INTRODUCTION
Nature is widely regarded as a remarkable source of inspiration in drug design. Species of Arthropoda and Reptilia are fascinating and valuable sources for finding compounds with potential therapeutic applications in the animal kingdom [1]. From the venom of various organisms, a wide array of bioactive compounds has been isolated and found to have specific therapeutic potential [2]. Organisms from which bioactive compounds have been isolated include snakes, scorpions, cone snails, sea anemones, octopuses, and bees [1,3–7]. Snake venom has been used for decades to prepare various pharmacological substances [8,9]. Snakes use the glandular secretion [venom] to paralyze and consume their prey. It is also used as an effective tool for defense and existence [10]. This lethal formulation contains biomolecules such as amino acids, carbohydrates, lipids, proteins, and peptides [11].
Snake venom is a mixture of biologically significant substances that have been studied for their medicinal potential. In recent years, there has been an increased demand to explore the anticancer properties of different venom compounds. Snake venom comprises 90%–95% of proteins and peptides; the remaining 5%–10% constitute nonproteinaceous components, including lipids and carbohydrates [11,12]. The enzymatic components include phospholipase A2 (PLA2), L-amino acid oxidases (LAAOs), endonucleases, hyaluronidases, metalloenzymes, and serine proteinases. The nonenzymatic components include inhibitors of proteases, vascular endothelial growth factor (VEGF), three-finger toxins, C-type lectins, and mycotoxins [2]. Even though these enzymatic and nonenzymatic components play a part in envenomation, they are used to prepare various pharmacologically essential substances to treat different conditions, including cancer.
The anticancer mechanism of snake venom components remains an active area of research. Studies have shown that these bioactive molecules can induce cytotoxic effects on cancer cells, leading to their apoptosis. Furthermore, they have exhibited specific mechanisms of action, including the production of reactive oxygen species, which contribute to their cytotoxicity. The pharmacological importance of these substances is due mainly to their high stability [13]. These components have shown promising efficacy against various cancer cells, making them potential candidates for the production of new anticancer agents.
This review analyses recent research on the therapeutic potential of snake venom in cancer treatment. The following section discusses the different snake venom components and their potential as novel anticancer agents, providing insights into their mechanisms of action and their impact on cancer cell lines. The challenges and opportunities in translating snake venom-based therapies into clinical applications are also discussed.
Snake venom components
The components of venom differ from one species of snake to another. The venom of poisonous snakes is more potent than that of other venomous organisms [14]. Snake venom contains more than 100 bioactive compounds that exert diverse effects on cells and tissues. The different bioeffects such as neurotoxicity, cytotoxicity, or hemotoxicity depend upon the type of snake species [15]. Bites of elapids, including cobra [Naja sp.] and krait [Bungarus sp.], induce neurotoxicity, cardiotoxicity, and cytotoxicity. Hemotoxicity is caused by the bite of the Viperidae group of snakes, including pit vipers and true vipers, respectively [15].
The chemicals that cause neurotoxic, cardiotoxic, and cytotoxic effects include phospholipase A2 (PLA2s) [16] and 3-finger toxins [3FTxs] [17]. These proteins are the dominant proteins during envenomation. Other proteins that are present in elapid venom include snake venom metalloproteinases (SVMPs) [18], serine proteases (SVSPs) [19], and LAAOs [20], as depicted in Figure 1. These components make up 6% of the snake venom proteins.
 | Figure 1. The various components of snake venom. [Click here to view] |
Hemotoxicity and myotoxicity are induced by PLA2s, SVMPs [19,20], and toxins of SVSPs [19] and smaller proportions of SV-LAAOs [18,20,21], c-type lectins [22–24], and some natriuretic proteins, respectively [25,26]. The functions and mechanisms of these proteins are described briefly in Table 1.
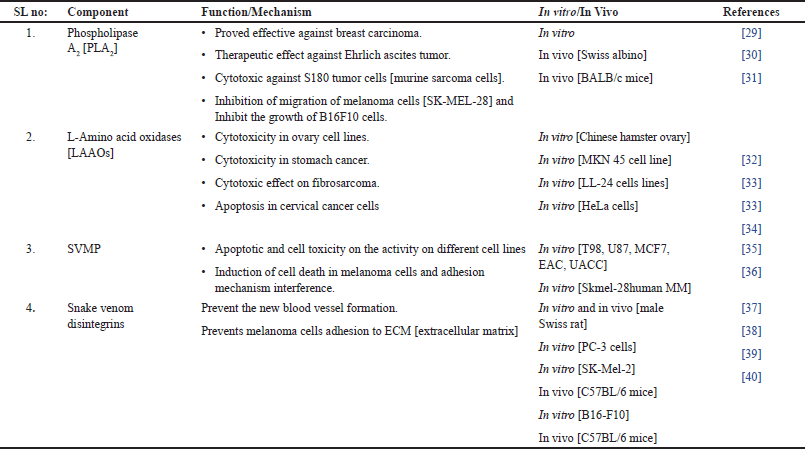 | Table 1. The various components and functions of snake venom. [Click here to view] |
Chemistry of major snake venom-derived components and their functions in cancer therapy
Major toxins isolated from venom possess different properties and thus induce various effects on the victim. Some of the toxins and their properties are as follows.
Phospholipase A2 [PLA2]
The first type of PLA2 identified was called secreted PLA2. It is abundant in snake venom, scorpions, and in the tissues of certain mammals [27]. Snake venom PLA2 is 13 to 15 kDa in molecular weight, contains a histidine residue at its catalytic site, and houses the divalent metal ion, calcium [Ca2+] in its active site. PLA2 contains six conserved disulfide bonds [28]. It catalyzes the reaction by attacking the sn-2 bond by removing an H+ ion[proton] from the water. A histidine/aspartic acid dyad activates water molecules in a Ca2+-dependent manner.
According to Kang et al., PLA2 contains amino acids such as histidine 48, aspartic acid49, tyrosine52, and aspartic acid99 [18]. Histidine 48 and aspartic acid 49 house a molecule of water attached to their side chains. This conserved H2O molecule helps in the process of catalysis [18]. The catalytic mechanism of PLA2 involves hydrolysis of the phospholipid molecules and polarization of the bound H2O molecules, which leads to the development of an intermediate by attacking the sn-2 bond. This process is catalyzed by histidine 48 and aspartic acid 99 [41]
PLA2 is divided into two major groups: active PLA2 and inactive PLA2. There are 14 different PLA2 groups among the members of both the Viperidae and Elapidae families [42]. According to Gutierrez and Lomonte, the catalytic activity of PLA2 can be divided into high [Asp49 PLA2] and low [Lys 49 PLA2] [43]. PLA2 displays a variety of pharmaceutical actions. Its exceptional capacity to target a particular tissue is due to highly specialized receptors that bind with high affinity, and the binding is not dependent on the catalytic sites [44], according to Berg et al. PLA2 activity occurs in two different stages. In the first stage, the enzyme specificity is maintained when PLA2 binds to the membrane. The second stage corresponds to the enzymatic efficiency of PLA2 due to the occurrence of chemical reactions [45]. PLA2s of Viperidae snakes have evolved and are divided into enzymes and catalytically inactive proteins. The effect of PLA2 on the victim’s body may vary according to the type of venom. In the neurotoxic venom of krait [Bungarus sp.], the PLA2 Kunitz-type protein [heterodimeric protein] constitutes one of the components of bungarotoxin. Beta bungarotoxin moves through the blood to reach its target membrane in the presynaptic neuromuscular junction and binds to K+ channels, thus trapping the peptide in a specific location. The neurotoxic effect is further enhanced when bungarotoxin opens K+ channels, initiating phospholipid hydrolysis. This hydrolysis increases neurotoxicity [46–48]. In general, the use of PLA2 in the treatment of several diseases is highly diverse.
PLA2 in cancer therapy
Many studies and shreds of evidence support the anticancer potential of snake venom. The snake venom toxin emphasized for its anticancer potential is snake venom PLA2 [49].
The cytotoxic and apoptotic mechanisms of PLA2 occur in cancer tissues through the liberation of reactive oxygen species (ROS) when PLA2 interacts with the cell membrane. The generated ROS leads to high oxidative stress in cells, thus activating specific apoptotic pathways. Benati et al. [50] reported that the interaction of PLA2 with the membrane led to the stimulation of apoptotic promoter proteins such as BAD and caspase 3, which caused cell death. Additionally, this protein led to the downregulation of antiapoptotic proteins such as Bcl2 and c-FLIP, thus promoting apoptosis. PLA2 also promotes apoptosis through the induction of DNA damage and micronuclei formation. Like other natural agents, PLA2 promotes apoptosis in a dose- and time-dependent manner [51]. The main factor involved in promoting cell death is the binding of the vascular endothelial growth factor receptor (VEGFR) and the C-C-terminus of PLA2, thus causing antiangiogenesis in tumor cells [52,53].
These molecules have been proven to prevent the development and progression of specific tumor cells. PLA2 from species such as Cerastes cerastes venom (CC) has been shown to inhibit the properties of cancer cells, thus inhibiting their growth [54]. The venom of these species inhibits the migration and adhesion of cancerous cells, as well as angiogenesis [55,56]. The metastatic activity of Ehrlich ascites tumors, leukemia [Jurkat], and breast cancer cell lines was inhibited via the SV PLA2 isolated from the Bothrops jararacussu sp. [30]. Venom-derived PLA2 from the species Crotalus durissus terrificus showed antitumor activity in vitro on erythroleukemic cells of murine origin [57]. PLA2 showed cytotoxic and apoptotic activity from the venom of the krait species Bungarus fasciatus.
The cytotoxic activity of heterodimeric elemental PLA2 from Crotalus durissus terrificus was found to inhibit the metastatic properties of different cancerous cell lines, such as U373 glioma cells and GAMG human glioblastoma cells. The IC50 value of PLA2 in the U373 and GAMG cell lines was 30.2 μg/ml [58]. TX-1, a type of PLA2 isolated from the Viperidae species Bothrops jararacussu, exhibited a cytotoxic effect on the SK-BR-3 and MCF-7 human breast cancer cell lines. The IC50 cytotoxicity values were 81.2 μg/ml and 104.35 μg/ml, respectively [59]. drsPLA2 is a type of PLA2 obtained from the venom of Eastern Russell’s Viper [Daboia russelii siamensis], and it was found to induce cytotoxicity in SK-MEL-28 human skin melanoma cells at an IC50 of 0.90 μg/ml [60]. The mechanism of action of PLA2 in cancer cells is depicted in Figure 2.
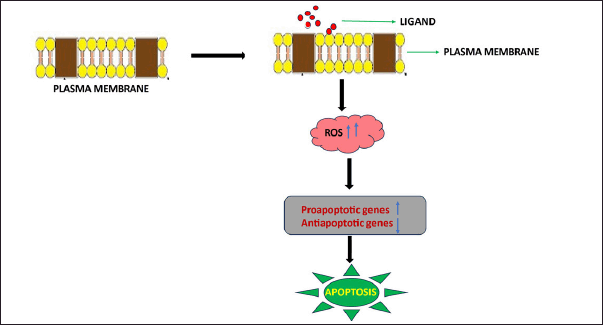 | Figure 2. Mechanism of the cytotoxicity of snake venom PLA2 in tumor cells. PLA2 protein interacts with the plasma membrane of tumor cells, thus causing membrane damage. This interaction leads to an increase in reactive oxygen species (ROS), thus causing oxidative stress. This mechanism stimulates the upregulation of proapoptotic genes [caspase 3 and Bad] and the downregulation of antiapoptotic genes [Bcl2 and Bcl-XL], thus stimulating apoptosis. [Click here to view] |
Specific clinical trials have been performed to prove the antitumor activity of crotoxin. Crotoxin was administered intramuscularly for 30 days in patients with solid tumors refractory to conventional therapy in a phase I clinical trial at a very low dosage. In 23 patients, 35 cycles of crotoxin administration were evaluated. In this study, no deaths were observed. Patients with various types of carcinomas responded differently, thus resulting in disease reduction [61]. Hence, PLA2 from snake venom can be used in cancer therapy to treat different kinds of cancer, as it has proven to be an effective pharmacological agent.
Snake venom metalloproteinases (SVMPs)
Snake venom metalloproteinases, abbreviated as SVMPs, are specific types of peptides that possess multiple domains and many biological activities, such as the induction of hemorrhage, lysis of fibrinogen and fibrin, promotion of cell death, and prevention of blood clot formation. Because of their diverse pathological activities, these proteins are responsible for changes following snake bite. These proteins are present mainly in the Viperidae and Crotalinae venoms [62].
SVMPs are zinc-dependent proteinases that contribute to envenomation symptoms after a snake bite [63]. Most SVMPs are hemorrhagic and have been further classified into subcategories depending on the number of domains. The P-III SVMPs are the largest, oldest, and most complex enzymes. Loss of domains from these categories results in the formation of P-I and P-II enzymes. Only P-III SVMPs are present in the venom of elapid members. Each of the three SVMPs comprises a catalytic domain responsible for hemorrhage. The function of the catalytic domain is the hydrolytic attack of collagen IV, fibrinogen, and other coagulation factors [64,65]. Continuous hydrolysis disrupts the standard mechanism of clotting [66,67]. In addition to the catalytic domain, P-II SVMPs contain a specific disintegrin domain that notably prevents clot formation. Prevention of clotting occurs by binding to a protein that helps in blood clotting [αIIBβ3 integrin] and inhibiting the same protein [68]. Compared to P-II SVMPs, P-III SVMPs house a catalytic domain, a disintegrin domain with an ECD motif [Glu-Cys-Asp], and a cysteine-rich domain that helps in substrate recognition and binding [69]. Unlike P-III SVMPs, P-1 V SVMPs contain C-type lectins [70]. These domains of SVMPs are involved in inflammation through the release of TNF-α, IL-1β, and IL-6. These observations prove that these domains are actively involved in SVMP-induced hyperalgesia [63].
SVMPs in cancer therapy
Snake venom metalloproteases (SVMPs) are ubiquitously distributed in Crotalidae and Viperidae venom. These compounds are essential for inducing envenomation symptoms such as hemorrhage and alterations in blood clotting mechanisms [71]. In addition, certain SVMPs have been proven to have anticancer or apoptotic properties.
The mechanism of cytotoxicity involves mechanisms such as certain metalloproteins, such as jararhagin, a 50 kDa protein isolated from Bothrops jararaca, which is a multidomain protein with a zinc metal-dependent part and a cysteine-rich domain within it. This metalloproteinase works like other groups of metalloproteinases by inducing cell death in tumor cells [72]. The mechanism of apoptosis involves decreased cell viability caused by changes in cellular morphology, genetic makeup, and reduced adhesion [72]. Continuous apoptosis of the cancer cells indicated inactivation of the catalytic domain and further activation of the caspase 3 pathway, DNA fragmentation, condensation of the chromatin, and cell cycle arrest. All these changes contribute to programmed cell death, as depicted in Figure 3. These cell death mechanisms were observed in B16F10 and Sk-Mel-28 cells [73]. .
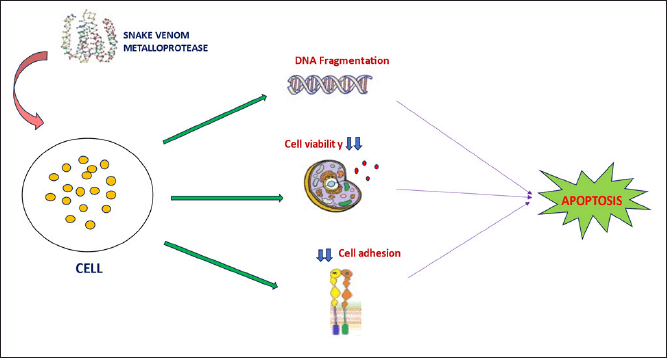 | Figure 3. Mechanism of SVMP action on cancer cells. When SVMP is added to cancer cells, it causes fragmentation of the genetic material, thus leading to genomic instability. SVMPs also reduce cell adhesion to the extracellular matrix, reducing tumor cell viability. All these factors contribute to caspase three-mediated apoptosis in SVMP-treated cancer cells. [Click here to view] |
A type of P-III SVMP called Leucurolysin-B [leuc-B], which was isolated from Bothrops leucurus , exhibited antitumor activity against different types of cancers, such as glioblastoma, carcinoma of the breast, and Ehrlich ascites carcinoma, and the inhibition concentration 50 [IC50] value of Leucurolysin-B against these cancer cell lines was found to be in the range of 0.2 to 0.6 µM. These results indicate that this metalloproteinase is highly effective against certain tumor cell lines, making it more potent than the commonly used anticancer agent cisplatin [35].
Another P-III SVMP, hemorrhagic VaH4, was cytotoxic to HeLa cells at a very low concentration of approximately 7.5 nM. Notably, VaH4 induced specific morphological changes in HeLa cells associated with apoptosis [74]. Bothropoidins, a type of metalloproteinase, have anticancer effects on MDA-MB-231 breast cancer cells. Although bothropoidins are cytotoxic to breast cancer cell lines, these toxins do not inhibit all kinds of breast cancer cells, such as MCF10A cells. Hence, these SVMPs are thought to inhibit only preferred cancer cell lines [75].
The function of SVMPs is their hemorrhagic activity and their capacity to induce abnormalities in various steps of clotting, thus causing systemic hemorrhage, resulting in the coagulation of victims and prey [64]. Hemorrhage caused by the action of snake metalloproteinases occurs in two different steps, such as cleavage of the basement membrane and other adhesion proteins associated with it. This process causes weakening of the capillary vessels. In the second stage of action, the detachment of endothelial cells from the walls of the basement membrane occurs, resulting in capillary wall disruption and the leakage of blood from capillary vessels that have become weak and thin [76,77]. Hence, SVMPs are considered important classes of proteins that result in snake venom toxicity. There is no supporting preclinical evidence to date to prove its antiangiogenic properties.
Disintegrins
Disintegrins are tiny peptides rich in cysteine and contain the main peptide sequence Lys-Gly-Asp or Lys-Thr-Ser. The molecular weight of disintegrins typically varies from 5 kilodaltons to 10 kilodaltons [78]. Venoms of Viperidae, Elapidae, and Colubridae contain disintegrins [79]. Snake venom disintegrins are classified on the basis of the number of disulfide bonds and amino acid residues present. In this way, disintegrins have been classified as short disintegrins with four disulfide bonds and 51 residues, medium disintegrins with six disulfide bonds and 70 different amino acid residues, and long disintegrins with seven disulfide bonds and 84 different amino acid residues. All these classifications account for various types of monomeric disintegrins. The structure of dimeric disintegrins appears to be more complicated, with 67 different residues and ten cysteine bonds in the 4 and 2 intrachain disulfide linkages [80]. The monomeric disintegrins include the RGD (arginine–glycine–aspartic acid) motif. It constitutes the most prominent family of disintegrins. Although RGD domains are present in monomeric disintegrins, some dimeric disintegrins also possess these domains [81]. Unlike monomeric disintegrins, heteromeric disintegrins possess an MLD domain [methionine?leucine?aspartic acid]. Snake venom disintegrins are known for their various functions, including the antitumor potential as mentioned later.
Disintegrins in cancer therapy
Disintegrins connect with integrins via glycoprotein receptors found on the cell membrane. It plays a significant role in cell?matrix interactions. Snake venoms with RGD sequences can display selective apoptotic activity or inhibit angiogenic activity via receptors. According to Akhtar et al. [78] some disintegrins have antitumor properties by preventing tumor cell adhesion to the extracellular matrix by blocking integrins 51 and v3 [78]. Disintegrins induce antitumor activity in different tumor cell lines through cytotoxicity and apoptosis, as shown in Figure 4.
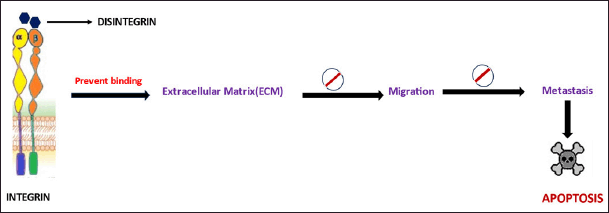 | Figure 4. Cytotoxic mechanism of disintegrins isolated from venom. Disintegrins prevent the attachment of cancer cells to the ECM, thus preventing migration and metastasis. Snake venom disintegrins promote apoptosis through a caspaseindependent pathway. [Click here to view] |
Snake venom disintegrins, such as Lebein, have demonstrated their potential as antineoplastic agents by inducing apoptosis in melanoma cells. Lebein effectively inhibits melanoma cell proliferation, promoting a shift toward a more differentiated cell state. Prevention of metastasis is achieved by blocking the phosphorylation of extracellular signal-regulated kinase (ERK) and increasing the expression of microphthalmia-associated transcription factor (MITF). The interaction of cancer cells with Lebein leads to the detachment of melanoma cells and reduces their invasive ability, concurrently increasing the level of E-cadherin expression. Furthermore, Lebein triggers apoptosis through a caspase-independent pathway characterized by increased levels of apoptosis-inducing factor (AIF), BCL-2-associated X protein [BAX], and Bim while downregulating B-cell lymphoma-2 [BCL-2] [82]. Salmosin, another disintegrin ubiquitously distributed in Agkistrodon halys brevicaudus snake venom, has also demonstrated cytotoxic properties. It achieves this by inhibiting angiogenesis, suppressing tumor growth, and inducing apoptosis in cancer cells. Salmosin interacts with integrin alpha [v]beta [3] on the cell surface, causing the disassembly of focal adhesions and the detachment of cells from the extracellular matrix (ECM). This disruption of cell?matrix interactions triggers apoptosis in some cells, such as endothelial cells. Importantly, salmosin selectively targets actively proliferating cells that express relatively high levels of integrin alpha[v]beta [3], making it a promising candidate for effective anticancer therapy. The mechanism of action of salmosin involves competition with the ECM for direct binding to integrin alpha[v]beta [3], ultimately leading to disruption of focal contacts and cellular detachment. This interference with integrin-mediated signaling pathways, such as those dependent on focal adhesion kinase (FAK), contributes to the induction of apoptosis in cancer cells [83].
A specific type of disintegrin called obtustatin obtained from the Vipera lebetina obtusa and Macrovipera lebetina obtuse venom was found to have antiangiogenic effects on both melanoma and sarcoma cell lines. Oncostatic activity of obtustatin is believed to be achieved through the suppression of α1β1 integrin [84].
Lino et al. [85] studied the antiapoptotic property of DisBa-01, a disintegrin isolated from the species Bothrops alternatus. Its antiangiogenic properties have been investigated against cell lines such as L929 fibroblasts and 4T1BM2 breast tumor cells [85]. DisBa-01 arrested the division of breast cancer cells at the synthesis phase. In tumor cells undergoing apoptosis, there is a tremendous increase in the expression of marker proteins specific for autophagy. When HUVECs are treated with fibronectin or vitronectin, specific processes mediated by angiogenesis are inhibited, thus preventing the division of cancer cells or metastasis [86].
Although snake venom disintegrins have been proven to be antiproliferative agents, many preclinical studies have not been performed. Additionally, snake venom disintegrins such as eptifibatide and tirofiban have been approved by the FDA for use as antiplatelet drugs [87].
L-amino acid oxidase [LAAO]
Snake venom LAAO (SV-LAAO) is one of the least studied enzymes among snake venom components. SV-LAAO is a heterodimer containing FAD that helps deaminate L-amino acids to specific α keto acids with concomitant release of large amounts of hydrogen peroxide and ammonia [88,89] as depicted in Figure 5. It belongs to the family of NAD[P]/FAD-dependent oxidoreductases and contains flavin adenine dinucleotide (FAD), flavin-containing monoamine oxidases (MAOs), linoleic acid isomerase, and polyamine oxidase [90]. .
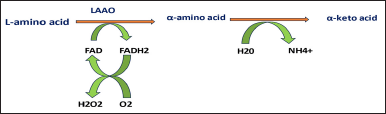 | Figure 5. Illustration of the mechanism by which SV-LAAO induces LAAO. [Click here to view] |
Each subunit of the LAAO is made of subunits of 50–70 kDa molecular weight. The enzyme possesses three subunits: a helical domain, a substrate-binding domain, and a FAD-binding domain [91]. The GG motif and the FAD-binding motif, which have a continuous repeat of three glycine residues [Gly] residues, make up the FAD-binding domain.
The substrate is bound to a pocket of seven β-pleated strands, forming the substrate-binding domain. The protein’s active site is located adjacent to the flavin cofactor, near which the helical domain forms a funnel-shaped pathway leading to the protein’s core region. This shape of the helical domain facilitates maintaining the orientation of amino acids entering the funnel. The proper orientation is maintained by encouraging the electrostatic interaction of amino groups with the carboxylic groups of substrates [20,92]. Histidine and arginine residues [His 223, Arg 322] are present in the active site of LAAO and play essential roles in the catalytic activity of LAAO [20]. In addition, the LAAO active site consists of many amino acid residues, including arginine 90, histidine 223, phenylalanine 227, lysine 326, tyrosine 372, and tryptophan 375. The active site also houses a specially conserved water molecule near the FAD domain and Lysine 326 [93].
Although least studied, these snake venom components are ubiquitously distributed in the venom of all snake species and contribute to envenomation [88]. During envenomation, these enzymes are responsible for several physiological and pathological changes, such as the induction of apoptosis [88], the development of edema [94], and the aggregation or inhibition of platelet activities [95,96], leading to hemorrhage and anticoagulation effects [97]. A study performed by Oliveira et al. in 2019 revealed the LAAO from Bothrops spp. of the Viperidae family causes autophagy, apoptosis, and necrosis in normal human skin cells. LAAO from snake venom also has antibacterial [98], leishmanicidal [96,99] and antifungal activities. In addition to these functions, it has anticancer [100] and anti-HIV [101] activities and can be used to treat these diseases.
These enzymes are not only limited to snake venom but also found in insects [102] and bacteria [103], as well as in several species of fungi, some species of green algae [104], several plants [105], and mammals. The yellow color of the venom (light to dark) indicates the presence of LAAO. This yellow color is due to the oxidation of FAD [92]. These enzymes are active in their dimerized form. LAAO appears to be stable at room temperature but becomes inactive when exposed to very low temperatures ranging from -5°C to -60°C, except for the venoms of Ophiophagus hannah and Calloselasma rhodostoma, respectively.
SV-LAAO in cancer therapy
In many studies, the effect of SV-LAAO on cancer cells has been identified and proven that these proteins have effective anticancer potential on tumor cells. The antitumor mechanism employed by this protein involves the generation of ROS such as superoxide anions, hydrogen peroxide, and hydroxyl radicals [106]. The primary mechanism of LAAO cytotoxicity may involve the release of hydrogen peroxide because of LAAO activity [107]. H2O2 release produces ROS that result in membrane damage, thus resulting in apoptosis. The generated ROS contributes to the intrinsic apoptotic pathway by mediating the release of cytochrome C from the mitochondria. The release of cytochrome C leads to the activation of caspase 9, thus promoting the apoptosis of the cells. In addition to caspase 9 activation, LAAO activates caspase eight, thus promoting apoptosis through the extrinsic pathway [108,109].
Figure 6 illustrates the anticancer mechanism of SV-LAAO.
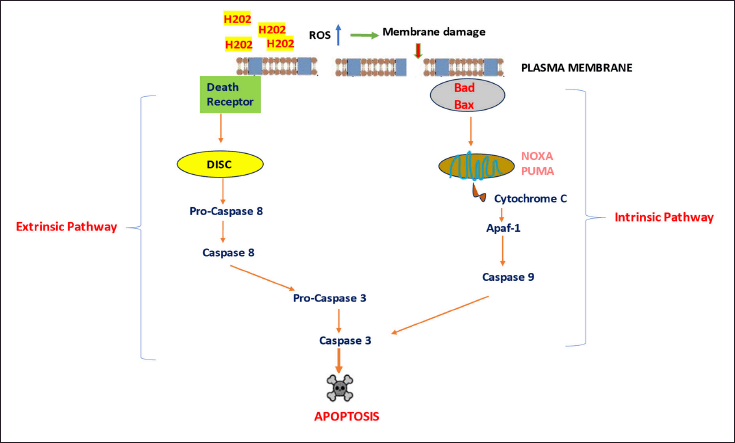 | Figure 6. Summary of the cytotoxic effects of SVLAAO on cancer cells. SVLAAO promotes apoptosis in tumor cells via both extrinsic and intrinsic pathways. Excessive hydrogen peroxide generated by LAAO results in the release of ROS, which act with the plasma membrane of cancer cells, thus causing apoptosis. Apaf-1 = apoptotic protease-activating factor-1; Bad = BCL2-associated agonist of cell death; Bax = Bcl-2-associated X protein; DISC = Deathinducing signaling complex; NOXA = Phorbol-12-myristate-13-acetate-induced protein 1; PUMA = p53-upregulated modulator of apoptosis; ROS = Reactive oxygen species. [Click here to view] |
Abdelkafi-Koubaa et al. reported that LAAO isolated from the CC decreased the viability of U87 cells at different concentrations, and its IC50 value was 2.6 nM [110]. Hence, CC-LAAO affected the overall viability of glioblastoma cell lines. LAAO from the species Agkistrodon acutus, known as ACTX-8, when introduced into cervical cancer cell lines [HeLa cells], results in DNA fragmentation followed by externalization of phosphatidylserine, thus inducing apoptosis. Further analysis revealed that apoptosis was induced through caspase-dependent pathways and the involvement of apoptosis-inducing factors (AIFs) [34]. Activation of the JNK cascade resulted in A549 lung cancer cell death when A549 cells were treated with ACTX-6 from Agkistrodon acutus [111].
In addition to the solid tumors mentioned above, LAAO also has antitumor effects on other tumors. LAAO isolated from Bothrops induced caspase-mediated apoptotic activity in rat pheochromocytoma tumors [PC-12 cells], B16F10 cells [mouse melanoma], HL-60 cells, and Jurkat cells. Furthermore, LAAO has been shown to control the apoptotic regulatory process by changing the expression of microRNAs such as miR-145, miR-26a, miR-142-3p, miR-21, miR-130a, and miR-146a in BCR-ABL cells. miRNAs control specific targets of microRNAs, such as Bcl-2, CIAP-2, and MCL-1. According to Teixeira et al. [112], SV LAAO can be used as an agent to treat particular types of cancer [112].
OTHER SNAKE VENOM TOXINS WITH ANTICANCER PROPERTIES
In addition to the toxins mentioned earlier, some other toxins of snake venoms have certain antitumor activities. Vixapatin derived from the venom of Vipera xantina palestinae is a C-type lectin that prevents angiogenesis and adhesion in a wide variety of cancer cells, such as rat C6 glioma cells and human dermal microvascular endothelial cells (HDMECs) [113]. De Castro Damasio et al. [114] reported that C-type lectin (BJcuL) purified from Bathrops jaracassu venom induced cell death in the HT29 cell line by activating the external apoptosis pathway. Another type of CTL, daboialectin, which is isolated from Russell’s viper, causes cytoskeletal changes and apoptosis in A549 cells [115]. Hammouda et al. [82] reported that macrovipectin (CTL from Microvipera lebetinus) promoted the apoptosis of melanoma cells (SK-Mel-28). The combination of macrovipectin and cisplatin enhanced the apoptotic activity of SK-Mel 28 cells by preventing the integrin-dependent adhesion and migration of the cells. Nasulysin 1, which belongs to the SVMP category, is a potent toxin derived from hognose pit viper (Porthidium nasutum) venom that has been shown to have apoptotic activity in JURKAT cells and K562 cells. The rate of death in normal cells was lower than that in cancer cells. Nasulysin 1 induces apoptosis by activating caspase 3, resulting in DNA fragmentation and chromatin condensation [116]. A potent snake venom serine protease (SVSP), collinein-1, was isolated from Crotalus durissus collilineatus venom and exhibited antitumor activity in the breast cancer cell line MCF7. However, its activity was reduced in normal breast epithelial cells (MCF10A) [117].
METHODS OF SNAKE VENOM ADMINISTRATION
While it has been established that snake venom and its various components cause apoptosis in tumor cells, delivering these substances to the target site is a significant challenge. Direct administration of venom or its components into the body is not feasible, as it can trigger envenomation symptoms upon contact with the bloodstream, which may prove fatal for healthy cells [118]. These can be circumvented by using targeted drug delivery strategies as mentioned later.
These challenges can lead to a promising strategy to attach these venom components to monoclonal antibodies (mAbs) that target specific cancer cell sites [119]. However, the use of mABs in drug delivery presents several challenges, including undesirable side effects and the need for frequent dosing due to their short half-life under certain conditions. Additionally, the use of mABs can result in the aggregation of misfolded proteins, diminishing their therapeutic effectiveness and causing adverse effects. These limitations restrict patient accessibility to this approach [120].
Drug targeting can be achieved via the use of specific drug carriers to deliver the drug to particular locations. These drug delivery systems are called carrier vectors, as they enable the transport, retention, and targeting of specific drug molecules. The primary type of drug carrier or drug delivery system is nanoparticles. This system involves the use of specific nanosized vesicles with sizes ranging from 1 to 100 nm [121]. These nanosized materials are considered more efficient because they have a higher surface area-to-volume ratio and are regarded as potent vehicles for targeted drug delivery. These nanoparticles are classified on the basis of various strategies, such as structural aspects of the synthesized components. On the basis of these criteria, they can be divided into liposomes, dendrimers, micelles, inorganic nanoparticles, and polymeric nanoparticles [121]. The different types of vehicles used for snake venom delivery are shown in Table 2.
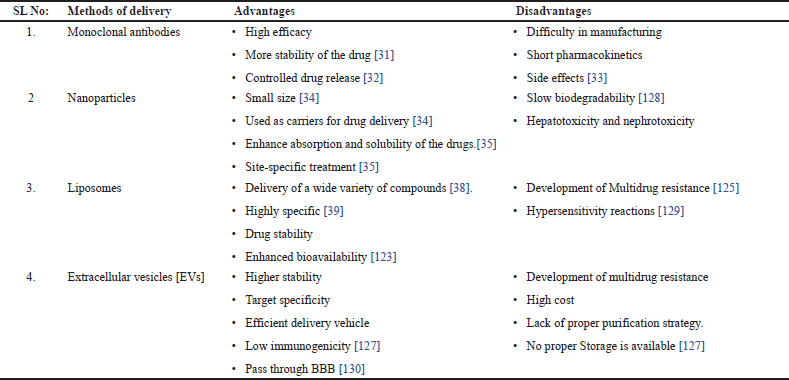 | Table 2. Methods of delivery [Click here to view] |
One such type of nanoparticle commonly used is liposomes. These materials have been extensively examined as drug delivery vehicles owing to their capacity to encapsulate both hydrophilic and hydrophobic drugs, thus providing controlled release and target-specific delivery. Liposomes are small, spherical nanovesicles composed of one or more layers of phospholipids that encapsulate an inner aqueous solution. They can exist unilamellarly, consisting of a single phospholipid bilayer, or multilamellarly, with multiple concentric phospholipid bilayers. This unique structure enables liposomes to effectively enclose hydrophilic and hydrophobic drugs, with water-soluble drugs trapped within the aqueous core and fat-soluble drugs integrated into the phospholipid bilayers [122].
Liposomes present advantages such as increased therapeutic efficacy, reduced dosing frequency, and increased bioavailability [123]. Various approaches have been explored to actively direct liposomal drug delivery systems to specific cells and tissues. These approaches encompass the targeting of surface, transmembrane, and internal cell receptors and direct cell targeting [124]. Liposomes have also been investigated for the delivery of drugs from the nose to the brain, where they can circumvent the blood?brain barrier and transport drugs to the brain through intranasal administration [125]. Moreover, liposomes combined with high-intensity focused ultrasound have demonstrated potential for targeted drug delivery and improved the efficacy of anticancer drugs while simultaneously minimizing damage to normal tissue [126].
Another type of nanoparticle for drug delivery is extracellular vesicles. These are considered novel carriers that help target drug delivery to specific locations. These vesicles also treat cancer, nerve diseases, and wound healing. Hence, these nanoparticles bound by lipids are considered novel drug delivery systems that can carry different types of cargo, including amino acids, proteins, and nucleic acids. The difficulties associated with producing, storing, and loading such as biotherapeutics can be overcome via the use of an appropriate delivery system. One possible benefit of using EVs to deliver therapeutic agents over synthetic vehicles is the involvement of endogenous cellular machinery for producing the proper cargo and sorting it inside EVs [127]. Another advantage of using EVs is their ability to cross physical barriers, such as the blood?brain barrier (BBB), which is one of the hindrances that limits the transportation of certain therapeutics and cargo to the central nervous system to treat conditions related to it [118,131]. .
Another advantage of using EVs is their ability to reduce the harmful effects of foreign chemicals when they are administered as delivery vehicles. EVs have been proven to cause few or no immunogenic reactions, mainly due to their biological origin. The nonreplicative and nonmutagenic nature of EVs prevents neoplasia formation. This makes them different from virus-derived vehicles. The reduced toxicity observed in in vivo tests of EV therapies has supported these advantages [127].
To effectively target these vesicles to the target sites, they must be loaded with the cargo. Cargo loading can be performed during EV biogenesis [endogenous loading] or loading after EV isolation [exogenous loading] [132]. The most commonly used technique is loading through the exogenous pathway, which includes various methods, such as electroporation [133], sonication, incubation, and freeze?thaw cycles [134].
In addition to these nanovesicles, other nanoparticles, such as solid lipid nanoparticles (SL)NPs, polymer-based NPs, magnetic NPs, silica NPs, and carbon nanomaterials, are used for effective drug delivery for diseases ranging from microbial infections to cancer therapy [135].
Jimenez Canale et al. [136] reported that chitosan nanoparticles loaded with Crotalus molossus venom exhibited cytotoxic and apoptotic effects on T47D breast cancer cells. Silica nanoparticles loaded with the venom of Vipera ammodytes transcaucasiana (Transcaucasian sand viper) induced cytotoxicity in U87MG and SHSY5Y cancer cells [137]. Walterinnesia aegyptia venom-loaded silica nanoparticles were found to have cytotoxic and apoptotic effects on prostate cancer cells [138].
Limitations of the current targeting systems
Although nanoparticle-based drug delivery methods are emerging for the treatment of various diseases, in addition to their advantages, these methods have several disadvantages, including their limited ability to target specific sites, susceptibility to multidrug resistance in antitumor treatments, and potential for high drug toxicity [139]. Additionally, biological barriers such as the ECM and mucus gels pose substantial challenges by hindering the diffusion and distribution of nanoparticles to their intended target cells. According to TP et al. (2024), nanoparticles induce successful drug delivery, and their long-term safety is thought to be a significant concern [140].
Even if nanoparticles target drugs at a specific location, the nanoparticles must cross significant physiological barriers, which may hinder the movement of the particles [141]. In conclusion, these biological barriers within target tissues present formidable obstacles for nanoparticle-based drug delivery systems, emphasizing the pressing need to develop strategies to overcome these challenges and enhance therapeutic outcomes. These limitations apply to almost all nanoparticles, including EVs. Although Evs are used as novel therapeutics in nanomedicine and technology, there are various limitations to considering Evs as a successful therapeutic strategy.
The primary obstacles in this field are the lack of clinical-grade purification techniques appropriate for mass production and the inadequate understanding and standardization of factors impacting EV generation. According to Whitford et al. [142], the manufacturing cost of EV-based therapeutics is very high, which also adds to the limitations of using EVs as therapeutics. Sterile creation, large-scale, and practical synthesis of significant numbers of EVs with therapeutic loads for clinical testing are necessary to create clinical-grade EVs [143]. Another problem with the use of EVs is storage. Improper storage at different temperatures results in the degradation or loss of functional proteins or biomolecules in the EV membrane [144].
Another main challenge in using EVs is their drug loading efficiency. Many methods exist that involve loading cargo into EVs. EV loading can be performed in different ways, such as via liposomes, but the loading efficiency is greater than that of EVs, which is one of the significant drawbacks in drug loading efficiency [132]. The main reason for this is the lack of space within the EVs due to the presence of remnants from a place of origin. Different methods for loading drugs to circumvent these barriers are being used. Specifically, a preloading method that includes a transfection process and coincubation is performed during the formation of EVs. In this method, cargo loading coincides with the formation of EVs [145]. Other methods, such as electroporation [146], sonication [147], and freeze?thaw cycles [148], are considered post-loading methods. Another efficient loading method is the production of nanoparticles from engineered cells [149]. Overall, the challenges of using nanoparticles as delivery vehicles are the absence of specific safety data for large mammalian models [150], ineffective delivery to specific target sites, accumulation in nontarget organs, and lower cellular uptake, thus resulting in lower therapeutic efficiency [151].
CONCLUSION AND FUTURE PERSPECTIVES
Snake venom components have proven to offer immense potential as novel anticancer agents. The mechanisms of action, cytotoxic effects, and apoptosis-inducing capabilities of these bioactive molecules against different cancer cell lines are known through a comprehensive analysis of various studies. Various studies have shown that snake venom components possess inherent cytotoxic properties and can selectively target cancer cells, making them attractive drug delivery systems for novel anticancer therapies.
However, significant challenges and opportunities exist in translating snake venom-based therapies into clinical applications. The major challenge is the side effects posed by the direct use of venom components, which can lead to life-threatening situations. To overcome this, developing delivery systems and formulations for snake venom components holds promise, thus enhancing their therapeutic potential. The delivery systems can be of different types, such as metal nanoparticles, lipid nanoparticles, and extracellular vesicles, as listed in Table 2. The use of these inert delivery systems thus helps reduce side effects and significantly crosses various biological barriers [152,153].
The synthesis of snake venom-encapsulated delivery systems, such as EVs, holds significant promise for cancer treatment. These vesicles offer numerous advantages, including targeted drug delivery, enhanced stability, and controlled release of therapeutic substances. Future application of snake venom-encapsulated nanoparticles in cancer treatment opens exciting possibilities for improved efficacy and reduced side effects. This new formulation will help achieve targeted delivery to specific sites, thus reducing off-target effects and enhancing drug effectiveness. These compounds can also treat cancer via combination therapy, improving treatment outcomes. The new formulation can be designed to escape the standard immunological mechanisms of the body. Reduced immunogenicity and increased biocompatibility make it more suitable for clinical studies. The future of these new formulations in cancer treatment relies on rigorous preclinical and clinical studies. These studies provide valuable insights into the safety, efficacy, and optimal dosage regimens of these novel formulations. Additionally, long-term studies are needed to assess the potential side effects and evaluate the overall impact on patient outcomes.
In addition to the use of venom carriers, the use of novel techniques such as AI-mediated drug delivery and synthetic venom peptides also contributes to anticancer therapy. The use of artificial intelligence in the design of anticancer drugs is gaining importance because of its lower research expenses [154]. . AI-mediated drug delivery enhances the efficiency of drug delivery to target sites without involving healthy tissues [155]. Owing to the development of these techniques, more potent and promising tools for designing anticancer medications in much easier, faster, and affordable ways are needed.
Kalle Moebius et al. [156] explained synthetic peptides as excellent tools for mimicking certain locations of protein fragments. They can be produced as precise replicas of protein fragments through a variety of chemical modifications, such as the addition of a wide variety of nonproteinogenic amino acids or changes to the peptide backbone. Such alterations not only broaden the chemical and structural variety of peptides but also improve their proteolytic stability, which raises the possibility that they could be used as therapeutic options. One such example is the LZ1 peptide derived from the venom of Bungarus fasciatus, which suppresses the growth of pancreatic cancer cells by targeting nucleolin on the cell surface, thus making the development of therapeutic drugs against pancreatic cancer possible [157]. In addition to snake venom, venom peptides from other organisms also play important roles in anticancer therapy. LVTX-8 (amphipathic, alpha-helical) from Lycosa vittate spider mites promotes the apoptosis of lung cancer cells. Conopeptides obtained from Conus inscriptus (marine snails) exhibit cytotoxicity against HeLa-HPV 16-associated cell lines, thus acting as potent anticancer agents [157].
In conclusion, the future prospects of the use of snake venom-encapsulated carriers in cancer treatment are highly promising. With targeted delivery, combination therapy, and the ability to overcome drug resistance, these nanoparticles can potentially revolutionize cancer treatment. Further research and preclinical and clinical trials are necessary to explore the therapeutic potential of snake venom-encapsulated nanoparticles fully and pave the way for their integration into routine clinical practice.
ACKNOWLEDGMENT
We express our sincere gratitude to Kasturba Medical College, Manipal, for providing access to the necessary resources and research tools required to complete this review. We also thank all the authors for their valuable insights and discussions, which greatly assisted the research. Additionally, we acknowledge the support. No external funding was received for this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Utkin YN. Animal venom studies: Current benefits and future developments. World J Biol Chem. 2015;6(2):28. CrossRef
2. Munawar A, Ali SA, Akrem A, Betzel C. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018;10(11):474. CrossRef
3. Undheim EAB, Georgieva DN, Thoen HH, Norman JA, Mork J, Betzel C, et al. Venom on ice: first insights into Antarctic octopus venoms. Toxicon. 2010 Nov;56(6):897–913. CrossRef
4. King GF. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther. 2011 Nov;11(11):1469–84. CrossRef
5. Ruder T, Ali SA, Ormerod K, Brust A, Roymanchadi ML, Ventura S, et al. Functional characterization on invertebrate and vertebrate tissues of tachykinin peptides from octopus venoms. Peptides (NY). 2013;47:71–6. CrossRef
6. Dutertre S, Jin AH, Vetter I, Hamilton B, Sunagar K, Lavergne V, et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat Commun. 2014;5:3521. CrossRef
7. Koh CY, Kini RM. From snake venom toxins to therapeutics--cardiovascular examples. Toxicon. 2012 ;59(4):497–506. CrossRef
8. Simoes-Silva R, Alfonso J, Gomez A, Holanda RJ, Sobrinho JC, Zaqueo KD, et al. Snake venom, a natural library of new potential therapeutic molecules: challenges and current perspectives. Curr Pharm Biotechnol. 2018 Jun 21;19(4):308–35. CrossRef
9. Otvos RA, Heus F, Vonk FJ, Halff J, Bruyneel B, Paliukhovich I, et al. Analytical workflow for rapid screening and purification of bioactives from venom proteomes. Toxicon. 2013 Dec 15;76:270–81. CrossRef
10. Liu S, Yang F, Zhang Q, Sun MZ, Gao Y, Shao S. “Anatomical” view of the protein composition and protein characteristics for gloydius shedaoensis snake venom via proteomics approach. Anat Record. 2011 ;294(2):273–82. CrossRef
11. Georgieva D, Arni RK, Betzel C. Proteome analysis of snake venom toxins: pharmacological insights. Expert Rev Proteomics. 2014 Dec;5(6):787–97. CrossRef
12. Ediss.sub.hamburg. Analysis of the low molecular weight peptides of selected snake venoms. [cited 2022 Jul 28]. Available from: https://ediss.sub.uni-hamburg.de/handle/ediss/4696
13. Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nat Rev Drug Discov 2003;2(10):790–802. CrossRef
14. Tu AT. Overview of snake venom chemistry. Adv Exp Med Biol. 1996;391:37–62. CrossRef
15. Oliveira AL, Viegas MF, da Silva SL, Soares AM, Ramos MJ, Fernandes PA. The chemistry of snake venom and its medicinal potential. Nat Rev Chem. 2022;6(7):451–69. CrossRef
16. Gutiérrez JM, Lomonte B. Phospholipases A2: unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon. 2013 Feb 1;62:27–39. CrossRef
17. Kini RM, Koh CY. Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases. Biochem Pharmacol. 2020 Nov 1;181:114105. CrossRef
18. Kang TS, Georgieva D, Genov N, Murakami MT, Sinha M, Kumar RP, et al. Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J. 2011 Dec 1;278(23):4544–76. CrossRef
19. Ullah A, Masood R, Ali I, Ullah K, Ali H, Akbar H, et al. Thrombin-like enzymes from snake venom: Structural characterization and mechanism of action. Int J Biol Macromol. 2018 Jul 15;114:788–811. CrossRef
20. Hiu JJ, Yap MKK. Cytotoxicity of snake venom enzymatic toxins: phospholipase A2 and l-amino acid oxidase. Biochem Soc Trans. 2020 Apr 29;48(2):719–31. CrossRef
21. Tan KK, Bay BH, Gopalakrishnakone P. L-amino acid oxidase from snake venom and its anticancer potential. Toxicon. 2018 Mar 15;144:7–13. CrossRef
22. Arlinghaus FT, Eble JA. C-type lectin-like proteins from snake venoms. Toxicon. 2012 Sep 15;60(4):512–9. CrossRef
23. Morita T. Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon. 2005 Jun 15;45(8):1099–114. CrossRef
24. Lu Q, Navdaev A, Clemetson JM, Clemetson KJ. Snake venom C-type lectins interacting with platelet receptors. Structure–function relationships and effects on haemostasis. Toxicon. 2005 Jun 15;45(8):1089–98. CrossRef
25. Vink S, Jin AH, Poth KJ, Head GA, Alewood PF. Natriuretic peptide drug leads from snake venom. Toxicon. 2012 Mar 15;59(4):434–45. CrossRef
26. Sridharan S, Kini RM, Richards AM. Venom natriuretic peptides guide the design of heart failure therapeutics. Pharmacol Res. 2020 May 1;155:104687. CrossRef
27. Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta (BBA). 2000 Oct 31;1488(1–2):1–19. CrossRef
28. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009 Apr ;50(Suppl):S237. CrossRef
29. Chwetzoff S, Tsunasawa S, Sakiyama F, Ménez A. Nigexine, a Phospholipase A2 from cobra venom with cytotoxic properties not related to esterase activity. J Biol Chem. 1989 Aug;264(22):13289–97. CrossRef
30. Roberto PG, Kashima S, Marcussi S, Pereira JO, Astolfi-Filho S, Nomizo A, et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A 2 from Bothrops jararacussu Venom. Protein J. 2004;23(4):273–85. CrossRef
31. Kennedy AR. Chemopreventive agents: protease inhibitors. Epidemiol Data Suggest. Pharmacol. Ther. 1998;78:167–209. CrossRef
32. Young A, Byung MI, Lee MU, Yeong ’, Kim S. Characterization and cytotoxicity of L-amino acid oxidase from the venom of king cobra (Ophiopkagus hannah). Int J Biochem Cell Biol. 1997;29:911–9. CrossRef
33. Zhang L, Wu WT. Isolation and characterization of ACTX-6: A cytotoxic L-amino acid oxidase from Agkistrodon acutus snake venom. Nat Prod Res. 2008 Apr 15;22(6):554–63. CrossRef
34. Zhang L, Wei LJ. ACTX-8, a cytotoxic l-amino acid oxidase isolated from Agkistrodon acutus snake venom, induces apoptosis in Hela cervical cancer cells. Life Sci. 2007 Mar 6;80(13):1189–97. CrossRef
35. Lm G, Sanchez EF, Sg S, Santos RG. Tumor cytotoxicity of Leucurolysin-B, a P-III snake venom metalloproteinase from Bothrops leucurus. J Venomous Anim Toxins Include Trop Dis. 2012;18(1):24–33. CrossRef
36. Ârio M, Âsar C, Ãa C, Maria DA, Moura-Da-Silva AM, Pizzocaro KF, et al. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Available from: www.elsevier.com/locate/toxicon
37. Higuchi DA, Almeida MC, Barros CC, Sanchez EF, Pesquero PR, Lang EAS, et al. Leucurogin, a new recombinant disintegrin cloned from Bothrops leucurus (white-tailed-jararaca) with potent activity upon platelet aggregation and tumor growth. Toxicon. 2011 Jul;58(1):123–9. CrossRef
38. Swaim MW, Chiang2 HS, Huang2 TF. Characterisation of platelet aggregation induced by PC-3 human prostate adenocarcinoma cells and inhibited by venom peptides, trigramin and rhodostomin. Eur J Cancer. 1996 Apr;32A(4):715-21. CrossRef
39. Chung KH, Kim SH, Han K yeon, Sohn YD, Chang SI, Baek KH, et al. Inhibitory effect of salmosin, a Korean snake venomderived disintegrin, on the integrin αv-mediated proliferation of SK-Mel-2 human melanoma cells. J Pharm Pharmacol. 2010 Feb 18;55(11):1577–82. CrossRef
40. Soszka T, Knudsen KA, Lucia Beviglia J, Rossi C, Poggi A, Niewiarowski S, et al. Inhibition of murine melanoma cell-matrix adhesion and experimental metastasis by albolabrin, an RGD-containing peptide isolated from the venom of Trimeresurus albolabris. Exp Cell Res. 1991 Sep;196(1):6–12. CrossRef
41. Google Books. Handbook of Venoms and Toxins of Reptiles. [cited 2023 Jan 5]. Available from: https://books.google.co.in/books?hl=en&lr=&id=x_vME799de4C&oi=fnd&pg=PA173&dq=Doley+R,+Zhou+X,+Kini+RM.+Snake+venom+phospholipase+A2+enzymes.+In:+Handbook+of+venoms+and+toxins+of+reptiles.+CRC+Press/Taylor+and+Francis+Group%3B+2009.+p.+173%E2%80%93205.&ots=PzK50HB-Qg&sig=lrutuXBZH9Sll2tOfgFwD-i6as0&redir_esc=y#v=onepage&q&f=false
42. Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011 Oct 12;111(10):6130–85. CrossRef
43. Gutiérrez J, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995 Nov 1;33(11):1405–24. CrossRef
44. Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003 Dec 1;42(8):827–40. CrossRef
45. Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzymology: the secreted phospholipase A2-paradigm. Chem Rev. 2001 Sep;101(9):2613–53. CrossRef
46. Rowan EG. What does β-bungarotoxin do at the neuromuscular junction? Toxicon. 2001 Jan 1;39(1):107–18. CrossRef
47. Doley R, Kini RM. Protein complexes in snake venom. Cell Mol Life Sci. 2009 Jun 4;66(17):2851–71. CrossRef
48. Kwong PD, McDonald NQ, Sigler PB, Hendrickson WA. Structure of beta 2-bungarotoxin: potassium channel binding by Kunitz modules and targeted phospholipase action. Structure. 1995;3(10):1109–19. CrossRef
49. Rodrigues R, Izidoro LF, de Oliveira Jr. R, Soares A, Rodrigues V, Sampaio S. Snake venom phospholipases A2: a new class of antitumor agents. Protein Pept Lett. 2009 Aug 1;16(8):894–8. CrossRef
50. Benati RB, Costa TR, Cacemiro MDC, Sampaio SV, De Castro FA, Burin SM. Cytotoxic and pro-apoptotic action of MjTX-I, a phospholipase A2 isolated from Bothrops moojeni snake venom, towards leukemic cells. J Venom Anim Toxins Incl Trop Dis. 2018 Dec 20;24(1):40. CrossRef
51. Marcussi S, Santos PRS, Menaldo DL, Silveira LB, Santos-Filho NA, Mazzi M V., et al. Evaluation of the genotoxicity of Crotalus durissus terrificus snake venom and its isolated toxins on human lymphocytes. Mutat Res Genet Toxicol Environ Mutagen. 2011 Sep 18;724(1–2):59–63. CrossRef
52. Lomonte B, Angulo Y, Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. CrossRef
53. Fujisawa D, Yamazaki Y, Lomonte B, Morita T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochem J. 2008 May 1;411(3):515–22. CrossRef
54. Kessentini-Zouari R, Jebali J, Taboubi S, Srairi-Abid N, Morjen M, Kallech-Ziri O, et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo. Lab Investig. 2010;90:510–9. CrossRef
55. Bazaa A, Limam I, Kessentini-Zouari R, Kallech-Ziri O, El Battari A, Braguer D, et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. Available from: www.plosone.org
56. Bazaa A, Luis J, Srairi-Abid N, Kallech-Ziri O, Kessentini-Zouari R, Defilles C, et al. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 2009 May 1;28(4):188–93. CrossRef
57. Corin RE, Viskatis LJ, Vidal JC, Etcheverry MA. Cytotoxicity of crotoxin on murine erythroleukemia cells in vitro. Investig New Drugs. 1993;11(1):11–15 CrossRef
58. Muller SP, Silva VAO, Silvestrini AVP, de Macedo LH, Caetano GF, Reis RM, et al. Crotoxin from Crotalus durissus terrificus venom: in vitro cytotoxic activity of a heterodimeric phospholipase A2 on human cancer-derived cell lines. Toxicon. 2018;156:13–22. CrossRef
59. Bezerra PHA, Ferreira IM, Franceschi BT, Bianchini F, Ambrósio L, Cintra ACO, et al. BthTX-I from Bothrops jararacussu induces apoptosis in human breast cancer cell lines and decreases cancer stem cell subpopulation. J Venom Anim Toxins Incl Trop Dis. 2019 Jul 29;25:e20190010. CrossRef
60. Khunsap S, Pakmanee N, Khow O, Chanhome L, Sitprija V, Suntravat M, et al. Purification of a phospholipase A 2 from Daboia russelii siamensis venom with anticancer effects. J Venom Res. 2011;2:42–51.
61. Jain D, Kumar S. Snake venom: a potent anticancer agent. Asian Pac J Cancer Prev. 2012 ;13(10):4855–60. CrossRef
62. Fox JW, Serrano SMT. Timeline of key events in snake venom metalloproteinase research. J Proteomics. 2009 Mar 6;72(2):200–9. CrossRef
63. Ferreira BA, Deconte SR, de Moura FBR, Tomiosso TC, Clissa PB, Andrade SP, et al. Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. Int J Biol Macromol. 2018 ;119:1179–87. CrossRef
64. Markland FS, Swenson S. Snake venom metalloproteinases. Toxicon. 2013 Feb ;62:3–18. CrossRef
65. Gutiérrez JM, Escalante T, Rucavado A, Herrera C. Hemorrhage caused by snake venom metalloproteinases: a journey of discovery and understanding. Toxins. 2016;8(4):93. CrossRef
66. Sanchez EF, Flores-Ortiz RJ, Alvarenga VG, Eble JA. Direct fibrinolytic snake venom metalloproteinases affecting hemostasis: structural, biochemical features and therapeutic potential. Toxins. 2017 ;9(12):392. CrossRef
67. Kini RM, Koh CY. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins. 2016;8(10):284. CrossRef
68. Woodside DG, Vanderslice P. Inflammation and regulation by integrin cell adhesion antagonists. Transl Inflam. 2019 Jan 1;2019:43–68. CrossRef
69. Olaoba OT, Karina dos Santos P, Selistre-de-Araujo HS, Ferreira de Souza DH. Snake venom metalloproteinases (SVMPs): a structure-function update. Toxicon X. 2020 Sep 1;7:100052. CrossRef
70. Onyango OK. Isolation and characterization of snake venom proteins and peptides from members of Viperidae and Elapidae snake families from Kilifi County. 2018 [cited 2022 Aug 16]. Available from: http://localhost/xmlui/handle/123456789/4764
71. Calderon LA, Sobrinho JC, Zaqueo KD, De Moura AA, Grabner AN, Mazzi M V, et al. Antitumoral activity of snake venom proteins: new trends in cancer therapy. 2014 [cited 2023 Sep 16]. Available from: CrossRef
72. Maria AA, da Silva LGL, Correia CC, Ruiz GRG. Antiproliferative effect of the Jararhagin toxin on B16F10 murine melanoma. BMC Complement Altern Med. 2014 Nov 18;14(1):446. CrossRef
73. Ma R, Mahadevappa R, Kwok HF. Venom-based peptide therapy: insights into anti-cancer mechanism. Oncotarget. 2017; 2017:8. CrossRef
74. Leonardi A, Sajevic T, Kova?i? L, Punger?ar J, Lang Balija M, Halassy B, et al. Hemorrhagin VaH4, a covalent heterodimeric P-III metalloproteinase from Vipera ammodytes ammodytes with a potential antitumour activity. Toxicon. 2014 Jan 1;77:141–55. CrossRef
75. Guimarães D de O, Lopes DS, Azevedo FVPV, Gimenes SNC, Silva MA, Achê DC, et al. In vitro antitumor and antiangiogenic effects of Bothropoidin, a metalloproteinase from Bothrops pauloensis snake venom. Int J Biol Macromol. 2017 Apr 1;97:770–7. CrossRef
76. Escalante T, Rucavado A, Fox JW, Gutiérrez JM. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J Proteomics. 2011 ;74(9):1781–94. CrossRef
77. Gutiérrez JM, Rucavado A, Escalante T, Díaz C. Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005 ;45(8):997–1011. CrossRef
78. Akhtar B, Muhammad F, Sharif A, Anwar MI. Mechanistic insights of snake venom disintegrins in cancer treatment. Eur J Pharmacol. 2021;899:174022. CrossRef
79. Galán JA, Sánchez EE, Rodríguez-Acosta A, Soto JG, Bashir S, McLane MA, et al. Inhibition of lung tumor colonization and cell migration with the disintegrin crotatroxin 2 isolated from the venom of Crotalus atrox. Toxicon. 2008 Jun 1;51(7):1186–96. CrossRef
80. McLane MA, Joerger T, Mahmoud A. Disintegrins in health and disease. Front Biosci. 2008 May 1;13(17):6617–37 CrossRef
81. Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013 Feb 1;62:40–9. CrossRef
82. Hammouda MB, Montenegro MF, Sánchez-Del-Campo L, Zakraoui O, Aloui Z, Riahi-Chebbi I, et al. Lebein, A snake venom disintegrin, Induces apoptosis in human melanoma cells. Toxins (Basel). 2016 Jul 5;8:7. CrossRef
83. Hong SY, Lee H, You WK, Chung KH, Kim DS, Song K. The snake venom disintegrin salmosin induces apoptosis by disassembly of focal adhesions in bovine capillary endothelial cells. Biochem Biophys Res Commun. 2003 Mar 14;302(3):502–8. CrossRef
84. Ghazaryan N, Movsisyan N, Macedo JC, Vaz S, Ayvazyan N, Pardo L, et al. The antitumor efficacy of monomeric disintegrin obtustatin in S-180 sarcoma mouse model. Invest New Drugs. 2019 Oct 1;37(5):1044–51. CrossRef
85. Lino RLB, dos Santos PK, Pisani GFD, Altei WF, Cominetti MR, Selistre-de-Araújo HS. Alphavbeta3 integrin blocking inhibits apoptosis and induces autophagy in murine breast tumor cells. Biochim Biophys Acta. 2019 Dec 1;1866(12):118536. CrossRef
86. Danilucci TM, Santos PK, Pachane BC, Pisani GFD, Lino RLB, Casali BC, et al. Recombinant RGD-disintegrin DisBa-01 blocks integrin α v β 3 and impairs VEGF signaling in endothelial cells. Cell Commun Signal. 2019 Mar 20;17(1):27. CrossRef
87. Chan YS, Cheung RCF, Xia L, Wong JH, Ng TB, Chan WY. Snake venom toxins: toxicity and medicinal applications. Appl Microbiol Biotechnol. . 2016 ;100(14):6165–81. CrossRef
88. Costal-Oliveira F, Stransky S, Guerra-Duarte C, Naves de Souza DL, Vivas-Ruiz DE, Yarlequé A, et al. L-amino acid oxidase from Bothrops atrox snake venom triggers autophagy, apoptosis and necrosis in normal human keratinocytes. Sci Rep. 2019 ;9(1):781. CrossRef
89. Ullah A, Masood R, Spencer PJ, Murakami MT, Arni RK. Crystallization and preliminary X-ray diffraction studies of an L-amino-acid oxidase from Lachesis muta venom. Acta Crystallogr F Struct Biol Commun. 2014 ;70(Pt 11):1556–9. CrossRef
90. Kazianis S, Della Coletta L, Morizot DC, Johnston DA, Osterndorff EA, Nairn RS. Overexpression of a fish CDKN2 gene in a hereditary melanoma model. Carcinogenesis. 2000 ;21(4):599–605. CrossRef
91. Hanukoglu I. Proteopedia: Rossmann fold: a beta-alpha-beta fold at dinucleotide binding sites. Biochem Mol Biol Educ. 2015 May 1;43(3):206–9. CrossRef
92. Pawelek PD, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A. The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J. 2000 Aug 15;19(16):4204–15. CrossRef
93. Ullah A. Structure–function studies and mechanism of action of snake venom L-amino acid oxidases. Front Pharmacol. 2020 Feb 25;11:110. CrossRef
94. Lazo F, Vivas-Ruiz DE, Sandoval GA, Rodríguez EF, Kozlova EEG, Costal-Oliveira F, et al. Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom. Toxicon. 2017 ;139:74–86. CrossRef
95. Takatsuka H, Sakurai Y, Yoshioka A, Kokubo T, Usami Y, Suzuki M, et al. Molecular characterization of l-amino acid oxidase from Agkistrodon halys blomhoffii with special reference to platelet aggregation. Biochim Biophys Acta. 2001 Jan 12;1544(1–2):267–77. CrossRef
96. Izidoro LFM, Ribeiro MC, Souza GRL, Sant’Ana CD, Hamaguchi A, Homsi-Brandeburgo MI, et al. Biochemical and functional characterization of an l-amino acid oxidase isolated from Bothrops pirajai snake venom. Bioorg Med Chem. 2006 Oct 15;14(20):7034–43. CrossRef
97. Toyama MH, Toyama DDO, Passero LFD, Laurenti MD, Corbett CE, Tomokane TY, et al. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon. 2006 Jan ;47(1):47–57. CrossRef
98. Rey-Suárez P, Acosta C, Torres U, Saldarriaga-Córdoba M, Lomonte B, Núñez V. MipLAAO, a new L-amino acid oxidase from the redtail coral snake Micrurus mipartitus. PeerJ. 2018 ;2018(6):e4924. CrossRef
99. Tempone AG, Spencer PJ, Lourenço CO, Rogero JR, Nascimento N, Andrade HF. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its L-amino acid oxidase. Biochem Biophys Res Commun. 2001 ;280(3):620–4. CrossRef
100. Costa TR, Menaldo DL, Zoccal KF, Burin SM, Aissa AF, Castro FAD, et al. CR-LAAO, an L-amino acid oxidase from Calloselasma rhodostoma venom, as a potential tool for developing novel immunotherapeutic strategies against cancer. Sci Rep. 2017 ;7(1):1–12. CrossRef
101. Sant’Ana CD, Menaldo DL, Costa TR, Godoy H, Muller VDM, Aquino VH, et al. RETRACTED: Antiviral and antiparasite properties of an l-amino acid oxidase from the Snake Bothrops jararaca: Cloning and identification of a complete cDNA sequence. Biochem Pharmacol. 2008 Jul 15;76(2):279–88. CrossRef
102. NUUTINEN JT, Timonen S. Identification of nitrogen mineralization enzymes, l-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol Res. 2008 Dec 1;112(12):1453–64. CrossRef
103. Arima J, Sasaki C, Sakaguchi C, Mizuno H, Tamura T, Kashima A, et al. Structural characterization of l-glutamate oxidase from Streptomyces sp. X-119-6. FEBS J. 2009 ;276(14):3894–903. CrossRef
104. Schriek S, Kahmann U, Staiger D, Pistorius EK, Michel KP. Detection of an L-amino acid dehydrogenase activity in Synechocystis sp. PCC 6803. J Exp Bot. 2009 Mar ;60(3):1035. CrossRef
105. Nishizawa T, Aldrich CC, Sherman DH. Molecular analysis of the rebeccamycin L-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243. J Bacteriol. 2005 Mar ;187(6):2084–92. CrossRef
106. Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000 Feb 1;32(2):157–70. CrossRef
107. Singh M, Sharma H, Singh N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion. 2007 Dec;7(6):367–73. CrossRef
108. Burin SM, Ayres LR, Neves RP, Ambrósio L, De Morais FR, Dias-Baruffi M, et al. L-amino acid oxidase isolated from bothrops pirajai induces apoptosis in BCR-ABL-positive cells and potentiates imatinib mesylate effect. Basic Clin Pharmacol Toxicol. 2013 Aug;113(2):103–12. CrossRef
109. Alves RM, Antonucci GA, Paiva HH, Cintra ACO, Franco JJ, Mendonça-Franqueiro EP, et al. Evidence of caspase-mediated apoptosis induced by l-amino acid oxidase isolated from Bothrops atrox snake venom. Comp Biochem Physiol. 2008;151(4):542–50. CrossRef
110. Abdelkafi-Koubaa Z, Elbini-Dhouib I, Souid S, Jebali J, Doghri R, Srairi-Abid N, et al. Pharmacological investigation of CC-LAAO, an L-Amino acid oxidase from Cerastes cerastes snake venom. Toxins. 2021 ;13(12):904. CrossRef
111. Zhang L, Cui L. A cytotoxin isolated from Agkistrodon acutus snake venom induces apoptosis via Fas pathway in A549 cells. Toxicol in Vitro. 2007 Sep 1;21(6):1095–103. CrossRef
112. Teixeira TL, Oliveira Silva VA, da Cunha DB, Polettini FL, Thomaz CD, Pianca AA, et al. Isolation, characterization and screening of the in vitro cytotoxic activity of a novel L-amino acid oxidase (LAAOcdt) from Crotalus durissus terrificus venom on human cancer cell lines. Toxicon. 2016 Sep 1;119:203–17. CrossRef
113. Momic T, Cohen G, Reich R, Arlinghaus FT, Eble JA, Marcinkiewicz C, et al. Vixapatin (VP12), a C-type lectin-protein from Vipera xantina palestinae venom: Characterization as a novel anti-angiogenic compound. Toxins (Basel). 2012 Oct;4(10):862–77. CrossRef
114. De Castro Damasio D, Nolte S, Polak LP, Brandt AP, Bonan NB, Zischler L, et al. The lectin BJcuL induces apoptosis through TRAIL expression, caspase cascade activation and mitochondrial membrane permeability in a human colon adenocarcinoma cell line. Toxicon. 2014;90:299–307. CrossRef
115. Pathan J, Mondal S, Sarkar A, Chakrabarty D. Daboialectin, a C-type lectin from Russell’s viper venom induces cytoskeletal damage and apoptosis in human lung cancer cells in vitro. Toxicon. 2017 Mar 1;127:11–21. CrossRef
116. Bonilla-Porras AR, Vargas LJ, Jimenez-Del-Rio M, Nuñez V, Velez-Pardo C. Purification of nasulysin-1: A new toxin from Porthidium nasutum snake venom that specifically induces apoptosis in leukemia cell model through caspase-3 and apoptosis-inducing factor activation. Toxicon. 2016 Sep 15;120:166–74. CrossRef
117. Boldrini-França J, Pinheiro-Junior EL, Peigneur S, Pucca MB, Cerni FA, Borges RJ, et al. Beyond hemostasis: a snake venom serine protease with potassium channel blocking and potential antitumor activities. Sci Rep. 2020 Dec 1;10(1):4476. CrossRef
118. Bittenbinder MA, van Thiel J, Cardoso FC, Casewell NR, Gutiérrez JM, Kool J, et al. Tissue damaging toxins in snake venoms: mechanisms of action, pathophysiology and treatment strategies. Commun Biol. 2024;7(1):358 CrossRef
119. Zhao Y sheng, Yang H ling, Liu C zheng. [Inhibitory effects of immunotargeting of Chinese cobra cytotoxin and iodine-131 against nasopharyngeal carcinoma cells in vitro]. Nan Fang Yi Ke Da Xue Xue Bao. 2008 Jul 1;28(7):1235–6.
120. Awwad S, Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018 Sep 1;10(3):83. CrossRef
121. Chenthamara D, Subramaniam S, Ramakrishnan G, Krishnaswamy S, Essa MM, Lin FH, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23:20. CrossRef
122. Suralkar AR, Khedkar CS, Zanwar NR, Chandak CC, Gandhi SJ. Liposomes as a novel drug delivery system. GSC Biol Pharm Sci. 2022 Sep 30;20(3):336–43. CrossRef
123. Vatankhah M, Dadashzadeh S, Mahboubi A, Haeri A, Jandaghi Alaee K, Mostafavi Naeini SB, et al. Preparation of multivesicular liposomes for the loco-regional delivery of Vancomycin hydrochloride using active loading method: drug release and antimicrobial properties. J Liposome Res. 2023; CrossRef
124. Law LH, Huang J, Xiao P, Liu Y, Chen Z, Lai JHC, et al. Multiple CEST contrast imaging of nose-to-brain drug delivery using iohexol liposomes at 3T MRI. J Control Release. 2023 Feb 1;354:208–20. CrossRef
125. Nel J, Elkhoury K, Velot É, Bianchi A, Acherar S, Francius G, et al. Functionalized liposomes for targeted breast cancer drug delivery. Bioactive Mater. 2023;24:401–37. CrossRef
126. Namakshenas P, Mojra A. Efficient drug delivery to hypoxic tumors using thermosensitive liposomes with encapsulated anti-cancer drug under high intensity pulsed ultrasound. Int J Mech Sci. 2023 Jan 1;237:107818. CrossRef
127. Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi S EL, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev. 2020 Jan 1;159:332–43. CrossRef
128. Almeida H, Silva AC. Nanoparticles in ocular drug delivery systems. Pharmaceutics. 2023;15:1675. CrossRef
129. Baldrick P. Nonclinical testing evaluation of liposomes as drug delivery systems. Int J Toxicol. 2023 Mar-Apr;42(2):122–34. CrossRef
130. Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng. 2016 Dec 1;9(4):509–29. CrossRef
131. Jimidi Bhaskar S. A Recent advances in drug delivery to brain-a review. Asian J Pharm Res. 2024;14(1):668–87. CrossRef
132. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery . Adv Drug Deliv Rev. 2016;106:148–156. CrossRef
133. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. CrossRef
134. Reddy SK, Ballal AR, Shailaja S, Seetharam RN, Raghu CH, Sankhe R, et al. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics. 2023;13(7):2241–55. CrossRef
135. Siddhardha B, Dyavaiah M, Kasinathan K. Model organisms to study biological activities and toxicity of nanoparticles. Springer Singapore; 2020. 1–466 p. CrossRef
136. Jimenez-Canale J, Fernández-Quiroz D, Teran-Saavedra NG, Diaz-Galvez KR, Gallegos-Tabanico A, Burgara-Estrella AJ, et al. Cytotoxic activity of Crotalus molossus molossus snake venom-loaded in chitosan nanoparticles against T-47D breast carcinoma cells. Acta Biochim Pol. 2022;69(1):233–43.
137. Çelen Ç, Keçeciler C, Kar?? M, Göçmen B, Yesil-Celiktas O, Nalbantsoy A. Cytotoxicity of silica nanoparticles with transcaucasian nose-horned viper, Vipera ammodytes transcaucasiana, Venom on U87MG and SHSY5Y Neuronal Cancer Cells. Appl Biochem Biotechnol. 2018 Oct 1;186(2):350–7. CrossRef
138. Badr G, Al-Sadoon MK, Rabah DM, Sayed D. Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles induce apoptosis and growth arrest in human prostate cancer cells. Apoptosis. 2013 Mar;18(3):300–14. CrossRef
139. Chen D, Liu X, Lu X, Tian J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Frontiers Media S.A.; 2023. CrossRef
140. Srinivas T, Parvathi D, Devi UA. Advancements in nanoparticle applications for targeted drug delivery: benefits and implications. Int J Biol Res. 2024 Jul 26;11(2):50–6. CrossRef
141. Ani AC, Emencheta SC, Orah KJ, Upaganlawar AB, Prajapati BG, Oranu CK, et al. Targeted nanotechnology-based formulations. Alzheimer’s disease and advanced drug delivery strategies. Elsevier; 2023. p. 347–59. CrossRef
142. Whitford W, Guterstam P. Exosome manufacturing status. Future Med Chem. 2019 ;11(10):1225–36. CrossRef
143. Kishore BK, Park F, Ecelbarger CM, Lv LL, Liu BC, Tang TT, et al. Extracellular vesicles: opportunities and challenges for the treatment of renal diseases. Front Physiol. 2019 ;1:226.
144. Lee JC, Ray RM, Scott TA. Prospects and challenges of tissue-derived extracellular vesicles. Molecular Therapy. Cell Press; 2024. CrossRef
145. Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H, et al. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15(5):2938–44. CrossRef
146. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014 ;35(7):2383–90. CrossRef
147. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016 ;12(3):655–64 CrossRef
148. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015 ;207:18–30. CrossRef
149. Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, et al. In vitro and in vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019 ;19(1):19–28. CrossRef
150. Feng T, Huang X, Ni R, Suen WLL, Chau Y. Nanoparticles for drug delivery targeting neurodegeneration in brain and eye. Nanomater Drug Deliv Ther. 2019; 2019:149–83. CrossRef
151. Yang P, Ren J, Yang L. Nanoparticles in the new era of cardiovascular therapeutics: challenges and opportunities. Int J Mol Sci. 2023; 2023:24. CrossRef
152. Alkufi H, Salman A, Taher S. Principles and Advantages of New Drug Delivery Technologie. J Complem Med Res. 2023;14(3):6. CrossRef
153. Hami Z. A Brief review on advantages of nano-based drug delivery systems. Ann Milit Health Sci Res. 2021 Mar 6;19(1):541–69. CrossRef
154. Wang L, Song Y, Wang H, Zhang X, Wang M, He J, et al. Advances of artificial intelligence in anti-cancer drug design: a review of the past decade. Pharmaceuticals. 2023;16:253. CrossRef
155. Tan P, Chen X, Zhang H, Wei Q, Luo K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin Cancer Biol. 2023 Feb 1;89:61–75. CrossRef
156. Moebius K, Eichler J. HIV-derived peptide mimics. Drug Discov Today Technol. 2009 Jan 1;6(1–4):e19–25. CrossRef
157. Díaz-Gómez JL, Martín-Estal I, Rivera-Aboytes E, Gaxiola-Muñíz RA, Puente-Garza CA, García-Lara S, et al. Biomedical applications of synthetic peptides derived from venom of animal origin: a systematic review. Biomed Pharmacother. 2024;170:116015. CrossRef