INTRODUCTION
Diabetes mellitus (DM) is a persistent metabolic condition that causes severe damage to vital organs. Projections suggest that the global prevalence of DM will exceed 300 million individuals by 2025 [1]. While there is no cure for diabetes, managing it through a balanced diet, regular exercise, and proper medication can keep it under control [2]. Diabetic nephropathy (DN), an important complication of DM, has become a health concern and the primary cause of chronic kidney disease in developed countries [3]. The prevalence of DN is approximately 15%–25% in patients with type 1 and 30%–40% in those with type 2 diabetes. DN progression is influenced by multiple factors, including elevated blood glucose levels, hypertension, abnormal lipid levels, release of inflammatory cytokines, and oxidative stress [4].
The pathology of DN includes fibrosis arising as a result of excessive deposition of the extracellular matrix (ECM) by myofibroblasts. Although the origin of kidney fibroblasts is not well defined, resident fibroblasts, pericytes, fibrocytes, and those arising from the epithelial-mesenchymal transition (EMT) may generate fibroblasts. EMT is a potential mechanism in chronic renal insufficiency pathogenesis because injured renal tubular cells can transform into mesenchymal cells, contributing to fibrosis [5]. Growth factors such as transforming growth factor-β (TGF-β) and basic fibroblast growth factor-2 can induce epithelial cells to lose epithelial characteristics, gain mesenchymal traits, increase ECM components, and produce matrix metalloproteinases [6].
EMT was initially linked to fibrogenesis approximately 15 years ago, following the discovery of renal tubular epithelial cells that exhibited abnormal expression of fibroblast-specific protein-1 (FSP-1) [7]. In inflamed and fibrotic kidneys, the concurrent upregulation of FSP-1 in the interstitium and damaged tubular epithelial cells is considered key evidence for the transition of these epithelial cells into mesenchymal cells and their subsequent migration into the interstitial space as (myo)fibroblasts [8]. Thus, the FSP-1 protein serves as a valuable marker for identifying EMT and detecting myofibroblasts in diseased kidneys [9].
Despite the presence of pharmaceutical interventions that impede the advancement of DN, there is growing interest in incorporating herbal remedies to prevent the onset of this complication [10]. A recent recommendation by the WHO Expert Committee emphasized the significance of investigating plant-derived hypoglycaemic agents, as traditional medicinal plants are known to have fewer adverse effects than synthetic pharmaceuticals [11]. India possesses a diverse repository of medicinal flora. Some of these botanical sources have been subjected to scientific scrutiny, including Strychnos potatorum, a moderate-sized tree species. High performance thin layer chromatography analysis conducted by our research group demonstrated the presence of terpenoids, tannins, flavonoids, phenols, and alkaloids in the ethanolic extract of Strychnos potatorum seeds. Gas chromatography-mass spectrometry analysis revealed the presence of 17 metabolites, including Imidazole 2-fluoro-1-triacetylribofuranosyl, Dispiro bicyclo heptane, Thiophene,2,5-dihydro, 2,3,5,6-Diacetone-4-O-methylmannitol, 1,4, Hexadiene,4-methyl, Pthalic acid, 2-Pyridicarbonitrile, and 3-nitro (data not provided) consistent with previous studies [12]. Notably, all S. potatorum samples contained the alkaloid diaboline. In Ayurveda, its seeds are employed in conditions such as strangulation, urinary incontinence, and disorders of the head and neck [13]. According to the Unani medical system, seeds are beneficial for the management of liver and renal diseases, gonorrhoea, and colic. The seeds are bitter, with astringent effects on the bowels, aphrodisiac, tonic, and diuretic. Acute and chronic toxicity studies in Wistar albino mice and rats using aqueous extract and powder of S. potatorum seeds demonstrated safety at doses of up to 2,000 mg/kg body weight. Long-term toxicity assessments revealed that administration of 100–200 mg/kg of the same over 90 days did not cause significant changes compared with the control group [14].
The antidiabetic and nephroprotective effects of S. potatorum have been extensively documented in the literature [2,15]. Despite extensive research on S. potatorum, its effects on DN remain unclear. Furthermore, the plant’s potential to regulate kidney markers, especially FSP-1, has not been extensively explored. This study aimed to evaluate the effect of the ethanolic extract of S. potatorum seeds (EESP) on DN by measuring the expression of FSP-1, which inhibits EMT and restores the slit diaphragm in the renal tissue. The effects of EESP were compared with those of the standard drug metformin (MET), which has established antifibrotic properties.
MATERIALS AND METHODS
Preparation of plant extract
The seeds of S. potatorum, in their dried form, were obtained from a nearby marketplace and subsequently verified and authenticated by the Siddha Central Research Institute, Arumbakkam (reference number 339.18082201 dated 18/08/2022). Four kilograms of desiccated S. potatorum seeds were coarsely milled. Two kilograms of the coarse powder were soaked in 95% ethanol (5:1 v/w) and left undisturbed at 20°C for 2 days. After filtration, the resulting liquid was transferred to the extraction chamber of a Soxhlet apparatus. Four extraction cycles were conducted per hour over a 12-hours period out to allow better equilibration between the solvent and the sample and to maximize the extraction of soluble compounds resulting in a higher yield. Following the extraction process, a rotary evaporator was used to eliminate the solvent, resulting in a dehydrated extract with a mass of approximately 500 g (yield 25% w/w). The resulting powder was stored in a sealed container for further analysis [16].
Experimental animals
Prior approval was obtained from the Institutional Animal Ethics Committee (IAEC 1/ Proposal:105/A. Lr: 78, Dt: 13.02.2023). Thirty adult male Wistar albino rats (250–300 g) were maintained at an institutional animal facility. One week, the animals were left undisturbed to acclimatize to the laboratory settings. The animals were maintained at a temperature of 25°C ± 1°C and subjected to a 12-hours light-dark cycle and relative humidity levels ranging from 30% to 70%. During the entire experiment, the rats were housed in polypropylene cages (3 per cage) with corncobs as bedding material and provided with continuous and unlimited access to both food and water. The research was conducted in accordance with the guidelines established by the Committee for the Purpose of Control and Supervision of Experiments on Animals. The study included 30 rats, which were divided into five groups, each containing six animals.
I - Control
II - EESP (500 mg/kg body weight)
III - DN
IV - DN + EESP (500 mg/kg body weight)
V - DN + MET (50 mg/kg body weight)
Induction of diabetes
Experimental diabetes was induced according to the procedure outlined by Punithavathi et al. [17]. Initially, fasting blood glucose levels were assessed in overnight-fasted animals to ensure that they were within the normal range before diabetes induction. To induce diabetes, a single intraperitoneal injection of streptozotocin (STZ) was administered at a dose of 50 mg/kg body weight in a cold citrate buffer (0.1M, pH 4.5). To prevent mortality from hypoglycemia, diabetes-induced animals were administered a 20% glucose solution for two days. The bedding material was replaced daily to maintain appropriate hygiene standards. Diabetes was confirmed in animals whose fasting blood glucose reached or exceeded 250 mg/dl, 72 hours after STZ administration.
Administration of experimental drugs
For 30 days, 500 mg/kg body weight EESP was administered through oral gavage to animals in groups II and IV. Concurrently, animals in group V were orally administered MET (50 mg/kg body weight) for the same duration.
Sample collection
Upon completion of the experiments, the animals were humanely euthanized using halothane [18], and their kidney tissues were dissected. One kidney was preserved in a 10% formaldehyde solution for histopathological assessment, and the other was stored at −80°C for gene and protein expression analyses. Retroorbital venous blood samples (2 ml) were collected and stored at 4°C for biochemical analysis.
Physical and biochemical parameters
Body weight was recorded at weekly intervals. Fasting blood glucose levels were quantified with a glucometer using tail-vein blood samples. Serum urea and creatinine levels (Jaffee method) were determined using kits procured from Cusa Bioscience (USA) [19,20]. Other parameters, including total cholesterol, triglycerides, albumin, and proteins, were analyzed using enzymatic colorimetric methods using commercial kits (Life Span Biosciences, India). A rat insulin ELISA kit (Cal Biotech, USA) was used to evaluate serum insulin levels [2].
Histopathology and Immunohistochemistry (IHC)
4-μm paraffin sections were taken from formalin-fixed kidney samples and stained with Masson’s trichrome to examine collagen deposition, fibrosis, and ECM. Kidney tissue sections (8 µm) underwent antigen retrieval with hot citrate buffer (0.01 mol/l, pH 6.0) at 85°C for 5 minutes. Nonspecific binding was inhibited by treating the slides with 5% normal goat serum for 1 hour at room temperature. Immunohistochemical protocols were adapted from the study by Tsai et al. [21]. Briefly, the sections were incubated overnight at 4°C with a 1:1,000 dilution of rabbit monoclonal anti-FSP-1 primary antibody (Abcam, USA, #ab220213). The sections were then exposed to a secondary antibody, anti-rabbit IgG, at a dilution of 1:1,000 for one hour at 37°C. Following hematoxylin counterstaining, slides were examined and photographed under a microscope (Olympus BX51, Japan). The intensity of blue color-stained collagen fibers (renal fibrosis) and brown-colored FSP-1 expression were quantified from Masson’s trichrome and IHC slides, respectively, using the deconvolution plugin in ImageJ software according to the methods described by Sharun et al. and Crowe et al. [22,23]. Ten random sections were selected for each animal in each group. Five random fields were examined in each section. Fifty fields were observed per animal.
Gene expression
Total RNA was isolated from kidney tissues preserved at 80°C using TRIzol reagent (Invitrogen, USA). By utilizing 0.5 µg of total RNA, cDNA was synthesized using the QI adenosine monophosphate (AMP) DNA Mini kit (Qiagen, USA) and amplified in a polymerase chain reaction (PCR) system (ABI PRISM 7000, USA) using the following primers: Fsp-1 forward 5?-CTGGCCACATTCATCAACTG-3´, reverse 5´-CTTCTTCAGGGCCTTGTTGTAG-3´; β-Actin forward 5´-GCTTCTGGGTTCCGATGATA-3´, reverse 5´-CCTGGCACACCATCATCTTG-3´. Agarose gel electrophoresis was used to separate and visualize the PCR products. The intensity of the resulting bands was quantified using Gel Pro Analyzer software (version 4.0), implementing the ΔCT method for analysis. β-Actin was used as an endogenous control. FSP-1 gene expression across different experimental groups was standardized relative to β-Actin expression.
Western blot analysis
Homogenized kidney samples were lysed, quantified, and subjected to western blot analysis, following established protocols. Using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the protein samples underwent separation and were then transferred to a polyvinylidene difluoride membrane. The samples were then blocked with 5% calf serum albumin before being exposed to primary rabbit monoclonal antibodies targeting FSP-1 (Abcam, USA, #ab220213) and GAPDH (Abcam, USA, #ab199554) at a 1:1,000 dilution for 2 hours. Following the washing step, the blots underwent incubation with a secondary antibody labeled with HRP for 1 hour at ambient temperature. An enhanced chemiluminescence reagent was used to visualize protein expression, and the intensity of the bands was quantified through densitometry using the BIO-1D image analysis software.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was conducted using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test to assess significant differences among the experimental groups. A p value below 0.05 was considered statistically significant. All analyses were performed using SPSS software (version 29.0.2.0).
RESULTS
Effect on body weight
Following diabetic induction, groups III–V animals exhibited weight loss in the first week. Untreated diabetic rats gradually lost weight during the study period. However, after treatment with EESP, at the end of the experiment, group IV animals gained significantly more weight (p = 0.02) than did group III (Fig. 1). MET treatment (Group V) produced a better effect (p = 0.005) than EESP, with body weight approaching that of the control animals by the end of the study.
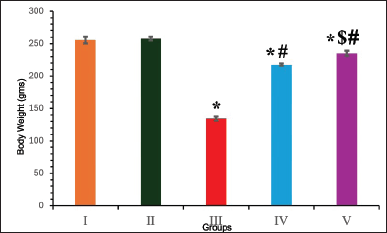 | Figure 1. Body weight of animals in different groups at the end of 4th week. Results are expressed as mean ± SD (n = 6). Superscript indicates a significant difference between groups based on Tukey’s post-hoc test following one-way ANOVA (p ≤ 0.05). *Compared with group I; #compared with group III; $compared with group IV. [Click here to view] |
Effect on blood glucose and insulin
In the DN group, a significant elevation in blood glucose levels (298.67 mg/dl) and a decline in insulin secretion (11.38 ng/dl) were noted, in contrast to the control group. Conversely, in the DN groups treated with EESP and MET, these levels were effectively restored to near-normal (Fig. 2). The results indicated that MET exerted a more pronounced effect than that of EESP (p = 0.001). This observation suggests that EESP is capable of controlling hyperglycemia by increasing insulin levels.
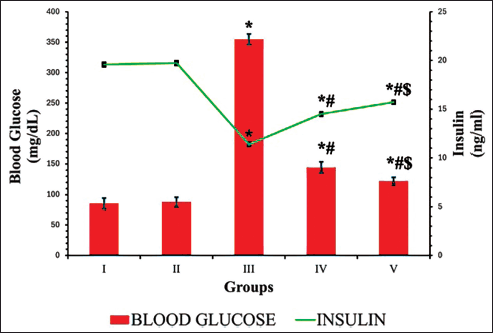 | Figure 2. Fasting blood glucose and insulin levels at the end of 4th week. Results are expressed as mean ± SD (n = 6). Superscript indicates a significant difference between groups based on Tukey’s post-hoc test following one-way ANOVA (p ≤ 0.05). *Compared with group I; #compared with group III; $compared with group IV. [Click here to view] |
Effect on other biochemical parameters
It is evident from Table 1 that no discernible difference was observed between groups I and II. Conversely, the untreated diabetic group III demonstrated significantly elevated levels of serum urea, creatinine, blood urea nitrogen (BUN), total cholesterol, and triglycerides and reduced serum albumin and protein levels when juxtaposed with the control group. The administration of EESP and MET reversed these alterations, which were subsequently determined to be statistically significant compared with those in group III. Interestingly, EESP treatment resulted in a more pronounced reduction in urea (p = 0.004), creatinine (p = 0.003), BUN (p = 0.04), and albumin (p = 0.002) levels than MET treatment. However, MET treatment was more effective in reducing triglyceride levels (p = 0.04) than ESSP. This observation indicates that EESP has the potential to ameliorate the disturbances in protein metabolism and renal function attributed to diabetes and DN.
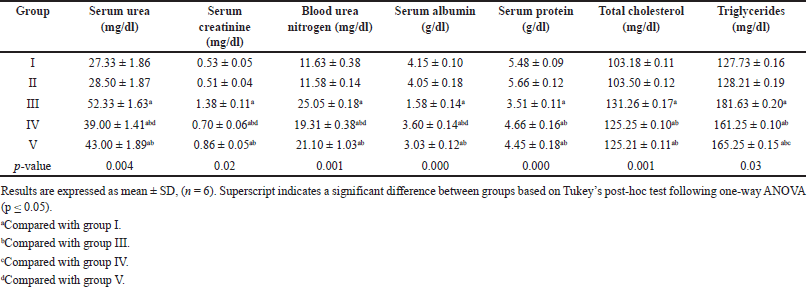 | Table 1. Results of serum biochemical parameters in different groups at the end of 4 weeks. [Click here to view] |
Trichrome staining and FSP-1 IHC
Trichome staining was employed to show collagen deposition in the renal tissue, wherein collagen fibers appeared blue, nuclei stained black, and the cytoplasm displayed a red/pink hue, as depicted in Figure 3. Remarkable increases in collagen content (87%), periglomerular fibrosis, and interstitial spaces were observed in untreated DN rats (Figs. 3.a3 and 4b). Collagen deposition, thickening, and vacuolization of the glomerular basement membrane indicate mesangial expansion. Conversely, the diabetes-induced group treated with EESP and MET displayed 42% and 30% fibrosis, respectively, with near-normal glomerular structures and reduced distribution of collagen fibers within the renal interstitium (Figs. 3.a4,a5 and 4b), showing restoration of normal architecture and offset mesangial expansion. Furthermore, FSP-1 expression was very high in untreated diabetic rats (Group III), supporting EMT, which was validated by fibrosis seen in trichrome staining (Figs. 3.b3 and 4a). Diabetic rats treated with EESP had a lower expression of FSP-1, indicating the protective effect of S. potatorum in halting EMT and subsequent fibrosis (Fig. 3.b4). Comparable results were observed in group V animals treated with MET.
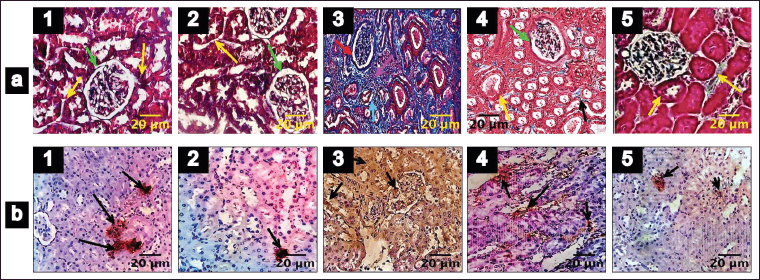 | Figure 3. Trichrome staining and FSP-1 IHC of renal tissues (Magnification 400X). 1–5 indicates groups I–V. (a) Trichrome staining of the renal tissues. (b) FSP-1 IHC. Scale bar = 20 µm, n = 6. Green arrow: renal corpuscles; yellow arrow: dilated renal tubules; orange arrow: normal distribution of collagen fibers in the renal interstitium; red arrow: collagen fibers deposited in renal corpuscles; magenta arrow: widening of interstitium and fibrosis; cyan arrow: atrophy of renal tubules; black arrow: widening of interstitium is reduced and less collagen fibrosis; Black arrow in b: FSP-1 positive cells. [Click here to view] |
Effect on Fsp-1 gene expression
The animals in Group III displayed a notable increase in Fsp-1 expression under diabetic conditions (p = 0.03). Nonetheless, the mRNA expression of Fsp-1 declined in the diabetic group treated with EESP (group IV), leading to a significant reduction in gene expression (p = 0.003) compared to that in group III. Similarly, the DN + MET group showed a significant reduction (p = 0.004) in the expression of Fsp-1 relative to that in the DN-untreated and EESP-treated groups. Groups I and II exhibited minimal Fsp-1 expression (Fig. 5).
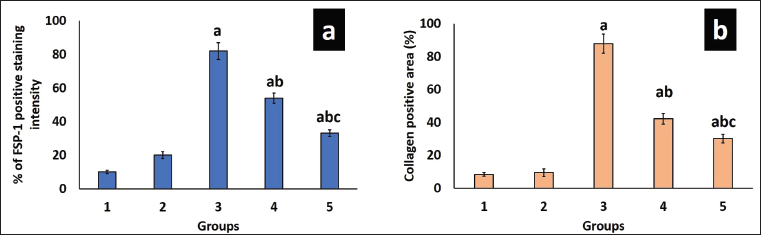 | Figure 4. Analysis of FSP-1 and collagen staining intensity. (a) shows the staining intensity of FSP-1 expression in various groups. (b) shows the staining intensity of collagen fibers seen in Masson’s trichrome slides of different groups. Values are represented as the mean ± SD (n = 6). Superscripts indicate significant differences between groups based on Tukey’s post-hoc test following one-way ANOVA (p ≤ 0.05). a = comparison with group I; b = comparison with group III; c = comparison with group IV. [Click here to view] |
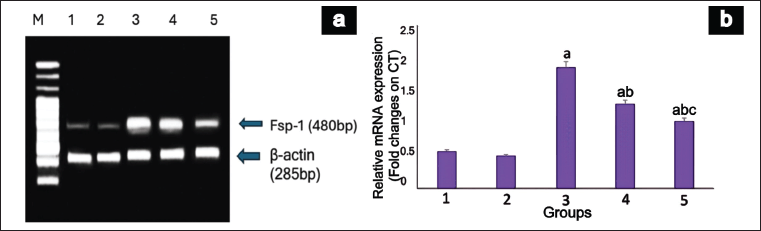 | Figure 5. mRNA expression of Fsp-1 gene in rat kidney. (a) shows a representative gel image of Fsp-1 expression in the various groups. M: Marker Lane (100-1,000 Bp), 1: Group I, 2: Group II, 3: Group III, 4: Group IV, 5: Group V. (b) shows the relative Fsp-1 expression levels in the different groups. Values are represented as the mean ± SD (n = 6). Superscripts indicate a significant difference between groups based on Tukey’s post-hoc test following one-way ANOVA (p ≤ 0.05). a = comparison with group I; b = comparison with group III; c = comparison with group IV. [Click here to view] |
Effect on FSP-1 western blot
Western blot analysis of FSP-1 protein revealed a notable discrepancy among the various groups, as illustrated in Figure 6. Protein expression was significantly upregulated (p = 0.02) in group III when juxtaposed with group I. Notably, the animals in groups IV and V exhibited remarkably diminished levels of FSP-1 protein compared to group III. MET demonstrated a more pronounced reduction (p = 0.004) in FSP-1 protein expression than EESP, suggesting a stronger protective effect against renal fibrosis in DN.
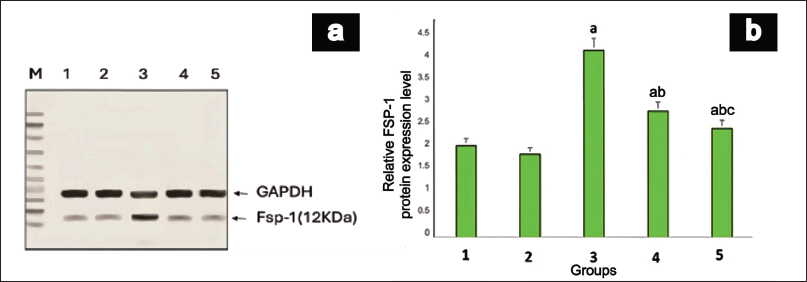 | Figure 6. FSP-1 Protein expression in rat kidney. (a) shows a representative gel image of FSP-1 protein expression in the various groups. Lane M: Marker Lane (10-100kDa); 1: Group I, 2: Group II, 3: Group III, 4: Group IV, 5: Group V. (b) shows the relative FSP-1 protein expression levels in different groups. Values are represented as the mean ± SD (n = 6). Superscripts indicate significant differences between groups based on Tukey’s post-hoc test following one-way ANOVA (p ≤ 0.05). a = comparison with group I; b = comparison with group III; c = comparison with group IV. [Click here to view] |
DISCUSSION
Our research examined the possible nephroprotective properties of EESP in rats with STZ-induced DN. Rodents are the predominant species used in experimental research on nephropathy. Among rodents, excluding genetically modified models, mice exhibit resistance to the development of DN. Rats, being larger animals, provide sufficient tissue (renal and blood) for analyses and possess genes susceptible to diseases such as hypertension and cardiovascular and renal disorders, thus mimicking multifactorial human diseases [24]. Therefore, rats were used in the present study.
The glucose transporter type 2 transporter, found in the plasma membrane of pancreatic beta cells, liver, and kidneys, facilitates the entry of STZ into these cells. STZ, in addition to selectively destroying beta cells, induces damage to renal tissue [25]. Furthermore, the activation of protein kinase C (leading to increased vascular permeability and abnormal ECM production) [26], accumulation of advanced glycation end-products (triggers inflammatory responses and damage to glomeruli and tubules) [27], mesangial expansion, and podocyte injury have been documented in this model [28]. Consequently, the STZ model was used to induce DN and assess the nephroprotective role of EESP.
According to Brenna et al., STZ-induced diabetes is linked to a reduction in body mass resulting from heightened hyperglycemia, hyperinsulinemia, muscle wasting, and loss of protein from various bodily tissues [29]. In this investigation, the weight of untreated diabetic rats gradually decreased, indicating an enhanced catabolism of tissue proteins. In contrast, diabetic rats treated with EESP and MET showed a significant gain in body weight, indicating a reduction in muscle tissue deterioration caused by hyperglycemia.
One of the primary findings of this study was that diabetic rats treated with EESP experienced a significant decrease in blood sugar levels and an enhancement in insulin production. This hypoglycaemic effect aligns with previous studies reporting the antidiabetic properties of S. potatorum seed extracts [20,30]. The observed increase in insulin levels suggested that EESP may have insulin-stimulating or insulin-mimicking properties. This antidiabetic effect is likely attributable to the presence of phytochemicals, including diabolines, flavonoids, and other substances that possess antioxidant and anti-inflammatory characteristics [31,32]. The antioxidant potential of EESP was validated by Mishra et al., who reported a significant increase in antioxidant enzymes, including reduced glutathione, glutathione peroxidase, glutathione-S-transferase, catalase, and superoxide dismutase, in the renal tissues of STZ-nicotinamide-induced diabetic rats [20].
Diabetes induces alterations in amino acid metabolism, including increased muscle catabolism, decreased protein synthesis, and enhanced hepatic gluconeogenesis by utilizing amino acids for glucose production [2]. This resulted in reduced total protein levels in the diabetic rats. Oral administration of EESP effectively regulated muscle catabolism due to insulin deficiency, and restored protein levels to near-normal values, demonstrating its pharmacological efficacy. Furthermore, chronic hyperglycemia leads to significant perturbations in protein metabolism, resulting in a negative nitrogen balance. This subsequently leads to elevated levels of blood urea and serum creatinine, which serve as critical diagnostic indicators of renal dysfunction. Blood urea and serum creatinine levels were effectively normalized by EESP administration in diabetic rats, suggesting a notable improvement in renal function.
The antidiabetic effects (reduction in blood glucose levels and increase in insulin secretion) and improvement in renal function markers (normalization of BUN and serum creatinine levels) observed in the present study are consistent with previous studies on glabridin (from licorice), Molineria recurvata, and Desmodium caudatum extracts in STZ-induced DN models. The beneficial effects of these interventions have been attributed to their antioxidant and anti-inflammatory properties [33–35].
Histopathological analysis revealed that EESP treatment attenuated the key structural changes associated with DN, including mesangial expansion, glomerular basement membrane thickening, and glomerular sclerosis. These improvements in renal histology suggest that EESP may interfere with the pathological processes driving DN progression, such as ECM accumulation and fibrosis. This study revealed the significant impact of EESP on the expression of FSP-1, a key indicator of EMT, which is involved in the development of renal fibrosis associated with DN [5]. The significant reduction in FSP-1 gene and protein expression in EESP-treated diabetic rats suggests that EESP inhibits EMT in diabetic kidneys. Structural improvement and inhibition of EMT have resulted in functional enhancement in EESP-treated DN rats, as evidenced by biochemical parameters. Although the limited duration of the study precluded complete normalization of these parameters, it underscores the necessity for long-term investigations to evaluate the sustained effects and safety of EESP on DN.
Excess ECM proteins result from an imbalance in renal ECM dynamics, where production surpasses degradation [36]. In pathological states, activated fibroblasts/myofibroblasts significantly contribute to interstitial ECM accumulation in kidneys [37]. Studies have revealed that approximately 12% of fibroblasts in mouse kidney fibrosis originate from the bone marrow, whereas approximately 30% arise via EMT in tubular epithelial cells [38]. Hence, EMT is pivotal in renal fibrosis, denoting a phenotypic shift in epithelial cells marked by the loss of cell-cell and cell-basement membrane interactions and structural polarity, transforming them into spindle-shaped mesenchymal-like cells [39].
Furthermore, fibrosis is frequently associated with chronic inflammation, which may enhance fibroblast activation and FSP-1 expression [40]. Research has shown that MET, which is known for its anti-inflammatory effects, can reduce EMT in various fibrosis models. This is achieved through activation of the AMP-activated protein kinase (AMPK) pathway and suppression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). In turn, these inhibit TGF-β signaling and downregulate FSP-1 expression, leading to diminished fibrosis and enhanced renal function [40–42]. Thus, EESP may exert a similar anti-inflammatory effect in attenuating fibrosis.
Mitochondrial dysfunction, triggered by hyperglycemia, is another key factor in DN. This process disrupts the electron transport chain, leading to an increase in reactive oxygen species (ROS) production and a decrease in adenosine triphosphate synthesis. Radix Astragali, berberine, salidroside, resveratrol, astragaloside IV, polydatin, betulinic acid, obacunone, and curcumin exhibit protective effects against mitochondrial dysfunction in DN [43,44]. Similar to these natural compounds, the role of EESP in enhancing biogenesis and maintaining mitochondrial homeostasis in DN warrants further investigation.
EESP exhibits significant antifibrotic properties, comparable to MET, by modulating FSP-1 expression and mitigating renal fibrosis, highlighting its potential as a therapeutic option for DN [40–42]. Similarly, other natural compounds, including glabridin and extracts from M. recurvata and D. caudatum, have been shown to suppress the vascular endothelial growth factor/Ak strain transforming/extracellular signal-regulated kinase (VEGF/Akt/ERK) pathway and downregulate fibrosis-related markers such as TGF-β, α-smooth muscle actin (α-SMA), collagen-1, fibronectin, and vimentin. Curcumin, berberine, and astragaloside IV have demonstrated efficacy in mitigating renal fibrosis through distinct mechanisms [33–35]. Additionally, curcumin reduced fibrosis in high glucose-induced HK-2 cells by downregulating F-actin, α-SMA, fibronectin, and collagen I via ROS and TGF-β modulation. Berberine and astragaloside IV alleviated fibrosis in ureteric obstruction models by reducing inflammation, apoptosis, and regulating EMT markers [45–47]. The antioxidant properties of EESP may enable it to share similar pathways and mechanisms with other phytochemicals in alleviating fibrosis in DN, warranting further exploration.
Few naturally occurring compounds that influence the EMT have been documented in the literature. 25-O-methylalisol F (MAF), a triterpenoid, Astragaloside IV, a saponin, Atractylenolide 1, Chrysin, a flavonoid derived from Alismatis rhizome, Astragalus membranaceus, Atractylodis macrocephalae, and Pleurotus ostreatus, respectively, have been demonstrated to inhibit EMT by suppressing the renin-angiotensin system, TGFβ1/Suppressor of Mothers Against Decapentaplegic (Smad), and Wingless-related integration site (Wnt)/β-catenin signaling pathways [5,48–50]. Although these studies examined EMT in DN, the specific focus on FSP-1 impeding renal fibrosis was less prevalent, with the exception of MAF and chrysin.
Several natural products, including Tripterygium, a Chinese medicinal preparation, and injections of Radix Astragali, derived from the dried root of Astragalus mongholicus or A. membranaceus, have completed clinical trials with positive outcomes against glomerulosclerosis and fibrosis. However, these substances have demonstrated adverse effects including hepatic dysfunction, gastrointestinal disturbances, hyperkalemia, cough, dizziness, cephalgia, menstrual irregularities, and reproductive complications [43,51]. Consequently, the long-term effects of EESP should be evaluated with caution in order to observe the potential occurrence of adverse reactions.
Although this study has not fully elucidated the precise mechanisms through which EESP exerts its protective effects on the kidneys, several possibilities can be proposed based on the observed results and the existing literature. The antioxidant properties of EESP constituents may help reduce oxidative stress, which is a key driver of DN pathogenesis [32,52]. Additionally, the anti-inflammatory effects of EESP components can contribute to reduced renal inflammation and fibrosis [32,52]. Furthermore, the inhibition of FSP-1 expression by EESP suggests the potential modulation of the signaling pathways involved in EMT and fibrosis. These may include the p38 mitogen-activated protein kinase, NF-κB, TGF-β/Smad, Wnt/β-catenin, AMPK, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR), and Notch1 pathways, which have been implicated in DN progression and are known targets of other natural compounds with antifibrotic effects [48–50,53]. However, these are extrapolations based on existing phytochemical evidence rather than direct mechanistic validation for EESP. Further studies are required to elucidate these mechanisms.
Specific pathways influenced by other compounds can provide valuable insights into how EESP can complement existing treatments for DN. For instance, compounds such as MET are known to activate the AMPK pathway, which plays a crucial role in reducing fibrosis by inhibiting the TGF-β signaling pathway and downregulating FSP-1 expression. Similarly, natural compounds such as glabridin and extracts from M. recurvata have been shown to suppress the VEGF/Akt/ERK pathway, leading to reduced fibrosis and improved renal function. EESP, with its antioxidant and anti-inflammatory properties, can act synergistically with these pathways to enhance therapeutic effects. By downregulating FSP-1 expression, EESP inhibits EMT, thereby reducing renal fibrosis and restoring normal kidney function. This complementary action could provide a more comprehensive approach for managing DN and improving patient outcomes when used along with treatments such as MET or other natural compounds.
The findings of this study are consistent with previous research on the anti-diabetic and nephroprotective properties of EESP and provide novel insights into the potential mechanisms of action, particularly regarding the inhibition of EMT and renal fibrosis through the modulation of FSP-1. This study suggests that EESP exhibits a combination of antidiabetic and antifibrotic effects, representing a more comprehensive, multi-targeted therapeutic approach in animal models. With subsequent clinical trials, EESP may be considered a potential candidate for evaluation as a complementary therapy to existing treatments and could be integrated into clinical practice.
Limitations
The limitations of this study include the absence of a direct assessment of oxidative stress markers and inflammatory mediators, which could have provided additional insights into EESP’s mechanisms of action. Moreover, investigation of the particular cellular pathways involved in the observed effects would enhance our understanding of the nephroprotective actions of EESP. Furthermore, challenges regarding the translation of these findings from animal models to human subjects, potential adverse effects due to long-term administration, and interactions with other pharmacological agents were not addressed.
CONCLUSION
This study demonstrated that EESP exhibited significant nephroprotective effects in a rat model of DN, likely through a combination of antidiabetic and anti-fibrotic actions. The ability of EESP to inhibit FSP-1 expression and potentially modulate EMT is a novel finding that warrants further investigation. These results suggest that EESP has potential as a complementary or alternative treatment for DN. Future studies should focus on elucidating the precise molecular mechanisms underlying these effects and evaluating the long-term safety and efficacy of EESP in clinical settings.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work received no specific grants from any funding agency in public, commercial, or non-profit organizations.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental protocol was approved by the Institutional Animal Ethics Committee of the Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, India (Approval No.: IAEC 1/ Proposal:105/A. Lr: 78, Dt: 13.02.2023). This study was performed in accordance with the principles of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
DATA AVAILABILITY
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Mestry SN, Dhodi JB, Kumbhar SB, Juvekar AR. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med. 2017;7(3):273–80.
2. Dhasarathan P, Theriappan P. Evaluation of antidiabetic activity of Strychonous potatorum in alloxan induced diabetic rats. J Med Med Sci. 2011;2(2):670–4.
3. Giralt-López A, Molina-Van den Bosch M, Vergara A, García-Carro C, Seron D, Jacobs-Cachá C, et al. Revisiting experimental models of diabetic nephropathy. Int J Mol Sci. 2020;21(10):3587.
4. Kikuchi Y, Yamada M, Imakiire T, Kushiyama T, Higashi K, Hyodo N, et al. A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol. 2007;192(3):595–603.
5. Kang MK, Park SH, Choi YJ, Shin D, Kang YH. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med. 2015;93:759–72.
6. Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61(5):1714–28.
7. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130(2):393–405.
8. Kuncio GS, Neilson EG, Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. 1991;39(3):550–6.
9. Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123:335–46.
10. Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complementary Altern Med. 2011;2011(1):323171.
11. Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethnopharmacol. 1998;61(2):101–10.
12. Arunthathi K. Exploring anti-diabetic compounds from the ethanolic extraction of Strychnos potatorum seeds: ligand- based design, molecular dynamics. YMER. 2023;22(01):435–44.
13. Yadav KN, Kadam PV, Patel JA, Patil MJ. Strychnos potatorum: phytochemical and pharmacological review. Phcog Rev. 2014;8(15):61.
14. Sanmugapriya E, Venkataraman S. Toxicological investigations on Strychnos potatorum Linn seeds in experimental animal models. Phcog Rev. 2006;52(4):339–43.
15. Varghese R, Moideen MM, Suhail MJM, Dhanapal CK. Nephroprotective effect of ethanolic extract of Strychnos potatorum seeds in rat models. Res J Pharm Biol Chem Sci. 2011;2(3):521–9.
16. Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17(6):300–12.
17. Punithavathi VR, Prince PS, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol. 2011;650(1):465–71.
18. Government of India. Compendium of CPCSEA 2018 [Internet]. New Delhi: Government of India; 2018 [cited 2025 Feb 11]. Available from: https://ccsea.gov.in/WriteReadData/userfiles/file/Compendium%20of%20CPCSEA.pdf
19. Langenfeld NJ, Payne LE, Bugbee B. Colorimetric determination of urea using diacetyl monoxime with strong acids. PLoS One. 2021;16(11):e0259760.
20. Mishra SB, Verma A, Vijayakumar M. Preclinical valuation of anti-hyperglycemic and antioxidant action of Nirmali (Strychnos potatorum) seeds in streptozotocin-nicotinamide-induced diabetic Wistar rats: a histopathological investigation. Biomark Genomic Med. 2013;5(4):157–63.
21. Tsai GY, Cui JZ, Syed H, Xia Z, Ozerdem U, McNeill JH, et al. Effect of Nacetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Exp Ophthalmol. 2009;37(2):223–31.
22. Sharun K, Banu SA, Mamachan M, Subash A, Mathesh K, Kumar R, et al. Comparative evaluation of masson’s trichrome and picrosirius red staining for digital collagen quantification using ImageJ in rabbit wound healing research. JEBAS. 2023;11(5):822–33.
23. Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9(24):e3465.
24. Noshahr ZS, Salmani H, Khajavi Rad A, Sahebkar A. Animal models of diabetes-associated renal injury. J Diabetes Res. 2020;2020:9416419.
25. Badole SL, Jangam GB. Animal models of diabetic cardiomyopathy. In: Watson RR, Dokken BB, editors. Glucose intake and utilization in pre-diabetes and diabetes. Amsterdam, Netherlands: Academic Press; 2015. pp. 181–90.
26. Carpenter L, Cordery D, Biden TJ. Inhibition of protein kinase C δ protects rat INS-1 cells against interleukin-1β and streptozotocin-induced apoptosis. Diabetes. 2002;51(2):317–24.
27. Bendayan M. Immunocytochemical detection of advanced glycated end products in rat renal tissue as a function of age and diabetes. Kidney Int. 1998;54(2):438–47.
28. Gross ML, Ritz E, Schoof A, Adamczak M, Koch A, Tulp O, et al. Comparison of renal morphology in the Streptozotocin and the SHR/N-cp models of diabetes. Lab Invest. 2004;84(4):452–64.
29. Brenna Ø, Qvigstad G, Brenna E, Waldum HL. Cytotoxicity of streptozotocin on neuroendocrine cells of the pancreas and the gut. Dig Dis Sci. 2003;48:906–10.
30. Sanmugapriya E, Venkataraman S. Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. J Ethnopharmacol. 2006;105(1–2):154–60.
31. Singh AK, Dhar DN. Studies on the chemical constituents of the seeds of Strychnos potatorum L. Part I. Planta Med. 1977;32(08):362–7.
32. Al-Tameme HJ, Hadi MY, Hameed IH. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. J Pharmacognosy Phytother. 2015;7(10):238–52.
33. Tan H, Chen J, Li Y, Li Y, Zhong Y, Li G, et al. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol Med. 2022;28(1):58.
34. Dey P, Kundu A, Lee HE, Kar B, Vishal V, Dash S, et al. Molineria recurvata ameliorates streptozotocin-induced diabetic nephropathy through antioxidant and anti-inflammatory pathways. Molecules. 2022;27(15):4985.
35. Lin HH, Tseng CY, Yu PR, Ho HY, Hsu CC, Chen JH. Therapeutic effect of Desmodium caudatum extracts on alleviating diabetic nephropathy mice. Plant Foods Hum Nutr. 2024;79(2):374–80.
36. Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. JASN. 2003;14(5):1358–73.
37. Badid C, Desmouliere A, Babici D, Hadj-Aissa A, McGregor B, Lefrancois N, et al. Interstitial expression of α-SMA: an early marker of chronic renal allograft dysfunction. Nephrol Dial Transplant. 2002;17(11):1993–8.
38. Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. JASN. 2004;15(1_suppl):S55–7.
39. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol. 1999;277(1):C1–9.
40. Ganesan D, Albert A, Paul E, Ananthapadmanabhan K, Andiappan R, Sadasivam SG. Rutin ameliorates metabolic acidosis and fibrosis in alloxan induced diabetic nephropathy and cardiomyopathy in experimental rats. Mol Cell Biochem. 2020;471:41–50.
41. Kim KH, Seol HJ, Kim EH, Rheey J, Jin HJ, Lee Y, et al. Wnt/β-catenin signaling is a key downstream mediator of MET signaling in glioblastoma stem cells. Neuro-oncology. 2013;15(2):161–71.
42. Cheng D, Xu Q, Wang Y, Li G, Sun W, Ma D, et al. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J Transl Med. 2021;19:1–8.
43. Xue HZ, Chen Y, Wang SD, Yang YM, Cai LQ, Zhao JX, et al. Radix astragali and its representative extracts for diabetic mephropathy: efficacy and molecular mechanism. J Diabetes Res. 2024;2024(1):5216113.
44. Zhang PN, Zhou MQ, Guo J, Zheng HJ, Tang J, Zhang C, et al. Mitochondrial dysfunction and diabetic nephropathy: nontraditional therapeutic opportunities. J Diabetes Res. 2021;2021(1):1010268.
45. Noonin C, Thongboonkerd V. Curcumin prevents high glucose-induced stimulatory effects of renal cell secretome on fibroblast activation via mitigating intracellular free radicals and TGF-β secretion. Biomed Pharmacother. 2024;174:116536.
46. Tan E, Gao Z, Wang Q, Han B, Shi H, Wang L, et al. Berberine ameliorates renal interstitial inflammation and fibrosis in mice with unilateral ureteral obstruction. Basic Clin Pharmacol Toxicol. 2023;133(6):757–69.
47. Zhang L, Liu W, Li S, Wang J, Sun D, Li H, et al. Astragaloside IV alleviates renal fibrosis by inhibiting renal tubular epithelial cell pyroptosis induced by urotensin II through regulating the cAMP/PKA signaling pathway. PLoS One. 2024;19(5):e0304365.
48. Chen H, Yang T, Wang MC, Chen DQ, Yang Y, Zhao YY. Novel RAS inhibitor 25-O-methylalisol F attenuates epithelial-to-mesenchymal transition and tubulo-interstitial fibrosis by selectively inhibiting TGF-β-mediated Smad3 phosphorylation. Phytomedicine. 2018;42:207–18.
49. Wang E, Wang L, Ding R, Zhai M, Ge R, Zhou P, et al. Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol Res. 2020;157:104831.
50. Guo Y, Xiao Y, Zhu H, Guo H, Zhou Y, Shentu Y, et al. Inhibition of proliferation-linked signaling cascades with atractylenolide I reduces myofibroblastic phenotype and renal fibrosis. Biochem Pharmacol. 2021;183:114344.
51. Huang WJ, Liu WJ, Xiao YH, Zheng HJ, Xiao Y, Jia Q, et al. Tripterygium and its extracts for diabetic nephropathy: efficacy and pharmacological mechanisms. Biomed Pharmacother. 2020;121:109599.
52. Ganesan T, Subban M, Christopher Leslee DB, Kuppannan SB, Seedevi P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers Biorefin. 2024;14(13):14547–58.
53. Ding T, Zhao T, Li Y, Liu Z, Ding J, Ji B, et al. Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine. 2021;86:153562.