INTRODUCTION
YA-KAE-KASAI-LIN-KRA-BUE (KLB) is a traditional Thai medicine formulation documented in the Phaetsat Songkhro volume I scripture. This remedy, named after the condition, it treats, has historically been used to address a variety of health issues related to “Krasai,” a Thai term for decline or decay often associated with illness. Historically, KLB has been employed for conditions ranging from chronic liver diseases with elevated liver transaminase levels to liver cancer accompanied by ascites. The name itself highlights the deep understanding of the human body and its ailments embedded in traditional Thai medicine practices [1,2]. KLB is composed of the resin of Garcinia hanburyi, along with potassium nitrate and potash alum elements, which are heat-treated following a traditional method known as satu. Satu-treated extracts are one of the Thai traditional medicine methods for reducing possible toxicity before formulation. However, scientific evidence supporting this practice is still lacking. The formulation includes five herbs: Senna siamea, Strychnos lucida, Smilax corbularia, and Smilax glabra [3]. The chemical substances, including phenolic and flavonoid contents of plant compounds, have been extensively researched. Phenolic compounds exhibit a wide spectrum of pharmacological activities, including antioxidant and anti-inflammatory effects, as well as potent inhibitory impacts on chronic diseases such as cancer [4–6]. Antioxidants react quickly and may help in the prevention of presently incurable illnesses. They can interfere with radical forms, resulting in decreased free radical activity, improved general health, cell regeneration, and enhanced anticancer processes [7]. The KLB formulation, folk medicine was written to treat chronic diseases called “Krasai”. However, there is no scientific evidence to support this claim. The KLB formulation, a form of folk medicine, is written to treat chronic diseases referred to as “Krasai.” However, scientific evidence supporting this claim remains absent. Generally, “satu” is a traditional method employed to reduce potential toxicity before the use of medicine; however, no reports exist regarding its effectiveness concerning cancerous cell specificity after satu treatment [2]. This study focuses on evaluating the antioxidant activity, total phenolic content (TPC), total flavonoid content (TFC) of KLB, and anti-cancer activity on HepG2 cells. FTIR spectroscopy will be employed to analyze cancer cells exposed to KLB, while gas chromatography-mass spectrometry (GC-MS) analysis will be utilized for screening and identifying active components to determine reliable molecules.
MATERIALS AND METHODS
Plant collection and extraction
The five herbs and three material elements of KLB formulation were identified following Thai herbal pharmacopoeia 2020 [1] and Plant list.org. Satu is a Thai detoxification method that employs moderate heat, based on traditional techniques documented in the Phaetsat Songkhro Volume I scripture. Non-satu does not utilize the satu method. This process was authenticated by the herbal medicine unit. The resin of G. hanburyi, along with potassium nitrate and potash alum, undergoes a heat treatment detoxification process known as satu. For the satu of the resin of G. hanburyi, the following steps are taken:
1. Grind or pound G. hanburyi thoroughly.
2. Wrap it in seven layers of lotus or galangal leaves and squeeze lime juice over it.
3. Once the mixture becomes moldable, grill it until crispy.
The satu method can decrease the toxicity of gambogic acid before the medicine is used. Potash alum and potassium nitrate were melted over moderate heat, mixed with the other ingredients, and dried at 60°C. The KLB extract was prepared using 95% ethanol at a ratio of 1:3 (w/v), following a traditional Thai process. The solution was then filtered and dried using a rotary evaporator. The KLB extracts were stored at −20°C until use.
Total phenolic contents
The TPC in the KLB extracts was quantified using Folin-Ciocalteu method [4]. All extracts were diluted by adding 1 mg/ml in a 96-well microplate mix with 10% (w/v). Folin-Ciocalteu reagent, then distilled water and 100 µl of 7.5% Na2CO3 solution. The resulting sample was incubated in the dark at room temperature for 30 minutes. The absorbance was read at 765 nm. TPC was estimated using an analytical standard curve of gallic acid (linearity: 5–320 µg/ml; R2 = 0.9,991), and results were expressed as mg of gallic acid equivalents/g of extracts (mg GAE/g extracts).
Total flavonoid contents
The TFC was determined by aluminium chloride colorimetric assay, adapted from a previous study [4]. KLB (1 mg/ml) extract was added in a 96-well plate with 10% AlCl3.6H2O. The solution was incubated for 15 minutes in the dark at room temperature and the absorbance was measured at OD 415 nm. The setup was quercetin standard curve (linearity: 0.78125–50 µg/ml; R2 = 0.9998) and the result was expressed as mg quercetin equivalents/g of extracts (mgQE/g extracts).
Antioxidant assay
DPPH assay
The 2,2-diphenyl picrylhydrazyl (DPPH) radical scavenging activity was used to evaluate the antioxidant assay of KLB extracts. The DPPH test was according to [4]. Briefly, 0.4 mM DPPH solution and KLB extract in different concentrations (10–640 µg/ml) were added and incubated in the dark at room temperature for 30 minutes, and absorbance was measured at 517 nm. The antioxidant effect was calculated using an analytical curve of Trolox (linearity: 6.25–200 µM; R2 = 0.9991), and the result was expressed as 50% inhibition on DPPH (IC50 value). Scavenging (%) was calculated.
ABTS assay
The ABTS (2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid) was performed following [5]. A solution of 7 mM ABTS in water and 2.45 mM K2PO4 was incubated in the dark at room temperature for 12–16 hours before use, adjusting the OD with methanol to 0.7 ± 0.02. A 50 µl sample of KLB extracts at different concentrations (0–640 µg/ml) was added to the ABTS working solution. The mixture was incubated in the dark at room temperature for 15 minutes and the absorbance was evaluated at 734 nm. Trolox was used as the standard substance, and percent inhibition was calculated.
Metal chelating activity (MCA) assay
The MCA was measured using the method proposed by [6]. The KLB extracts were diluted to 0–640 µg/ml and mixed with 0.1 mM FeSO4 and 0.25 mM ferrozine. The mixture was incubated in the dark at room temperature for 10 minutes, and the absorbance was measured at 562 nm. The chelating activity was calculated.
GC-MS analysis
GC–MS was performed with a GCMS-QP2020, Japan according to [7] with slight modification. A silica fused TR-5 MS column (30 m × 0.25 mm inner diameter × 0.25 µm film thickness) was utilized. For GC-MS operation, an electron ionization system with an ionization energy of 70 eV was used, with a fragments scan range of 35–550 amu. Carrier gas (99.99% helium gas) was used at a constant flow rate of 1.33 ml/minute. The diluted extract (1 µl) was injected into the injector with a split ratio of 5:1 at an injector temperature of 250°C. The ion source temperature was set at 280°C. The MS source and quadrupole were held at 230°C and 150°C, respectively. Heating rates for the GC-MS column were set to 100°C (1 minute), 20°C/minute to 150°C (2 minutes), 10°C/minute to 200°C (2 minutes), and 10°C/minute to 250°C (hold 1 minute).
Cytotoxic assay
Following [8], the impact of KLB on HepG2 cell viability was assessed using the SRB assay. Briefly, 1 × 104 cells/well of the HepG2 cell line were maintained and seeded into 96-well culture plates. The cells were cultured for 24 hours, and then exposed to KLB formulation at concentrations ranging from 0.0125 to 10 µg/ml. Gemcitabine was used as a positive control at 0.625–10 µM (or 0.1645–2.6324 µg/ml) and 0.05% DMSO as a negative control for 24, 48, and 72 hours. After treatment, cells were stained with a 0.4% SRB solution while being shielded from light, and absorbance was measured at 510 nm using a microplate reader spectrophotometer to determine the IC50 value.
Cell cycle assay
HepG2 cell viability 1 × 105 cells/well was assessed following [9], treating with IC50 concentrations KLB satu and non satu extracts. Gemcitabine at 0.75 µM (0.20 µg/ml) served as a positive control, and 0.05%DMSO in 1%RPMI was used as a negative control. The cells were cultured for the next day, then trypsinized using 0.25% trysin and fixed in 70% ethanol at 4°C. The sample was centrifuged to remove the 70% ethanol and then 10 µl of PI were added, and 0.1 mg/ml RNase (BD Biosciences, USA) were added. After 30 minutes, the sample was incubated, and then flow cytometry was used for analysis.
Apoptosis assay
According to the protocols provided [10]. HepG2 cells (1 × 105 cells/well) were treated with IC50 concentrations of KLB (satu and non satu) and gemcitabine as a positive control, while 0.05%DMSO/1%RPMI was used as a negative control. The cells were permitted to attach overnight. They were then trypsinized and washed with PBS. The FITC-Annexin V/PI stain (Sigma-Aldrich, Germany) was applied, and allowed to sit at room temperature in the dark for 15 minutes. Following that, cells were examined using flow cytometry (FACSCanto II, BD Biosciences, UK).
FTIR analysis
The effect of KLB on HepG2 was assessed according to [11]. HepG2 cell density 1 × 106 cells/well was seeded and treated with concentrations KLB (satu and non satu) as per the apoptosis assay. The samples were treated for 48 hours. They were fixed cells with 4% formaldehyde. The sample was centrifuged with 0.9% normal saline and deionized water. Then 2 µl of sample was dropped on the IR window and dried in a desiccator. The samples were analyzed at 4 cm-¹ resolution within a range of 4,000–400 cm-¹. Following that, samples were analyzed at Synchrotron Light Research Institute (Public Organization).
Statistical analysis
Results were expressed as mean ± SD. All the assays were run in triplicate trials. The statistical analysis used was SPSS (Version 26). Variance ANOVA and Duncan’s multiple range test were used. There were significant differences when p < 0.05.
RESULTS
Phenolic and flavonoid contents
The total phenolic and total flavonoid content of extracts (Table 1). From TPC ethanolic extracts obtained, the highest TPC value was found in S. siamea, KLB (satu) and S. corbularia expressed as 337.21 ± 0.67, 334.63 ± 3.92 and 326.77 ± 4.45 mgGAE/g extracts, respectively. Similarly, results of total flavonoid contents for ethanolic extracts were found in S. siamea, KLB (satu) and KLB (non satu) at 58.27 ± 0.90, 41.13 ± 1.46, and 22.53 ± 0.59 mgQE/g extracts, respectively.
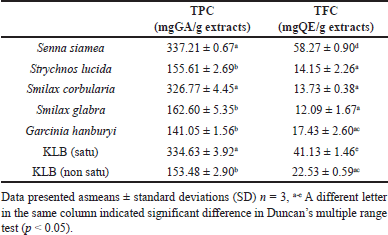 | Table 1. Total phenolic & total flavonoid constituents of KLB recipe. [Click here to view] |
Determination of antioxidants on KLB formula
The results of DPPH, ABTS, and MCA are shown in Figure 1 and Table 2. The ethanolic extract of G. hanburyi (non satu) (IC50 15.06 ± 6.92 μg/ml), S. siamea (IC50 21.24 ± 2.69 μg/ml) and S. glabra (IC50 37.95 ± 5.13 μg/ml), respectively, presence higher DPPH radical scavenging activity more effectiveness than other plants with significant differences (Fig. 1). The highest antioxidant activity by ABTS was found in ethanolic extracts of S. siamea and S. lucida with IC50 value of 37.32 ± 1.76 and 41.79 ± 2.05 μg/ml, respectively, and KLB (satu) IC50 47.52 ± 2.91 μg/ml compare with other plant with significant differences (Fig. 1). When compared to Trolox (a positive control), it was determined that the components in the KLB formulation have even less scavenging efficacy (p < 0.05). A high metal chelating ability was found in the ethanolic extract of G. hanburyi (satu), S. glabra, and S. lucida with IC50 values of 19.14 ± 0.61, 24.52 ± 0.57, and 44.02 ± 4.18 μg/ml, respectively (Fig. 1).
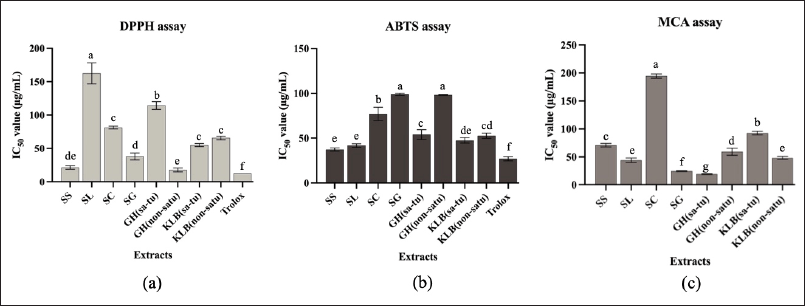 | Figure 1. (a) DPPH, (b) ABTS and (c) MCA of KLB formulation. [Click here to view] |
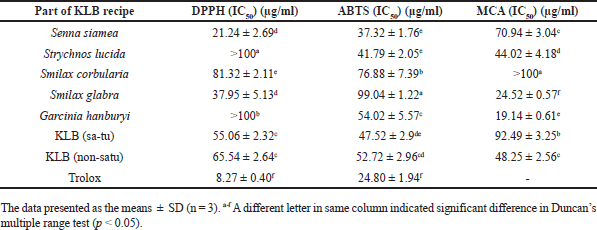 | Table 2. DPPH radical scavenging activities expressed as 50% inhibitory concentration (IC50) of KLB extract [Click here to view] |
Identification of chemical compounds by GC–MS
KLB (satu/non satu treated extracts processes) formulation extract. The chemical compounds were determined by GC–MS analysis. The ethanolic extract compounds are shown in Tables 3 and 4 and Figure 2a and b. KLB satu in Table 3 and Figure 2a indicates a total of 10 compounds with the maximum peak areas (%) for alpha.-Cadinol, 1-Pentadecene, and 2,4-Di-tert-butylphenol with values of 28.47%, 16.57%, and 12.68%, respectively. The chromatogram profiles of KLB (non satu) was showed a total of 18 compounds and presented the highest peak areas (%) for 2,4-Di-tert-butylphenol, Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, ethyl ester and Lidocaine with values of 15.70%, 11.47%, and 10.38%, respectively (Table 4 and Fig. 2b). The number of toxic compounds in ethanolic extracts of KLB (satu) was 3 compounds and non satu was 14 compounds, respectively. Beyond comparison to the non satu approach (Fig. 3), the satu method (Fig. 3) demonstrated a reduced number of harmful compounds.
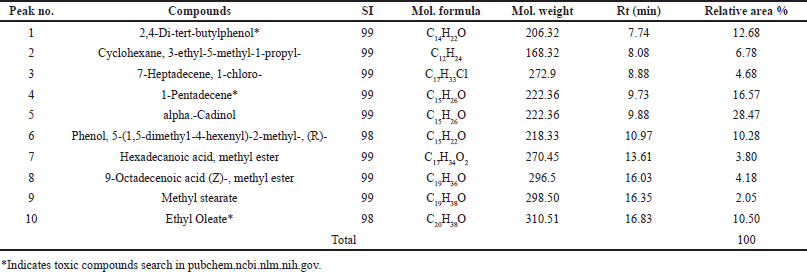 | Table 3. Compounds identified in ethanolic extracts of KLB (satu) using GC-MS. [Click here to view] |
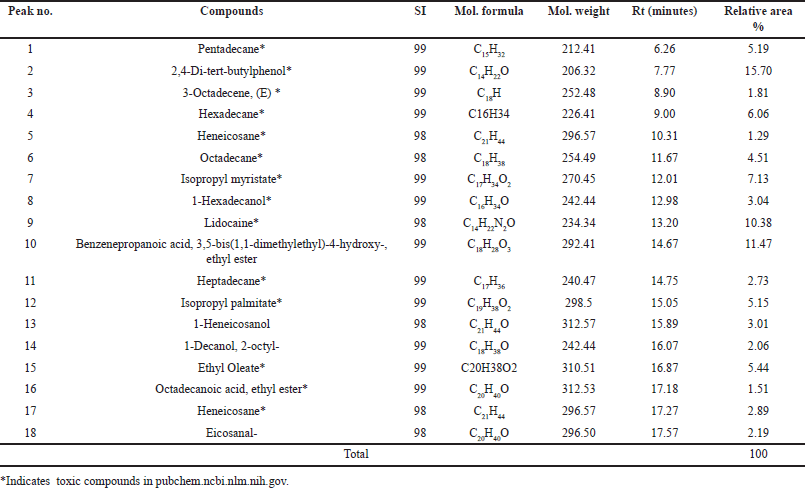 | Table 4. Compounds identified in the crude ethanolic extracts of KLB (nonsatu) using GC-MS. [Click here to view] |
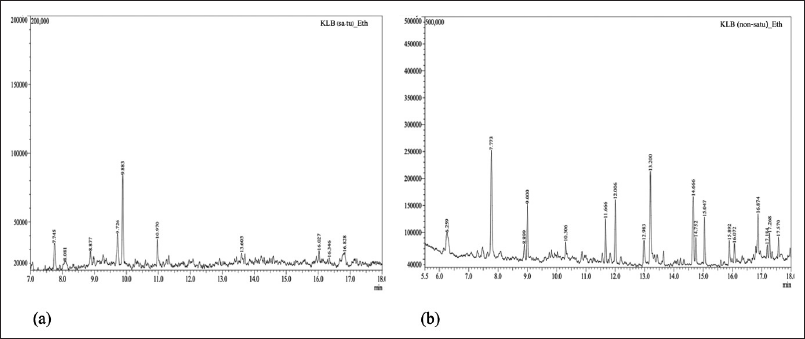 | Figure 2. GC-MS chromatogram of KLB formulation extract; (a) satu and (b) nonsatu. [Click here to view] |
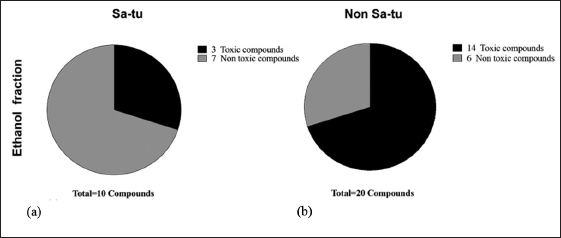 | Figure 3. Percentage of chemical compounds of extract (a) KLB (satu), b) KLB (nonsatu). [Click here to view] |
Cytotoxic activity of KLB on hepatocellular carcinoma cell
An investigation into the cytotoxic effects of KLB extracts on HepG2 as shown in Table 5 and Figure 4a–c. The ethanolic KLB sa-tu/non-satu extracts of show a strong effect cytotoxicity reported concentrations and time-dependent decrease potential in cytotoxicity on HepG2 cell (Fig. 4a and b). Gemcitabine has great potential when treated by long-time dependence (Fig. 4) The half-maximal inhibitory concentrations (IC50) of ingredient KLB extracts present the corresponding incubation periods.
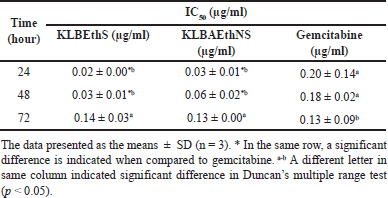 | Table 5. Whole cytotoxic of KLB formulation of different 3-time dependents. [Click here to view] |
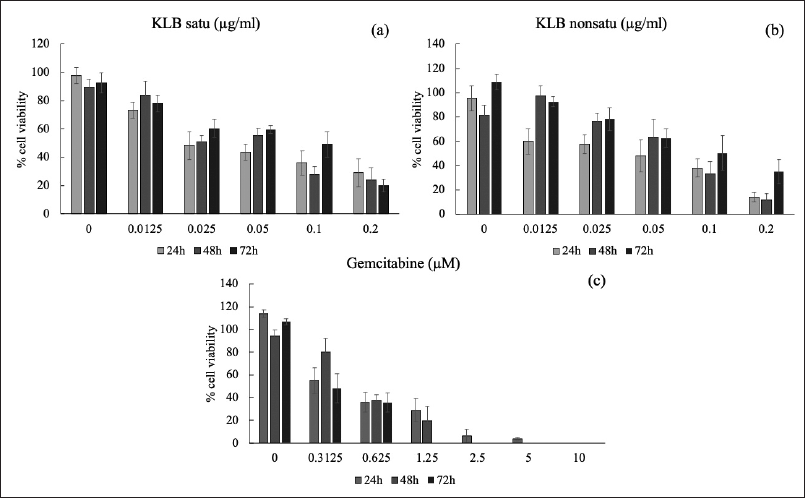 | Figure 4. Cytotoxic effect of KLB (a) satu/ (b) nonsatu extract and (c) gemcitabine on HepG2. [Click here to view] |
KLB exerts cell cycle
The KLB cell cycle analysis was also investigated in this study (Table 6 and Fig. 5). The results revealed that the HepG2 cells after treatment showed KLB satu (c), KLB non satu (d), and gemcitabine (b) were inhibited progression S phase and G2/M phase and significantly compared to the control group (a).
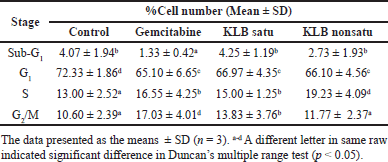 | Table 6. The cell cycle percentage of HepG2 cells treated with the KLB formulation. [Click here to view] |
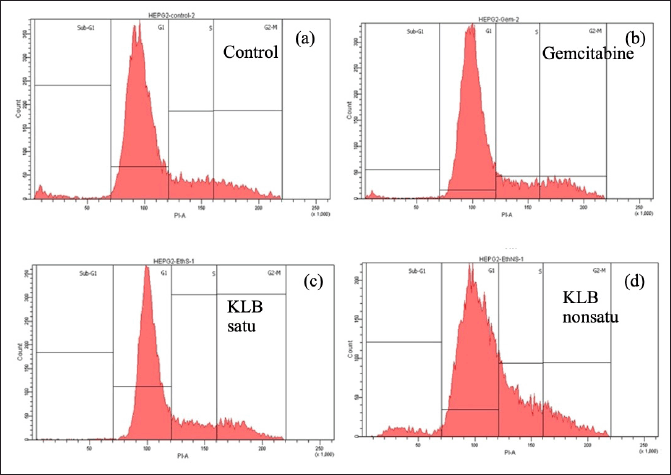 | Figure 5. Cell cycle distribution exposed of KLB on HepG2 cells. [Click here to view] |
Effects of KLB on liver cancer cell apoptosis
The Annexin V assay revealed that sulforaphane undergoes early and late stages of apoptosis. The effect of KLB on the cell of HepG2 for induce apoptosis was found apoptotic population of KLB satu 58.93% ± 2.13% while KLB non satu 70.00% ± 4.45% and gemcitabine 49.70% ± 1.98%. The results show that KLB non satu extract can induce apoptosis nearly gemcitabine as shown in Figure 6.
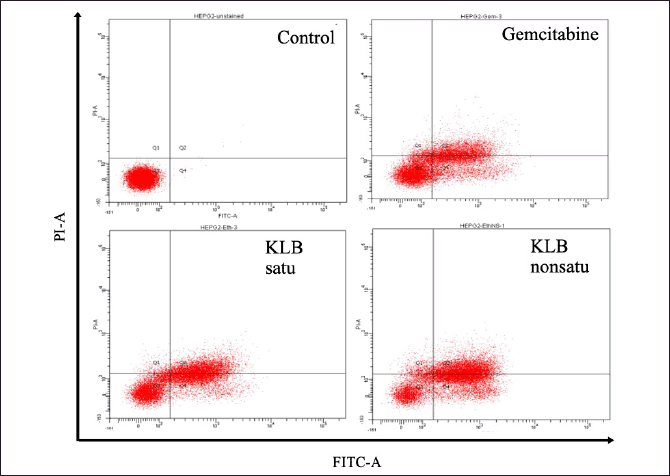 | Figure 6. Effect of KLB extracts on HepG2 induction of apoptosis, distribution apoptosis by chromatogram of quadrant of apoptosis cell. [Click here to view] |
FTIR spectra
As a result of the approach, the KLB formula on HepG2 cells is stronger. After screening all FTIR extract spectra, we decide which spectra to analyze according to the Principal component analysis (PCA) score plot (Fig. 7 a–c). The studies of average FTIR spectra of HepG2 under treatment KLB satu/non satu ethanolic extracts. The intensity of lipid CH2, CH2 stretching modes 2,852 (CH3) cm-1and 2,923 (CH2) cm-1(Fig. 7), respectively, was observed samples. Additionally, the Amide I band associated with the lipid head-group (C=C) stretching at 1,654 (C=C) cm-1 and 1,635 (C=C) cm-1(Fig. 7), respectively. Nucleic acid band show stretching at 1,239 (PO2-) cm-1, 1,168 (PO2-) cm-1, 1,087 (PO2-) cm-1, and 1,056 (C-C) cm-1 (Fig. 7). Those to wavenumber (cm-1) scale indicates an energy form, and as frequency represents one-half of wavelength, there is a direct correlation between energy and frequency. E=hv is the energy ratio.
The most effective grouping among confirmed treatments was attained by PCA is frequently used to minimize the quantity of high-dimensional collinear data sets to analyze the main variations pattern. Since>2 samples from two independent experiments were included in the analysis. PCA revealed that spectra of samples taken treatment were intermingled, generating one big cluster PC1 versus PC2 and PC3 score plot revealing that proteins may be mostly to blame for discriminating and PC1, PC2, and PC3 explained 22%, 12%, and 9% of the total variance, respectively. PCA score plots; principal components (PC 1, 2, and PC3) within 95% confidence ellipse. PCA loading at variables 3000 and 900 cm-1 as shown in Figure 7, indicating the high content of α-helix in the secondary structure of protein and nucleic acid. The α-helix band of protein in control HepG2 and KLB treatment presented higher intensity. KLB satu exhibited the lowest lipid ester band intensity. Interestingly, KLB and Gemcitabine treatment seemed to have effects on Amide I band of HepG2 at 1,635 cm1 which supports apoptosis results. These results show that FTIR spectroscopy coupled to machine learning is a suitable tool to discriminate healthy cell, KLB treated cell, and Gemcitabine based on the metabolic fingerprints of their group of cells.
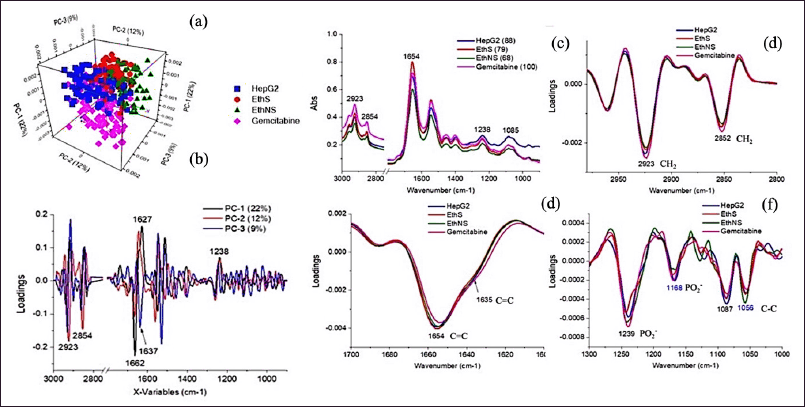 | Figure 7. PCA score plot of HepG2 cells calculated from second derivative spectra under treatment of KLB satu/non satu ethanolic extracts, Gemcitabine, and untreated cell (HepG2). Biochemical changes from each group classified as per their PC1 versus PC2 and PC3 score plot (a–c). Average second derivative spectra (8 spectra smoothed normalization from triplicate samples) in the (d) lipid (3,000–2,800 cm–1), (e) protein (1,700–1,450 cm–1), and (f) nucleic acid (1,300–1,000 cm–1) HepG2 cell. [Click here to view] |
DISCUSSION
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality globally. In various regions, diverse therapeutic approaches exist, including traditional medicine practices. Thai Ayurvedic medicine, passed down through generations, offers alternative remedies that are often unexamined scientifically. This study evaluates the pharmacological properties of the Thai traditional remedy, the KLB formula, to bridge the gap between traditional knowledge and scientific research. The results indicate that both the satu and nonsatu extracts of KLB contain significant phenolic and flavonoid compounds, known for their antioxidant properties. Previous reports have shown that ethanolic extraction effectively concentrates these beneficial compounds, demonstrating a strong correlation between their presence and free radical scavenging activity [12].
The total phenolic content and TFC of KLB were notably high, particularly in S. siamea, G. hanburyi (nonsatu), and KLB (satu). These compounds are essential as they can directly participate in antioxidant activity, thereby potentially mitigating the oxidative stress associated with degenerative diseases [4]. TPC of ethanolic extracts was the highest in S. siamea, KLB (satu), and S. corbularia, respectively, whereas the highest TFC was observed of G. hanburyi (nonsatu), S. siamea, and KLB (satu), respectively. The reported total phenolic content of S. siamea and G. hanburyi which was the highest, demonstrated the presence of carbohydrates, glycosides, flavonoids, tannins, and phenolic compounds, according to phytochemical analysis, the phenolic and flavonoid molecules have other numerous beneficial biological properties [13,14] and KLB phenolics and flavonoids are antioxidants that protect against a variety of diseases and can help reduce morbidity and mortality associated with degenerative diseases. Plants with higher phenolic and flavonoid contents than formula may have a significant and noticeable reduction in TPC and TFC potential.
Assessing antioxidant activity is crucial for understanding the medicinal potential of plant extracts. Our study employed DPPH, ABTS, and metal chelating assays to evaluate the antioxidant capacity of the KLB formulation. The results indicated that G. hanburyi (nonsatu) and S. siamea exhibited the highest radical scavenging activity, suggesting that individual plant extracts may contribute more effectively to antioxidant capacity than the combined formula. The identification of metal-chelating abilities in these extracts further highlights their therapeutic potential [15,16]. Thus, color reduction measures can be used in a metal chelating test to estimate the chelating activity of a coexisting chelator. Fe2+, a transition metal ion, may transfer single electrons, allowing it to create and promote a wide spectrum of radical reactions, even those that begin with relatively non-reactive radicals [6].
The GC-MS analysis of the KLB formula revealed various bioactive compounds with reported antifungal, anticancer, antioxidant, antimicrobial, and antiviral activities [17]. Importantly, the toxicological profile showed that the satu extract contained fewer toxic compounds compared to the non satu extract, underscoring the potential for safer applications in traditional medicine.
The review of studies toxicity was shown to be persistent in ethanol extracts of KLB (satu) was 3 compounds, respectively, while toxic compounds were identified in 14 compounds of KLB (non satu). So, compared to the nonsatu approach, the satu method could reduce toxicity in KLB formula. The sample reveals many extract layers with chemicals. Increased peaks and overlaps will come from more complex sample materials sending more signals to the chromatographic separation process than usual [18]. According to the findings of this study, the KLB formula includes a variety of bioactive chemicals that can be used for medical and pharmaceutical applications. This plant has powerful antioxidant effects, which might be attributable to its high total phenolic and flavonoid content, according to the most effective biological screening current research was the first in vitro experiment to create data that can be used as scientific proof to support the use of KLB in the treatment of chronic diseases that eventually lead to cancer claim traditional medical reported. Plants with strong antioxidant activity and cause radical scavenging activity of free radical oxygen and other species involved with cancer cell growth may be the greatest choice for these cytotoxic effects on cancer [19].
In the current study, the mechanism of the KLB formula to reduce the human liver cancer cell line (HepG2) was investigated. According to recent research, there are 4 main categories of cytotoxicity for plant extracts: high efficiency (IC50 20 µg/ml), medium efficiency (IC50 >20–100 µg/ml), poor efficiency (IC50 >100 µg/ml), and non-displayed performance (IC50 >1,000 µg/ml) [20].
The present study’s in vitro experiments provide scientific validation for the traditional claims associated with KLB, particularly regarding its effects on chronic diseases and cancer. Gamboge in G. hanburyi according to reports, secretes P53 protein alters the morphology of liver cancer cells, and activates caspases to activate apoptosis in cancer cells. The observed cytotoxic effects on HepG2 cells suggest that the herbal ingredients, including G. hanburyi, may play a critical role in inducing apoptosis by influencing the expression of key regulatory proteins [21,22]. This aligns with traditional uses of these herbs in treating liver ailments, reinforcing the relevance of historical knowledge in contemporary scientific discourse.
Past of study S. glabra on HepG2. This could result in DNA cleavage, a decrease in cytochrome C an increase in caspase-3 activity, and therefore cell death [23]. It demonstrates the toxicity of the herbs in KLB by demonstrating how liver cancer cells death. When compared Gemcitabine has cytotoxic follow dose-time dependent. The outcomes are similar to previous research on HepG2 cells, with an IC50 of 2.97 ± 0.32 µM [24]. Per the past studies, the gemcitabine test on HepG2 cells remained constant for 64 hours. When this occurred, the drug would respond. This study found that cells react to medications by dying off more frequently after 24, 48, and 72 hours. Among these, the Thai formula Pra-Sa-Prao-Yhai was found strong toxic on HepG2 cell and IC50 values of 79.55 µg/ml. Tein-5 and Ben-ja-kul remedies IC50 values of 89.61 and 87.19 µg/ml, respectively [25]. Other formulations were indicated cytotoxic potential lower than that of KLB extracts (satu and non satu) on HepG2 and KLB highly efficient similarly gemcitabine and same as benja ammarit formula IC50 0.22 µg/ml [26]. Results of KLB extracts in cytotoxicity on HepG2 cells tend to be more promising at 24 hours.
The cell cycle is a process of cell reproduction that controls how living things, including both normal cells and cancer cells, grow and develop. Moreover, the study’s findings indicate that KLB treatment resulted in significant cell cycle arrest in HepG2 cells, particularly in the S and G2/M phases. This inhibition suggests that KLB extracts may interfere with DNA synthesis and repair, paralleling the mechanisms of conventional chemotherapeutics [20,27]. Gemcitabine shows cell cycle arrest on HepG2 S and G2/M phase increase ~3% when compared to the control. The increase in the proportion of cells accumulating in the G2/M phase, indicates that chromosomes have completed replication, and DNA repair (nuclear and cell division phase) [28]. The G1/S phase transition gives it a crucial role as a checkpoint for cell cycle development in inhibiting the replication and destruction of damaged DNA [29]. The primary regulatory mechanism through which cells undergo apoptosis in various contexts, such as inadequate DNA damage repair, is identified in this study. Among the different groups, KLB non satu is the most potent in inducing apoptosis. Typical morphological and biochemical indicators of apoptosis include condensed chromatin in cells, the formation of apoptotic bodies, the presence of a hypodiploid peak, and DNA laddering observed through agarose gel electrophoresis [30]. Past study ingredients of KLB down-regulation of NF-κB and Bcl-2 protein expression, and up-regulation of Bax protein expression are two members of the Bcl-2 family and play different roles in programmed cell death [31].
Using FTIR micro-spectroscopy, we tracked biochemical changes in HepG2 cells treated with KLB, revealing alterations in lipid, protein, and nucleic acid structures. These changes, indicative of apoptosis, corroborate the traditional understanding of the KLB formula’s medicinal efficacy. The accumulation of lipid content, the raising of β-pleated sheet intensity, and α-helix protein shifting confirm the induction of apoptosis via pro-apoptotic proteins [32]. KLB satu decreased lipid content while also decreasing nucleic acid/DNA intensity. Nucleic acid/DNA intensity reduction 1,625 cm-1 (C=C) cross-β structure (Amide I) was showed on KLB non satu and gemcitabine has a lower signal than control. The PCA loading plots 1,654 (C=C)cm-1 protein, α-helix structure, and 1,635 (C=C)cm-1 protein structures (β-sheet) were found signal of KLB satu nearly gemcitabine; therefore, a shift is lower frequency also supporting change in human apoptotic proteins cause the N-C terminus of the peptide chain of P53 has the ability to break down the membrane of cancer cells [32]. At signally 1,238 (PO2-) Ribose structure dynamics A-to B-form transitions in DNA and 1,083 (PO2-)cm-1 symmetric stretching (DNA/RNA) found gemcitabine and KLB non satu were lowest signal, and showed PCA loading plots 1083 (PO2-)cm-1 symmetric stretching and 1,239 (PO2-)cm-1 double stranded DNA (ds-DNA) and 1,168 (PO2-)cm-1 ribose-phosphate were dawn signaling and 1087 (PO 2-)cm-1 single stranded DNA (ss-DNA) and 1,056 (C-C)cm-1. A stretching C–O deoxyribose were an increase signal this result DNA break down sensibility of KLB as good as Gemcitabine on HepG2 causes cell death and DNA damage. The structural dynamics of nucleic acids and lipids highlighted the potential for KLB to induce DNA damage, similar to that of Gemcitabine, providing a compelling argument for the integration of traditional formulations into modern cancer therapies [33].
CONCLUSION
In conclusion, this study illustrates the potential of the KLB formula as a viable treatment option for HCC, validating the empirical wisdom of Thai traditional medicine through scientific investigation. The connection between its bioactive components and their therapeutic effects reinforces the importance of preserving and exploring traditional medical knowledge in contemporary healthcare contexts.
ACKNOWLEDGMENT
Foremost, the authors would like to express gratitude to Rajamangala University of Technology Isan, Sakon Nakhon campus, Thailand for providing laboratory facilities. The authors thank their colleagues who provided insight and expertise that greatly assisted the research. The authors thank Dr. Chatchanok Nukulkit and Dr. Pongsathorn Tongkrasee for their guidance and assistance with the site measurement. Finally, they are also immensely grateful to Ms. Martha Maloi Eromine and Mr. Praphat Manuelo Ruengthanoo for the English grammar check of the manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Hepatocellular carcinoma cell line (ATTC®, HB8065, lot no.70039681) was purchased from Biomedia Co., Ltd. This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ministry of Public Health. The Announcement to Define the Thailand National Traditional Medicine Textbook and Thailand National Traditional Pharmacopoeia. Bangkok, Thailand: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, Royal Thai Government Gazette.; 2016.
2. Songpol C, Aimmanas A, Pranee C, Somkiat P, Songpol P, Rungtip J. Toxicity study on a Thai traditional medicine: Ya-Kae-Ka-Sai-Lin-Kra-Bue. J Thai Trad Alt Med. 2004;3(1):42.
3. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel). 2018;5(3):93. CrossRef
4. Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8(4):96. CrossRef
5. Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73(2):239–44. CrossRef
6. Wong FC, Yong AL, Ting EPS, Khoo SC, Ong HC, Chai TT. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran J Pharm Res. 2014;13(4):1409.
7. Yapasert R, Sripanidkulchai B, Teerachaisakul M, Banchuen K, Banjerdpongchai R. Anticancer effects of a traditional Thai herbal recipe Benja Amarit extracts against human hepatocellular carcinoma and colon cancer cell by targeting apoptosis pathways. J Ethnopharmacol. 2020;254:112732. CrossRef
8. Zhang Y, Wu J, Xu W, Gao J, Cao H, Yang M, et al. Cytotoxic effects of Avermectin on human HepG2 cells in vitro bioassays. Environ Pollut. 2017;220:1127–37. CrossRef
9. Zhang XH, Zou ZQ, Xu CW, Shen YZ, Li D. Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Mol Med Rep. 2011;4(2):273–7. CrossRef
10. Cui H, Bashar MA, Rady I, El-Naggar HA, El-Maoula A, Lamiaa M, et al. Antiproliferative activity, proapoptotic effect, and cell cycle arrest in human cancer cells of some marine natural product extract. Oxid Med Cell Longev. 2020;2020:7948705. CrossRef
11. Junhom C, Weerapreeyakul N, Tanthanuch W, Thumanu K. FTIR microspectroscopy defines early drug resistant human hepatocellular carcinoma (HepG2) cells. Exp Cell Res. 2016;340(1):71–80. CrossRef
12. Nguyen QV, Eun JB. Antioxidant activity of solvent extracts from Vietnamese medicinal plants. J Med Plant Res. 2011;5(13):2798–811.
13. Koffi C, Soleti R, Nitiema M, Mallegol P, Hilairet G, Chaigneau J, et al. Ethanol extract of leaves of Cassia siamea lam protects against diabetes-induced insulin resistance, hepatic, and endothelial dysfunctions in ob/ob Mice. Oxid Med Cell Longev. 2019;2019:6560498. CrossRef
14. Panthong A, Norkaew P, Kanjanapothi D, Taesotikul T, Anantachoke N, Reutrakul V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J Ethnopharmacol. 2007;111:335–40. CrossRef
15. Dalcorso G, Manara A, Piasentin S, Furini A. Nutrient metal elements in plants. Metallomics. 2014;6:1770–88.
16. Murthy HN, Dalawai D, Dewir YH, Ibrahim A. Phytochemicals and biological activities of Garcinia morella (Gaertn.) Desr.: a review. Molecules. 2020;25(23):1–15.
17. Zhao X, Chen R, Shi Y, Zhang X, Tian C, Xia D. Antioxidant and anti-inflammatory activities of six flavonoids from Smilax glabra Roxb. Molecules. 2020;25(22):5295. CrossRef
18. Khan M, Khan Yusufzai S, Kimin L, Jabi N. Determination of chemical composition, total flavonoid content, total phenolic content and antioxidant capacity of various crude extracts of Manihot esculenta crantz leaves. Int J Res Appl Sci Eng Technol. 2018;6:2433–43. CrossRef
19. Khalighi-Sigaroodi F, Ahvazi M, Hadjiakhoondi A, Taghizadeh M, Yazdani D, Khalighi-Sigaroodi S, et al. Cytotoxicity and antioxidant activity of 23 plant species of leguminosae family. Iran J Pharm Sci. 2012;11(1):295–302.
20. Domnic G, Jeng Yeou Chear N, Abdul Rahman SF, Ramanathan S, Lo KW, Singh D, et al. Combinations of indole based alkaloids from Mitragyna speciosa (Kratom) and cisplatin inhibit cell proliferation and migration of nasopharyngeal carcinoma cell lines. J Ethnopharmacol. 2021;279:114391. CrossRef
21. Mu R, Lu N, Wang J, Yin Y, Ding Y, Zhang X, et al. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur J Cancer Prev. 2010;19(1):61–7. CrossRef
22. Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53(3):153–9. CrossRef
23. Sa F, Gao JL, Fung KP, Zheng Y, Lee SM, Wang YT. Anti-proliferative and pro-apoptotic effect of Smilax glabra Roxb. extract on hepatoma cell lines. Chem Biol Interact. 2008;171(1):1–14. CrossRef
24. Hawry?kiewicz A, Ptaszy?ska N. Gemcitabine peptide-based conjugates and their application in targeted tumor therapy. Molecules (Basel, Switzerland). 2021;26(2):364. CrossRef
25. Mahavorasirikul W, Viyanant V, Chaijaroenkul W, Itharat A, Na-Bangchang K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med. 2010;10(1):1–8. CrossRef
26. Kaewnoonual N, Pradidarcheep W. Effect of benja-ammarit dispensatory for treatment of hepatocellular carcinoma in rat. Bangkok, Thailand: Srinakharinwirot University; 2019.
27. Choi HJ, Lim DY, Park JHY. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,3’-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009;9(1):39. CrossRef
28. Haneef J, M P, Thankayyan R SK, Sithul H, Sreeharshan S. Bax translocation mediated mitochondrial apoptosis and caspase dependent photosensitizing effect of Ficus religiosa on cancer cells. PLOS One. 2012;7(7):e40055. CrossRef
29. Satyanarayana A, Hilton MB, Kaldis P. p21 Inhibits Cdk1 in the Absence of Cdk2 to Maintain the G1/S Phase DNA Damage Checkpoint. Mol Biol Cell. 2008;19(1):65–77. CrossRef
30. Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, et al. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64(12):1713–24. CrossRef
31. Samarakoon SR, Thabrew I, Galhena PB, Tennekoon KH. Modulation of apoptosis in human hepatocellular carcinoma (HepG2 cells) by a standardized herbal decoction of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes with anti-hepatocarcinogenic effects. BMC Complement Altern Med. 2012;12:25. CrossRef
32. Gasparri F, Muzio M. Monitoring of apoptosis of HL60 cells by Fourier-transform infrared spectroscopy. Biochem J. 2003;369(2):239–48. CrossRef
33. Holman HY, Martin MC, Blakely EA, Bjornstad K, McKinney WR. IR spectroscopic characteristics of cell cycle and cell death probed by synchrotron radiation based Fourier transform IR spectromicroscopy. Biopolymers. 2000;57(6):329–35. CrossRef