INTRODUCTION
Prostate cancer (PCa) is a leading cause of mortality among men, with an approximate lifetime risk of diagnosis reaching 12.5% [1]. The number of new cases is expected to rise from 1.4 million in 2020 to 2.9 million in 2040, accounting for 15% of all cancer cases [2]. A major challenge in PCa management is its tendency to develop resistance to multiple treatments [3–5]. This includes alterations in the androgen receptor (AR) pathway, such as AR amplification, mutations, and the emergence of AR splice variants that remain active even in the absence of androgens [6]. Additionally, cancer cells can produce their androgens or become androgen-independent, activate alternative growth pathways, and evade apoptosis [7,8]. This resistance leads to limited treatment options, increased morbidity and mortality, and a significant decline in patients’ quality of life.
Recent studies have highlighted the potential of curcumin in overcoming drug resistance and re-sensitizing cancer cells to chemotherapeutic drugs [9]. Curcumin is reported to increase doxorubicin efficacy in castration-resistant PCa treatment by enhancing apoptosis, inducing endoplasmic reticulum stress, and inhibiting survival pathways [10]. While PCa cells (DU145, LNCaP, and PC-3) are generally resistant to TRAIL, curcumin can make them more susceptible to TRAIL-induced apoptosis [11–13]. When used alongside radiation, curcumin markedly enhances the effectiveness of radiation by inducing apoptosis and increasing clonogenic inhibition. This synergy adjusts the Bax/Bcl2 ratio and triggers the activation of cytochrome c, caspase-9, and caspase-3, demonstrating that curcumin effectively increases the sensitivity of PCa cells to radiation [14]. In addition to being used in combination therapies, curcumin exhibits potent effects when used as a single agent. Studies have shown that curcumin lowers AR expression, prevents AR from binding to the androgen response element on the prostate-specific antigen (PSA) gene, and reduces PSA levels in LNCaP cells [15]. It enhances the expression of microRNA miR-30a-5p and reduces the expression of PCNA clamp-associated factor, showing that curcumin can suppress the malignant biological behaviors of PCa [16]. However, despite the potential benefits of these combination therapies, patients undergoing chemotherapy frequently suffer from mineral deficiencies in magnesium, potassium, sodium, zinc, and iron, which are crucial for maintaining various physiological functions [17]. Significant metabolic damage might still occur if mineral consumption exceeds the recommended daily amount [18]. These deficiencies can lead to complications such as diarrhea, electrolyte imbalances, and chemotherapy-induced peripheral neuropathy (CIPN) [19,20]. The disruption of these minerals can exacerbate the patient’s overall health condition, leading to additional treatments and longer recovery times.
Concentrated marine mineral (CMM) solutions, rich in essential minerals such as magnesium, potassium, and calcium, have been proposed as adjunct therapies to address the mineral deficiencies caused by chemotherapy [21]. These solutions are even suggested to have a role in inhibiting the metastatic potential of breast cancer [22]. Additionally, the complexation between curcumin and certain minerals can improve solubility and enhance cellular uptake [23]. Consequently, combining curcumin and CMM might yield more optimal cancer therapeutic effects while minimizing the side effects of co-administered chemotherapy drugs. The potential synergistic effects of combining curcumin with CMM have not been thoroughly explored, prompting us to investigate how this combination influences cancerous and normal cells compared to standard treatments. This study aims to compare the cytotoxic effects of curcumin and CMM and their combination against prostate cancer cells (DU145) and normal kidney cells (HEK293) relative to cisplatin. This research explored the mechanisms of action of curcumin and CMM, particularly in terms of gene expression changes related to cancer cell proliferation and survival, such as Cyclin-D1, Wnt3a, and C-Myc. Additionally, we aim to identify the optimal ratios of curcumin and CMM that maximize anticancer efficacy while minimizing toxicity to normal cells.
METHODS
Materials
In this study, we used DU145 and HEK-293 cell lines from the Cellular and Molecular Biology Laboratory, Faculty of Pharmacy, Universitas Padjadjaran. We also used WST-8, PBS, fetal bovine serum (Sigma), Dulbecco’s modified eagle medium (DMEM) with high glucose (Sigma), penicillin-streptomycin (Sigma), TrypLE trypsin (Gibco), phosphate buffer saline 10X (Lonza), KAPA SYBR FAST One-Step Qrt-PCR Master Mix (2x) Universal (Kapa Biosystem), and SensiFASTTM SYBR® No-ROX One-Step Kit (Meridian Bioscience). Curcumin used in this study was obtained from Punca Loka Nusantara, with a purity of less than 95%. The sea mineral concentrate was sourced from Pamekasan, Madura, with sampling points located approximately 500–750 m from the coastline and at a depth of about 1–1.1 m [24,25].
Suspension preparation
In preparing the combination of sea mineral concentrate and curcumin, CMM and curcumin were weighed according to the amounts specified in Table 1. Curcumin was then dissolved using Span 60, and CMM and distilled water were added. The mixture was stirred and heated until a homogeneous solution was formed, and then distilled water was added to make up to 30 ml.
 | Table 1. Suspension formula of curcumin and CMM mix. [Click here to view] |
In vitro cytotoxicity
Cell culture
The DU145 prostate cancer cell line and the HEK-293 normal cell line were grown in DMEM with 10% FBS, 100 IU/ml penicillin, and 10 µg/ml streptomycin, maintained at 37°C in a 5% CO2 environment. The flasks were incubated until cells reached 80%–90% confluence (~48–72 hours). Cells were harvested by adding 1–2 ml of 0.25% trypsin for 3–5 minutes at 37°C. The trypsinized cells were transferred to conical tubes, and DMEM was added to 10 ml. Cells were centrifuged for 5 minutes at 2,000 rpm. Following removal of the supernatant, the pellet was resuspended in 1 ml of medium, and cell numbers were measured with a hemocytometer.
Cell treatment
The test samples were assessed for their effects on the DU145 prostate cancer cell line and the HEK-293 normal cell line using the WST-8 assay. The cells were grown in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were plated in 96-well plates and incubated at 37°C with 5% CO2 for 24 hours. After this incubation period, the medium was replaced with fresh culture medium, and the samples, in varying concentrations, were introduced, with cisplatin as a positive control and DMSO as a blank. Following another 24 hours incubation, WST-8 reagent was added, and the plates were incubated for 2–4 hours. Absorbance was then measured at 450 nm using a Tecan Infinite spectrophotometer. The cell viability rate was calculated based on Equation 1.
Quantitative real-time PCR (qRT-PCR) analysis
RNA Isolation
Cell isolation using GeneZolTM follows the protocol below. Cells that have undergone seeding and treatment were subsequently detached and harvested by adding 750 µl of Ribozol to the surface of the 6-well plate. Pipetting was performed to resuspend the Ribozol and ensure the cells were uniformly detached from the plate surface. Cells harvested with Ribozol were then transferred to a 1.5 ml microtube for storage at −80°C or for immediate isolation.
The next step was phase separation, where 100 µl of chloroform was added to the cell-Ribozol mixture and vortexed for 30 minutes until homogeneous. The lysate was then centrifuged at 12,000–16,000 g for 15 minutes. After centrifugation, three distinct phases were observed: an aqueous phase (transparent), containing RNA; an interphase (white), containing DNA; and an organic phase (pink), consisting of Ribozol, chloroform, organic molecules like lipids, carbohydrates, and proteins separated after cell lysis. The aqueous phase containing RNA was transferred to a new sterile 1.5 ml microtube, while the remaining microtube was added with 100 µl of nuclease free water (NFW) and centrifuged again at the same speed and duration. The newly formed aqueous phase was combined with the previous one in the same microtube.
Next, RNA precipitation was performed by adding isopropanol in a volume equal to the volume of the aqueous phase obtained. The microtube was vortexed and homogenized by inverting several times, then incubated for 10 minutes. After incubation, the microtube was centrifuged at 12,000–16,000 g for 10 minutes. The RNA pellet was formed at the bottom and sides of the tube. The pellet was washed with 500 µl of ethanol, vortexed, and centrifuged at 12,000–16,000 g for 5 minutes. This RNA washing process was repeated 2–3 times. After the final wash, the supernatant was carefully removed without disturbing the pellet, and the pellet was air-dried for 5–10 minutes. The pellet should not be over-dried to maintain its solubility.
The final step was RNA resuspension or pellet dissolution using 20–50 µl of NFW, depending on the pellet size obtained. If a large pellet was visible, a larger volume of NFW was used for RNA dissolution, while a smaller pellet required less NFW. After adding NFW, the microtube was vortexed for 15 seconds until homogeneous and then incubated at 55°C–60°C for 10–15 minutes to ensure the RNA was fully dissolved in NFW. The isolated RNA sample was then stored at −70°C until used.
Gene expression analysis
The reverse transcription reaction and quantitative analysis were carried out using two kits, namely KAPA SYBR FAST One-Step Qrt-PCR Master Mix (2x) Universal and SensiFASTTM SYBR® No-ROX One-Step Kit with a volume of 20 μl for each reaction. The primer sequences used (Macrogen) can be seen in Table 2.
 | Table 2. Primer PCR used in the gen expression analysis. [Click here to view] |
A final volume of 20 µl KAPA Biosystem (without RNA template) was prepared using PCR-grade water. KAPA SYBR FAST qPCR Master Mix (2X) was added to reach a 1X concentration, accounting for 10 µl. Optional 10 mM dUTP was added at a final concentration of 0.2 mM, contributing 0.4 µl. The forward and reverse primers, each at a concentration of 200 µM, were added in volumes of 0.4 µl. Additionally, 50X KAPA RT Mix was incorporated at a 1X concentration, totaling 0.4 µl. Template RNA (1 pg) was added, contributing 2 µl to the mixture.
The Meridian Bioscience kit (without template RNA) was made by mixing the SensiFASTTM SYBr® No-ROX One-Step Mix at a final concentration of 1X in 10 µl total volume, forward and reverse primers at 400 µM each (0.8 µl each), reverse transcriptase (0.2 µl), RiboSafe RNAse Inhibitor (0.4 µl), and PCR-grade water added to reach a final volume of 18 µl. Template RNA, ranging from 1 pg to 1 µg, was added at 2 µl.
Thermal cycling
The reaction mixture, totaling 18 μl, was aliquoted into PCR tubes. Subsequently, 2 μl of template RNA (ranging from 1 pg to 1 µg RNA per 20 µl) was added to each tube. For the KAPA Biosystem protocol, reverse transcription was conducted at 42°C for 5 minutes to synthesize cDNA. This was followed by enzyme activation at 95°C for 3 minutes to prepare the enzymes for PCR. Denaturation was performed at 95°C for 1–3 seconds to separate the DNA strands. Annealing, extension, and data collection were carried out, with annealing occurring at 60°C for ≥ 20 seconds. On the other hand, using the Meridian Bioscience protocol, reverse transcription was conducted at 45°C for 10 minutes. Enzyme activation followed at 95°C for 2 minutes to ensure optimal enzyme performance. Denaturation was then performed at 95°C for 5 seconds to denature the DNA. Annealing occurred at 60°C for 10 seconds, followed by extension at 72°C for 5 seconds to elongate the DNA strands.
Statistical analysis
The data are expressed as mean ± standard error of the mean. Statistical analysis was performed using GraphPad Prism software version 9.0.0. One-way analysis of variance followed by Tukey’s post hoc test was conducted to assess statistical significance. These analyses evaluated the differences in % survival rate and gene expression values across the groups tested. A p-value of < 0.05 was considered statistically significant.
RESULTS
In vitro cytotoxicity of individual material
The results in Table 3 indicated that curcumin and CMM exhibited better inhibitory activity than cisplatin against DU145 prostate cancer cells, categorizing it as highly active. Compared to their effects on DU145 cells, higher IC50 values of curcumin and CMM against HEK293 cells indicate reduced cytotoxic effects, suggesting that these test samples exert less influence on normal cells than the reference control. Cisplatin demonstrated the lowest IC50 value on HEK293 cells, lower than that observed on DU145 cells, highlighting its significant cytotoxic impact on normal kidney cells. Conversely, CMMs exhibited the highest IC50 value at 49.36 ppm, indicating minimal impact on HEK293 normal cells.
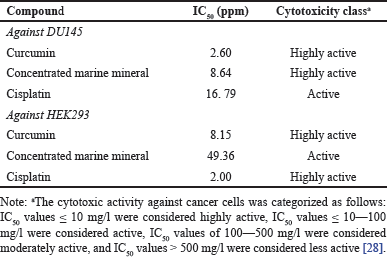 | Table 3. Cytotoxicity of curcumin, CMM, and cisplatin against DU145 and HEK293 cells. [Click here to view] |
In vitro toxicity of mixed curcumin and concentrated marine mineral
In the IC50 test, it was observed that curcumin exhibited stronger effects individually compared to CMM. However, in combination form, the suspension of curcumin and CMM resulted in higher cytotoxicity on prostate cancer cells across all mixture ratios compared to the single curcumin group at all concentrations (12.5, 25, and 50 ppm). This indicates that combining curcumin and CMM therapy is superior to curcumin alone. Increasing the ratio of curcumin in the mixture did not show better toxic effects. However, increasing the concentration of CMM in the mixture ratio demonstrated more potent toxic effects in several formulations across different ratios of curcumin concentrations (Fig. 1A).
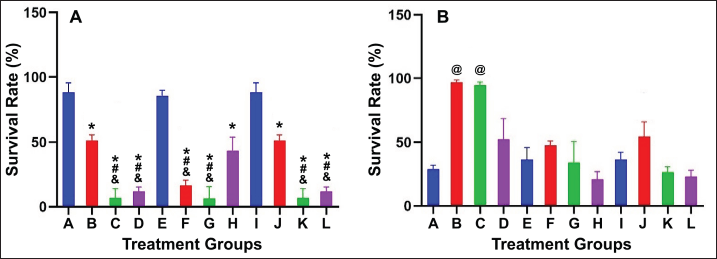 | Figure 1. Cell survival rate (%) of (A) DU145 and (B) HEK293 treated with curcumin and CMM suspension. CMM stands for a CMM. [Click here to view] |
In HEK293 cells, toxic effects were observed in all single curcumin groups at various concentrations. However, cell survival rates significantly increased in the mixture of 12.5 ppm curcumin and 100–500 ppm CMM. This pattern was also observed in groups B, F, and J (Fig. 1B). This indicates that adding CMM can reduce curcumin’s toxic effects on normal cells. Adding more CMM (>500 ppm) did not improve normal cell safety. Therefore, using CMM in combinations needs to ensure appropriate mixture ratios.
Cytotoxic selectivity index
The selectivity of anticancer drugs can be measured by calculating the IC50 of the compound/mixture on normal cells divided by its IC50 value on cancer cells, with a selectivity index >10 indicating high selectivity [29]. Based on Table 4, it can be seen that the selectivity of the suspension of curcumin and CMM mixture significantly increased compared to its single treatments, both at 100 and 500 ppm CMM. The high selectivity of the combined test substances indicates the potential of marine mineral concentrate and curcumin combination as safe chemopreventive agents.
 | Table 4. Cytotoxic selectivity index of curcumin, CMM, and their combination. [Click here to view] |
Downregulation of Cyclin-D, Wnt3a, and C-Myc
Based on IC50 observations and cell survival rates, the optimal dose for killing prostate cancer cells while maintaining normal cell viability was 12.5 ppm curcumin and 100 ppm CMM. However, the dose used at this stage was reduced to ensure more apparent gene expression observations so that the cells do not die and continue expressing their genes. Therefore, the combination dose set was 2.5–5 ppm for curcumin and 5–12.5 ppm for CMM.
In Figure 2, it can be seen that all test samples and positive controls show the ability to reduce the expression of Cyclin-D, Wnt3a, and C-Myc to near-normal values (healthy cells). For Cyclin-D and C-Myc (Fig. 2A and C), although there was a significant decrease, the cisplatin treatment group remains significantly higher compared to the normal control group, with the cisplatin group also substantially higher than all other test groups in terms of C-Myc gene expression. Only the single curcumin group (2.5 and 5 ppm), the single CMM group at 5 ppm, and the combination of 5 ppm curcumin with both doses of CMM (5 and 12.5 ppm) experienced a decrease in the expression of all tested genes. It did not show significant differences from the normal control values. Based on this finding, effective mixture dosage is CMM 5 ppm + curcumin 5 ppm.
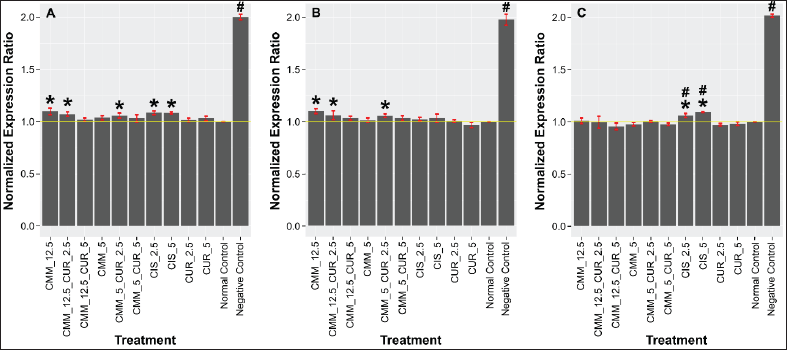 | Figure 2. Impact of curcumin, concentrated deepsea mineral (CMM), and their combination on (A) Cyclin-D1, (B) Wnt3a, and (C) C-Myc level in normalized value. [Click here to view] |
DISCUSSION
Cancer therapy generally can cause mineral deficiencies in the body, leading to side effects associated with these deficiencies, such as diarrhea and other electrolyte disorders [30,31]. The minerals commonly deficient include magnesium, potassium, sodium, zinc, iron, and others [30,32]. Therefore, some studies have been conducted incorporating a single type of mineral in chemotherapy to prevent related side effects. A diet rich in magnesium and calcium during chemotherapy has been reported to reduce the potential for CIPN [19].
CMM contains abundant minerals, with as much as 10.88% pure magnesium that can be isolated [24]. CMM can be obtained through appropriate concentration procedures in a safe, consumable, sterilizable, and non-toxic form [25]. Besides addressing various mineral deficiencies occurring during chemotherapy, CMM also contains several anticancer minerals, such as boron [33,34] and manganese [35,36], which can work synergistically with chemotherapy for both the prevention and treatment of cancer. With these advantages, chemotherapy combined with CMM administration will produce more optimal effects with fewer side effects.
Against prostate cancer (DU145), CMM alone was able to inhibit cell growth by 50% (IC50) with a concentration of only 8.64 ppm (Table 3), categorizing it as a very active anticancer agent, similar to curcumin (IC50 = 2.6 ppm). These results suggest that both CMM and curcumin possess significant cytotoxic effects against prostate cancer cells, making them promising candidates for further development as therapeutic agents. The low IC50 values indicate that these compounds are effective at relatively small concentrations, which is crucial in reducing potential side effects in clinical settings [37]. Moreover, the high potency of CMM, while slightly less than curcumin, still places it in the very active category, showcasing its potential as a powerful natural anticancer compound. Both CMM and curcumin are much more robust than the positive control, cisplatin (IC50 = 16.79 ppm), a widely used chemotherapy drug. Cisplatin’s higher IC50 in DU145 cells suggests that higher drug concentrations are required to achieve the same cytotoxic effect as CMM or curcumin. This is a critical finding, implying that CMM and curcumin could provide anticancer effects at lower doses, potentially reducing the toxic side effects typically associated with higher dosages of conventional chemotherapy like cisplatin. The ability of natural compounds like CMM and curcumin to outperform traditional chemotherapeutic agents, in this context, highlights the potential for developing less harmful but effective treatments for prostate cancer.
In contrast, in normal kidney cells (HEK293), cisplatin alone demonstrated a strong toxic effect, showing the smallest IC50 value compared to curcumin and CMM. This suggests that cisplatin exerts significant cytotoxicity on cancer and healthy, non-cancerous cells. The strong toxicity profile of cisplatin in normal cells correlates with known clinical side effects, such as nephrotoxicity, where kidney damage occurs due to cisplatin treatment [38]. The toxicity in kidney cells from cisplatin therapy causes excessive excretion of minerals, leading to deficiencies and long-term renal complications [39].
On the other hand, CMM and curcumin exhibited much higher IC50 values in HEK293 cells, with CMM having the highest IC50, indicating that it is less toxic to normal cells than cisplatin. The lower toxicity of CMM in non-cancerous cells implies a better therapeutic index, meaning that CMM can target cancer cells more selectively while sparing normal cells. This selective cytotoxicity is biologically relevant, as it highlights the potential of CMM as a safer therapeutic option with fewer off-target effects, particularly in minimizing damage to vital organs such as the kidneys. The high tolerance of normal cells to CMM further underscores its promise as an anticancer agent with reduced systemic toxicity, a critical aspect in developing effective yet safe cancer treatments.
In combination with CMM, the cytotoxicity of curcumin significantly increased against prostate cancer cells, while toxicity to normal cells decreased. This indicates that CMM can enhance the toxic selectivity of curcumin, as shown in Table 4. Increasing the amount of curcumin in the mixture did not show an increase in toxicity, but increasing the amount of CMM (up to ≤ 500 ppm) increased its toxicity. This suggests that CMM can improve the chemotherapy efficiency of curcumin. Several mechanisms might coincide, including the complexation between curcumin and minerals in CMM. It has been reported that curcumin complexes with several minerals can increase its selectivity toward mouse tumor cells [40,41]. Gallium in CMM that can form complexes with curcumin and curcuminoids has also been reported to significantly increase cell uptake [42].
Curcumin, CMM, and cisplatin alone were shown to significantly reduce the expression of Cyclin-D1, Wnt3a, and C-Myc in prostate cancer cells. This downregulation is particularly notable, as these genes play vital roles in the proliferation and survival of cancer cells [43] , making them critical targets in cancer therapy. The observed reduction in gene expression can even reach levels comparable to those in normal cells when prostate cancer cells are treated with a combination of curcumin and CMM. Notably, a 5 ppm curcumin and 5 ppm CMM (1:1) produced the most optimal suppression effect for all the genes tested, underscoring the potential synergistic effect between these two compounds in targeting multiple oncogenic pathways.
Cyclin-D1, Wnt3a, and C-Myc are critical regulators involved in the proliferation and survival of cancer cells, particularly in prostate cancer. Cyclin-D1 plays a crucial role in the transition from the G1 to the S phase of the cell cycle, and its overexpression is linked to uncontrolled cell proliferation in various cancers [44] . The downregulation of Cyclin-D1 by natural compounds like curcumin and CMM suggests that these agents may induce cell cycle arrest, limiting cancer cell growth while avoiding the side effects of traditional chemotherapeutics. Similarly, Wnt3a, a pivotal component of the Wnt/β-catenin pathway, is frequently dysregulated in prostate cancer, leading to enhanced tumor growth and metastasis [45] . The reduction of Wnt3a expression by curcumin and CMM highlights their potential to disrupt Wnt signaling, thereby inhibiting tumorigenesis. Moreover, the downregulation of C-Myc, a critical oncogene associated with aggressive prostate cancer, further underscores the anticancer potential of curcumin and CMM. By targeting C-Myc, these compounds may decrease cancer cells’ proliferative and metabolic activity, offering a multi-faceted approach to suppress tumor progression [46].
The study highlights the importance of combination therapy, as the 5 ppm curcumin and 5 ppm CMM ratio produced the most robust suppression of all three genes tested. This synergistic effect likely arises from the ability of curcumin and CMM to target multiple pathways simultaneously, which may be more effective than higher doses of either compound used alone. For instance, CMM at higher doses (12.5 ppm) alone did not significantly inhibit the expression of Cyclin-D1 and Wnt3a (p > 0.05), underscoring the dose-dependent nature of CMM’s therapeutic effects. However, when combined with 5 ppm curcumin, the downregulation of these genes became significant, highlighting the advantage of combination therapy in achieving more potent anticancer effects with lower doses. This dose dependency and the synergistic relationship between curcumin and CMM are biologically significant because they point to a potential therapeutic strategy that maximizes efficacy while minimizing toxicity. Lower effective doses can reduce the risk of adverse effects commonly seen with higher concentrations of chemotherapy agents like cisplatin. Additionally, targeting multiple oncogenic pathways—Cyclin-D1, Wnt3a, and C-Myc—through combination therapy provides a multi-faceted attack on cancer cells, reducing the likelihood of resistance developing and improving the overall treatment outcome.
Furthermore, the fact that these natural agents can achieve significant gene suppression at relatively low doses highlights their potential as low-toxicity alternatives to traditional chemotherapies. Their ability to selectively target cancer cells while exhibiting reduced cytotoxicity in normal cells (as evidenced by their higher IC50 values in normal cells) further underscores the promise of curcumin and CMM as components of prostate cancer therapy. Future studies could explore their role in combination with other therapies to enhance treatment efficacy while minimizing side effects, potentially leading to more holistic and targeted cancer treatment regimens. Although these are preliminary results, an in vivo test and clinical studies are necessary to validate our findings.
CONCLUSION
In conclusion, this study demonstrates the potential of combining curcumin and CMM as an effective treatment strategy for prostate cancer. Both compounds show significant cytotoxic effects against DU145 prostate cancer cells, with curcumin exhibiting similar potency to cisplatin but less toxic to normal cells. Combining curcumin and CMM enhances their anticancer effects while reducing harm to healthy cells, suggesting improved therapeutic selectivity compared to cisplatin. Additionally, the synergistic downregulation of key cancer-related genes (Cyclin-D1, Wnt3a, and C-Myc) further supports the efficacy of this combination in targeting multiple pathways involved in prostate cancer progression.
Future studies should focus on optimizing the curcumin-CMM combination, exploring different ratios to maximize the therapeutic benefits while minimizing side effects. In vivo animal model validation is also necessary to confirm these findings in a more complex biological setting. Additionally, further investigation into the molecular mechanisms behind the gene suppression observed in this study will provide deeper insights into how curcumin and CMM work together to combat cancer, which could lead to the development of more targeted therapies.
Integrating natural compounds like curcumin with mineral-rich supplements such as CMM represents a promising new direction for prostate cancer treatment. These findings lay the groundwork for future research, potentially leading to safer, more effective therapeutic options that reduce toxicity while enhancing treatment outcomes for prostate cancer patients.
LIST OF ABBREVIATIONS
AR, Androgen receptor; CIPN, Chemotherapy-induced peripheral neuropathy; CMM, Concentrated marine mineral; DMEM, Dulbecco’s modified eagle medium; NFW, Nuclease free water; Pca, Prostate cancer; PSA, Prostate-specific antigen
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
We want to acknowledge that this work is supported and funded by the Hibah Kerjasama Dalam Negeri. Research Grants of the Direktorat Akademik Pendidikan Tinggi Vokasi, Direktorat Jendral Pendidikan Vokasi, Kementrian Pendidikan, Kebudayaan, Riset, dan Tekhnologi (DIPA-023.18.1.690524/2023).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Barsouk A, Padala SA, Vakiti A, Mohammed A, Saginala K, Thandra KC, et al. Epidemiology, staging and management of prostate cancer. Med Sci. 2020 Jul 20;8(3):28. CrossRef
2. James ND, Tannock I, N’Dow J, Feng F, Gillessen S, Ali SA, et al. The Lancet Commission on prostate cancer: planning for the surge in cases. Lancet. 2024 Apr;403(10437):1683–722. CrossRef
3. Kalathil AA, Guin S, Ashokan A, Basu U, Surnar B, Delma KS, et al. New pathway for cisplatin prodrug to utilize metabolic substrate preference to overcome cancer intrinsic resistance. ACS Cent Sci. 2023 Jul 26;9(7):1297–312. CrossRef
4. Catapano J, Luty M, Wróbel T, Pudelek M, Piwowarczyk K, Kedracka-Krok S, et al. Acquired drug resistance interferes with the susceptibility of prostate cancer cells to metabolic stress. Cell Mol Biol Lett. 2022 Dec 18;27(1):100. CrossRef
5. Nakazawa M, Paller C, Kyprianou N. Mechanisms of therapeutic resistance in prostate cancer. Curr Oncol Rep. 2017 Feb 22;19(2):13. CrossRef
6. Jernberg E, Bergh A, Wikström P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr Connect. 2017 Nov;6(8):R146–61. CrossRef
7. Le TK, Duong QH, Baylot V, Fargette C, Baboudjian M, Colleaux L, et al. Castration-resistant prostate cancer: from uncovered resistance mechanisms to current treatments. Cancers (Basel). 2023 Oct 19;15(20):5047. CrossRef
8. Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002 Mar;9(1):61–73. doi: https://doi.org/10.1677/erc.0.0090061 CrossRef
9. Das S. Phytochemicals modify the action of cancer cells mitochondrial drug-resistance mechanism. Sci Pharm. 2023 Jul 14;2(3):79–105. CrossRef
10. Mathur A, Abd Elmageed ZY, Liu X, Kostochka ML, Zhang H, Abdel-Mageed AB, et al. Subverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells. PLoS One. 2014 Aug 14;9(8):e103109. CrossRef
11. Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007 Oct 4;2:10. CrossRef
12. Deeb D, Xu YX, Jiang H, Gao X, Janakiraman N, Chapman RA, et al. Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther. 2003 Jan;2(1):95–103.
13. Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004 Jul;3(7):803–12. CrossRef
14. Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004 Feb 26;23(8):1599–607. CrossRef
15. Ide H, Lu Y, Yamaguchi R, Muto S, Horie S. Abstract 226: Chemopreventive potential of curcumin in prostate cancer. Cancer Res. 2014 Oct 1;74(19_Supplement):226. CrossRef
16. Pan L, Sha J, Lin W, Wang Y, Bian T. Curcumin inhibits prostate cancer progression by regulating the miR-30a-5p/PCLAF axis. Exp Ther Med. 2021 Jul 7;22(3):969. CrossRef
17. Venturelli S, Leischner C, Helling T, Renner O, Burkard M, Marongiu L. Minerals and cancer: overview of the possible diagnostic value. Cancers (Basel). 2022 Feb 28;14(5):1256. CrossRef
18. Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer. 2002 Sep;2(9):694–704. CrossRef
19. Wesselink E, Winkels R, Van Baar H, Geijsen A, Van Zutphen M, Van Halteren H, et al. Dietary intake of magnesium or calcium and chemotherapy-induced peripheral neuropathy in colorectal cancer patients. Nutrients. 2018 Mar 23;10(4):398. CrossRef
20. Workeneh BT, Uppal NN, Jhaveri KD, Rondon-Berrios H. Hypomagnesemia in the cancer patient. Kidney360. 2021 Jan;2(1):154–66. CrossRef
21. Mohd Nani SZ, Majid FAA, Jaafar AB, Mahdzir A, Musa MN. Potential health benefits of deep sea water: a review. Evidence-Based Complement Altern Med. 2016;2016:1–18. CrossRef
22. Kim S, Chun SY, Lee DH, Lee KS, Nam KS. Mineral-enriched deep-sea water inhibits the metastatic potential of human breast cancer cell lines. Int J Oncol. 2013 Nov;43(5):1691–700. CrossRef
23. Kotagale NR, Charde PB, Helonde A, Gupta KR, Umekar MJ, Raut NS. Studies on bioavailability enhancement of curcumin. Int J Pharm Pharm Sci. 2020 Feb; 12(2):20–5. CrossRef
24. Sriwidodo, Abd. Kakhar Umar, Ega Megawati, Maria Elvina Tresia Butarbutar, Nasrul Wathoni, Syafika Alaydrus. Physicochemical characterization of concentrated mineral and magnesium isolate of sea water pamekasan madura. Int J Res Pharm Sci. 2020 Dec 21;11(SPL4):2154–7. CrossRef
25. Alaydrus S, Umar AK, Sriwidodo S, Diantini A, Wathoni N, Amalia R. Characterization and acute oral toxicity of concentrated minerals of Pamekasan Madura seawater. J Adv Pharm Technol Res [Internet]. 2021;12(3):305–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34345612 CrossRef
26. Jiang X, Li S, Qiu X, Cong J, Zhou J, Miu W. Curcumin inhibits cell viability and increases apoptosis of SW620 human colon adenocarcinoma cells via the caudal type homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit. 2019;25. doi: https://doi.org/10.12659/MSM.918364 CrossRef
27. Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, et al. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol. 2022;6(65):eabc6424. CrossRef
28. Gusungi DE, Maarisit W, Hariyadi H, Potalangi NO. Studi Aktivitas Antioksidan Dan Antikanker Payudara (MCF-7) Ekstrak Etanol Daun Benalu Langsat Dendrophthoe pentandra. Biofarmasetikal Trop. 2020 May 11;3(1):166–74. CrossRef
29. Indrayanto G, Putra GS, Suhud F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst Excip Relat Methodol. 2021;46:273–307. CrossRef
30. Awan A, Basulaiman B, Stober C, Clemons M, Fergusson D, Hilton J, et al. Oral magnesium supplements for cancer treatment-induced hypomagnesemia: results from a pilot randomized trial. Heal Sci Reports. 2021 Dec 14;4(4):e443. CrossRef
31. Oronsky B, Caroen S, Oronsky A, Dobalian VE, Oronsky N, Lybeck M, et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol. 2017 Nov 20;80(5):895–907. CrossRef
32. Ezoe Y, Mizusawa J, Katayama H, Kataoka K, Muto M. An integrated analysis of hyponatremia in cancer patients receiving platinum-based or nonplatinum-based chemotherapy in clinical trials (JCOG1405-A). Oncotarget. 2018 Jan 19;9(5):6595–606. CrossRef
33. Kulkarni S, Bhandary D, Singh Y, Monga V, Thareja S. Boron in cancer therapeutics: an overview. Pharmacol Ther. 2023 Nov;251:108548. CrossRef
34. Scorei RI, Popa R. Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anticancer Agents Med Chem. 2010 May 1;10(4):346–51. CrossRef
35. Robbins D, Zhao Y. Manganese superoxide dismutase in cancer prevention. Antioxid Redox Signal. 2014 Apr;20(10):1628–45. CrossRef
36. Wang Y, Zhong D, Xie F, Chen S, Ma Z, Yang X, et al. Manganese phosphate-doxorubicin-based nanomedicines using mimetic mineralization for cancer chemotherapy. ACS Biomater Sci Eng. 2022 May 9;8(5):1930–41. CrossRef
37. Abdel-Tawab M. Considerations to be taken when carrying out medicinal plant research—what we learn from an insight into the IC50 values, bioavailability and clinical efficacy of exemplary anti-inflammatory herbal components. Pharmaceuticals. 2021 May 6;14(5):437. CrossRef
38. Aldossary SA. Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J. 2019 Mar 28;12(1):07–15. CrossRef
39. Hayati F, Hossainzadeh M, Shayanpour S, Abedi-Gheshlaghi Z, Beladi Mousavi SS. Prevention of cisplatin nephrotoxicity. J Nephropharmacology. 2016;5(1):57–60.
40. Mohammadi K, Thompson KH, Patrick BO, Storr T, Martins C, Polishchuk E, et al. Synthesis and characterization of dual function vanadyl, gallium and indium curcumin complexes for medicinal applications. J Inorg Biochem. 2005 Nov;99(11):2217–25. CrossRef
41. Pucci D, Bellini T, Crispini A, D’Agnano I, Liguori PF, Garcia-Orduña P, et al. DNA binding and cytotoxicity of fluorescent curcumin-based Zn(ii) complexes. Medchemcomm. 2012;3(4):462. CrossRef
42. Ferrari E, Benassi R, Sacchi S, Pignedoli F, Asti M, Saladini M. Curcumin derivatives as metal-chelating agents with potential multifunctional activity for pharmaceutical applications. J Inorg Biochem. 2014 Oct;139:38–48. CrossRef
43. Liao DJ, Thakur A, Wu J, Biliran H, Sarkar FH. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog. 2007;13(2):93–158. CrossRef
44. Montalto FI, De Amicis F. Cyclin D1 in Cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 2020 Dec 9;9(12):2648. CrossRef
45. Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol. 2012 Aug 19;9(8):418–28. CrossRef
46. Truica MI, Burns MC, Han H, Abdulkadir SA. Turning up the heat on MYC: progress in small-molecule inhibitors. Cancer Res. 2021 Jan 15;81(2):248–53. CrossRef