INTRODUCTION
Traditional medicine has evolved over centuries across various countries with diverse techniques, such as Traditional Chinese Medicine in China, Kampo therapy in Japan, Traditional Korean Medicine including Sasang in Korea, and Traditional Medicine in Indonesia. Consequently, there are multiple clinical practices, each with its own set of concepts, diagnostic evaluation procedures, structures, rationales for diagnosis, and corresponding treatment approaches [1]. Traditional medicine in Indonesia is a form of cultural heritage, both tangible and intangible, that shapes the identity of a community or society. It is passed down through generations in the form of creativity, practices, and values, which are preserved for the future [2,3].
Indonesia’s cultural heritage includes its culinary traditions, featuring both traditional foods and beverages. These foods are prepared exclusively with local ingredients and methods, showcasing rich regional distinctions [4]. Due to Indonesia’s abundance of spices, the country offers a wide variety of traditional beverages made from local resources. While many share similar ingredients and preparation techniques, each region brings its own unique qualities and names. This variation is influenced by different socio-cultural backgrounds, local conditions, and available resources. Traditional beverages offer a diverse range of flavors, aromas, and health benefits.
Bali Province is one of the regions in Indonesia known for its culinary culture and cultural tourism, which attracts many visitors. Traditional medicine, referred to as “Usadha,” has emerged as a significant component of Balinese traditional healing practices. Various traditional Balinese beverages, known as “Loloh,” are prepared using spices and plants and have been empirically validated as remedies by the Balinese community [5]. These beverages possess functional qualities and can be used experimentally to treat various health disorders. Traditional drinks contain active compounds that promote beauty, health, and body care. The general public is increasingly aware of the benefits associated with a return to nature.
One of the plants processed into traditional beverages is Beluntas leaves (Pluchea indica L.), which belongs to the herbaceous family Asteraceae and typically grows wild in dry, rocky areas or is cultivated as medicinal plants. Empirically, Beluntas is often used in traditional medicine to eliminate body and breath odor, enhance appetite, address digestive disorders, relieve pain from rheumatism, bone pain, and lower back pain, reduce fever, and manage irregular menstruation and leukorrhea. Due to its potential to boost the immune system, some literature reports its benefits as an immunostimulant against COVID-19 [6] and for anti-hyperglycemia, dyslipidemia, and obesity [7]. The presence of phytochemicals such as alkaloids, flavonoids, tannins, essential oils, and chlorogenic acid is strongly associated with the mechanisms by which Beluntas leaves may reduce the risk of chronic diseases such as diabetes, cancer, obesity, and cardiovascular diseases.
Despite the potential of P. indica, there are still relatively few publications that comprehensively address its phytochemical composition and bioactivity concerning various diseases. Therefore, a review investigating the phytochemicals present in P. indica extracts is warranted. This review also emphasizes the numerous health benefits associated with P. indica, including cholesterol reduction, blood sugar regulation, anticancer properties, antibacterial effects, antioxidant activity, and anti-inflammatory characteristics. The findings from this review can provide valuable information for researchers interested in exploring P. indica extracts further, particularly regarding the development of standardized herbal medicines, phytopharmaceuticals, and its economic potential as a local resource in Indonesia.
METHODS
An extensive literature study was conducted from 2000 to 2024 and literature was obtained from Google Scholar and scientific databases such as Scopus, PubMed, Crossref, and Web of Science, along with publications from Taylor and Francis, Elsevier, Wiley, Thieme, Bentham, Sage, SpringerLink, and Wiley–Blackwell. The most searched keywords are “Traditional medicine”, “Bioactive compounds”, “Phytochemistry”, “Extraction”, “Pluchea indica”, “Bioactivity”, “Therapeutic use”, “Antibacterial activity”, “antiviral activity”, “antifungal activity”, “anticancer activity”, “antioxidant activity”, “antihyperlipidemic activity”, “clinical studies”. Current research focuses on the traditional use of P. indica, its nutritional composition, phytochemical profile, health benefits, and its applications in both preclinical and clinical studies. The therapeutic uses of P. indica and its potential in new drug discovery have been discussed. Articles in languages other than English were ignored. Other literatures that were left out included those in ecology and social dimensions. Chemical structure illustrations of some research results were performed using Chedraw from Pubchem (https://pubchem.ncbi.nlm.nih.gov/).
TRADITIONAL USE OF P. INDICA IN SEVERAL COUNTRIES
Pluchea indica, locally known as Beluntas in Indonesia, is an herbaceous plant from the Asteraceae family. It grows wild in dry, rocky areas with relatively hard soil or is intentionally cultivated as a medicinal plant, known as “TOGA” (Tanaman Obat Keluarga) in Indonesia. P. indica is commonly found in coastal regions, where it thrives naturally. Beyond Indonesia, Beluntas leaves are also used in traditional medicine in various other countries. For example, in Thailand, Beluntas leaves are processed into herbal tea and marketed as a health drink to treat diabetes, tumors, hypertension, cystitis, and wounds [8]. In Malaysia, herbal practitioners believe that P. indica leaves can treat dysentery, rheumatism, leukorrhea, breath, and body odor, as well as boils and ulcers [9]. In India, P. indica, also known as Indian Camphorweed, has been noted in Ayurvedic medicine for its anti-diabetic and anti-rheumatic properties. Recent studies further underscore the efficacy and safety of P. indica as a dietary supplement or remedy for preventing various diseases [10]. All of the parts of the Beluntas plants are in Figure 1.
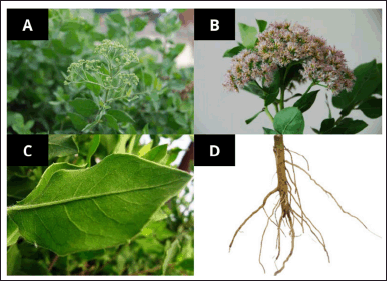 | Figure 1. Beluntas parts, (A) overall parts of Beluntas, (B) flower and stem parts, (C) Leaf part, and (D) root part [10] . [Click here to view] |
The use of P. indica as a traditional medicinal preparation varies across different regions of Indonesia and has been empirically validated in each area. Pluchea indica is known by various local names in Indonesia, such as Bluntas (Bali), Baruntas (Sunda), Luntas (Java), Baluntas (Madura), Lamutasa (Makassar), and Lenabou (Timor). Internationally, it is referred to as Luan Yi in China, Phatpai in Vietnam, and Marsh Fleabane in English. Traditionally, P. indica leaves are prepared as herbal beverages by boiling or steeping fresh or dried leaves. For wound healing, the fresh leaves are typically crushed and applied directly to the affected area [11].
NUTRITIONAL COMPOSITION OF P. INDICA
Pluchea indica leaves are rich in calcium, vitamin C, dietary fiber, and β-carotene [12]. The calcium content in P. indica leaves is seven times higher (251 mg/100 g), and the β-carotene content is twice as high (1,225 g/100 g) compared to basil leaves (32 mg/100 g and 812 g/100 g, respectively) [13]. In detail, the mineral content of P. indica leaves in μg/100 ml concentrations are potassium (78.68 ± 0.66), calcium (913.55 ± 9.12), sodium (704.35 ± 2.33), phosphorus (78.68 ± 0.66), magnesium (27.75 ± 0.30), zinc (0.64 ± 0.01), iron (0.01 ± 0.00), manganese (1.23 ± 0.01), selenium (104.95 ± 0.64), copper (0.30 ± 0.00), and chromium (47.39 ± 2.47) [14], respectively. The pounded leaves of P. indica emit a distinctive aroma and are characterized by their sweet, astringent, and fragrant flavor. In several Asian countries, fresh or boiled P. indica leaves are used as a side dish, often incorporated into chili paste to enhance the spiciness of dishes [15]. The fragrant aroma and spicy flavor of P. indica make it a popular ingredient in various traditional dishes, such as Kang Ped (spicy coconut milk soup), yum (spicy and sour salad), and Bothok (steamed meat mixed with P. indica leaves).
PHYTOCHEMICALS POTENTIAL IN P. INDICA
Pluchea indica is also highly advantageous for development into standardized herbal products and even phytopharmaceuticals due to the diverse phytochemicals present in its various parts. However, it is crucial to note that the quantity of these phytochemicals can vary greatly, influenced by environmental factors, genotypes, or a combination of both. Pluchea indica contains a wide range of phytochemicals that can be categorized into several key classes. These classes include flavonoids, alkaloids, tannins, steroids, phenolic acids, and phytosterols. The leaves of P. indica are particularly rich in flavonoids, which are organic antioxidants. Traditional medicinal plants often contain varying levels of flavonoids, with the highest concentration typically found in the leaves. Flavonoids, known as potent antioxidants, possess anti-cancer, anti-allergic, anti-inflammatory, and anti-carcinogenic properties, while also supporting the immune system and protecting digestive system function [16,17]. Several notable phytochemical structures in P. indica including vanillin, syringaldehyde, esculetin, (+)-isolariciresinol, caryolane-1,9β-diol, stigmasterol, fraxinellone, clovane-2α,9β-diol, and ethyl caffeate (Fig. 2).
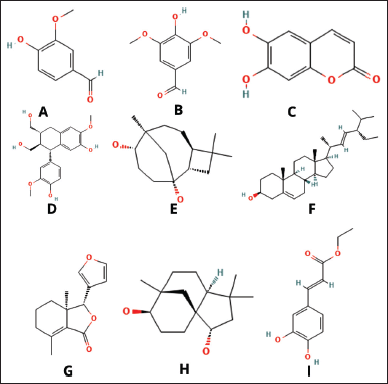 | Figure 2. Phytochemical structure of P. indica, (A) vanillin, (B) syringaldehyde, (C) esculetin, (D) (+)-isolariciresinol, (E) caryolane-1,9β-diol, (F) stigmasterol, (G) fraxinellone, (H) clovane-2α,9β-diol, and (I) ethyl caffeate [16] . [Click here to view] |
Phenolic content
It is known that the presence of phenolic compounds with antioxidant characteristics can prevent or reduce the progression of diseases related to oxidative stress [18]. Total phenolic content refers to the various phenolic compounds present in an extract, and calculating the levels of these compounds is crucial due to their significant correlation with antioxidant activity [19]. Reactive oxygen species (ROS) can be scavenged by phenolic components exhibiting strong antioxidant activity. These reactive chemical species are commonly associated with cardiovascular diseases, cancer, and neurological disorders [20,21]. Research reports indicate that gamma irradiation applied to P. indica leaf extracts can increase antioxidant activity, and total phenolic content, and reduce microbial contamination during storage [22]. Herbal tea made from P. indica leaves has been commercially marketed in Indonesia and Thailand and exhibits high phenolic compound content based on phytochemical testing [23]. Comparative studies indicate that the total phenol content in the essential oil of P. indica is higher than that of the essential oil from basil leaves, with respective values of 275.21 mg GAE/l of oil and 209.30 mg GAE/l of oil [24]. Phenolic compounds, such as caffeoylquinic acid derivatives, have been successfully validated in P. indica leaf extracts using high-performance liquid chromatography (HPLC). Quantitatively, the ultrasonic extraction method employing 50% ethanol achieved the highest concentration of caffeoylquinic acid derivatives from P. indica leaf extracts sourced from various regions in Thailand [25]. In addition to Thailand, the phenolic components of P. indica leaves have also been analyzed from miscellaneous locations in Malaysia. These phenolic components include 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid [26]. Remarkably, the maturity stages of P. indica leaves reveal varying total phenol content, with extracts from young leaf buds displaying significantly higher phenolic levels compared to other maturity stages [26].
Flavonoids
Flavonoids are a class of active polyphenolic compounds found in plants, derived from the core structure of 2-phenylchromone. These phytochemicals exhibit a C6-C3-C6 structure, characterized by two aromatic rings connected by a three-carbon bridge. In plant physiology, flavonoids are regarded as UV-B absorbers, protecting against UV radiation in response to “excess light” stress [27]. Due to their widespread presence in many plants, they are crucial for meeting human dietary needs [28]. Flavonoids are currently classified into several categories, including flavonols, flavanols, flavones, anthocyanidins, flavanonols, flavanones, and chalcones, based on their structural characteristics [29]. These natural herbal products exhibit anti-inflammatory properties, natural antioxidant activity, and therapeutic effects against cardiovascular diseases, with minimal side effects and safety concerns [30]. Flavonoids have been documented in P. indica across various regions, including Asia, the Middle East, and the Americas, demonstrating their capacity to manage inflammation [31]. Compounds such as quercetin, kaempferol, myricetin, luteolin, and apigenin, along with caffeic acid, anthocyanins [32], and essential oils, have also been identified in the extracts of P. indica leaves [23]. UV spectrum analysis revealed that flavonoid compounds in P. indica leaf extracts absorb at wavelengths of 240–285 nm (band I) and 300–560 nm (band II) [22]. Notably, P. indica exhibits a higher flavonoid content compared to Pluchea sagittalis, which is linked to its antinociceptive effects [33]. Several vegetable plants in Indonesia, including P. indica, contain flavonoids at a level of 6.39 mg/100 g fw, which is lower than that of Cosmos Caudatus (52.19 mg/100 g fw) and Polyscias Pinnata (52.19 mg/100 g fw) [32]. The report indicates that there are no significant differences in flavonoid concentrations concerning the geographical elevation at which P. indica is grown. However, the study noted that the choice of solvent in the ethyl acetate fraction yielded a higher flavonoid content compared to methanol, distilled aquades, and n-butanol [34].
Alkaloids
Alkaloids are natural compounds typically characterized by one or more ring structures that possess at least one nitrogen atom in a basic form. These low molecular weight structures are predominantly found in plants and are generally synthesized from amino acids. Over 20,000 distinct alkaloids have been reported, with approximately 20% of all plant species producing alkaloids as secondary metabolites. This class of compounds exhibits greater structural diversity than other secondary metabolites [35]. Despite their potentially harmful effects, plants containing alkaloids have long been utilized in the production of stimulants, sedatives, narcotics, insecticides, aphrodisiacs, and various pharmaceuticals [36]. Plants that contain alkaloids have been integrated into everyday diets. The alkaloid content within the genus Pluchea, which is part of the Asteraceae family, varies and has primarily been reported qualitatively in several studies [37,38,23]. However, the mechanisms of action of alkaloid compounds in these plants exhibit parallels with certain diseases. For example, alkaloids demonstrate cytotoxic properties during active mitosis, particularly in the G2 and M phases, and can act as inhibitors of topoisomerase. Traditional plants rich in alkaloids can be isolated, and their efficacy is compared with commonly used medications, such as vincristine and vinblastine, for human treatments [39].
Tannins
Tannins, also known as tannic acid, are water-soluble polyphenols discovered in various plant-based foods. Research suggests that while tannins enhance digestive efficiency, they may decrease the efficiency of converting digested nutrients into new body components. The consumption of tannin-rich foods, such as betel nuts and herbal teas, has been associated with the treatment of degenerative diseases such as cancer, indicating that tannins may possess anticarcinogenic properties. Traditional remedies in Asia often use plant extracts containing tannins to alleviate clinical symptoms of diarrhea and inflammation [40–42]. Tannins have also been found in P. indica extracts, as reported in several studies. The bioactive uses of tannins from P. indica have been shown to influence glutamic acid levels in the sperm of male white rats, analyzed through HPLC. The decrease in glutamic acid in the P. indica extract group of rats was measured at 1,341.40 mg per 100 g, which positively impacted sperm quality [43]. Quantitative analysis revealed that the tannin content in the Pluchea species ranges from 8.7 to 20 mg TAE/g, revealing the therapeutic potential of these plants [44].
Saponins
Saponins are amphipathic glycosides featuring a triterpene or steroid structure linked to one or more hydrophilic sugar moieties, such as glucuronic acid, glucose, galactose, rhamnose, and xylose. Most saponins are classified as Monodesmosides or Bidesmosides, indicating the presence of one or two sugar chains at different positions [45,46]. Steroidal saponins describe some of the most significant active compounds, drawing considerable interest due to their pharmacological properties. Researchers are particularly focused on several steroidal saponins as potential therapeutic agents [47]. Qualitative investigations into the presence of saponins in P. indica leaf extracts have been conducted in multiple studies. Extraction methods, including maceration, percolation, and Soxhlet extraction, have confirmed the presence of saponins across these techniques [48]. Furthermore, P. indica leaf extract, utilized as a natural insecticide against Spodoptera litura, exhibited saponin content when the leaves were macerated for 72 hours [49].
HEALTH BENEFITS OF P. INDICA
Anti-hiperlipidemia
Hyperlipidemia is characterized by elevated levels of total cholesterol and/or triglycerides in the blood, along with decreasing levels of high-density lipoprotein (HDL), which predisposes individuals to the development of atherosclerosis [50]. It is also expressed as an imbalance of lipids or lipoproteins in the bloodstream, mainly caused by dietary disorders, obesity, diabetes, and genetic factors [51]. Current studies have reported the effectiveness of P. indica extracts as anti-hyperlipidemic agents. The phytochemicals present in P. indica, including total phenolics, flavonoids, and several derivatives of caffeoylquinic acid and gamma-gurjunene, play an essential role in inhibiting hyperlipidemia in high-fat diet (HFD)-induced mice. This study is further supported by histological findings, which demonstrate that the average area and amount of perigonadal fat adiposity in the P. indica tea group (400 and 600 mg/kg orally) were significantly lower compared to the control group [7]. The leaf extracts of P. indica (administered at doses of 100 and 300 mg/kg/day) for 6 weeks, combined with a high-fat high-fructose diet for 10 weeks, demonstrated a significant increase in fasting blood glucose, oral glucose tolerance, insulin and leptin levels, lipid profiles, and hepatic triglyceride content. The extracts notably downregulated genes involved in lipid synthesis while upregulating genes associated with fatty acid oxidation, thereby effectively preventing dyslipidemia [12]. Furthermore, P. indica tea, at concentrations between 250 and 1,000 μg/ml, was shown to enhance pancreatic lipase activity and inhibit adipogenesis in 3T3-L1 cells in vitro [52]. Singdam et al. [12] discovered that phenolic compound from the extract of P. indica leaf alleviates dyslipidemia and hepatic steatosis in high-fat fructose diet rats. The major phenolic compounds found in the leaf of P. indica are tannic acid, rutin, quercetin, gallic acid, isoquercetin, and catechin (Fig. 3) [12].
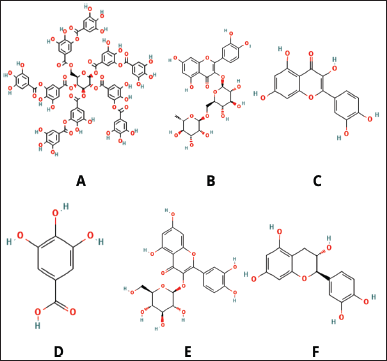 | Figure 3. Notable phytochemical compounds related to anti-hiperlipidemia, (A) tannic acid, (B) rutin, (C) quercetin, (D) gallic aicd, (E) isoquercetin, and (F) catechin. Sources: https://pubchem.ncbi.nlm.nih.gov/[12] . [Click here to view] |
Anti-diabetic
Hyperglycemic crises, such as diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state, are severe complications of diabetes that carry a significant risk of mortality if not efficiently and effectively managed. For example, DKA has become a fatal cause of death among children and adults under 58 years of age with type 1 diabetes, accounting for more than half of the fatalities in pediatric diabetes patients [53]. In contrast, type 2 diabetes mellitus (T2DM) inhibits weight loss in individuals with excess body weight. Various factors underlie these distinct clinical findings, including energy conservation resulting from improved blood glucose management and reduced glucosuria, hyperinsulinemia frequently observed in individuals with T2DM, the potential use of anti-diabetic drugs that may be obesogenic, and contributions from multiple physiological systems [54].
A clinical study involving 45 participants with prediabetes revealed that daily consumption of P. indica tea for 12 weeks significantly increased hyperglycemia and reduced serum triglyceride levels by 109.22 ± 5.21 mg/dl and LDL-C levels by 122.20 ± 3.67 mg/dl compared to the placebo group [55]. Furthermore, oral administration of ethanol extract from P. indica, starting at a dose of 50 mg/kg and increasing to 100 mg/kg after two weeks, resulted in decreased hyperglycemia and restored the histology of Langerhans islets in streptozotocin-induced diabetic rats. The mechanism of action of P. indica extract in hyperglycemic rats involves the reduction of inflammatory response markers such as IFN-γ, TNF-α, and IL-1β, along with the inhibition of caspase-3, caspase-8, and caspase-9. Additionally, it affects the phosphorylation of signal transducer and activator of transcription 1, nuclear factor-κBp65, and inducible nitric oxide synthase. Moreover, the extract was shown to regulate the ability of β-cell proliferation mediated by Bcl-2 and Ki67 [56].
Anti-cancer agents
Cancer is a fatal degenerative disease that poses a significant public health challenge due to its high prevalence and the growing number of affected patients [57,58]. Natural compounds, such as those derived from P. indica, have been shown to exhibit bioactive properties in various studies. Notably, substantial reductions in cell viability and proliferation have been reported in breast and cervical cancer cells treated with ethanol and aqueous extracts of P. indica. The proposed mechanism of action involves an increase in intracellular ROS levels, which negatively correlates with cancer cell viability. It implies that intracellular oxidative stress may contribute to the anti-cancer effects of P. indica [59]. Further research reports indicate that the aqueous extract from P. indica leaves and roots inhibits the proliferation, viability, and migration of GBM8401 and HeLa cells over 48 hours. In GBM8401 and HeLa cells treated with P. indica extract, the phosphorylation of p53 and p21 proteins was observed, while phosphorylated AKT expression decreased in HeLa cells treated with the aqueous extract [60].
The molecular mechanisms behind the anti-cancer properties of P. indica extract have also been reported to inhibit human nasopharyngeal carcinoma cells (NPC-TW01 and NPC-TW04) in a dose-dependent manner. The apoptosis mechanism induced by P. indica extract involves the regulation of pro-apoptotic Bax proteins and Bcl-2, increasing the Bax/Bcl-2 protein ratio, with similar effects seen on the p53 protein [61]. The ethyl acetate fraction of P. indica exhibits strong anti-inflammatory effects by inhibiting NO production and iNOS expression through the NF-κB pathway suppression [62]. iNOS production can lead to oxidative stress, resulting in DNA damage and promoting cancer cell growth [63]. Computational investigation of P. indica with pinoresinol, syringaresinol, and plucheoside showed the inhibition mechanism targeting peroxisome proliferator-activated receptor gamma in the cancer cell. The notable phytochemical compounds for its anti-cancer activity from Beluntas showed on Figure 4. Molecular dynamics analysis also showed that the complex of phytochemical compounds with receptor stable in the cell with RMSF <2 Å [64]. It means that those compounds can be useful as anti-cancer drug candidates.
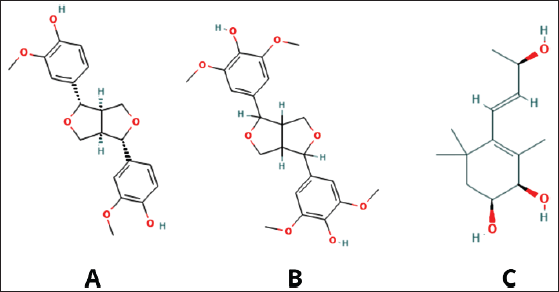 | Figure 4. Notable phytochemical compounds related to anti-cancer activity, (A) pinoresinol, (B) syringaresinol, and (C) plucheoside. Sources: https://pubchem.ncbi.nlm.nih.gov/ [64] . [Click here to view] |
Anti-bacterial activity
Pathogenic bacteria exist in various forms. When pathogenic microorganisms transfer from their source to humans through different products and food channels, they provoke several human diseases known as foodborne illnesses, and these microorganisms are referred to as foodborne pathogens [65–67]. Zoonotic transmission is mainly attributed to the presence of foodborne pathogens in animal products such as eggs, dairy products, meat, poultry, and others [68]. Common foodborne pathogens include Listeria monocytogenes, Escherichia coli, Campylobacter jejuni, Clostridium botulinum, Bacillus subtilis, Salmonella typhi, and Staphylococcus aureus, which provoke infections such as campylobacteriosis, listeriosis, salmonellosis, and E. coli infections in humans [69]. Natural compounds have long been reported to possess antibacterial properties, offering various mechanisms of action in their bioactivity.
The antibacterial potential of P. indica extract against E. coli and B. subtilis has been reported using different extraction methods. The highest inhibition against E. coli and B. subtilis was achieved using P. indica extract prepared through Soxhlet extraction, compared to two other methods—maceration and percolation [48]. Fermented P. indica leaf extract supplemented with the cell-free supernatant of S. cerevisiae revealed enriched antibacterial activity against E. coli and S. aureus, with inhibition zone diameters ranging from 3.85 to 4.81 mm and 4.63 to 5.12 mm, respectively [70]. A deodorant roll-on containing P. indica extract was tested for its antibacterial activity against S. epidermidis, revealing that the extract successfully inhibited the growth of S. epidermidis. Moreover, the deodorant roll-on stored at 28°C for 8 weeks maintained stable color, odor, and homogeneity, and did not irritate [71]. The antibacterial mechanism of P. indica extract is often attributed to the influence of its phytochemical compounds. Herbal compounds like those found in P. indica, and their formulations with other herbs, have been shown to damage cell membranes and walls, inhibit nucleic acid and protein synthesis, and increase intracellular osmotic pressure [72].
Anti-inflammatory activity
Herbal plants have consistently played a crucial role in human healthcare through their various biologically active compounds, which often display anti-inflammatory properties [73]. Inflammation is recognized as a protective process that helps maintain physiological balance within the body [74]. This response involves a complex series of changes in tissues aimed at eliminating the initial cause of cell injury, which may result from contagious agents or their metabolites (microorganisms and toxins), as well as physical agents (radiation, burns, and trauma), or chemical substances (caustic agents) [75]. For instance, the methanol fraction of the chloroform extract of P. indica root has demonstrated anti-inflammatory activity in several inflammation models. The extract inhibits inflammatory processes by blocking carrageenan, histamine, serotonin, hyaluronidase, and sodium urate. It also suppresses protein exudation and leukocyte migration, as well as granuloma formation and joint edema [76]. Inflammation provoked by hyperglycemia can be alleviated utilizing the ethanol extract of P. indica in streptozotocin-induced rats. Markers associated with liver inflammation, including interleukin-6, tumor necrosis factor-α, NF-κB p65, transforming growth factor-β1, and protein kinase C, were reduced following treatment with P. indica extract at a dose of 100 mg/kg for 8 weeks [77]. The ethyl acetate fraction of P. indica ethanol leaf extract exhibited anti-inflammatory activity in acute inflammatory phases, as observed in ethyl phenylpropionate-induced ear edema and carrageenan-induced paw edema in rat models [62].
ROLE OF P. INDICA IN CLINICAL STUDIES
The clinical evidence for the efficacy of P. indica remains relatively limited compared to in vitro and in vivo studies. A clinical study involving 45 participants with pre-diabetes treated with P. indica tea, consumed once daily for 12 weeks, demonstrated antihyperglycemic and antidyslipidemic effects in the participants. The results also showed that P. indica tea significantly reduced triglyceride and LDL-c levels compared to the placebo. Notably, the administration of P. indica tea significantly increased HDL-c levels to 57.56 ± 3.05 mg/dl, compared to only 46.44 ± 2.47 mg/dl in the placebo group [55]. Based on these findings, clinical trials involving P. indica leaf extracts are urgently needed to develop standardized herbal medicinal products and phytopharmaceuticals supported by scientific validation.
TOXICOLOGICAL STUDY OF P. INDICA
Toxicity studies are vital for the drug development process involving natural products. Our review has revealed several reports on the toxicological properties of P. indica. Notably, methanol extracts from micropropagated P. indica leaves demonstrated diuretic activity in Wistar albino rats. The acute oral toxicity study established a Lethal Dose of 2.825 mg/kg body weight [78]. Furthermore, the oral glucose tolerance test investigating the efficacy of P. indica tea in mitigating hyperglycemia was conducted in vivo. The toxicity evaluation of P. indica leaf tea administered at a dosage of 600 mg/kg/day, alongside a high-fat diet, indicated that serum creatinine, alanine transaminase, alkaline phosphatase, and complete blood count levels in the treatment group with a HFD and P. indica tea did not exhibit significant differences compared to the normal control diet, which similarly showed no significant disparities when compared to the normal control group (without a high-fat diet) [7].
FUTURE DIRECTIONS
People in tropical locations, notably Indonesia, have commonly employed P. indica-based medicines to treat a variety of ailments. Although various research indicates that this plant has economic and health promise, there is currently little information available on this commercially packaged product. The preceding portion of this research described the phytochemical composition of P. indica extract, including flavonoids, alkaloids, tannins, and steroids. This has great industrial application potential. Traditional medicine is highly helpful in building a green economy because of numerous variables such as time, energy usage, high extraction yields, little solvent use, low economic expenses, and environmental friendliness [79,80].
Pluchea indica’s pharmacological qualities, as detailed in the parts of this review, make it potentially useful in herbal therapy. According to the available data, P. indica can be packed as an extra supplement to be eaten daily in the form of herbal tea or capsules. This P. indica-based health supplement may be used as an antihyperglycemic and antidyslipidemic treatment with a low toxicity level up to a dose of 600 mg/kg/day [55]. The development of P. indica as a health supplement can be permitted by the US Food and Drug Administration or national authorities since requirements are less severe than those for manufactured pharmaceuticals. Further study into P. indica as a standardized herbal medication should concentrate on certain of its bioactivities to ensure its efficacy in treating specific ailments.
In general, this plant is abundant and often regarded as a weed, thus in order to raise its selling value, it must be developed as a raw material for medication. Using this plant as a base component in medication can provide both health and economic advantages. This plant grows quite easily and does not require special care, making it suitable for cultivation as part of local communities’ herbal medicine development efforts. A technological technique based on tissue culture can also be used to improve the extract’s biological activity [81], particularly its phenolic content. This plant still requires post-harvest management, particularly in terms of harvesting procedure, storage period, and basic handling. Interestingly, a clinical trial study on 45 people was done to determine the antihyperlipidemic and antiglycemic efficacy of this plant extract. Following the efficacy and effectiveness of this plant in clinical testing, it is now commercially accessible and ready for general use as a complimentary medication to synthetic pharmaceuticals. The findings of this study support the use of P. indica as a therapeutic agent in the treatment of antihyperlipidemic and antihyperglycemic conditions, as well as the possibility of testing it on other illnesses in the clinical phase.
CONCLUSION
Pluchea indica is an herbal plant widely utilized by communities for ethnomedicinal purposes across various regions, including Indonesia. As previously discussed, the biochemical uniqueness of P. indica lies in its distinctive bioactive compounds, which have the potential to lead to the discovery of herbal remedies and semi-synthetic drugs. However, the full potential of this plant has not yet been thoroughly explored, particularly through clinical testing. This review highlights that P. indica leaf extracts exhibit anticancer, antimicrobial, antidiabetic, anti-inflammatory, and antioxidant activities, contributing to biomedical applications. Therefore, substantial efforts and scientific validation are still required to unlock new opportunities in the development of the phytochemical potential of P. indica. Research findings in this area could significantly support the pharmaceutical industry by introducing new therapeutic agents. The use of P. indica as a natural remedy not only enhances medical sovereignty, but it can also assist farmers by encouraging the cultivation of plants for medicinal purposes. Clinical studies, both stages 1–3, are highly recommended in attempts to advance P. indica extracts in the biomedical sector.
ACKNOWLEDGMENTS
The author would like to thank the Doctoral Program in Medical Sciences, Faculty of Medicine, Udayana University, and the Nursing Profession Program, Stikes Bina Usada Bali for supporting the implementation of this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by the Ministry of Education, Culture, Research, and Technology (KEMDIKBUDRISTEK) of the Republic of Indonesia in 2022 through the Indonesian Education Scholarship Programme (BPI) with Contract Number: 02523/J5.2.3./BPI.06/9/2022.
CONFLICT OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Birch S, Alraek T, Bovey M, Lee MS, Lee JA, Zaslawski C, et al. Overview on pattern identification—history, nature and strategies for treating patients: a narrative review. Eur J Integr Med [Internet]. 2020 Apr;35:101101. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1876382019314283
2. Sevindik M, Uygun AE, Uysal I, Sabik AE. A comprehensive review on the biological activities, usage areas, chemical and phenolic compositions of Lycium barbarum used in traditional medicine practices. Sci Technol Indones [Internet]. 2025 Jan 1;10(1):173–82. Available from: https://sciencetechindonesia.com/index.php/jsti/article/view/1387
3. Sevindik M, Khassanov VT, Sevindik E, Uysal I, Mohammed FS. Cornelian cherry (Cornus mas L.): a comprehensive review on its usage areas, biological activities, mineral, phenolic and chemical contents and applications. Appl Fruit Sci [Internet]. 2024 Oct 24;66(5):2061–71. Available from: https://link.springer.com/10.1007/s10341-024-01151-3
4. Setyowati N, Masyhuri, Mulyo JH, Irham, Yudhistira B. The hidden treasure of wedang uwuh, an ethnic traditional drink from Java, Indonesia: Its benefits and innovations. Int J Gastron Food Sci [Internet]. 2023 Mar;31:100688. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878450X23000306
5. Widhiantara IG, Jawi IM. Phytochemical composition and health properties of Sembung plant (Blumea balsamifera): a review. Vet World [Internet]. 2021 May 17;14(5):1185–96. Available from: http://www.veterinaryworld.org/Vol.14/May-2021/17.html
6. Jha NK, Sharma C, Hashiesh HM, Arunachalam S, Meeran MN, Javed H, et al. β-Caryophyllene, a natural dietary CB2 receptor selective cannabinoid can be a candidate to target the trinity of infection, immunity, and inflammation in COVID-19. Front Pharmacol [Internet]. 2021 May 14;12:590201. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2021.590201/full
7. Sirichaiwetchakoon K, Lowe GM, Kupittayanant S, Churproong S, Eumkeb G. Pluchea indica (L.) Less. Tea ameliorates hyperglycemia, dyslipidemia, and obesity in high fat diet-fed mice. Evidence-Based Complement Altern Med [Internet]. 2020 Jun 3;2020:1–12. Available from: https://www.hindawi.com/journals/ecam/2020/8746137/
8. Chewchida S, Vongsak B. Simultaneous HPTLC quantification of three caffeoylquinic acids in Pluchea indica leaves and their commercial products in Thailand. Rev Bras Farmacogn [Internet]. 2019 Mar;29(2):177–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0102695X18304411
9. Roslida A, Erazuliana A, Zuraini A. Anti-inflammatory and Antinociceptive activities of ethanolic extract of Pluchea indica (L) less leaf. Pharmacologyonline. 2008;2:349–60.
10. Ibrahim SRM, Bagalagel AA, Diri RM, Noor AO, Bakhsh HT, Mohamed GA. Phytoconstituents and pharmacological activities of Indian Camphorweed (Pluchea indica): a multi-potential medicinal plant of nutritional and ethnomedicinal importance. Molecules [Internet]. 2022 Apr 7;27(8):2383. Available from: https://www.mdpi.com/1420-3049/27/8/2383
11. Buranasukhon W, Athikomkulchai S, Tadtong S, Chittasupho C. Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm Biol [Internet]. 2017 Jan 1;55(1):1767–74. Available from: https://www.tandfonline.com/doi/full/10.1080/13880209.2017.1326511
12. Singdam P, Naowaboot J, Senggunprai L, Boonloh K, Pannangpetch P. Pluchea indica Leaf extract alleviates dyslipidemia and hepatic steatosis by modifying the expression of lipid metabolism-related genes in rats fed a high fat-high fructose diet. Prev Nutr Food Sci [Internet]. 2022 Dec 31;27(4):384–98. Available from: http://www.pnfs.or.kr/journal/view.html?doi=10.3746/pnf.2022.27.4.384
13. Sudjaroen Y. Evaluation of ethnobotanical vegetables and herbs in Samut Songkram province. Procedia Eng [Internet]. 2012;32:160–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1877705812012829
14. Baharuddin SA, Shah NNAK, Yazan LS, Kadota K, Rashed AA, Yusof YA. Mineral composition and antioxidant properties of Plucheaindica (L.) Leaf Extract. Afr J BioSc. 2024;6(12):1–5.
15. Chan EWC, Ng YK, Wong SK, Chan HT. Pluchea indica: an updated review of its botany, uses, bioactive compounds and pharmacological properties. Pharm Sci Asia [Internet]. 2022;49(1):77–85. Available from: https://pharmacy.mahidol.ac.th/journal/journalabstract.php?jvol=49&jpart=1&jconnum=10
16. Mohammed FS, Sevindik M, Uysal ?, Çesko C, Koraqi H. Chemical composition, biological activities, uses, nutritional and mineral contents of cumin (Cuminum cyminum). Meas Food [Internet]. 2024 Jun;14:100157. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2772275924000248
17. El-Chaghaby GA, Mohammed FS, Rashad S, Uysal I, Koçer O, Lekesiz Ö, et al. Genus Hypericum: general properties, chemical contents and biological activities. Egypt J Bot [Internet]. 2023 Sep 21;64:1–26. Available from: https://ejbo.journals.ekb.eg/article_318135.html
18. Tan BL, Norhaizan ME, Liew WPP, Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol [Internet]. 2018 Oct 16;9:1162. Available from: https://www.frontiersin.org/article/10.3389/fphar.2018.01162/full
19. Blainski A, Lopes G, de Mello J. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules [Internet]. 2013 Jun 10;18(6):6852–65. Available from: http://www.mdpi.com/1420-3049/18/6/6852
20. Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med [Internet]. 2010;5(1):13. Available from: http://cmjournal.biomedcentral.com/articles/10.1186/1749-8546-5-13
21. Mohammed FS, Sevindik M, Uysal I, Sevindik E, Akgül H. A natural material for suppressing the effects of oxidative stress: biological activities of Alcea kurdica. Biol Bull [Internet]. 2022 Dec 24;49(S2):S59–66. Available from: https://link.springer.com/10.1134/S1062359022140102
22. Ernawati, Suryadi H, Mun’im A. Effect of gamma irradiation on the caffeoylquinic acid derivatives content, antioxidant activity, and microbial contamination of Pluchea indica leaves. Heliyon [Internet]. 2021 Aug;7(8):e07825. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405844021019289
23. Widyawati PS, Budianta TDW, Kusuma FA, Wijaya E. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts. Int J Pharmacogn Phytochem Res. 2014;6(4):850–5.
24. WidyaWati PS, Wijaya CH, Hardjosworo PS, Sajuthi D. Volatile compounds of Pluchea indica less and Ocimum basillicum Linn essential oiland potency as antioxidant. HAYATI J Biosci [Internet]. 2013 Sep;20(3):117–26. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1978301916301127
25. Kongkiatpaiboon S, Chewchinda S, Vongsak B. Optimization of extraction method and HPLC analysis of six caffeoylquinic acids in Pluchea indica leaves from different provenances in Thailand. Rev Bras Farmacogn [Internet]. 2018 Mar;28(2):145–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0102695X17306816
26. Vongsak B, Kongkiatpaiboon S, Jaisamut S, Konsap K. Comparison of active constituents, antioxidant capacity, and α-glucosidase inhibition in Pluchea indica leaf extracts at different maturity stages. Food Biosci [Internet]. 2018 Oct;25:68–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2212429218300853
27. Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol [Internet]. 2011 Feb;168(3):204–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0176161710003755
28. Ribeiro D, Freitas M, Lima JLFC, Fernandes E. Proinflammatory pathways: the modulation by flavonoids. Med Res Rev [Internet]. 2015 Sep;35(5):877–936. Available from: https://onlinelibrary.wiley.com/doi/10.1002/med.21347
29. Lu MF, Xiao ZT, Zhang HY. Where do health benefits of flavonoids come from? Insights from flavonoid targets and their evolutionary history. Biochem Biophys Res Commun [Internet]. 2013 May;434(4):701–4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X1300661X
30. Salaritabar A, Darvishi B, Hadjiakhoondi F, Manayi A, Sureda A, Nabavi SF, et al. Therapeutic potential of flavonoids in inflammatory bowel disease: a comprehensive review. World J Gastroenterol [Internet]. 2017;23(28):5097. Available from: http://www.wjgnet.com/1007-9327/full/v23/i28/5097.htm
31. Srivastava P, Shanker K. Pluchea lanceolata (Rasana): chemical and biological potential of Rasayana herb used in traditional system of medicine. Fitoterapia [Internet]. 2012 Dec;83(8):1371–85. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0367326X12002109
32. Andarwulan N, Batari R, Sandrasari DA, Bolling B, Wijaya H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem [Internet]. 2010 Aug;121(4):1231–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814610001135
33. Figueredo SM, do Nascimento FP, Freitas CS, Baggio CH, Soldi C, Pizzolatti MG, et al. Antinociceptive and gastroprotective actions of ethanolic extract from Pluchea sagittalis (Lam.) Cabrera. J Ethnopharmacol [Internet]. 2011 Jun;135(3):603–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378874111001395
34. Yuliani, Soemarno, Yanuwiadi B, Leksono A. Total phenolic and flavonoid contents of pluchea indica less leaves extracts from some altitude habitats. Int J ChemTech Res. 2015;8(4):1618–25.
35. van Rootselaar S, Peterse E, Blanco-Ania D, Rutjes FPJT. Stereoselective Mannich reactions in the synthesis of Enantiopure Piperidine Alkaloids and derivatives. European J Org Chem [Internet]. 2023 Jun 6;26(22). Available from: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejoc.202300053
36. Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol [Internet]. 2008 Jun 1;59(1):735–69. Available from: https://www.annualreviews.org/doi/10.1146/annurev.arplant.59.032607.092730
37. Ahmed SA, Kamel EM. Phenolic constituents and biological activity of the genus Pluchea. Der Pharma Chem. 2013;5(5):109–14.
38. Hussain H, Al-Harrasi A, Abbas G, Rehman NU, Mabood F, Ahmed I, et al. The Genus Pluchea: phytochemistry, traditional uses, and biological activities. Chem Biodivers [Internet]. 2013 Nov;10(11):1944–71. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cbdv.201200140
39. Zehnder • A, Graham • J, Reavill • DR, McLaughlin A. Neoplastic diseases in avian species. In: Speer B, editor. Current therapy in Avian medicine and surgery [Internet]. Oakley, CA: Elsevier; 2016. pp. 107–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9781455746712000124
40. Baba A, Hoshino T, Ogawa S, Takara T. Improvement of glucose metabolism and safety of acacia bark-derived proanthocyanidins in healthy Japanese adults: a randomized, double-blind, Placebo-controlled, Parallel-group Trial. Funct Foods Heal Dis [Internet]. 2021 Sep 3;11(9):431. Available from: https://ffhdj.com/index.php/ffhd/article/view/822
41. Li Y, Zhu L, Guo C, Xue M, Xia F, Wang Y, et al. Dietary intake of hydrolyzable Tannins and condensed Tannins to regulate lipid metabolism. Mini-Reviews Med Chem [Internet]. 2022 Jul;22(13):1789–802. Available from: https://www.eurekaselect.com/199592/article
42. Ren Y, Zhang X, Li T, Zeng Y, Wang J, Huang Q. Galla Chinensis, a Traditional Chinese Medicine: comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J Ethnopharmacol [Internet]. 2021 Oct;278:114247. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378874121004748
43. Susetyarini RE. The level of glutamic acid in the semen of male white rat (Ratus norwegicus) after being treated with Tannin of PlucheaIndica. Procedia Chem [Internet]. 2015;14:152–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1876619615000236
44. Qasim M, Abideen Z, Adnan MY, Gulzar S, Gul B, Rasheed M, et al. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. South African J Bot [Internet]. 2017 May;110:240–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0254629916305142
45. Renda G, Gökkaya ?, ?öhreto?lu D. Immunomodulatory properties of triterpenes. Phytochem Rev [Internet]. 2022 Apr 18;21(2):537–63. Available from: https://link.springer.com/10.1007/s11101-021-09785-x
46. Sharma P, Tyagi A, Bhansali P, Pareek S, Singh V, Ilyas A, et al. Saponins: extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem Toxicol [Internet]. 2021 Apr;150:112075. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0278691521001083
47. Xie H, Shi X, Zhao D, Wang B, Jin Y, Li X. A review: the structures and bioactivities of steroidal saponins from Allium macrostemon Bulbus. Phytochem Lett [Internet]. 2023 Oct;57:210–26. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1874390023001635
48. Lestari KAP, Pranoto PP, Sofiyah S, Musyirah M, Pratiwi FI. Antibacterial activity of Beluntas (Pluchea indica L.) leaves extract using different extraction methods. J Ris Biol dan Apl [Internet]. 2020 Sep 29;2(2):49. Available from: https://journal.unesa.ac.id/index.php/risetbiologi/article/view/6629
49. Muta’ali R, Purwani KI. Effect of Beluntas (Pluchea indica) leaf extract on mortality and development of Spodoptera litura F Larvae. J Sains dan Seni ITS. 2015;4(2):19–25.
50. Liu J, Rajendram R, Zhang L. Effects of oleanolic acid and maslinic acid on glucose and lipid metabolism. In: Olives and Olive Oil in health and disease prevention [Internet]. Elsevier; 2010. pp. 1423–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123744203001583
51. Sudhakaran S, Bottiglieri T, Tecson KM, Kluger AY, McCullough PA. Alteration of lipid metabolism in chronic kidney disease, the role of novel antihyperlipidemic agents, and future directions. Rev Cardiovasc Med. 2018;19:77–88.
52. Sirichaiwetchakoon K, Lowe GM, Thumanu K, Eumkeb G. The effect of Pluchea indica (L.) Less. Tea on Adipogenesis in 3T3-L1 Adipocytes and Lipase Activity. Evidence-Based Complement Altern Med [Internet]. 2018 Jul 12;2018:1–13. Available from: https://www.hindawi.com/journals/ecam/2018/4108787/
53. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Prim [Internet]. 2020 May 14;6(1):40. Available from: https://www.nature.com/articles/s41572-020-0165-1
54. Bays HE. Why does type 2 diabetes mellitus impair weight reduction in patients with obesity? A review. Obes Pillars [Internet]. 2023 Sep;7:100076. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2667368123000220
55. Sirichaiwetchakoon K, Churproong S, Kupittayanant S, Eumkeb G. The effect of Pluchea indica (L.) Less. Tea on blood glucose and lipid profile in people with prediabetes: a randomized clinical trial. J Altern Complement Med [Internet]. 2021 Aug 1;27(8):669–77. Available from: https://www.liebertpub.com/doi/10.1089/acm.2020.0246
56. Nopparat J, Nualla-ong A, Phongdara A. Ethanolic extracts of Pluchea indica (L.) leaf pretreatment attenuates cytokine-induced β-cell apoptosis in multiple low-dose streptozotocin-induced diabetic mice. PLoS One [Internet]. 2019 Feb 19;14(2):e0212133. Available from: https://dx.plos.org/10.1371/journal.pone.0212133
57. Mailankody S, Bajpai J, Budukh A, Swaminathan R, Dikshit R, Dhimal M, et al. Epidemiology of rare cancers in India and South Asian countries—remembering the forgotten. Lancet Reg Heal—Southeast Asia [Internet]. 2023 May;12:100168. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2772368223000288
58. Yazar M, Sevindik M, Polat AO, Koçer O, Ku?çu Karatepe H, Uysal ?. General properties, biosynthesis, pharmacological properties, biological activities and daily uses of luteolin. Prospect Pharm Sci [Internet]. 2024 Dec 27;22(4):146–54. Available from: https://prospects.wum.edu.pl/index.php/pps/article/view/286
59. Iawsipo P, Poonbud R, Somtragool N, Mutapat P, Meejom A. Pluchea indica tea-leaf extracts exert anti-cancer activity by inducing ROS-mediated cytotoxicity on breast and cervical cancer cells. Br Food J [Internet]. 2022 Nov 3;124(12):4769–81. Available from: https://www.emerald.com/insight/content/doi/10.1108/BFJ-05-2021-0497/full/html
60. Cho JJ, Cho CL, Kao CL, Chen CM, Tseng CN, Lee YZ, et al. Crude aqueous extracts of Pluchea indica (L.) Less. inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death. BMC Complement Altern Med [Internet]. 2012 Dec 26;12(1):265. Available from: https://bmccomplementalternmed.biomedcentral.com/articles/10.1186/1472-6882-12-265
61. Kao CL, Cho J, Lee YZ, Cheng YB, Chien CY, Hwang CF, et al. Ethanolic extracts of Pluchea indica induce apoptosis and antiproliferation effects in human nasopharyngeal carcinoma cells. Molecules [Internet]. 2015 Jun 22;20(6):11508–23. Available from: http://www.mdpi.com/1420-3049/20/6/11508
62. Buapool D, Mongkol N, Chantimal J, Roytrakul S, Srisook E, Srisook K. Molecular mechanism of anti-inflammatory activity of Pluchea indica leaves in macrophages RAW 264.7 and its action in animal models ofinflammation. J Ethnopharmacol [Internet]. 2013 Mar;146(2):495–504. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378874113000251
63. Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol [Internet]. 2012;2012:1–11. Available from: http://www.hindawi.com/journals/bmri/2012/623019/
64. Meida, Warno Utomo S, Petala Patria M. Analysis of natural formaldehyde formation on several types of marine fish circulating in Jakarta. E3S Web Conf [Internet]. 2020 Nov 25;211:02020. Available from: https://www.e3s-conferences.org/10.1051/e3sconf/202021102020
65. Bhunia AK. Introduction to foodborne pathogens. In: Foodborne microbial pathogens. food science text series. New York, NY: Springer; 2048. Pp. 1–23. Available from: http://link.springer.com/10.1007/978-1-4939-7349-1_1
66. Mohammed FS, Uysal ?, Sevindik M. A review on antiviral plants effective against different virus types. Prospect Pharm Sci [Internet]. 2023 Apr 13;21(2):1–21. Available from: https://prospects.wum.edu.pl/index.php/pps/article/view/128
67. Permatasari AAAP, Wiradana PA, Sari NKY, Widhiantara IG, Rosiana IW, Sandhika IMGS, et al. Antioxidant capacity, cytotoxicity, and bacterial contamination of brown macroalgae simplicia. J Pengolah Has Perikan Indones. 2024;27(10).
68. Bintsis T. Foodborne pathogens. AIMS Microbiol [Internet]. 2017;3(3):529–63. Available from: http://www.aimspress.com/article/10.3934/microbiol.2017.3.529
69. Farid N, Waheed A, Motwani S. Synthetic and natural antimicrobials as a control against food borne pathogens: a review. Heliyon [Internet]. 2023 Jun;9(6):e17021. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405844023042287
70. Rismawati R, Dwi Jatmiko Y, Widyarti S. Antibacterial activity of Pluchea indica leaf extract was increased after being fermented with Saccharomyces cerevisiae and added with its cell-free supernatant. Biotropika J Trop Biol [Internet]. 2022 Aug 1;10(2):111–6. Available from: https://biotropika.ub.ac.id/index.php/biotropika/article/view/1308
71. Komala O, Wiendarlina IY, Rizqiyana N. Antibacterial activity roll on deodorant with Pluchea indica (L.) leaf extract against Staphylococcus epidermidis (Evans 1916 ) in vitro. IOP Conf Ser Earth Environ Sci [Internet]. 2019 Jun 1;293(1):012031. Available from: https://iopscience.iop.org/article/10.1088/1755-1315/293/1/012031
72. Liang J, Huang X, Ma G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv [Internet]. 2022;12(45):29197–213. Available from: http://xlink.rsc.org/?DOI=D2RA02389J
73. Nardi G, Anuário A de F, Freire C, Megiolaro F, Schneider K, Perazzoli MA, et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacognosy Res [Internet]. 2016;8(5):42. Available from: https://www.phcogres.com/article/2016/8/5/1041030974-8490178642
74. Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MRJ, et al. Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nat Immunol [Internet]. 2017 Nov 18;18(11):1175–80. Available from: https://www.nature.com/articles/ni.3828
75. Jang CH, Kim YY, Seong JY, Kang SH, Jung EK, Sung CM, et al. Clinical characteristics of pediatric external auditory canal cholesteatoma. Int J Pediatr Otorhinolaryngol [Internet]. 2016 Aug;87:5–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165587616301203
76. Sen T, Chaudhuri AKN. Antiinflammatory evaluation of a Pluchea indica root extract. J Ethnopharmacol [Internet]. 1991 May;33(1–2):135–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/037887419190172A
77. Nopparat J, Nualla?Ong A, Phongdara A. Treatment with Pluchea indica (L.) Less. leaf ethanol extract alleviates liver injury in multiple low?dose streptozotocin?induced diabetic BALB/c mice. Exp Ther Med [Internet]. 2020 Jun 11;20:1385–96. Available from: http://www.spandidos-publications.com/10.3892/etm.2020.8877
78. Pramanik KC, Biswas R, Mitra A, Bandyopadhyay D, Mishra M, Chatterjee TK. Tissue culture of the plant Pluchea indica (L.) Less. and evaluation of diuretic potential of its leaves. Orient Pharm Exp Med [Internet]. 2007 Jun 30;7(2):197–204. Available from: http://koreascience.or.kr/journal/view.jsp?kj=E1OGB9&py=2007&vnc=v7n2&sp=197
79. Ahmad I, Hikmawan BD, Mun’im A, Sulistiarini R. Peperomia pellucida (L.) Kunth herbs: A comprehensive review on phytochemical, pharmacological, extraction engineering development, and economic promising perspectives. J Appl Pharm Sci [Internet]. 2022;13. Available from: https://japsonline.com/abstract.php?article_id=3803&sts=2
80. Widhiantara IG, Putri Permatasari AAA, Rosiana IW, Sari NKY, Sudyadnyana IMGS, Wiradana PA, et al. The role of biopolymers as candidates for promoting health agents: a review. J Appl Pharm Sci [Internet]. 2022;13(1):42–55. Available from: https://japsonline.com/abstract.php?article_id=3819&sts=2
81. Dias MI, Sousa MJ, Alves RC, Ferreira ICFR. Exploring plant tissue culture to improve the production of phenolic compounds: a review. Ind Crops Prod [Internet]. 2016 Apr;82:9–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0926669015306063