, trends and challenges. Acta Pharm Sin B. 2022;12(7):2969–89. CrossRef
32. Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124(3):453–62. CrossRef
33. Burris HA, Patel MR, Cho DC, Clarke JM, Gutierrez M, Zaks TZ, et al. A phase 1, open-label, multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with pembrolizumab in subjects with unresectable solid tumors (Keynote-603). J Cli Oncol. 2024;37(15_Suppl):2523. CrossRef
34. Shim K, Jo H, Jeoung D. “Cancer/Testis Antigens as targets for RNA-based anticancer therapy. Int J Mol Sci. 2023;24(19):14679. CrossRef
35. Rudge SR, Ladisch MR. Industrial challenges of recombinant proteins. Adv Biochem Eng Biotechnol. 2020;171:1–22. CrossRef
36. Almási T, Guey LT, Lukacs C, Csetneki K, Vokó Z, Zelei T. Systematic literature review and meta-analysis on the epidemiology of methylmalonic acidemia (MMA) with a focus on MMA caused by methylmalonyl-CoA mutase (mut) deficiency. Orphanet J Rare Dis. 2019;14(1):84. CrossRef
37. Jiang L, Berraondo P, Jericó D, Guey LT, Sampedro A, Frassetto A, et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018;24(12):1899–909. CrossRef
38. Hofmann L, Hose D, Grießhammer A, Blum R, Döring F, Dib-Hajj S, et al. Characterization of small fiber pathology in a mouse model of Fabry disease. Elife. 2018;7:e39300. CrossRef
39. Liou TG. The clinical biology of cystic fibrosis transmembrane regulator protein: its role and function in extrapulmonary disease. Chest. 2019;155(3):605–16. CrossRef
40. Rowe SM, Zuckerman JB, Dorgan D, Lascano J, McCoy K, Jain M, et al. Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study. J Cyst Fibros. 2023;22(4):656–64. CrossRef
41. Elangkovan N, Dickson G. Gene therapy for Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021;8(s2):S303–16. CrossRef
42. Shen G, Liu J, Yang H, Xie N, Yang Y. mRNA therapies: pioneering a new era in rare genetic disease treatment. J Control Release. 2024;369:696–721. CrossRef
43. Collén A, Bergenhem N, Carlsson L, Chien KR, Hoge S, Gan LM, et al. VEGFA mRNA for regenerative treatment of heart failure. Nat Rev Drug Discov. 2022;21(1):79–80. CrossRef
44. Finnson KW, Arany PR, Philip A. Transforming growth factor beta signaling in cutaneous wound healing: lessons learned from animal studies. Adv Wound Care. 2013;2(5):225–37. CrossRef
45. Leng Q, Chen L, Lv Y. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10(7):3190–205. CrossRef
46. Chabanovska O, Galow AM, David R, Lemcke H. Mrna—A game changer in regenerative medicine, cell-based therapy and reprogramming strategies. Adv Drug Deliv Rev. 2021;179:114002. CrossRef
47. Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81(11):678–99. CrossRef
48. Prieve MG, Harvie P, Monahan SD, Roy D, Li AG, Blevins TL, et al. Targeted mRNA therapy for ornithine transcarbamylase deficiency. Mol Ther. 2018;26(3):801–13. CrossRef
49. Bergsma AJ, In ‘t Groen SL, Verheijen FW, van der Ploeg AT, Pijnappel WWMP. From cryptic toward canonical Pre-mRNA splicing in pompe disease: a pipeline for the development of antisense oligonucleotides. Mol Ther Nucleic Acids. 2016;5(9):e361. CrossRef
50. August A, Attarwala HZ, Himansu S, Kalidindi S, Lu S, Pajon R, et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat Med. 2021;27(12):2224–33. CrossRef
51. Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8:14630. CrossRef
52. Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371(6525):145–53. CrossRef
53. Rajlic IL, Guglieri-Lopez B, Rangoonwala N, Ivaturi V, Van L, Mori S, et al. Translational kinetic-pharmacodynamics of mRNA-6231, an investigational mRNA therapeutic encoding mutein interleukin-2. CPT Pharmacometrics Syst. Pharmacol. 2024;13(6):1067–78. CrossRef
54. Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493–502. CrossRef
55. Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10(3):194–200. CrossRef
56. Leggat DJ, Cohen KW, Willis JR, Fulp WJ, deCamp AC, Kalyuzhniy O, et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science. 2022;378(6623):eadd6502. CrossRef
57. Hwang IY, Koh E, Kim HR, Yew WS, Chang MW. Reprogrammable microbial cell-based therapeutics against antibiotic-resistant bacteria. Drug Resist Updat. 2016;27:59–71. CrossRef
58. Liao HC, Liu SJ. Advances in nucleic acid-based cancer vaccines. J Biomed Sci. 2025;32(1):10. CrossRef
59. Shariati A, Khani P, Nasri F, Afkhami H, Khezrpour A, Kamrani S, et al. mRNA cancer vaccines from bench to bedside: a new era in cancer immunotherapy. Biomark Res. 2024;12(1):157. CrossRef
60. Montefiori DC, Roederer M, Morris L, Seaman MS. Neutralization tiers of HIV-1. Curr Opin HIV AIDS. 2018;13(2):128–36. CrossRef
61. Mu Z, Haynes BF, Cain DW. HIV mRNA vaccines-progress and future paths. Vaccines (Basel). 2021;9(2):134. CrossRef
62. Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines. 2021;6(1):50. CrossRef
63. Jones RP, Lee LYW, Corrie PG, Danson S, Vimalachandran D. Individualized cancer vaccines versus surveillance after adjuvant chemotherapy for surgically resected high-risk stage 2 and stage 3 colorectal cancer: protocol for a randomized trial. Br J Surg. 2023;110(12):1883–4. CrossRef
64. Lui KO, Zangi L, Silva EA, Bu L, Sahara M, Li RA, et al. Driving vascular endothelial cell fate of humanmultipotent Isl1 heart progenitors with VEGF modified mRNA. Cell Res. 2013;23:1172–86. CrossRef
65. Jaques R, Shakeel A, Hoyle C. Novel therapeutic approaches for the management of cystic fibrosis. Multidiscip Respir Med. 2020;15(1):690. CrossRef
66. Robinson E, MacDonald KD, Slaughter K, McKinney M, Patel S, Sun C, et al. Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther. 2018;26(8):2034–46. CrossRef
67. Dong Y, Anderson DG. Opportunities and challenges in mRNA therapeutics. Acc Chem Res. 2022;55(1):1. CrossRef
68. Zhang Y, Hu Y, Tian H, Chen X. Opportunities and challenges for mRNA delivery nanoplatforms. J Phys Chem Lett. 2022;13(5):1314–22. CrossRef
69. Zhong Z, Mc Cafferty S, Combes F, Huysmans H, De Temmerman J, Gitsels A, et al. mRNA therapeutics deliver a hopeful message. Nano Today. 2018;23:16–39. CrossRef
70. Zadory M, Lopez E, Babity S, Gravel SP, Brambilla D. Current knowledge on the tissue distribution of mRNA nanocarriers for therapeutic protein expression. Biomater Sci. 2022;10 (21):6077–115. CrossRef
71. Minnaert AK, Vanluchene H, Verbeke R, Lentacker I, De Smedt SC, Raemdonck K, et al. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: getting the message across. Adv Drug Deliv Rev. 2021;176:113900. CrossRef
72. Bernard MC, Bazin E, Petiot N, Lemdani K, Commandeur S, Verdelet C, et al. The impact of nucleoside base modification in mRNA vaccine is influenced by the chemistry of its lipid nanoparticle delivery system. Mol Ther Nucleic Acids. 2023;32:794–806. CrossRef
73. Tani H. Recent advances and prospects in RNA drug development. Int J Mol Sci. 2024;25(22):12284. CrossRef
74. Blakney AK, Ip S, Geall AJ. An Update on self-amplifying mRNA vaccine development. Vaccines (Basel). 2021;9(2):97. CrossRef
75. Zhou W, Jiang L, Liao S, Wu F, Yang G, Hou L, et al. Vaccines’ New Era-RNA Vaccine. Viruses. 2023;15(8):1760. CrossRef
76. Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28(3-4):117–29. CrossRef
77. Brito LA, Kommareddy S, Maione D, Uematsu Y, Giovani C, Berlanda Scorza F, et al. Self-amplifying mRNA vaccines. Adv Genet. 2015;89:179–233. CrossRef
78. Aledhari M, Rahouti M. Gene and RNA editing: methods, enabling technologies, applications, and future directions. arXiv preprint arXiv:2409.09057, 2024. CrossRef
79. Qamar M, Tanvir K, Akbar S, Ghani U, Ali H, Bilal M, et al. CRISPER-RNA Guided gene editing and implications in endogenous genes activation. Sch Int J Biochem. 2020;03(03):73–81. CrossRef
80. Salmaninejad A, Jafari Abarghan Y, Bozorg Qomi S, Bayat H, Yousefi M, Azhdari S, et al. Common therapeutic advances for Duchenne muscular dystrophy (DMD). Int J Neurosci. 2021;131(4):370–89. CrossRef
81. Magadum A. Modified mRNA therapeutics for heart diseases. Int J Mol Sci. 2022;23(24):15514. CrossRef
82. Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56(6):1671–9. CrossRef
83. Pena SA, Iyengar R, Eshraghi RS, Bencie N, Mittal J, Aljohani A, et al. Gene therapy for neurological disorders: challenges and recent advancements. J Drug Target. 2020;28(2):111–28. CrossRef
84. Wu Y, Rakotoarisoa M, Angelov B, Deng Y, Angelova A. Self-assembled nanoscale materials for neuronal regeneration: a focus on BDNF protein and nucleic acid biotherapeutic delivery. Nanomaterials (Basel). 2022;12(13):2267. CrossRef
85. Dolgin E. CureVac COVID vaccine letdown spotlights mRNA design challenges. Nat Biotechnol. 2021;39:810–1. CrossRef
86. Verbeke R., Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2021;28:100766. CrossRef
87. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. CrossRef
88. Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–6. CrossRef
89. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22. CrossRef
90. Akilov OE, Kosaka S, O’Riordan K, Hasan T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp Dermatol. 2007;16(8):651–60. CrossRef
91. O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104(5):1604–9. CrossRef
92. Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495–500. CrossRef
93. Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–7. CrossRef
94. Butterworth MB. Role of microRNAs in aldosterone signaling. Curr Opin Nephrol Hypertens. 2018;27(5):390–4. CrossRef
95. Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94. CrossRef
96. Farshbaf A, Mohtasham N, Zare R, Mohajertehran F, Rezaee SA. Potential therapeutic approaches of microRNAs for COVID-19: challenges and opportunities. J Oral Biol Craniofac Res. 2021;11(2):132–7. CrossRef
97. Lei Y, Chen L, Zhang G, Shan A, Ye C, Liang B, et al. MicroRNAs target the Wnt/β?catenin signaling pathway to regulate epithelial?mesenchymal transition in cancer (Review). Oncol Rep. 2020;44(4):1299–313. CrossRef