INTRODUCTION
Tuberculosis is a global illness that remains difficult to control. The World Health Organization estimates the number of individuals diagnosed with tuberculosis will reach 10.6 million by 2021, an increase of 600,000 from 2020. The number of deaths caused by TB has also increased significantly from 1.3 million in 2020 to 1.6 million in 2021 [1]. India (28%), Indonesia (9.2%), China (7.4%), the Philippines (7.0%), Pakistan (5.8%), Nigeria (4.4%), Bangladesh (3.6%), and the Democratic Republic of the Congo (2.9%) are the countries with the most tuberculosis cases worldwide [2]. Mycobacterium tuberculosis infection has become a serious concern because it can cause death by a single infectious pathogen. This is worsened by the occurrence of mixed infections, commonly referred to as co-infections [3]. TB co-infection can be caused by bacteria, fungi, or viruses (Fig. 1) [3,4].
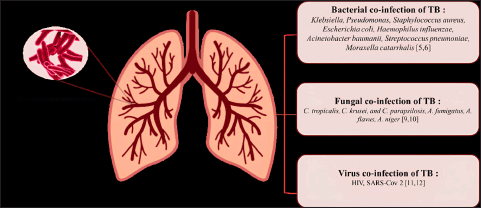 | Figure 1. Tuberculosis co-infection with pathogen microorganism. [Click here to view] |
Based on research conducted in Cambodia, the most common bacteria found in the sputum of tuberculosis patients are Klebsiella and Pseudomonas [5]. This is similar to research of pathogenic bacteria co-infection in tuberculosis patients in Nigeria, which found that Klebsiella and Pseudomonas we not the most dominant Gram-negative bacteria, while Staphylococcus aureus was the most common Gram-positive bacteria [6]. Other studies have shown the co-infection of tuberculosis with yeasts and molds. The study showed that TB-infected patients were able to be infected with the yeast Candida sp. In the samples, several candida species were even found such as Candida tropicalis, Candida krusei, and Candida parapsilosis [7]. Previous studies have also shown that about 15%–32% of TB-HIV infected patients are also positive for Candida [8]. Besides Candida, Aspergilus has also been reported as the most common co-infecting agent in TB patients. A study conducted in Ghaemshahr City, Iran, in 2018 reported that Aspergillus fumigatus was the dominant fungal species present in TB co-infections [9,10].
At this point, a lack of study has been conducted on the co-infection mechanisms between tuberculosis and other microorganisms. TB is a disease that can induce immune dysfunction. Consequently, enabling more infections induced by bacteria or fungi. Another case was identified in Bangladesh, where a tuberculosis patient co-infected with K. pneumoniae exhibited immunological dysfunction exacerbated by comorbidities such as diabetes mellitus, potentially worsening the patient’s condition (Fig. 2) [11].
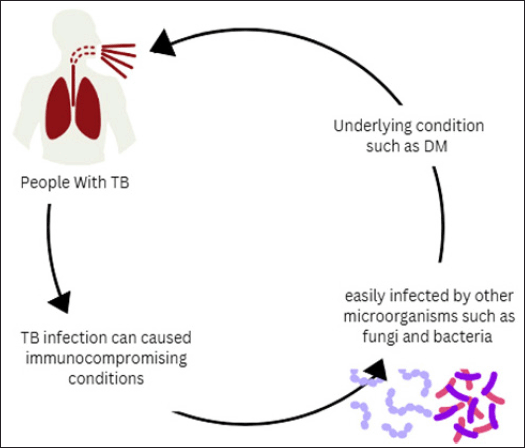 | Figure 2. TB co-infection with microorganism. [Click here to view] |
The most common bacterial co-infection of concern to the WHO is TB-HIV. The probability of developing active tuberculosis is 20–30 times greater in those with HIV compared to those without HIV. Co-infection with TB and HIV increases mortality rates and makes managing both illnesses increasingly challenging [12].
Mycobacterium tuberculosis is transmitted via airborne particles when an infected individual coughs, sneezes, speaks, laughs, or sings, dispersing little droplets containing pathogens into the environment. These droplets may be inhaled by individuals in nearby locations, resulting in tuberculosis infection [13]. Mycobacterium tuberculosis enters into the lungs and is detected by immune cells. It is mainly encountered by alveolar macrophages, the initial line of defense [14]. HIV infection significantly modifies the immunological environment, increasing risk to tuberculosis. The virus predominantly attacks CD4+ T cells, essential for initiating an efficient immunological response against M. tuberculosis. The decrease of these cells results in compromised macrophage activity, diminishing their capacity to phagocytose and eliminate bacteria effectively [15]. In addition, tuberculosis and HIV provoke persistent immunological activation, potentially resulting in further depletion of CD4+ T cells and other immune dysfunctions. This persistent stimulation may lead to heightened viral replication within granulomas, promoting a negative loop in which each infection worsens the impact of the other (Fig. 3) [16].
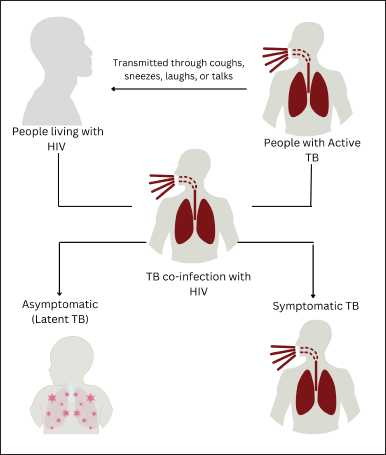 | Figure 3. The transmission of TB into TB co-infection with HIV. [Click here to view] |
Another viral infection that can cause death in TB patients is co-infection with SARS CoV-2. Research shows that TB patients with co-infection with SARS CoV-2 cause the potential latent TB to develop into TB infection [17]. These co-infections may result in longer treatment times and more serious lung damage and increase mortality rate.
Research related to TB co-infection microorganisms has not been studied in detail. To our knowledge, there are currently no bibliometric studies available on such research. Furthermore, it is essential to assess the global study overview of the scientific literature about TB co-infection microorganisms. The present investigation focused to deliver a thorough review of the published work on microorganism co-infection with tuberculosis over the past 29 years by visualizing international collaborations, evaluating the performance of prominent universities, examining the output of leading journals, analyzing the characteristics of frequently cited articles, and identifying promising research topics. The findings of this investigation enable academics and practitioners to evaluate the influence of their work through an illustrated evaluation of the research conditions in the field.
MATERIAL AND METHOD
The Scopus database was used to obtain all publications published over a 29-year period (1966–2024) on tuberculosis co-infection with microorganisms. In numerous domains, Scopus is considered as the primary resource for bibliometric analysis [18–20]. A bibliometric filter was extracted on July 12, 2024. To obtain papers on TB co-infection bacteria from the Scopus database, input the key phrases [“Tuberculosis” and “bacteria” and “co-infection”] in the “title” and “abstract” fields. The database maintained by Scopus identified 422 articles regarding TB co-infection. The data retrieved include document type, publication year, institutions, nations, journal titles, citations, and keywords. The acquired data were assessed utilizing VOSviewer [21].
RESULTS AND DISCUSSION
From 1966 to 2024, an overall of 422 articles concerning tuberculosis co-infection with microorganisms were published globally, averaging 14 documents annually. The literature on TB co-infection microorganisms from all around the world was thoroughly reviewed for this study. Our findings demonstrate that over the previous 29 years, researchers from all around the world have demonstrated a strong interest in TB microorganism co-infection. Research on co-infection began in 1966 (Fig. 3). This is because the 1960s also marked the onset of the HIV/AIDS epidemic. As the impact of HIV/AIDS became more apparent, researchers began to investigate the relationship between HIV and TB, which led to a significant increase in the study of co-infection with TB [22]. Research on TB co-infection microorganisms peaked in 2011 (Fig. 3). This was due to a number of factors, including an increase in TB-HIV case notifications and a high mortality rate from TB co-infection. For example, cases increased by 25.6% in Latin America and the Caribbean in 2011 [23,24]. In addition, the development of diagnostic technology has also influenced the high research interest in this sector. Diagnostic developments, such as interferon-gamma release assays and nucleic acid amplification tests, are needed to improve the accuracy of diagnosis in coinfected patients [25,26]. Research related to tuberculosis co-infection with microorganisms has also seen a significant increase by 2021. This is due to the COVID-19 pandemic that has significantly disrupted TB treatment services, including a decrease in the number of TB cases tested. This results in fewer TB cases identified, which in turn leads to an increase in undiagnosed and untreated TB cases, which ultimately contributes to higher TB-related deaths and more TB transmission in the community. Therefore, many researchers are interested in knowing the impact of COVID-19 on TB transmission and its clinical impact [27–29].
The leading category is research articles (n = 342; 81.04%), succeeded by reviews (n = 59; 13.9%), letters (n = 7; 1.6%), short surveys (n = 1; 0.2%), conference papers (n = 4; 0.9%), book chapters (n = 3; 0.7%), and errata (n = 2; 0.5%). English comprised the majority of the documents (n = 387; 91.7%), followed by Portuguese, German, and French (n = 6; 1.4%), and then Spanish and Chinese (n = 5; 1.2%), Turkish (n = 3; 0.7%), and Russian, Hungarian, and Czech (n = 2; 0.5%), with Japanese (n = 1; 0.2%) trailing in popularity. In contrast, the United States is the nation with the most publications (n = 94; 22.2%), followed by India (n = 73; 17.2%), the United Kingdom and South Africa (n = 40; 9.4%), and China (n = 33; 7.8%). Table 1 and Figure 4 indicate that the USA (n = 23) had the highest number of international collaborations, succeeded by the UK (n = 17), South Africa (n = 14), and Brazil (n = 12). The United States is a nation that takes a leading role in the case of global health. The United States has significant funds and resources dedicated to TB research, including organizations such as the National Institutes of Health and the Centers for Disease Control and Prevention. This resource supports extensive research on co-infection with TB [30].
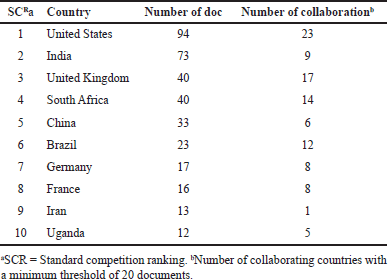 | Table 1. The involvement and collaboration of the 10 leading nations globally in the publication of tuberculosis co-infection with microorganisms from 1996 to 2024. [Click here to view] |
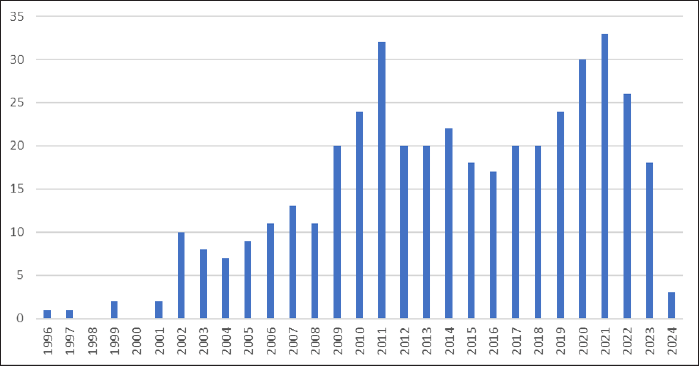 | Figure 4. A summary of research articles regarding TB co-infection with microorganisms from 1996 to 2024. A total of 422 papers were retrieved from the Scopus database. Since 2002, research on tuberculosis co-infection with microorganisms has become increasingly prolific, with the highest volume of publications occurring between 2011 and 2021. [Click here to view] |
India and China are also active in making publications related to TB co-infection, which has also triggered high cases of TB in India and China, and even India is the country with the highest TB cases in the world, followed by Indonesia and China [2]. UK and South Africa are also countries that are actively publicizing in the field of TB co-infection. Two of the ten universities with the most publications on TB co-infection are located in the United Kingdom. Similarly, South Africa is home to 4 of the 10 universities with the most publications related to TB co-infection (Table 2). This is because South Africa has one of the highest rates of TB coinfection in the world, with approximately 60% of HIV patients being co-infected with TB. The South African Tuberculosis Vaccine Initiative and the Centre for the AIDS Programme of Research in South Africa are two examples of the country’s well-established research infrastructure. These organizations produce a significant amount of papers and carry out in-depth research on the co-infection of HIV and TB (Table 1, Fig. 5) [31].
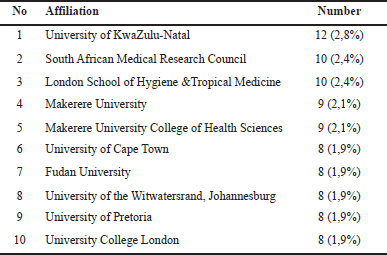 | Table 2. The most productive institutions in publications related to Tb co-infection with microorganism. [Click here to view] |
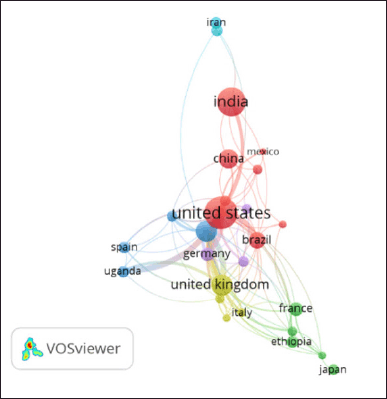 | Figure 5. Collaboration in mapping countries. Among the 27 countries, 6 published a minimum of 20 papers. The circle’s dimensions correlate with the number of foreign partnerships. [Click here to view] |
Using VOSviewer, the phrases are extracted and then categorized into color gradients ranging from blue to yellow, which correspond to the publishing year (Fig. 6). Early publications on TB co-infection were made by the US, Brazil, Belgium, and Mexico. Other nations, including India, Japan, and Canada then followed after. South Africa, the United Kingdom, Uganda, and Germany followed next. Recently, the latest research has been published by the following countries: China, Ethiopia, Italy, and Spain. China is a country with a fairly high burden of TB cases. For example, in the province of Hubei, TB outbreaks during the pandemic, highlighting the challenge of managing TB cases in the midst of COVID-19 responses. This obviously increases researchers’ interest in co-infecting TB with COVID-19. The study includes studies related to the clinical impact, detection, and characteristics of tuberculosis patients coinfected with COVID-19 [32–35]. Higher rates of mortality are linked to co-infections with SARS-CoV-2 and tuberculosis. In patients who tested positive for both TB and COVID-19, a global investigation found an 11% mortality rate, which is substantially higher than the anticipated mortality rates for COVID-19 without others. Additionally, according to reports from South Africa, patients with co-infections have a twice as great chance for fatalities in comparison to those with M. tuberculosis mono-infections [17]. A retrospective study conducted in Indonesia found that co-infection with SARS-CoV-2 and tuberculosis was linked to increased clinical severity and mortality. Serum hemoglobin levels and the number of co-morbidities were related to severity; 54 (0.7%) of 7,786 suspected TB patients had co-infection between TB and SARS-CoV-2, with 35 having rifampicin-sensitive TB and 19 having rifampicin-resistant TB, according to the study [36].
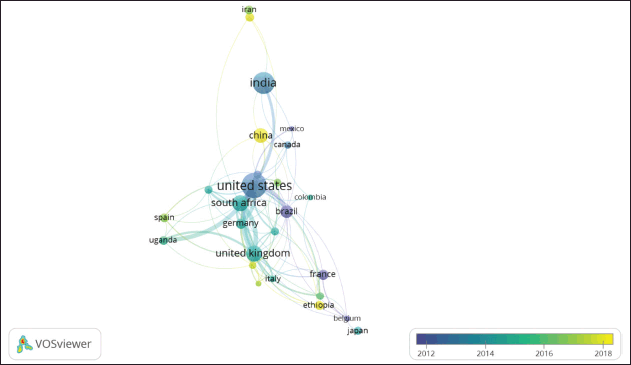 | Figure 6. Mapping of nations having ongoing publications regarding tuberculosis co-infection pathogens. Blue signifies older publications, whereas yellow denotes more current ones. [Click here to view] |
Table 3 presents the articles with the most citations for TB co-infection, together with the five most often cited articles in the Scopus database. The paper with the most citations has been published in Pulmonology, with a citation count of 181. A large number of studies define the clinical characteristics of TB-HIV co-infection and the dynamics associated with this co-infection. Additionally, it provides a resource and showcases significant improvements in the field of TB co-infection research (Table 3).
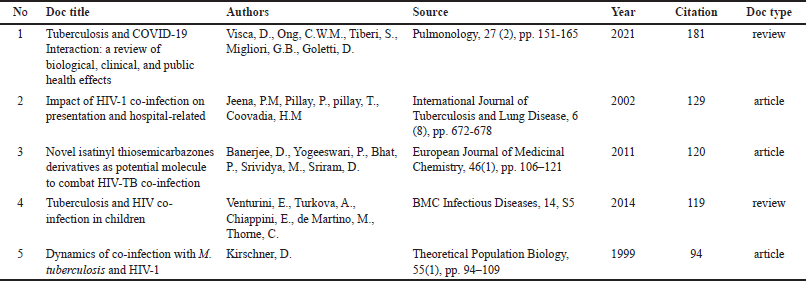 | Table 3. The highest article citation on TB co-infection.1 [Click here to view] |
Four distinct clusters, represented by the colors red, blue, yellow, and green (Fig. 7a). Cluster 1 (red color) includes terms such as child, adult, male, female, and sputum smear; cluster 2 (green color) includes human, bacterium culture, M. tuberculosis, tuberculosis, HIV, and HIV infection; cluster 3 (yellow color) includes article and co-infection; and cluster 4 (blue color) includes mycobacterium, enthambutol, isoniazid, and mixed infection classification. VOSviewer classifies the extracted phrases using a color gradient from blue to yellow, indicating publication years from old to new. The initial investigations on TB co-infection with microorganisms elucidated several crucial words, including sputum smear, HIV, acid fast bacterium, bacterium culture, and tuberculosis. Lately, emerging issues include co-infection and multiple infections (Fig. 7b). Initiation research related to TB co-infection is loaded with TB outbreak, TB identification, diagnosis, treatment, as well as clinical characteristics of patients [2,32,37–42]. Identification of tuberculosis co-infection microorganisms is important to support appropriate treatment for the patient, due to the mixed infection impact that can increase the severity of the patient’s disease [3,5,6–8,12,17,24,28,29,31,43,44]. Future research developments can focus on epidemiological studies and advanced diagnostic technologies and also focus on therapeutic management of combined infections due to exposure to bacteria and viruses, for example, in co-infections with HIV and SARS-CoV-2 is critical, given the increased severity and complexity of these infections [45–48].
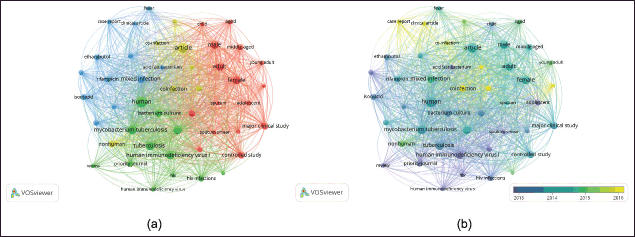 | Figure 7. Clustering of occurrence terms from VOSviewer derived from the abstracts and titles of research publications on tuberculosis co-infection with microorganisms. (a) Network visualization. (b) Overlay visualization. [Click here to view] |
CONCLUSION
This bibliometric analysis examines boundaries and identifies emerging trends in the field of tuberculosis co-infection with microorganisms over the previous 29 years. The analysis demonstrates that in terms of publications, citations, funding sources, and international collaborations, the United States is at the leading edge of this field of study. The university that contributes the most to this field and has produced the most articles is KwaZulu-Natal University. It was evident from the network of co-cited reference clusters and keywords that co-infection is a popular issue in studies. It also suggests that the co-infection of tuberculosis with microorganisms will be a crucial topic in establishing the precise diagnosis so that patients are provided effective treatment. However, bibliometric evaluation is generally more objective than conventional evaluations, and it nevertheless possesses many unavoidable limits. Initially, we exclusively incorporated the articles from the Scopus database. In addition, some new research will emerge as this study was taken until June 2024.
ACKNOWLEDGMENTS
We acknowledge the support of the Ministry of Education, Culture, Research, and Technology for Hibah Tesis Number 012/061026/PB/SP2H/AK.04/2024.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. WHO. WHO global tuberculosis report. Geneva, Switzerland: WHO; 2022.
2. World Health Organization. 2.1 TB incidence. Glob Tuberc Rep. 2022;2022:1–30.
3. Shu W, Chen W, Yao L, Sun M, Gao M, Wan Z, et al. A case series of co-infection in Mycobacterium tuberculosis and other pathogens: insights from nanopore sequencing. Egypt J Bronchol. 2024;18:19. CrossRef
4. Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8:1002464. CrossRef
5. Attia EF, Pho Y, Nhem S, Sok C, By B, Phann D, et al. Tuberculosis and other bacterial co-infection in Cambodia: a single center retrospective cross-sectional study. BMC Pulm Med. 2019;19:1–7. CrossRef
6. Abdulkadir B, Abubakar U, Abdullahi B, Owuna JE, Murtala R, Kabir K, et al. A survey of co-infection of some pathogenic bacteria with TB in patients attending Federal Medical Center Katsina, Nigeria. Bayero J Pure Appl Sci. 2020;12:209–14. CrossRef
7. Amala SE, Hanson A, Wokem GN. Candida co-infection with Mycobacterium tuberculosis in tuberculosis patients and antifungal susceptibility of the isolates. J Tuberc Res. 2020;8:53–65. CrossRef
8. Kali A, Charles MP, Joseph NM, Umadevi S, Kumar S, Easow JM. Prevalence of Candida co-infection in patients with pulmonary tuberculosis. Australas Med J. 2013;6:387–91. CrossRef
9. Amiri MRJ, Siami R, Khaledi A. Tuberculosis status and coinfection of pulmonary fungal infections in patients referred to reference laboratory of health centers Ghaemshahr City during 2007–2017. Ethiop J Health Sci. 2018;28:683–90. CrossRef
10. Hosseini M, Shakerimoghaddam A, Ghazalibina M, Khaledi A. Aspergillus coinfection among patients with pulmonary tuberculosis in Asia and Africa countries: a systematic review and meta-analysis of cross-sectional studies. Microb Pathog. 2020;141:104018. CrossRef
11. Hossain HT, Noor N, Akhter S, Ahmed M, Islam QT. Co-infection by Mycobacterium tuberculosis and Klebsiella pneumoniae in an elderly male with multiple co-morbidities: a rare entity with high mortality. Bangladesh J Med. 2022;34:52–7. CrossRef
12. World Health Organization (WHO). Co-infection of TB and HIV. Geneva, Switzerland: World Health Organization; 2019.
13. Stewart P, Patel S, Comer A, Muneer S, Nawaz U, Quann V, et al. Role of B cells in Mycobacterium tuberculosis infection. Vaccines. 2023;11:955. CrossRef
14. Hosseinian K, Gerami A, Bral M, Venketaraman V. Mycobacterium tuberculosis–human immunodeficiency virus infection and the role of T cells in protection. Vaccines. 2024;12:730. CrossRef
15. Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16:80–90. CrossRef
16. Hoerter A, Arnett E, Schlesinger LS, Pienaar E. Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection. Front Immunol. 2022;13:1014515. CrossRef
17. Chiok KR, Dhar N, Banerjee A. Mycobacterium tuberculosis and SARS-CoV-2 co-infections: the knowns and unknowns. IScience. 2023;26:106629. CrossRef
18. Burnham JF. Scopus database: a review. Biomed Digit Libr. 2006;3:1–8. CrossRef
19. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of pubmed, scopus, web of science, and google scholar: strengths and weaknesses. FASEB J. 2008;22:338–42. CrossRef
20. Kulkarni AV, Aziz B, Shams I, Busse JW. Comparisons of citations in web of science, scopus, and google scholar for articles published in general medical journals. JAMA. 2009;302:1092–6. CrossRef
21. Sofyantoro F, Yudha DS, Lischer K, Nuringtyas TR, Putri WA, Kusuma WA, et al. Bibliometric analysis of literature in snake venom-related research worldwide (1933–2022). Animals. 2022;12:1–20. CrossRef
22. Blattner WA. HIV epidemiology: past, present, and future. FASEB J. 1991;5:2340–8. CrossRef
23. Moreno R, Ravasi G, Avedillo P, Lopez R. Tuberculosis and HIV coinfection and related collaborative activities in Latin America and the Caribbean. Rev Panam Salud Publica/Pan Am J Public Heal. 2020;44:1–9. CrossRef
24. Komrower D, Thillai M. Tuberculosis and HIV co-infection. In: Davies PDO, editor. Clinical tuberculosis a practical handbook. London, UK: CRC Press; 2015. pp. 157–70.
25. Vittor AY, Garland JM, Gilman RH. Molecular diagnosis of TB in the HIV positive population. Ann Glob Heal. 2014;80:476–85. CrossRef
26. Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56:230–8. CrossRef
27. WHO. Tuberculosis deaths and disease increase during the COVID-19 pandemic. Geneva, Switzerland: World Health Organization; 2022.
28. Wang Q, Cao Y, Liu X, Fu Y, Zhang J, Zhang Y, et al. Systematic review and meta-analysis of tuberculosis and COVID-19 co-infection: prevalence, fatality, and treatment considerations. PLoS Negl Trop Dis. 2024;18:e0012136. CrossRef
29. Migliori GB, Casco N, Jorge AL, Palmero DJ, Alffenaar JW, Denholm J, et al. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2022;59:1–15. CrossRef
30. Center for Disease Control and Prevention (CDC). Tuberculosis (TB) TB and HIV coinfection. Atlanta, GA: CDC; n.d. 1–2 pp.
31. Olivier C, Luies L. WHO goals and beyond: managing HIV/TB co-infection in South Africa. SN Compr Clin Med. 2023;5:251. CrossRef
32. Li D, Peng X, Hou S, Li T, Yu XJ. A tuberculosis outbreak during the COVID-19 pandemic—Hubei Province, China, 2020. China CDC Wkly. 2021;3:562–5. CrossRef
33. Li J, Zhang Y, Liu JH, Zhao J, Yang S, Li Y. Impact of COVID-19 epidemic on tuberculosis control in Jingzhou city. Chinese J Public Heal. 2022;38:1340–4. CrossRef
34. Shen X, Sha W, Yang C, Pan Q, Cohen T, Cheng S, et al. Continuity of TB services during the COVID-19 pandemic in China. Int J Tuberc Lung Dis. 2021;25:81–3. CrossRef
35. Wang X, He W, Lei J, Liu G, Huang F, Zhao Y. Impact of COVID-19 pandemic on pre-treatment delays, detection, and clinical characteristics of tuberculosis patients in Ningxia Hui Autonomous Region, China. Front Public Heal. 2021;9:644536. CrossRef
36. Effendy L, Mertaniasih NM, Soedarsono S, Endraswari P. An initiative report on hospitalized pulmonary TB patients co-infected by SARS-CoV-2 during the COVID-19 pandemic from tertiary referral hospitals in Surabaya. Indones J Trop Infect Dis. 2023;11:112–20. CrossRef
37. Fournier P, Dubourg G, Raoult D. Clinical detection and characterization of bacterial pathogens in the genomics era. Genome Med. 2014;6:114.
38. SFDPH/TB Control. Provider information and guidelines for interpretation what is it?? GeneXpert (R) MTB/RIF 2010:8524.
39. WHO. Key facts tuberculosis. Geneva, Switzerland: WHO; 2023. 2–7 pp.
40. CDC. Tuberculosis (TB). Atlanta, GA: CDC; 2022. 1–7 pp.
41. World Health Organization (WHO). WHO consolidated guidelines on tuberculosis. Geneva, Switzerland: WHO; 2022.
42. Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2013 2014. Atlanta, GA: CDC; 2020. 9 p.
43. Liu J, Zhang Y, Cai J, Shao L, Jiang X, Yin X, et al. Clinical and microbiological characteristics of Klebsiella pneumoniae co-infections in pulmonary tuberculosis: a retrospective study. Infect Drug Resist. 2023;16:7175–85. CrossRef
44. Ishikawa S, Igari H, Yamagishi K, Takayanagi S, Yamagishi F. Microorganisms isolated at admission and treatment outcome in sputum smear-positive pulmonary tuberculosis. J Infect Chemother. 2019;25:45–9. CrossRef
45. Swanepoel J, van der Zalm MM, Preiser W, van Zyl G, Whittaker E, Hesseling AC, et al. SARS-CoV-2 infection and pulmonary tuberculosis in children and adolescents: a case-control study. BMC Infect Dis. 2023;23:442. CrossRef
46. Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56:764–5. CrossRef
47. Williams BD, Ferede D, Abdelaal HFM, Berube BJ, Podell BK, Larsen SE, et al. Protective interplay: Mycobacterium tuberculosis diminishes SARS-CoV-2 severity through innate immune priming. Front Immunol. 2024;15:1424374. CrossRef
48. Navasardyan I, Miwalian R, Petrosyan A, Yeganyan S, Venketaraman V. HIV–TB coinfection: current therapeutic approaches and drug interactions. Viruses. 2024;16:321. CrossRef