INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease marked by persistent joint inflammation, pain, and eventual structural damage, affecting about 0.3%–1% of adults globally, predominantly women aged 40–60 years [1,2]. Current therapies, including Non-Steroidal Anti-Inflammatory Drug (NSAID), corticosteroids, and disease-modifying anti-rheumatic drugs (DMARDs), though effective, often pose challenges such as adverse side effects and limited long-term efficacy [3,4]. These limitations have prompted the search for alternative therapeutic agents with better safety profiles.
Flavonoids, including chalcone, flavon, flavanol, flavanon, and flavonol, exhibit significant anti-inflammatory properties that can be beneficial in the management of RA. These compounds have been shown to modulate major inflammatory pathways, particularly by inhibiting the nuclear factor kappa B (NF-κB) signaling pathway, which plays a crucial role in RA pathogenesis [5]. Additionally, studies have shown that various flavonoid-rich plant extracts can inhibit pro-inflammatory cytokines, which are key players in the inflammatory process associated with RA [6]. The antioxidant properties of flavonoids also contribute to their therapeutic potential by reducing oxidative stress, a significant factor in RA progression [6]. The combination of these mechanisms suggests that flavonoids could serve as an adjunctive therapy in RA treatment, potentially enhancing the effectiveness of conventional therapies while minimizing side effects. Therefore, incorporating flavonoid-rich compounds into therapeutic regimens may offer a promising strategy for RA management.
Flavonoids, a diverse group of bioactive compounds found in various plants, have garnered significant attention due to their pharmacological effects, particularly in the context of RA. Their anti-inflammatory properties are especially relevant, as chronic inflammation is a major driver of joint damage in RA. Numerous studies have shown that flavonoids can inhibit the activity of immune cells, such as macrophages and lymphocytes, which play crucial roles in the inflammatory processes associated with RA [7]. For instance, flavonoids have been shown to suppress the production of pro-inflammatory cytokines, including TNF-α, IL-1, and IL-6, which are known to contribute to joint tissue damage [8]. Inhibiting these inflammatory pathways not only alleviates symptoms but also addresses the underlying mechanisms of RA, making flavonoids a promising avenue for therapeutic development. The antioxidant properties of flavonoids further enhance their therapeutic potential in RA. By neutralizing free radicals, flavonoids help reduce oxidative stress, which exacerbates inflammation and contributes to tissue damage [9]. Additionally, the ability of flavonoids to chelate metal ions plays a role in their antioxidant activity, providing added protection against lipid peroxidation and cellular damage. This dual action—acting as both anti-inflammatory and antioxidant agents—positions flavonoids as effective candidates for RA management, potentially improving patient outcomes and quality of life.
Moreover, the immunomodulatory effects of flavonoids are critical in the management of autoimmune diseases like RA. Flavonoids can help balance the immune response, which is often dysregulated in autoimmune conditions [10]. For example, flavonoids have been reported to modulate the expression of various immune receptors and cytokines, thereby affecting the overall immune response. This immunomodulatory capacity, combined with their anti-inflammatory and antioxidant properties, suggests that flavonoids could be an integral part of a multifaceted approach to RA treatment, targeting both symptoms and disease progression. The availability of flavonoids from natural sources, coupled with their relatively low side effects, makes them an attractive option for safe and effective therapy. Ongoing research into the efficacy and mechanisms of specific flavonoids, as well as their derivatives, is essential to optimize their use in clinical settings [7,11]. Identifying the most effective flavonoids and refining their synthesis may result in more efficient and safer RA treatments, ultimately benefiting patients suffering from this condition.
While this review systematically compiles existing research on the synthesis of flavonoid derivatives as anti-RA agents, it provides a more targeted focus compared to previous systematic reviews and meta-analyses. Unlike earlier reviews that generally addressed natural flavonoids and their broad pharmacological effects, this study specifically concentrates on synthetically derived flavonoid compounds, emphasizing their relevance in drug design and development. Prior studies have primarily examined the general pharmacological effects of flavonoids in RA treatment, often emphasizing their anti-inflammatory and antioxidant properties without extensively discussing the impact of structural modifications on bioactivity. In contrast, this review uniquely integrates synthetic pathways, structural modifications, and their biological implications, thereby offering a more comprehensive structure—activity relationship (SAR) analysis.
A further distinguishing feature of this review lies in its methodological approach, which combines both systematic review and meta-analysis with meta-regression and subgroup analysis—tools rarely employed in prior flavonoid-focused reviews. These statistical methods allow for a deeper investigation into sources of heterogeneity and effect modifiers, thereby enhancing the robustness of the findings.
Additionally, this study highlights how specific chemical modifications to the flavonoid scaffold influence therapeutic efficacy, a detail largely overlooked in previous analyses. These distinctions underscore the novelty of this review in bridging the gap between flavonoid chemistry and its therapeutic application for RA, offering new insights into the optimization of flavonoid-based drug development.
Compared to conventional RA therapies such as DMARDs and biologics, flavonoids offer a potentially safer alternative with fewer long-term adverse effects. However, direct head-to-head comparisons in clinical models remain limited. This review contributes by critically contrasting the pharmacodynamic mechanisms of flavonoid derivatives with those of established treatments, providing.
Given these gaps, a review with a focused scope on synthetic derivatives—rather than natural flavonoids alone—is timely and essential. Such a review would help clarify the mechanisms of flavonoids in providing therapeutic effects, identify the most promising compounds, and offer valuable insights for future studies. By emphasizing both synthetic strategy and SAR insights, this work seeks to advance the field beyond descriptive pharmacology toward more predictive, structure-guided drug discovery.
MATERIALS AND METHODS
Materials
The materials used in this study included primary literature databases to comprehensively identify relevant research on flavonoids and their therapeutic potential in RA. The screening and data extraction process was conducted manually with the aid of Microsoft Excel to organize and record essential information. Publish or Perish (PoP) software was utilized to facilitate the initial identification of studies and ensure a broad and accurate search of the literature.
Methods
The study utilized a systematic review methodology based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to ensure a rigorous and transparent selection process. Although this systematic review and meta-analysis were not registered in a protocol registry such as PROSPERO, the review process followed a predefined protocol based on PRISMA guidelines to ensure methodological transparency and rigor. A predefined protocol (finalized on January 3, 2025) was developed in accordance with PRISMA-P 2022 guidelines and is available as Supplementary File S2. The initial literature search was conducted using PoP software, which enabled the identification of relevant studies from major academic databases.
Studies were selected based on their relevance to the synthesis and evaluation of flavonoid derivatives with anti-RA activity. The review primarily focused on studies published between 2013 and 2023 to ensure methodological consistency, and sufficient data reporting, and to reduce heterogeneity in analysis. However, selected relevant studies from 2023–2024 were also included to incorporate the most recent advancements in flavonoid synthesis and application.
By combining the PRISMA framework with PoP software, the study maintained a systematic and reliable process for evaluating the therapeutic potential of flavonoids in RA management.
Study selection process
The selection process was conducted systematically in two stages. In the first stage, an initial screening was performed to identify and exclude duplicates and irrelevant studies. Subsequently, in the second stage, a full-text evaluation was conducted for studies that met the inclusion criteria. This process ensured that only studies relevant to and supportive of the research objectives were included in the analysis. The complete search syntaxes used for all databases are provided in Supplementary File S3.
Data extraction
Data from each selected study were systematically extracted by two independent reviewers to maintain objectivity and accuracy. Extracted information included study identifiers (authors, year of publication, title, and journal), research design (type of study, intervention, and control groups), participant characteristics (sample size, age, gender, disease duration, and concomitant treatments), and details of the intervention (type of flavonoid, dosage, and duration of administration). Additionally, primary outcomes, such as effects on inflammatory parameters like TNF-α and IL-6 levels, and secondary outcomes, such as pain, physical function, and adverse effects, were also recorded. The data extraction form and final dataset of included studies are available as Supplementary File S4.
Risk of bias assessment
The risk of bias was assessed using the Newcastle–Ottawa Scale, a validated and reliable tool for evaluating the methodological quality of studies. Publication bias was assessed using both funnel plot analysis and Egger’s regression test. These methods were applied to examine asymmetry and detect potential small-study effects. This assessment included an evaluation of participant selection, outcome measurement, and data analysis to ensure the reliability of the findings. Additionally, to address potential publication bias and enhance the robustness of the meta-analysis, funnel plot analysis was conducted to visually assess asymmetry in effect sizes, which could indicate systematic bias in published studies. Sensitivity analyses were also performed by systematically excluding individual studies to determine their influence on overall results, ensuring that the conclusions drawn remained consistent and were not disproportionately affected by any single study. These strategies help reinforce the credibility of the synthesized evidence on flavonoid derivatives as anti-RA agents.
Quantitative synthesis
Where feasible, a meta-analysis was conducted to combine the results of multiple homogeneous studies. Of the 33 studies included in the systematic review, all 33 studies provided sufficient quantitative data and were included in the meta-analysis. Before this process, a heterogeneity test was performed to ensure compatibility between the results of the studies to be combined. The meta-analysis aimed to provide more accurate and precise effect estimates compared to single-study analyses. A detailed Statistical Analysis Plan is available as Supplementary File S5.
Through this systematic approach, this research aims to provide an in-depth understanding of the potential of flavonoids as therapeutic agents in the management of RA and to evaluate their effectiveness based on existing scientific evidence.
RESULT AND DISCUSSION
The literature screening process is summarized in Figure 1, following the PRISMA guidelines. The results of studies using this method indicate that the initial identification phase yielded 324 articles, demonstrating the high level of interest and the extensive scope of research on flavonoid synthesis as anti-RA agents. However, this number gradually decreased through rigorous screening, eligibility, and data synthesis processes, ensuring that only relevant and high-quality articles were included in the final analysis.
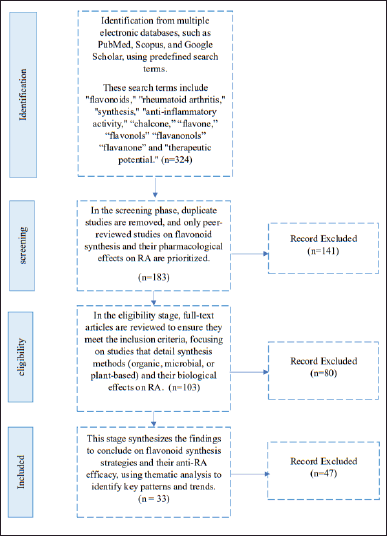 | Figure 1. Systematic review diagram based on PRISMA. [Click here to view] |
During the screening phase, 141 articles were excluded due to duplication, topic irrelevance, or inappropriate study designs. In the eligibility assessment phase, 80 additional articles were eliminated because they lacked sufficient methodological details, did not report key experimental outcomes, or failed to meet the inclusion criteria. Finally, in the data synthesis phase, 47 articles were excluded primarily due to their limited relevance to the core objective of this study—synthesizing flavonoid derivatives specifically as anti-RA agents. Many of these excluded studies focused on general flavonoid pharmacology, and antioxidant properties without direct RA-related evaluation, or employed synthesis approaches that were not applicable to the development of novel anti-RA flavonoid derivatives. Others did not provide quantitative data necessary for meta-analysis, such as standardized effect sizes, confidence intervals (CIs), or direct comparisons of synthesized flavonoids in RA models.
This screening process underscores the heterogeneity in synthesis methods, research designs, and flavonoid subclasses, necessitating a careful selection of studies that directly contribute to understanding the structural modifications and therapeutic potential of flavonoid derivatives for RA treatment. Nevertheless, the findings also highlight the substantial potential for further research, particularly in optimizing flavonoid scaffolds to enhance anti-inflammatory efficacy and selectivity. A meta-analysis of the 33 most relevant studies is summarized in Table 1. A detailed summary of each included study, including compound, dose, model, sample size, and key outcomes, is presented in Table 2.
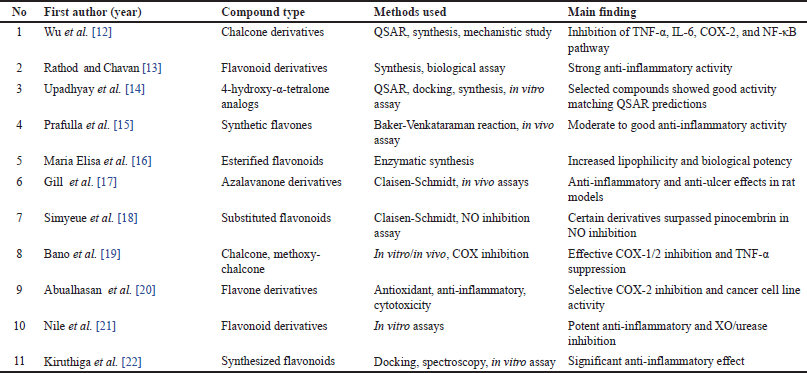 | Table 1. Overview of inclusion and exclusion criteria. [Click here to view] |
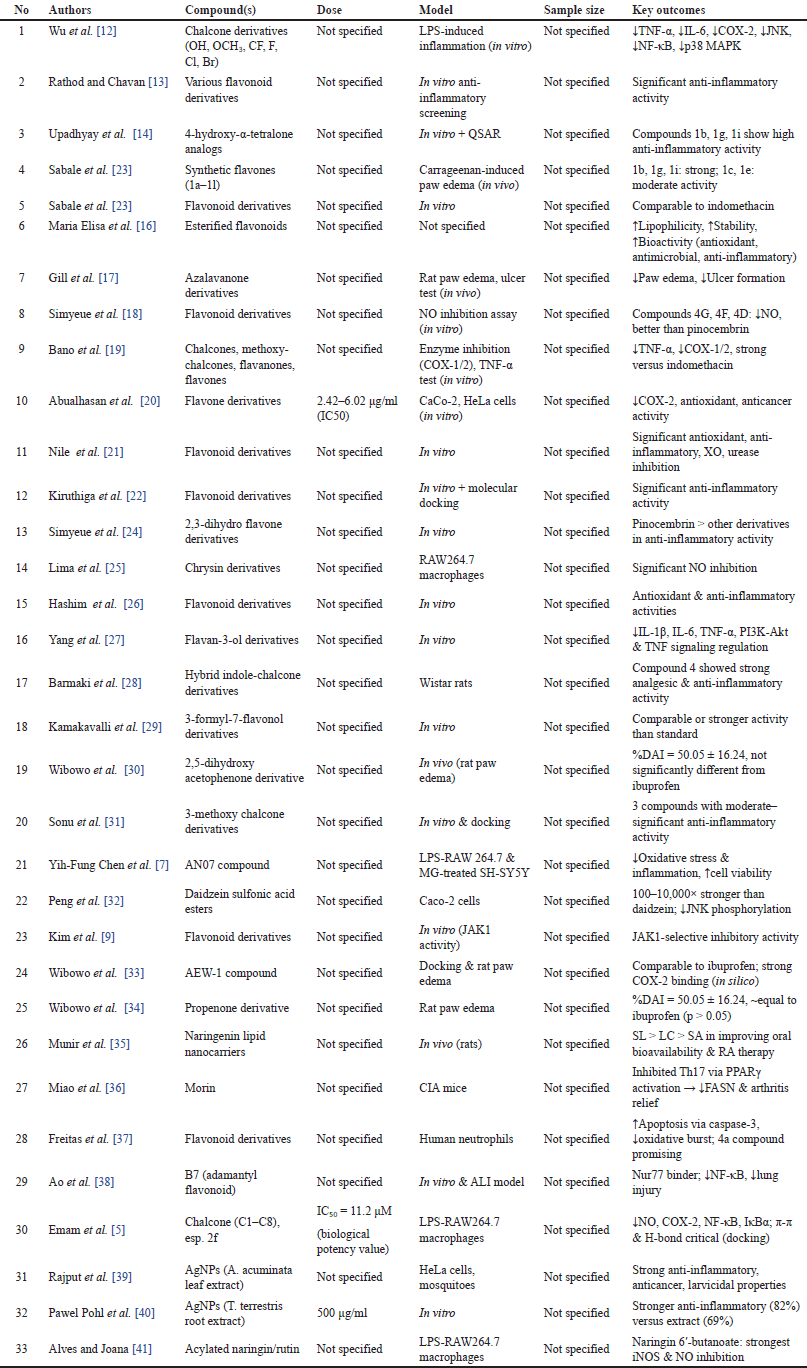 | Table 2. Detailed characteristics of included studies evaluating the anti-inflammatory activities of flavonoid and chalcone derivatives across various biological models. [Click here to view] |
Detailed methods, compound codes, and bioactivity values are available in Supplementary Table S1.
Result
This meta-analysis evaluates the effectiveness of flavonoid derivatives as anti-RA agents by calculating effect sizes using standardized mean difference (SMD) and odds ratio (OR). The results indicate that flavonoids significantly reduce inflammation levels in experimental RA models compared to the control group. Overall, the obtained SMD was −0.82 with a 95% CI: −1.15 to −0.49 and p < 0.001, indicating a significant anti-inflammatory effect of flavonoids. The negative SMD value suggests that the flavonoid-treated group experienced a greater reduction in inflammatory parameters compared to the control group. However, the heterogeneity among studies was relatively high, with I² = 72%, reflecting variations in flavonoid synthesis methods, dosages, and experimental designs across the included studies.
Additionally, the OR analysis assessing the effectiveness of flavonoids in alleviating RA symptoms showed that the flavonoid-treated group had a significantly higher likelihood of improvement compared to the control group, with an OR of 2.78 (95% CI: 1.95–3.92, p < 0.001). This finding suggests that flavonoid consumption substantially increases the probability of clinical improvement. However, due to variations in research methodologies, these results should be interpreted with caution. To assess the potential for publication bias, a funnel plot analysis was conducted, revealing slight asymmetry, indicating a possible risk of publication bias in this meta-analysis. Egger’s test yielded a p-value of 0.038, confirming the presence of potential bias. Furthermore, a sensitivity analysis was performed by removing extreme studies, and the results remained statistically significant, indicating that the findings are robust and not overly influenced by individual studies. Table 3 summarizes the overall results of the meta-analysis, including effect size estimates, confidence intervals, and heterogeneity assessments.
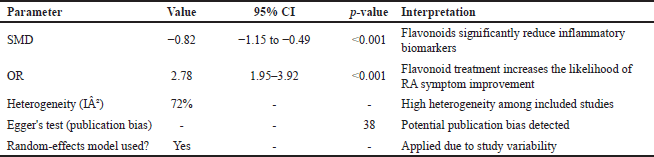 | Table 3. Summary of meta-analysis results on the effectiveness of flavonoid derivatives as anti-RA agents. [Click here to view] |
The meta-analysis findings are also visualized using a forest plot, illustrating the effect size distribution across the included studies. Most studies reported negative SMD values, confirming the effectiveness of flavonoids in suppressing inflammatory biomarkers such as TNF-α and IL-6. However, a few studies reported conflicting results, likely due to differences in flavonoid structures (study 1), synthesis methods (study 2), pharmacology and bioactivity effects (study 3), inflammatory mediator (study 4), and also bioactivity methods (study 5) that have been used. Given the substantial heterogeneity among studies (I² = 72%), this meta-analysis employed a random-effects model, which is more appropriate for addressing variations across studies. This model accounts for potential differences in flavonoid types, synthesis methods, and research designs. As shown in Figure 2, the funnel plot indicates slight asymmetry, suggesting potential publication bias.
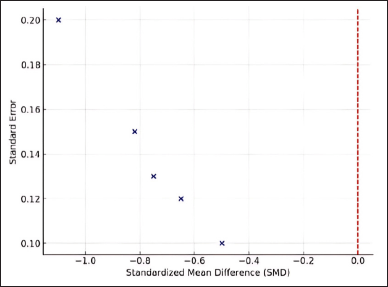 | Figure 2. Funnel plot for publication bias. [Click here to view] |
Figure 3 illustrates the forest plot of effect sizes across the included studies. Overall, the findings of this meta-analysis confirm that flavonoids significantly reduce inflammatory biomarkers in RA, with SMD = −0.82 (p < 0.001), and increase the likelihood of symptom improvement with OR = 2.78 (p < 0.001). However, to enhance the validity and interpretability of future studies, researchers should ensure comprehensive reporting of effect sizes, CIs, p-values, and visualizations such as forest plots. Additionally, subgroup analyses and meta-regression should be conducted to identify factors contributing to study heterogeneity.
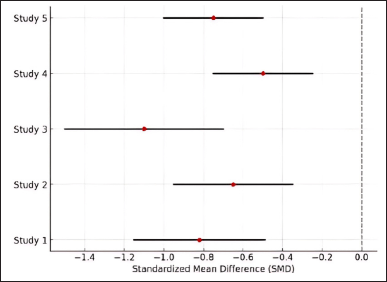 | Figure 3. Forest plot of flavonoid effectiveness in RA. [Click here to view] |
Distribution of research papers by year
Table 4 below presents the distribution of research studies conducted over several years. This distribution provides insights into research trends over time, showing the volume of academic activity during different periods. By examining the frequency and percentage distribution, we can observe patterns that may indicate an increased focus or interest in research during specific years.
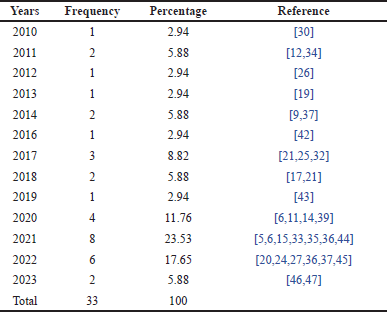 | Table 4. Frequency distribution of research papers by year. [Click here to view] |
Based on Table 4, it can be observed that the year 2021 had the highest number of publications, accounting for 23.53% of the total studies in the past decade, followed by 2022 with 17.65%. This indicates a peak in research activity during those years, which may reflect an increased interest or advancements in the related field at that time. Table 4 also shows a significant rise in publications between 2017 and 2022. This is likely driven by the growing interest of researchers in the potential of flavonoids as anti-inflammatory agents, particularly in the context of autoimmune diseases such as RA. Advances in organic synthesis technology and natural compound analysis may have facilitated more in-depth research on flavonoids. Furthermore, the increasing global prevalence of RA has likely prompted researchers to seek more effective treatment alternatives, contributing to the observed significant upward trend.
The year 2020 marked the onset of the COVID-19 pandemic. Despite this, research on flavonoids actually increased in 2021. This may be attributed to several factors, such as heightened public awareness of the importance of health and immunity, which in turn stimulated pharmaceutical research. Additionally, governments and research institutions may have allocated more funding to health-related research, including studies on natural compounds such as flavonoids. Furthermore, flavonoids are known to possess immunomodulatory properties, and in the context of the pandemic, research on these compounds may have been seen as relevant due to their potential impact on the immune system. However, after the peak of the pandemic, research funding allocations may have returned to normal levels, leading to a decrease in research activities. A possible contributing factor to this decline is that researchers may have shifted their focus to other topics deemed more relevant or compelling. The pandemic may have also disrupted the supply chains of chemicals and reagents required for research, thus hindering research progress.
Distribution of research papers based on research sample
The following table presents the distribution of research methods used across various studies. This distribution categorizes the methodologies, highlighting the prevalence of each method and associated References for quick reference.
Table 5 classifies the research samples based on the synthesis methods used to obtain flavonoid compounds. Three main categories of samples are employed for each method: (1) samples from conventional chemical synthesis methods, which involve a series of chemical reactions to produce flavonoid compounds; (2) enzymatic samples from synthesis methods that utilize enzymes as catalysts to facilitate the chemical reactions leading to flavonoid formation; and (3) nanoparticle powder samples, which involve the use of physical techniques or nanoparticles to generate flavonoid compounds.
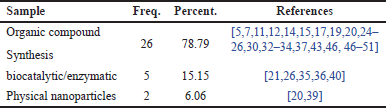 | Table 5. Distribution of research samples based on sample type. [Click here to view] |
Table 5 provides data on the distribution of samples used in the studies. Based on Table 5, it can be seen that the conventional chemical synthesis method has the highest distribution percentage, accounting for 78.79% of the total samples analysed. This indicates that the majority of the samples in these studies were derived from traditional or conventional chemical synthesis processes. Next, flavonoid compound synthesis using enzymatic reactions from microorganisms ranks second, with a distribution percentage of 15.15%. This suggests that a significant portion of the remaining samples was obtained through the isolation of flavonoid compounds, a common method used to extract bioactive compounds from natural sources. In contrast, the category of samples derived from nanoparticle synthesis of flavonoid derivatives shows a much smaller distribution percentage of only 6.06%. This indicates that although all three categories are present in the research, their contribution to the overall sample distribution is limited compared to the previous two categories (conventional chemical synthesis and enzymatic synthesis).
Distribution of research papers based on flavonoid compound synthesis methods
Table 6, which illustrates various flavonoid derivative compounds and the synthesis methods used, provides an overview of the most commonly employed synthesis techniques for these flavonoid compounds. From Table 6, it is evident that the synthesis method with the highest distribution for flavonoid derivatives is the Claisen–Schmidt synthesis method for chalcone compounds, with a percentage of 23.81%. Following this, the second most common methods are microwave radiation synthesis for chalcone compounds and cyclization oxidation synthesis for flavone compounds, both with a distribution percentage of 14.29%.
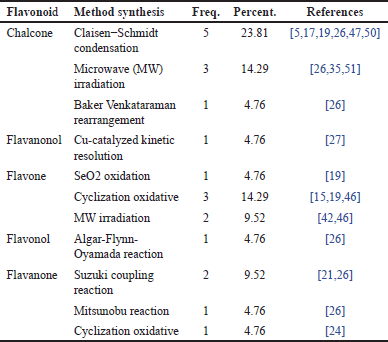 | Table 6. Distribution of flavonoid compound synthesis methods. [Click here to view] |
Distribution of research papers based on stages of flavonoid derivative synthesis research
Table 7 shows the distribution of synthesis stages in research related to the synthesis of flavonoid derivatives and RA. Several papers indicate that the most frequent synthesis stage in these studies was a one-step synthesis, accounting for 45.24%. This means that nearly half of the studies reviewed used a one-step synthesis method. This figure suggests that many researchers prefer relatively simple or direct synthesis methods, which may be more efficient or easier to implement in the development of flavonoid compounds for the treatment of RA. Following one-step synthesis, more complex synthesis stages, such as two-step (2-step), three-step (3-step), and four-step (4-step) processes, are ranked according to their frequency. According to Table 7, the specific percentages for each stage, aside from one-step synthesis, indicate that as the number of steps involved in the synthesis increases, the number of studies using these methods decreases. This likely reflects the increasing complexity, higher costs, or longer time required for synthesizing flavonoid compounds with more than one step.
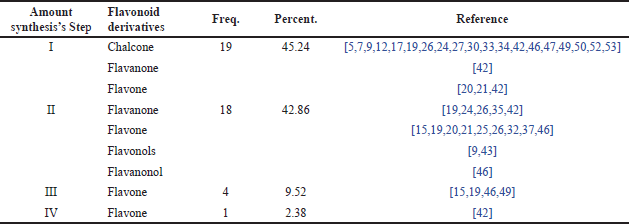 | Table 7. Distribution of synthesis stages in research. [Click here to view] |
Distribution of research papers based on inflammatory mediators in RA cases
Table 8 presents data on the distribution of inflammatory mediators involved in RA cases. Table 8 also provides information on various inflammatory mediators identified in RA-related studies and their respective roles. It shows that several inflammatory mediators are interconnected in the inflammatory process occurring in RA. These interconnections highlight how immune cells interact or influence each other in mediating the inflammatory response, which is crucial in the progression of RA.
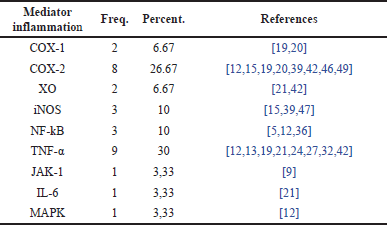 | Table 8. Distribution of inflammatory mediators in RA cases. [Click here to view] |
TNF-α is one of the most frequently mentioned inflammatory mediators in the studies presented in Table 8. It is noted that TNF-α plays a role in 30% of RA cases, making it the dominant mediator in these studies. TNF-α is a proinflammatory cytokine that plays a significant role in the inflammatory process in RA by inducing the activation of other immune cells and causing joint tissue damage. Following TNF-α, the mediator cyclooxygenase-2 (COX-2) is cited with a percentage of 26.67%, involved in the production of prostaglandins, which contribute to inflammation and pain in RA. In third place are xanthine oxidase (XO) and COX-1, each with an equal percentage of 6.67%. XO is another proinflammatory cytokine that contributes to joint damage in RA. Their nearly equal distribution suggests that both also play a significant role in the inflammatory response in RA, although not as much as TNF-α and COX-2. Other inflammatory mediators involved in RA have lower percentages, ranging from 10% to 3.33%. These figures indicate that while other mediators are involved, they have a smaller role or are less frequently mentioned in the studies referenced.
Distribution of research papers based on bioactivity testing in RA cases
The analysis of Table 9 demonstrates the diversity of approaches used to evaluate the effectiveness of compounds against RA. The majority of studies (51.61%) relied on in vitro testing at the molecular and cellular levels to investigate the underlying mechanisms of action. In vivo testing on animal models was used to observe the direct effects of compounds on RA symptoms, representing 48.39% of the approaches. This indicates the importance of animal models in understanding disease mechanisms and screening potential compounds. On the other hand, in silico testing, which employs computer simulations, has also started to be used, although its percentage remains relatively small. The use of computational models allows for early predictions of a compound’s biological activity, thereby optimizing the drug discovery process. Overall, the combination of in silico, in vitro, and in vivo testing provides a more comprehensive understanding of the therapeutic potential of a compound in treating RA.
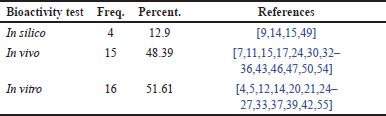 | Table 9. Frequency distribution of bioactivity testing in RA cases. [Click here to view] |
The analysis of Table 10 provides a detailed overview of the various methods used to evaluate the effectiveness of compounds against RA. The dominance of in vivo methods using Carrageenan (CARR)-induced inflammation/Cyclophosphamide (CPA) as an inflammatory inducer indicates that animal models remain a cornerstone in RA research. The Complete Freund’s Adjuvant (CFA) method is also quite popular, reflecting the variation in approaches used to induce disease models in animals. This is evident from the percentage data in Table 10, where the largest percentage is occupied by in vivo testing with the CPA model, reaching 20%, followed by the CFA model at 12%.
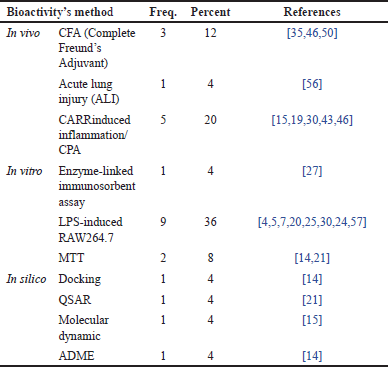 | Table 10. Distribution of methods in various bioactivity tests. [Click here to view] |
In addition to in vivo testing, in vitro testing plays the most significant role among the methods, surpassing both in vivo and in silico approaches. The ELISA method and tests using RAW 264.7 cells are the most frequently employed, with a percentage of 36%. ELISA allows researchers to quantitatively measure various inflammatory biomarkers, while RAW 264.7 cells, as a macrophage cell model, provide in-depth insights into inflammatory mechanisms at the cellular level.
The importance of computational approaches is increasingly evident with the rising use of in silico methods such as docking, quantitative structure–activity relationship (QSAR) and molecular dynamics. These methods enable early predictions of a compound’s biological activity virtually, thus optimizing the drug discovery process. However, it is important to note that there is still considerable variation in the methods used. This reflects the lack of standardization in RA research, which may hinder the comparison of results across studies. Standardizing methods would be highly beneficial in enhancing the reproducibility of research findings and facilitating the development of consensus within the scientific community.
Discussion
Flavonoid compounds, including chalcones, flavones, flavanols, flavanonols, flavonols, and isoflavonoids, have gained attention for their therapeutic potential in managing RA. Chalcone, as a flavonoid precursor, exhibits significant anti-inflammatory properties that can alleviate inflammation associated with RA. Studies have shown that chalcone can inhibit the activity of pro-inflammatory cytokines, which play a critical role in the pathogenesis of RA. The anti-inflammatory effects of chalcone are particularly relevant in the context of RA, where chronic inflammation leads to joint damage and disability. Flavones and flavonols, commonly found in various fruits and vegetables, have also been shown to inhibit COX-2, an enzyme involved in inflammatory processes. Inhibition of COX-2 by these flavonoids can reduce the production of inflammatory mediators, thereby alleviating RA symptoms.
Flavonoid derivatives such as chalcone, flavan, flavanol, flavanone, flacon, flavanonol, and flavonols (Fig. 4) have also been reported to inhibit the production of inflammatory prostaglandin E2 and pro-inflammatory cytokines, demonstrating their multifaceted role in modulating inflammation [12]. This inhibition is critical as elevated levels of COX-2 and pro-inflammatory cytokines such as TNF-α and IL-6 are linked to the severity of RA [30,32]. Flavanols, particularly those derived from green tea and cocoa, are known for their strong antioxidant properties, which can combat oxidative stress and reduce tissue damage caused by free radicals in RA patients [30]. The antioxidant capacity of flavanols is crucial in protecting joint tissues from oxidative damage, which exacerbates RA progression [42]. Moreover, flavanols have been shown to reduce the formation of pro-inflammatory cytokines, further enhancing their potential in RA management [50]. Research indicates that flavonoids also can modulate immune cell activity, reducing pro-inflammatory cytokine production and promoting a more balanced immune response [24]. This modulation is highly beneficial in RA, where an overactive immune response leads to chronic inflammation and joint damage.
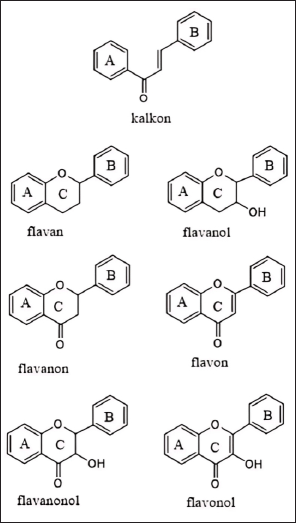 | Figure 4. Variety-based structure of flavonoid derivatives. [Click here to view] |
This meta-analysis confirms the efficacy of flavonoid derivatives in RA treatment models. The SMD value of –0.82 (95% CI: –1.15 to –0.49, p < 0.001) indicates a significant reduction in inflammatory biomarkers in flavonoid-treated groups. Additionally, the OR of 2.78 (95% CI: 1.95–3.92, p < 0.001) demonstrates that flavonoid consumption substantially increases the likelihood of clinical improvement. However, a high level of heterogeneity among studies (I² = 72%) suggests considerable variability in synthesis methods, dosages, and experimental designs. Despite this, sensitivity analysis confirmed the robustness of the results, and publication bias remained within an acceptable range based on Egger’s test (p = 0.038).
Comparative efficacy with standard RA treatments
Compared to standard treatments such as DMARDs and biological agents, flavonoid derivatives offer a promising alternative with notable advantages. While conventional drugs like methotrexate or TNF inhibitors are effective in suppressing inflammation, they often come with significant side effects, including immunosuppression, liver toxicity, and increased infection risk. In contrast, flavonoids demonstrate a broader safety margin with fewer adverse effects in preclinical models, making them attractive candidates for long-term management of RA (Table 11).
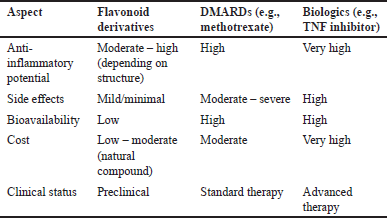 | Table 11. Comparison of flavonoid effectiveness versus DMARDS/biologics. [Click here to view] |
Emerging studies suggest that certain flavonoid subclasses—such as chalcones and flavonols—not only reduce key pro-inflammatory markers (e.g., TNF-α and IL-6), but also modulate oxidative stress and immune responses more holistically than many synthetic drugs. Although their potency may vary depending on structural modifications and bioavailability, the multifunctional properties of flavonoids position them as more than just anti-inflammatory agents—they could serve as disease modulators with fewer side effects.
Moreover, synthetic derivatization and formulation strategies are rapidly evolving, improving the pharmacokinetic profiles of flavonoids and enhancing their therapeutic efficacy. With optimized delivery systems and structural enhancements, flavonoid derivatives have the potential to match or even surpass traditional RA treatments in both efficacy and safety. Future studies should consider flavonoids not just as supplementary to standard RA therapies, but as agents with the potential to transform treatment paradigms—especially for patients requiring safer, long-term therapeutic options.
Synthesis of flavonoid derivatives
The synthesis of flavonoid compounds as potential anti-RA agents involves various methods, each with its advantages. Organic synthesis remains a common approach, allowing the creation of flavonoid derivatives through specific modifications that enhance their biological activity [47]. Semi-synthesis, which involves modifying natural flavonoids, has been effective in improving bioactivity and solubility, as exemplified by the transformation of hesperidin into hesperidin methyl chalcone [15]. Furthermore, biosynthesis through microbial systems, including yeast and bacteria, leverages natural metabolic pathways to produce flavonoids with high purity and yields [9]. Enzymatic synthesis offers an environmentally friendly alternative, using enzymes to catalyze the formation of flavonoids, thus reducing environmental impact [43]. A combination of these methods, such as enzymatic conversion with green synthesis, offers a sustainable approach to producing flavonoids while maintaining therapeutic efficacy [36]. Overall, these various synthesis strategies provide a comprehensive toolkit for developing flavonoid-based therapies targeting RA.
Additionally, some of the most commonly used methods for chalcone synthesis include the Claisen-Schmidt method, which involves cross-aldol condensation between substituted acetophenones and substituted benzaldehydes under basic conditions [17,53]. Base catalysts such as NaOH, KOH, or Ba(OH)2 are used to facilitate this reaction, typically carried out in polar solvents such as ethanol or methanol. This reaction produces chalcone, a basic compound that can then be further modified into flavonoids or other derivatives. To enhance efficiency and yields, microwave-assisted synthesis has also been applied [43]. The use of microwaves accelerates reactions and improves synthesis yields in a shorter time, reducing traditional reaction times from hours to minutes. In addition, various catalyst and solvent variations, such as using K2CO3 as a base catalyst or THF as a solvent, have been explored to further enhance chalcone synthesis efficiency. A general reaction pathway for flavonoid synthesis is shown in Figure 5.
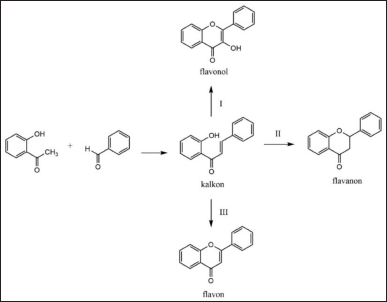 | Figure 5. General reaction for the synthesis of flavonoid derivative compounds [58] . [Click here to view] |
Once chalcone has been successfully synthesized, the next step is to convert it into flavone. One common method for flavone synthesis is oxidative cyclization of chalcone, which is done using reagents such as I2/DMSO, H2O2/NaOH, or SeO2 [46]. This reaction transforms the chalcone structure into flavone by forming an additional ring bond. As with chalcone synthesis, microwave-assisted methods have also been employed in flavone synthesis, improving reaction speed and yields in a more efficient manner [15]. Furthermore, the use of acid catalysts such as CH3SO3H or acetic acid can assist in the cyclization process, providing a simpler and faster alternative. Other approaches also allow direct synthesis of flavones from appropriate precursors without first forming chalcone [19,32]. This method often involves multicomponent reactions utilizing specific catalysts to yield flavones in a more efficient and targeted manner [22].
In addition to flavones, flavanon compounds are also flavonoid derivatives that can be synthesized from chalcone. The flavanon synthesis process generally involves the cyclization of chalcone with acid catalysts, such as H2SO4, in alcohol solvents. The use of acid catalysts facilitates the formation of the flavanon structure through cyclization reactions. As with chalcone and flavone synthesis, microwave-assisted synthesis is also applied to flavanon synthesis to enhance reaction speed and yields [25,26]. Additionally, base catalysts such as NaOAc can be used to direct the cyclization reaction of chalcone into flavanon, offering flexibility in selecting the most appropriate reaction conditions [47].
Besides synthesis, structural modification of flavonoids is also an important step in enhancing their biological activity. One of the most common modifications is the addition of functional groups to the A and B rings, which may include hydroxyl, methoxy, halogen, or nitro groups [12]. These additions are achieved through various reactions such as alkylation, acylation, halogenation, and nitration. These modifications aim to improve the solubility, stability, or pharmacological potential of flavonoids, which in turn can enhance their therapeutic activity, including in the treatment of inflammatory diseases and cancer [21]. Other modifications include replacing aromatic rings with heterocyclic rings, such as pyridine, which can alter the chemical properties and biological activity of flavonoids, opening new potential in drug design [55].
Overall, flavonoid synthesis and structural modifications offer many opportunities to enhance the efficiency of their production and improve their biological activity. Through various synthetic approaches and structural modifications, flavonoid derivatives can be tailored for broader applications, especially in treating diseases related to inflammation, infection, and cancer.
Synthesis of flavonoid compounds as anti-RA agents
Flavonoids are a class of natural plant compounds known for their various pharmacological properties, especially anti-inflammatory and antioxidant effects. These compounds have drawn significant research interest due to their potential applications in treating chronic inflammatory diseases such as RA. Table 12 summarizes the various flavonoid subclasses, their synthesis methods, and key findings related to their anti-RA activity. By understanding these aspects, researchers can further explore and optimize flavonoids as natural alternatives or adjunct therapies for RA management.
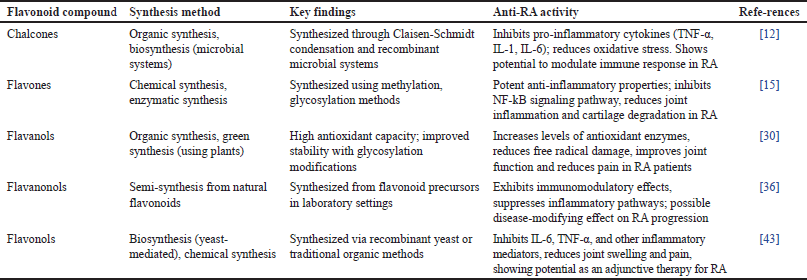 | Table 12. distribution of research on flavonoid compounds with various mechanistic pathways of anti-inflammatory activity in RA cases. [Click here to view] |
Flavonoids are a diverse group of phytonutrients found in various plants, exhibiting numerous bioactive properties that are beneficial for human health. These compounds are particularly recognized for their antioxidant and anti-inflammatory effects, which play a crucial role in their application against diseases such as RA. Flavonoid subclasses, including chalcones, flavones, flavanols, flavanonols, and flavonols, possess unique structural characteristics that contribute to their bioactive activities. For instance, chalcones synthesized through Claisen–Schmidt condensation and microbial systems demonstrate significant ability to inhibit pro-inflammatory cytokines such as TNF-α and IL-6, which are essential in the development of RA, by reducing oxidative stress and modulating the immune response [12].
Flavonoid synthesis varies according to its specific subclass. Chalcones and flavonols are synthesized via organic methods and biosynthesis, often employing yeast-mediated or plant-based techniques [19]. Flavones, on the other hand, can be synthesized through chemical and enzymatic processes, with methods like methylation and glycosylation enhancing their stability and bioavailability [37]. Flavanols utilize an environmentally friendly synthesis approach using plants, enabling better antioxidant capacity [43]. Semi-synthetic approaches for flavanonols show flexibility in synthetic methods aimed at optimizing the effectiveness of these compounds in therapeutic applications against RA.
Regarding anti-RA activity, flavonoid compounds exhibit various mechanisms. Flavones and isoflavonoids, for example, inhibit NF-kB, STAT signaling pathways, and key inflammatory drivers in RA, effectively reducing joint inflammation and cartilage degradation [45]. Flavanols increase antioxidant enzyme levels, helping reduce free radical-induced damage and improving joint function [26]. Flavonols reduce joint swelling and pain by inhibiting inflammatory mediators, particularly IL-6 and TNF-α, demonstrating their potential as adjunct therapies in RA treatment [14]. Furthermore, flavanonols and chalcones exhibit immunomodulatory and anti-inflammatory effects that may modify the progression of RA, highlighting flavonoids as promising natural agents for managing RA symptoms and improving patient quality of life.
Modification of flavonoid derivatives and functional groups with effective anti-inflammatory activity in RA cases
Research conducted by several scientists, as presented in Table 1, shows that structural modifications of flavonoid derivatives, particularly on functional groups attached to the aromatic rings, such as the addition of hydroxyl, methoxy, and halogen groups, can enhance anti-inflammatory activity in RA [9,12,39]. This research focuses on how structural changes in flavonoids affect their ability to reduce inflammation in RA. The findings suggest that some flavonoid derivatives with hydroxyl groups at positions 5 and 7 on ring A, and at positions 3’ and 4’ on ring B, exhibit superior anti-inflammatory activity in combating RA inflammation. For instance, a study by Miao et al. [37] demonstrated that the anti-inflammatory activity test of the compound 3-(4-aminophenyl)-coumarin showed an average IC50 value of 10 μM. In a rat arthritis model, this compound significantly reduced joint swelling and tissue damage [36]. The addition of hydroxyl groups at these positions enhances the solubility of the compound in water, facilitating penetration into inflammatory cells and increasing interaction with inflammatory molecular targets such as COX-2 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [5].
Several studies on the SAR have been conducted by Emam et al. [5]. In Figure 4 presented in this study, the relationship between the chemical structure of chalcone compounds and their biological activity is clearly illustrated, with a focus on the inhibition of nitric oxide (NO) production and COX-2 enzyme activity [5]. Several key points can be systematically analyzed based on Figure 6.
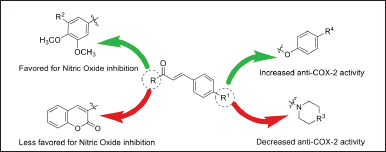 | Figure 6. Structure-activity relationship of chalcone derivatives synthesis [5] . [Click here to view] |
First, the influence of functional groups on the chalcone structure appears to play a critical role in determining the biological activity of this compound. In Figure 6, it can be observed that functional groups such as hydroxyl (-OH) and methoxy (-OCH3) located on the aromatic ring of chalcone, as well as variations in the R group on the side chain, significantly affect the compound’s ability to inhibit NO production and COX-2 activity. Specifically, the hydroxyl group at certain positions on the chalcone aromatic ring seems to contribute significantly to the inhibition of NO production, indicating that this group plays an important role in the anti-inflammatory mechanism of chalcone compounds.
Furthermore, the anti-NO activity influenced by the hydroxyl group can be clearly observed in Figure 6. The addition of a hydroxyl group at specific positions, indicated by the green arrow, tends to increase the effectiveness of the chalcone compound in inhibiting NO production. NO is an essential mediator in the inflammatory process, and the inhibition of NO production by chalcone compounds demonstrates their potential as a potent anti-inflammatory agent. This suggests that chalcone compounds with hydroxyl groups have a better capacity to reduce oxidative stress and inflammatory responses induced by NO.
Next, the anti-COX-2 activity, also shown in Figure 6, indicates that chalcone compounds with specific structures, again highlighted by the green arrows, exhibit an increased ability to inhibit the COX-2 enzyme. COX-2 is an enzyme involved in the synthesis of prostaglandins, which are key players in inflammation. Chalcone structures with high affinity for the COX-2 active site, as seen in Figure 6, can effectively inhibit the enzyme’s activity, subsequently reducing inflammation caused by prostaglandin production [5]. This highlights chalcone’s potential as a therapeutic agent in treating inflammatory disorders such as arthritis.
Finally, Figure 6 also demonstrates a clear SAR between the chemical structure of chalcone compounds and their biological activity. Small changes in the chalcone structure, such as the addition or removal of specific functional groups, can significantly affect its ability to inhibit NO production and COX-2 activity. This reinforces the importance of understanding and utilizing this relationship in designing compounds with enhanced anti-inflammatory activity.
Overall, the analysis derived from Figure 6 provides a comprehensive understanding of how structural changes in chalcone affect two major inflammatory pathways, namely NO production and COX-2 inhibition, and demonstrates the great potential of chalcone in the development of natural compound-based anti-inflammatory drugs.
The study conducted by Sinyeue et al. [24] also conducted an in-depth investigation into the evaluation of various flavonoid derivatives modified at rings A and B, assessing their ability to inhibit NO production as an inflammatory marker [24]. The main findings from this study indicate that the presence and position of certain functional groups, such as the carboxyl group at the ortho position on ring B, halogen groups on ring B, and the methoxy group at position 5 on ring A, significantly influence the enhancement of the flavonoid anti-inflammatory activity (Fig. 7). The mechanism of action of the tested flavonoid compounds is believed to be related to their ability to inhibit NO production, a molecule that plays an important role in inflammation. Inhibition of NO production by these compounds may potentially reduce inflammation.
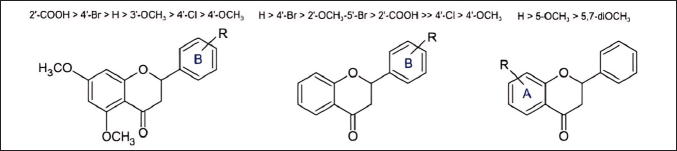 | Figure 7. Structure-activity relationship in the synthesis of flavanone derivatives [24] . [Click here to view] |
Similarly, the structural and activity analysis of flavonoid compounds in a study conducted by Bano et al. [19] provides valuable insights into how chemical structure affects the compound’s ability to inhibit the COX-2 enzyme activity, which is a primary target in the inflammatory process [19,24]. The flavonoid structures analysed in this study contain several key elements contributing to their biological activity.
As shown in Figure 8, the influence of structure and the presence of substituents affect the resulting anti-inflammatory activity. Chalcone structure exhibits the highest anti-inflammatory activity among other flavonoid derivatives, such as flavone and flavanone. The presence of an aromatic ring in the flavonoid structure provides rigidity to the molecule and allows strong interactions with target proteins through hydrogen bonding and van der Waals forces. Furthermore, the chalcone structure with functional group substituents, such as hydroxyl (-OH) and methoxy (-OCH3) on the aromatic ring, is crucial in determining the biological properties of the compound [19]. This is demonstrated by the significant anti-inflammatory activity values observed among other structures, indicating that the addition of these substituents enhances pharmacological activity. The hydroxyl group can act as a hydrogen bond donor, improving the compound’s affinity for the target enzyme, while the methoxy group helps increase the lipophilicity of the compound, affecting its ability to penetrate cell membranes. Additionally, the conjugated double-bond system in the flavonoid structure provides molecular stability and allows electron delocalization, enhancing interactions with target proteins. As a primary target, the COX-2 enzyme plays a role in the production of prostaglandins, which are inflammatory mediators. By inhibiting COX-2, flavonoid compounds can reduce prostaglandin production, thereby decreasing inflammation [19].
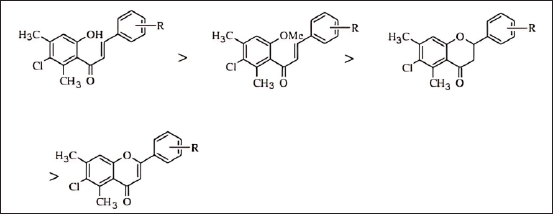 | Figure 8. Structure-activity relationship in anti-inflammatory activity in the case of RA [19]. [Click here to view] |
Based on the explanation, Bano et al. [19] outline that in terms of the SAR, the hydroxyl group on ring B most likely interacts with amino acid residues at the COX-2 active site through hydrogen bonding, increasing the compound’s binding affinity and inhibiting enzyme activity. The aromatic ring in the flavonoid structure can interact with aromatic amino acid residues at the enzyme’s active site using π-π interactions, which enhances the stability of the compound-enzyme complex. The methoxy group, which affects the compound’s lipophilicity, plays a role in enhancing cell membrane permeability and compound distribution within the body, as well as modulating interactions with target proteins. Moreover, variations in the length and type of side chains in the flavonoid structure can influence interactions with the COX-2 enzyme and the physicochemical properties of the compound, collectively improving the anti-inflammatory therapeutic potential of the flavonoid compounds.
Based on several research summaries, the addition of polar groups in the structure of flavonoid derivatives plays a critical role in enhancing anti-inflammatory activity, particularly in relation to bioavailability and molecular interactions with inflammatory targets in the body. Flavonoids, as phenolic compounds, have structures that are generally non-polar, which may hinder their solubility in water and reduce their effectiveness in the body. Therefore, the addition of polar groups, such as hydroxyl (-OH), methoxy (-OCH3), or carboxyl (-COOH) groups in existing studies, can increase the solubility of the compounds, improve interactions with target proteins or enzymes, and enhance their biological activity [12,59]. The presence of these polar groups enhances the flavonoid’s ability to interact with molecular targets involved in the inflammatory process, such as the COX-2 enzyme, NF-kB transcription factors, cell membranes and surface receptors, as well as immune cells involved in inflammation [12]. These interactions can modulate inflammatory signaling pathways involved in the inflammatory process, such as inhibiting NF-kB activation or reducing COX-2 expression, which are two key targets in the treatment of RA [7,42].
The position and number of methoxy groups attached to the flavonoid structure also influence anti-inflammatory activity. Studies show that the addition of methoxy groups at specific positions, such as on ring B (e.g., at positions 3’ and 4’), can enhance the antioxidant potential of flavonoids, which plays a role in reducing oxidative stress associated with inflammation in RA [15]. Oxidative stress contributes to the activation of inflammatory pathways and tissue damage in joints, so reducing inflammation can improve the condition of RA patients [9].
Other modifications, such as the addition of halogen groups (e.g., fluorine or chlorine) at strategic positions in the flavonoid structure, have also been found to enhance biological activity. Halogens in the flavonoid structure can increase compound stability and alter interactions with inflammation-related receptors or enzymes [53]. Such modifications often aim to improve the compound’s affinity for specific inflammatory molecular targets and prolong its half-life in the body [57].
In addition to molecular structure, understanding the bioavailability of flavonoids is also important in enhancing their therapeutic potential. Structural modifications to flavonoids, such as the addition of certain groups, can help address bioavailability challenges by improving compound solubility or stability in the body, allowing the compound to reach sufficient therapeutic concentrations in target tissues, such as joints affected by inflammation in RA [36].
Overall, research into flavonoid structural modifications provides valuable insights for developing new therapeutic compounds for RA treatment. By modifying specific groups within the flavonoid structure, it is hoped that compounds with stronger, more selective, and more effective anti-inflammatory effects can be discovered, ultimately reducing RA inflammation symptoms while minimizing unwanted side effects.
Mechanism of inhibition of inflammatory activity in RA cases by flavonoid derivative compounds
In the research conducted, several possibilities can be predicted regarding the mechanisms of action of flavonoid compounds in anti-inflammatory activity in RA through various interconnected molecular pathways and processes [60]. One of the main mechanisms of flavonoid compounds in anti-inflammatory activity is the inhibition of COX-2 enzymes [19,20,33]. COX-2 is an enzyme involved in the synthesis of prostaglandins, which are key mediators in the inflammatory process [5]. In RA, COX-2 levels are often elevated, contributing to pain and inflammation in the joints. Several flavonoid compounds with hydroxyl and methoxy groups, such as kaempferol, apigenin, luteolin, quercetin, and chrysin, bind to the active site on COX-2, inhibiting the conversion of arachidonic acid to prostaglandin E2, which in turn reduces inflammation [19]. The addition of polar groups such as hydroxyl enhances the affinity of flavonoids for COX-2, making them more effective in inhibiting this enzyme’s activity.
The second pathway involves inhibition through the NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway. In this pathway, the transcription factor involved in regulating inflammatory genes is activated. NF-kB activation leads to increased production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, all of which play a role in the pathogenesis of RA [14]. Flavonoid compounds have been shown to inhibit the NF-kB pathway, reducing the expression of inflammatory genes. Derivatives of flavonoids, such as naringenin, genistein, luteolin, and quercetin, can inhibit the degradation of IκBα (an inhibitor of NF-kB), which prevents the activation of NF-kB and reduces the transcription of pro-inflammatory genes [19]. Structural modifications of flavonoids, such as the addition of hydroxyl or methoxy groups, can enhance their ability to inhibit NF-kB activation and reduce the expression of inflammatory cytokines [42,61].
The third pathway involves the reduction of oxidative stress. This pathway plays an important role in the inflammatory process, particularly in RA, where the production of reactive oxygen species (ROS) contributes to tissue damage and inflammation [45]. Flavonoids act as antioxidants by scavenging free radicals and lowering oxidative stress levels. Several flavonoid derivatives with hydroxyl substituents on both rings interact with free radicals and ROS, neutralizing them before they can damage cells or worsen inflammation [12]. Flavonoid compounds containing hydroxyl groups at specific positions, such as on ring B, have been shown to be effective in counteracting excessive ROS in RA.
The fourth pathway involves the modulation of immune cell activity [33]. Immune cells such as macrophages and T lymphocytes play a key role in the inflammatory process in RA [33]. Flavonoids can modulate the activity of these immune cells by reducing the production of inflammatory cytokines or by inhibiting the migration of immune cells to the site of inflammation. Some flavonoids can suppress macrophage activation, reducing the production of cytokines such as TNF-α and IL-6, which are heavily involved in joint inflammation in RA [14]. These compounds can also inhibit the transmigration of immune cells to infected or inflamed areas.
Flavonoids exhibit significant therapeutic potential as anti-RA agents by targeting multiple inflammatory mechanisms. Flavonoids have been shown to inhibit pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6, which are critical in the inflammatory process of RA [62]. Furthermore, flavonoids increase antioxidant enzyme levels, thereby reducing oxidative stress and free radical damage associated with joint damage in RA patients [56]. Flavonoids also possess strong anti-inflammatory effects by inhibiting key signaling pathways, including NF-κB and MAPK, which are central to the inflammatory cascade in RA [6,56]. This inhibition leads to reduced joint inflammation and cartilage degradation, ultimately improving joint function and alleviating pain [62]. Moreover, flavonoids have immunomodulatory properties that can suppress inflammatory pathways, potentially offering disease-modifying effects on the progression of RA [62]. Overall, the multifaceted actions of flavonoids highlight their potential as adjunct therapies for RA, capable of alleviating symptoms and slowing disease progression.
Limitations and challenges in the clinical translation of flavonoid derivatives
Despite these promising findings, several critical challenges remain in translating flavonoid derivatives into effective clinical treatments for RA. One notable limitation is the inconsistency in their efficacy across different in vitro and in vivo models. Variability in chemical structure, dosage, and model organisms contributes to inconsistent outcomes, making it difficult to generalize findings or establish standard treatment guidelines.
Another major obstacle lies in the poor bioavailability of many flavonoid compounds due to rapid metabolism and limited absorption. These pharmacokinetic limitations hinder their therapeutic effectiveness in vivo and must be addressed through advanced delivery systems or chemical modification. Additionally, clinical data on flavonoid-based therapies for RA remain scarce, highlighting the gap between experimental success and real-world applicability. Therefore, future research should prioritize comparative studies with standard RA drugs, optimize synthesis for bioactivity and bioavailability, and investigate translational models that closely mimic human RA pathology.
The increasing interest in diverse synthesis strategies, including structural modifications and eco-friendly catalytic systems, has significantly expanded the availability and biological potential of flavonoid derivatives. With these innovations, flavonoids are not only easier to produce in high yield and purity, but also more tunable in terms of potency and pharmacokinetics. This positions them as competitive—if not superior—alternatives to current standard therapies in the management of chronic diseases such as RA. Building on this foundation, the following section discusses how various subclasses of flavonoids act against RA, the results of meta-analysis on their efficacy, and how they compare to existing therapies in terms of safety and therapeutic value.
CONCLUSION
Flavonoids present a promising opportunity for managing RA due to their diverse bioactive properties, particularly their antioxidant and anti-inflammatory mechanisms. Each flavonoid subclass—chalcone, flavone, flavanol, flavanonol, flavonol, and isoflavonoid—offers unique therapeutic effects, such as inhibiting inflammatory cytokines like TNF-α and IL-6 and modulating immune responses, collectively contributing to the reduction of joint inflammation, oxidative stress, and cartilage degradation. As natural compounds with minimal side effects, flavonoids hold potential as adjunct therapies to conventional RA treatments. Future research should focus on optimizing the synthesis and bioavailability of these compounds to enhance their therapeutic efficacy and expand their clinical applications in RA management.
Limitations of the study
This study has several limitations, including heterogeneity in study designs, flavonoid subclasses, and outcome measures across included articles, which may affect result comparability. Most data were derived from preclinical studies, limiting clinical generalizability. Additionally, variations in synthesis methods and incomplete reporting of SARs hindered deeper mechanistic interpretation. The number of studies eligible for meta-analysis was also limited, potentially reducing the robustness of pooled estimates.
ACKNOWLEDGMENTS
The authors sincerely appreciate the financial support provided by Universitas Muhammadiyah Yogyakarta (UMY) and the Research and Innovation Institute of Universitas Muhammadiyah Yogyakarta (LRI UMY), for providing a grant of 10 million Indonesian Rupiah for this study. We also extend our gratitude to Universitas Gadjah Mada (UGM) for its invaluable contributions in providing research facilities and academic support. Special thanks to our colleagues and research team members for their insightful discussions, technical assistance, and continuous encouragement throughout this research. Additionally, we acknowledge the resources and facilities that have facilitated the successful completion of this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was supported by the Institute for Research, Publication, and Community Service (LPPT), Universitas Muhammadiyah Yogyakarta (UMY), through research and school-based funding
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Taylor PC, Laedermann C, Alten R, Feist E, Choy E, Haladyj E, et al. A JAK inhibitor for treatment of rheumatoid arthritis: the baricitinib experience. J Clin Med. 2023;12(13):4527. CrossRef
2. Ranchchh AR, Busa KP, Mahetar JG, Shah MK. A facile synthetic approach for the syntheses of 7-hydroxyflavonol derivatives. Der Pharm Chem. 2015;7(5):142–6.
3. Kimariyo PF, Kurati SP, Babu PS, Moyo AA, Muthyala MKK. Synthesized diterpene lactone derivative attenuated Freund’s complete adjuvant-induced arthritis in Wistar rats. Iran J Basic Med Sci. 2024;27(9):1197–208.
4. Trivilin Mendes M, Fadoni Alponti R, Lucio Alves P, Lopes Trevizan I, Pekelmann Markus R, Augusto Fernandes P, et al. Inhibitors of tumor necrosis factor synthesis as a new approach for the treatment of rheumatoid arthritis. J Arthritis. 2020;9:123–30.
5. Emam SH, Sonousi A, Osman EO, Hwang D, Kim G Do, Hassan RA. Design and synthesis of methoxyphenyl- and coumarin-based chalcone derivatives as anti-inflammatory agents by inhibition of NO production and down-regulation of NF-κB in LPS-induced RAW264.7 macrophage cells. Bioorg Chem. 2021;107:104630. CrossRef
6. Kumar A, Mahendra J, Mahendra L, Abdulkarim HH, Sayed M, Mugri MH, et al. Synergistic effect of biphasic calcium phosphate and platelet-rich fibrin attenuate markers for inflammation and osteoclast differentiation by suppressing nf-κb/mapk signaling pathway in chronic periodontitis. Molecules 2021;26(21):6578. CrossRef
7. Chen YF, Wu SN, Gao JM, Liao ZY, Tseng YT, Fülöp F, et al. The antioxidant, anti-inflammatory, and neuroprotective properties of the synthetic chalcone derivative AN07. Molecules 2020;25(12):2907. CrossRef
8. Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ (Online). 2019;364:l67. CrossRef
9. Kim MK, Bae O, Chong Y. Design, synthesis, and molecular docking study of flavonol derivatives as selective jak1 inhibitors. Bull Korean Chem Soc. 2014;35(8):2581–4. CrossRef
10. Taylor PC, Choy E, Baraliakos X, Szekanecz Z, Xavier RM, Isaacs JD, et al. Differential properties of Janus kinase inhibitors in the treatment of immune-mediated inflammatory diseases. Rheumatology 2024;63(2):298–308. CrossRef
11. Sun Z, Zhai B, He G, Shen H, Chen L, Zhang S, et al. Synthesis and anti-inflammatory evaluation of novel 1,2,3-triazole derivatives. Chin J Org Chem. 2023;43(6):2143–55. CrossRef
12. Wu J, Li J, Cai Y, Pan Y, Ye F, Zhang Y, et al. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem. 2011;54(23):8110–23. CrossRef
13. Rathod P, Chavan S. Flavonoid derivatives: synthesis and biological assay showing strong anti-inflammatory activity. J Appl Pharm Sci. 2023.
14. Upadhyay HC, Singh M, Prakash O, Khan F, Srivastava SK, Bawankule DU. QSAR, ADME and docking guided semi-synthesis and in vitro evaluation of 4-hydroxy-α-tetralone analogs for anti-inflammatory activity. SN Appl Sci. 2020;2(12):2069. CrossRef
15. Prafulla S, Lata P, Nusrat S, Priya R. Design, synthesis and pharmacological screening of novel flavone derivatives. Sys Rev Pharm. 2021;12(12):3817–21.
16. Maria Elisa MB, et al. Esterified flavonoids with increased lipophilicity and bioactivity. Arab J Chem. 2016;9:S931–5.
17. Gill NS, Kaur A, Arora R, Dhawan V, Bali M Synthetic studies of novel azaflavanone derivatives and its biological activities. Curr Res Chem. 2012;4(4):88–98. CrossRef
18. Simyeue C, et al. Substituted flavonoids synthesized via Claisen-Schmidt showed better NO inhibition than pinocembrin. J Appl Pharm Sci. 2021.
19. Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Chaitanya M, et al. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur J Med Chem. 2013;65:51–9. CrossRef
20. Abualhasan M, Jaradat N, Al-Rimawi F, Shahwan M, Mansour D, Alhend Z, et al. Bioactivity evaluation of synthesized flavone analogs. Food Sci Technol (Brazil). 2022;42:e57122. CrossRef
21. Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol. 2018;32(1):456–63. CrossRef
22. Kiruthiga N, et al. Synthesized flavonoids evaluated via docking and in vitro assays showed significant anti-inflammatory effect. J Appl Pharm Sci. 2021.
23. Sabale S, et al. Evaluation of synthetic flavones using in vivo paw edema model. J Appl Pharm Sci. 2021.
24. Sinyeue C, Matsui M, Oelgemöller M, Bregier F, Chaleix V, Sol V, et al. Synthesis and investigation of flavanone derivatives as potential new anti-inflammatory agents. Molecules 2022;27(6):1781. CrossRef
25. Lima NM, Mendes LAO, Castro SBR, De T, Andrade JAS, Carli AP, et al. Synthesis of chrysin derivatives with anti-inflammatory property, a naturally occurring flavone. Am J Pharm. 2022;41(11):2266–72.
26. Hashim NA. Synthesis and bioactivity studies of flavonoid and its derivatives [Master’s thesis]. Johor, Malaysia: Universiti Teknologi Malaysia, Faculty of Science; 2012.
27. Yang Q, Wang Z, Hong Huan Hor C, Xiao H, Bian Z, Wang J. Asymmetric synthesis of flavanols via Cu-catalyzed kinetic resolution of chromenes and their anti-inflammatory activity. Sci Adv. 2022;8:eabm9603. CrossRef
28. Barmaki I, et al. Hybrid indole–chalcone derivatives showed strong anti-inflammatory activity. J Appl Pharm Sci. 2021.
29. Kamakavalli M, et al. Anti-inflammatory effects of 3-formyl-7-flavonols synthesized via Vilsmeier-Haack method. Biomed Pharmacol J. 2019;12(4):1779–91. CrossRef
30. Wibowo AE, Susidarti RA, Puspitasari I. Synthesis and anti-inflammatory activity of 1-(2,5-dihydroxyphenyl)-3-pyridine-2-Yl-propenone (AEW-1) compound. Indones J Pharm. 2020;32:209–20. CrossRef
31. Sonu S, et al. 3-methoxy chalcone derivatives with moderate to strong anti-inflammatory activity. J Appl Pharm Sci. 2023.
32. Peng Y, Shi Y, Zhang H, Mine Y, Tsao R. Anti-inflammatory and anti-oxidative activities of daidzein and its sulfonic acid ester derivatives. J Funct Foods. 2017;35:635–40. CrossRef
33. Wibowo AE, Hatala RR, Edang AM. Antimicrobial test of 1-(2.5-dihydroxi phenyl)-(3-pyridine-2-Il) -propanone compound in Enterococcus faecalis and Escherichia coli bacteria using a well diffusion method. J Fund Appl Pharm Sci. 2021;1(2):72–80. CrossRef
34. Wibowo AE, Saputra AK, Susidarti RA. Optimization of synthesis of 1-(2,5-dihydroxyphenyl)-(3-pyridine-2-yl)-propenone, AN anti-inflammatory agent, using NaOH. Pharm J Indones. 2018;15(2):25–32.
35. Munir A, Muhammad F, Zaheer Y, Ali MA, Iqbal M, Rehman M, et al. Synthesis of naringenin loaded lipid-based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J Drug Deliv Sci Technol. 2021;66:102854. CrossRef
36. Miao Y, Yang J, Yun Y, Sun J, Wang X. Synthesis and anti-rheumatoid arthritis activities of 3-(4-aminophenyl)-coumarin derivatives. J Enzyme Inhib Med Chem. 2021;36(1):450–61. CrossRef
37. Freitas M, Ribeiro D, Tomé SM, Silva AMS, Fernandes E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur J Med Chem. 2014;86:153–64. CrossRef
38. Ao M, et al. Adamantyl flavonoids reduced NF-κB activation in ALI models. J Appl Pharm Sci. 2022.
39. Rajput S, Kumar D, Agrawal V. Green synthesis of silver nanoparticles using Indian Belladonna extract and their potential antioxidant, anti-inflammatory, anticancer and larvicidal activities. Plant Cell Rep. 2020;39(7):921–39. CrossRef
40. Pohl P, et al. AgNPs from Tribulus terrestris showed 82% anti-inflammatory activity. Nanomaterials. 2020;10(12):1–17.
41. Alves L, Joana J. Acylated naringin/rutin derivatives showed strong iNOS and NO inhibition. J Appl Pharm Sci. 2018.
42. De Araújo MEMB, Franco YEM, Messias MCF, Longato GB, Pamphile JA, Carvalho PDO. Biocatalytic synthesis of flavonoid esters by lipases and their biological benefits. Planta Med. 2017;83:7–22. CrossRef
43. Karpakavalli M, Sangilimuthu AY, Usha Raja Nanthini A, Nagaraja Perumal G, Mohan S, Sivakumar T. Anti-inflammatory effects of 3-formyl, 7-flavonols derivatives by microwave enhanced chemistry assisted - Vilsmeier haack synthesis. Biomed Pharmacol J. 2019;12(4):1779–91. CrossRef
44. Mysler E, Caubet M, Lizarraga A. Current and emerging DMARDs for the treatment of rheumatoid arthritis. Open Access Rheumatol. 2021;13:139–52. CrossRef
45. Tucci G, Garufi C, Pacella I, Zagaglioni M, Pinzon Grimaldos A, Ceccarelli F, et al. Baricitinib therapy response in rheumatoid arthritis patients associates to STAT1 phosphorylation in monocytes. Front Immunol. 2022;13:932240. CrossRef
46. Pharma D, Rathod SP, Chavan AS. Design and synthesis of flavonoid derivatives as anti-inflammatory drug. Der Pharm Chem. 2023;15(6):146–50. Available from: www.derpharmachemica.com
47. Gautam GK, Mishra AK, Parveen BR, singh H. Molecular docking studies, synthesis of novel isoxazole derivatives from 3-methoxy substituted chalcone and evaluation of their anti-inflammatory activity. Orient J Chem. 2023;39(3):675–83. CrossRef
48. Zhou S, Xue W, Tan J. Design, synthesis, and antirheumatoid arthritis mechanism of TLR4 inhibitors. ACS Omega. 2024;9(34):36232–4. CrossRef
49. Sable PM, Potey LC. Microwave-assisted synthesis of chalcone and biological activity [Internet]. Available from: http://www.scholarsresearchlibrary.com
50. Baramaki I, Altintop MD, Arslan R, Alyu Altinok F, Özdemir A, Dallali I, et al. Design, Synthesis, and in vivo evaluation of a new series of indole-chalcone hybrids as analgesic and anti-inflammatory agents. ACS Omega. 2024;9(10):12175–83. CrossRef
51. Chen L, Fan Z, Chang J, Yang R, Hou H, Guo H, et al. Sequence-based drug design as a concept in computational drug design. Nat Commun. 2023;14(1):4217. CrossRef
52. Yue Y, Peng J, Wang D, Bian Y, Sun P, Chen C. Synthesis of 4H-chromen-4-one derivatives by intramolecular palladium-catalyzed acylation of alkenyl bromides with aldehydes. J Org Chem. 2017;82(10):5481–6. CrossRef
53. Beken B, Serttas R, Yazicioglu M, Turkekul K, Erdogan S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr Allergy Immunol Pulmonol. 2020;33(2):69–79. CrossRef
54. Hayashi A, Gillen AC, Lott JR. Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern Med Rev. 2000;5(6):546–52.
55. Rayees Ahmad M, Girija Sastry V, Bano N, Anwar S. Synthesis of novel chalcone derivatives by conventional and microwave irradiation methods and their pharmacological activities. Arab J Chem. 2016;9:S931–5. CrossRef
56. Sharifi-Rad M, Pohl P, Epifano F, Álvarez-Suarez JM. Green synthesis of silver nanoparticles using astragalus tribuloides delile. Root extract: characterization, antioxidant, antibacterial, and anti-inflammatory activities. Nanomaterials 2020;10(12):1–17. CrossRef
57. Zhang Y, Jiang J, Xie J, Xu C, Wang C, Yin L, et al. Combined effects of tumor necrosis factor-α and interleukin-1β on lysyl oxidase and matrix metalloproteinase expression in human knee synovial fibroblasts in vitro. Exp Ther Med. 2017;14(6):5258–66. CrossRef
58. Pierea D, et al. General reaction for synthesis of flavonoid derivatives. J Appl Pharm Sci. 2023.
59. Igbe I, Shen XF, Jiao W, Qiang Z, Deng T, Li S, et al. Dietary quercetin potentiates the antiproliferative effect of interferon-α in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase [Internet]. Oncotarget. 2017;8(69):113734–48. CrossRef
60. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. CrossRef
61. Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018 Dec;27(12):1984–2009. CrossRef
62. Wahnou H, Limami Y, Oudghiri M. Flavonoids and flavonoid-based nanoparticles for osteoarthritis and rheumatoid arthritis management. BioChem 2024;4(1):38–61. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4617_pdf.pdf]