INTRODUCTION
Snake venoms are complex mixtures of diverse bioactive compounds that aim to either kill or incapacitate the prey. Snake venoms usually have hemolytic, proteolytic, and cytotoxic properties, which facilitate the initiation of tissue digestion in the vicinity of the bite site. Up to 90% of the toxins found in snake venom are polypeptides, which include both enzymes and non-enzymatic proteins [1]. Both the composition and mode of action of venoms produced by snakes vary widely depending on the genus, furthermore even within the same species venom composition defers by the influence of such factors as age, sex, environmental conditions, and the type of available prey [2].
The two most relevant families of venomous snakes, Elapidae (elapids) and Viperidae (viperids, commonly referred to as vipers), include almost all medically important species. The major constituents of elapid venoms are phospholipases A2 (PLA2s) and three-finger toxins which play a dominant role in the venom action [3], although there are some other important toxins such as snake venom metalloproteinases (SVMPs), snake venom serine proteases (SVSPs), and L-amino acid oxidases (LAAOs), which together represent an average of 6% of elapid venom [4]. The other minor constituent of elapid venom (an average of ~5%) is a family of serine protease inhibitors, namely Kunitz-type peptides, which are potent and selective K+-channel blockers [1]. Viperid venoms are comprised mainly of PLA2s, SVMPs, and SVSPs, which together represent an average of ~70% of the whole venom proteome, while LAAOs, Kunitz-type peptides, C-type lectins, C-type lectin-like proteins, and natriuretic peptides are present in smaller concentration (up to 7% of the whole toxins). Variations in the chemical composition of elapid and viperid venoms cause distinct clinical effects: elapid venoms primarily trigger neurotoxic, cytotoxic, and cardiotoxic symptoms, whereas viper envenomation typically leads to myotoxic and hemotoxic effects [5].
Many toxins from the species belonging to the Viperidae family can influence various targets within the hemostatic system, causing coagulopathy and hemorrhage [6]. These targets include clotting factor activators and inhibitors, components of fibrinolytic cascade, platelets, and endotheliocytes. Components affecting hemostasis are also found among Elapidae, although they are uncommon. Since snake venoms are rich sources of new molecules that can affect hemostasis, they can be useful in the therapeutic area as potential tools for drug discovery or applications in both medicine and biotechnology.
A widely distributed venomous snake species within the Viperidae family is Vipera berus, commonly referred to as the common European adder. This species has an extensive range, occurring across much of Europe and extending to East Asia [7]. There are several recognized subspecies, two of which, namely Vipera berus berus and Vipera berus nikolskii, are the only venomous snakes that can be found on the territory of Ukraine. Vipera berus venom displays proteolytic, hemolytic, and cytotoxic properties, as well as fibrinolytic and anticoagulant activities, causing disruption of homeostasis [8]. Many efforts have been made to establish the nature of the specific components responsible for the biological effects of V. berus venom [8–11]. To date, the V. berus venom is well characterized; however, viper V. b. nikolskii is less studied. Furthermore, the primary characteristics of venom in subspecies across different regions and populations remain largely unexplored. Therefore, additional in vitro and in vivo studies may enhance the understanding of snake venom chemistry, thereby facilitating the development of novel therapeutic agents. Therefore, this study aimed to compare the in vivo hemostatic effects of V. b. berus and V. b. nikolskii venoms.
MATERIALS AND METHODS
Venom
The venoms of V. b. berus and V. b. nikolskii were obtained from the V.N. Karazin Kharkiv National University (Kharkiv, Ukraine). Lyophilized crude venoms were kept at –20°C. Before experiments, dried material was dissolved in 0.9% saline solution, and then centrifuged at 10,000 g for 15 minutes. The obtained supernatant was used in further experiments.
Experimental design
Thirty male white nonlinear rats (10 weeks old), weighing 200–220 g, were used for this study. The animals were housed in groups of up to five per cage under standard laboratory conditions, including a 12/12-hour light/dark cycle, a temperature of 22°C ± 2 °C, and a humidity level of 55% ± 5%. They had ad libitum access to water and standard laboratory rodent pellet food. After 5 days of acclimatization, the animals were randomly assigned into three experimental groups: 1—control group (n = 10): rats received a single intraperitoneal injection of 0.9% saline solution (0.5 ml); 2—Vbb group (n = 10): rats treated with a single intraperitoneal injection of V. b. berus venom at a dose of 1.576 μg/g of animal weight (in 0.5 ml); and 3—Vbn group (n = 10): rats treated with a single intraperitoneal injection of V. b. nikolskii venom at a dose of 0.972 μg/g of animal weight (in 0.5 ml). Rats were sacrificed 24 hours after venom injection, and blood samples were collected. All procedures involving venom injection and euthanasia were performed under ketamine–xylazine anesthesia (60 + 5 mg kg–1, i.p.) with basic monitoring.
The use of the laboratory animals was approved by the Biomedical Ethics Committee of the ESC “Institute of Biology and Medicine” of Taras Shevchenko National University of Kyiv (protocol No. 9 dated September 4, 2023). All experiments were performed in accordance with the “European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes” (Strasbourg, 1986) and Article 26 of the Law of Ukraine “On the Protection of Animals from Cruelty” (No. 3447-IV, 21.02.2006), as well as European Union Directive of 22 September 2010 (2010/63/EU) for the protection of animals used for scientific purposes.
Plasma sample preparation
Blood plasma was obtained using the standard protocol. Whole blood was collected into plastic tubes containing 3.8% (w/v) sodium citrate as an anticoagulant (9:1, vol/vol). The tubes were centrifuged at 900 g for 30 minutes at 4°C and the plasma was separated with plastic pipettes. All plasma samples were stored at –20°C until assayed.
Thrombin time (TT) determination
The TT was measured using an RT-2201C coagulation analyzer (Rayto, China) and a commercial TT assay kit (RENAU, Ukraine). All procedures were conducted in strict accordance with the manufacturer’s instructions. In brief, a 50 μl plasma sample was combined with 100 μl of thrombin (final activity: 3 U/ml) in a coagulometric cuvette, and the clotting time (in seconds) required for clot formation was recorded.
Fibrinogen concentration measurement
Fibrinogen concentration was determined spectrophotometrically by the technique previously described [12]. Briefly, the mixture of plasma (0.2 ml), thrombin (2 NIH units in 0.1 ml), 0.1 M phosphate buffer (1.7 ml, pH 7), and 0.04 M monoiodoacetic acid (0.1 ml) was prepared and incubated at +37°C for 30 minutes. The formed fibrin clot was removed, washed several times with cooled 0.13 M NaCl, and dissolved in 5 ml of 1.5% acetic acid.
The absorbance measurements were conducted at 280 and 320 nm for each sample. The concentration of fibrinogen (g/l) was calculated by means of the formula: (A280 – A320) ´ 255 / 1.506, where 255 represents the conversion factor for determining fibrinogen concentration in plasma from the sample volume, and 1.506 corresponds to the fibrin extinction coefficient at 280 nm.
Determination of plasma fibrin/fibrinogen degradation products (FDPs)
To measure the plasma levels of fibrin/FDPs, we used a method based on the ability of these molecules to prolong the time necessary for monomer fibrin polymerization [13]. The concentration of FDPs in plasma, which is directly proportional to the polymerization time, was calculated by means of a calibration curve prepared using the plasma samples with known FDP concentration and the monomer fibrin solution.
Plasmin activity assay
Plasmin activity was assessed using a previously described method [14]. In brief, plasma was diluted 1:50 in 0.05 M Tris-HCl buffer (pH 7.4). The reaction mixture, with a final volume of 250 μl, contained 0.05 M Tris-HCl buffer (pH 7.4), and the diluted plasma sample was preincubated at +37°C for 5 minutes. Subsequently, the chromogenic substrate S2251 (RENAU, Ukraine) was added to a final concentration of 3 mM. The absorbance of the samples was measured at a wavelength of 405 nm using a microplate spectrophotometer (BioTek Instruments, Inc., USA). The amount of free p-nitroaniline produced was directly proportional to plasmin activity.
α-2-Antiplasmin activity assay
The inhibitory potential of α-2-antiplasmin was assessed using a previously described method [14]. In brief, plasma was diluted 1:3 in 0.05 M Tris-HCl buffer (pH 7.4). The reaction mixture, with a final volume of 250 μl, contained 0.05 M Tris-HCl buffer (pH 7.4), a diluted plasma sample, and plasminogen. The mixture was preincubated at +37°C for 5 minutes, after which the chromogenic substrate S2251 (RENAU, Ukraine) was added to a final concentration of 3 mM. The absorbance of the samples was measured at a wavelength of 405 nm using a microplate spectrophotometer (BioTek Instruments, Inc., USA). The amount of free p-nitroaniline generated was inversely proportional to α-2-antiplasmin inhibitory activity.
Antithrombin III (AT III) activity assay
AT III activity was determined using a spectrophotometric technique [15]. Briefly, plasma was diluted 1:30 in 0.05 M Tris-HCl buffer (pH 7.4). The reaction mixture, with a final volume of 250 μl, contained 0.05 M Tris-HCl buffer (pH 7.4), a diluted plasma sample, thrombin, and heparin at a final concentration of 0.2 IU. The mixture was preincubated at +37°C for 5 minutes, after which the chromogenic substrate S2238 (RENAU, Ukraine) was added to a final concentration of 3 mM. The absorbance of the samples was assayed at a wavelength of 405 nm using a microplate spectrophotometer (BioTek Instruments, Inc., USA). The AT III activity was determined based on the amount of released p-nitroaniline, which was inversely proportional to the AT III activity in the plasma sample.
Heparin activity assay
Heparin activity was measured using a commercial chromogenic analysis kit (RENAU, Ukraine), following the manufacturer’s instructions. Plasma samples were diluted 1:5 in 0.05 M Tris-HCl buffer (pH 7.4) and mixed with an AT-III solution and factor Xa solution, then incubated at +37°C for 5 minutes. Subsequently, a chromogenic substrate was added, and the mixture was incubated for an additional 5 minutes at +37°C. The reaction was terminated by the addition of 50% acetic acid. The amount of p-nitroaniline released from the chromogenic substrate molecules was quantified at a wavelength of 405 nm by means of a microplate spectrophotometer (BioTek Instruments, USA). The amount of free p-nitroaniline generated was inversely proportional to heparin activity. The heparin activity in the control group was set at 100%, while the activity levels in venom-treated groups were expressed as percentages relative to the control.
Evaluation of functionally inactive prothrombin forms (FIPFs) in plasma
To evaluate the relative levels of FIPFs, we used a method based on the measurement of the difference between ecamulin time (ET) and prothrombin time (PT) [13]. ET is proportional to the levels of normal and FIPFs, while the PT represents the levels of normal thrombin. Therefore, the difference between ET and PT is proportional to the FIPFs in the plasma samples. The level of FIPFs in the control group of animals was set at 100%, while the FIPF levels in venom treated groups were given as percentages of control.
Statistical analysis
Statistical analyses were performed using Statistica software, version 12.0 (StatSoft Inc., USA). The Shapiro–Wilk test was applied to assess the data distribution. Since all data sets were determined to be distributed normally, means and standard deviation (M ± SD) were used as summary statistics. Data comparisons between groups were conducted using the Student’s t-test. A p-value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Toxins present in Viperidae venoms that target hemostasis contribute to a wide range of clinical and biological syndromes and may lead to severe complications, including potential fatalities. There is a broad range of clinically significant complications following viper envenoming, including incoagulability and local or systemic bleeding, which are among the major lethal factors. Thus, understanding the mechanisms of toxin-induced hemostasis alterations may shed light on fundamental biological processes associated with snakebite envenoming, which is important as a prerequisite to optimize current management strategies and search for novel therapeutic options.
The TT is a commonly employed screening test for assessing fibrinogen abnormalities and detecting the presence of inhibitors that affect thrombin activity, fibrin formation, and its polymerization [16]. Thus, to investigate the pro- and anticoagulant potential of viper toxins, we measured the TT in rats after the V. b. berus or V. b. nikolskii venoms injection. As can be seen from the obtained results (Fig. 1), the normal reference range of the TT test was from 17.1 to 19.3 seconds. Our preliminary study revealed the state of hypocoagulability in both groups of rats 24 hours after venom injection. Thus, the average TT values were 23.1 s in rats of the Vbb group (p = 0.033 vs. control) and 26.5 seconds in rats of the Vbn group (p < 0.001 vs. control). Since TT level reflects the conversion of fibrinogen to fibrin after the addition of thrombin reagent, our results could be associated with impairment of fibrinogen function caused by the presence of thrombin inhibitors or effectors of fibrin formation and polymerization. Increased clotting time in the TT test may be a risk factor for hemorrhage in experimental rats after venom injection.
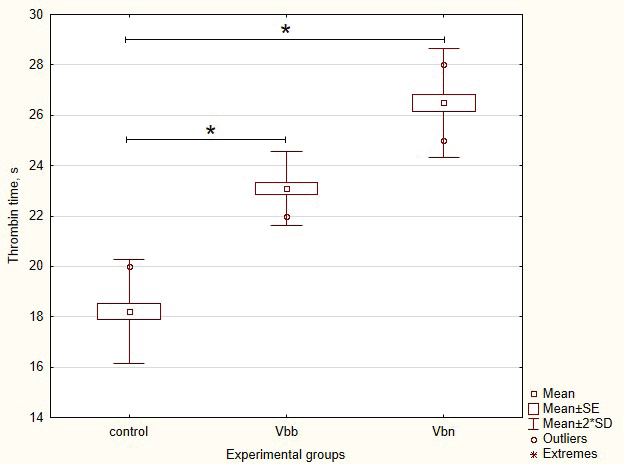 | Figure 1. Average values of TT in control rats and rats after 24 hours of intraperitoneal injection of V. b. berus (Vbb group) or V. b. nikolskii (Vbn group) venoms. [Click here to view] |
Table 1 shows the key parameters of the hemostatic system in blood plasma samples of the studied groups. No significant difference was observed in the mean fibrinogen concentration between the Vbb group and the control group (p = 0.813). In contrast, the mean fibrinogen concentration in the Vbn group was significantly lower compared to the control (p = 0.023). Furthermore, the mean levels of FDPs were significantly elevated in both venom-injected groups compared to the control (p < 0.05).
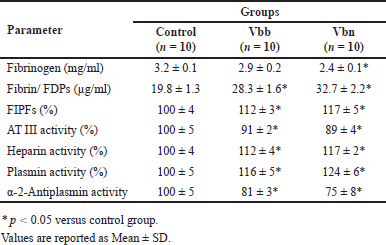 | Table 1. Basic characteristics of the hemostatic system in the studied groups. [Click here to view] |
The findings of the present study are supported by Dang et al. [17] who reported that Viperidae envenomation is accompanied by changes in coagulation status, decreased fibrinogen concentration, and increased D-dimer level. Similarly, another study reported coagulopathy following viper envenomation, characterized by prolonged activated partial thromboplastin time, reduced fibrinogen levels, and decreased activity of coagulation factors V, VIII, and X, with recovery occurring within 48 hours [18]. Additionally, Isbister et al. [18] have shown that the severity of clotting abnormalities correlates with venom concentrations.
FIPFs accumulation may serve as a biological marker of thrombophilia [19] since the plasma level of FIPFs increases during thrombotic processes, when thrombin cleaves prothrombin to form inactive fragments of prothrombin molecules. As can be seen from the results (Table 1), plasma FIPF levels were elevated in rats treated with either V. b. berus venom or V. b. nicolskii venom compared to controls. Such data may indicate an early stage of coagulation cascade activation. The study by Dineshkumar et al. [20] showed similar findings, reporting that thrombotic microangiopathy may occur due to Viperidae envenomation. However, it should be emphasized that thrombotic microangiopathy is a rare complication of snake envenoming [21]. The existence of such contradictory data further emphasizes the importance of venom composition variability across different subspecies and geographic regions, which may contribute to inconsistencies in reported results regarding the biological effects of Viperidae venom.
Among the serine proteinases that are present in snake venom, thrombin-like enzymes exist; they can affect fibrinogen molecules, making blood plasma unclottable. They are widely distributed within several species in the Viperidae family and show more than 67% of sequence similarity among themselves [22]. The other interesting fact about such snake thrombin-like enzymes is that most of them cannot be inhibited by thrombin inhibitors such as AT III and hirudin [6,23].
Antithrombin is one of the principal anticoagulant agents, which inhibits several key enzymes of the coagulation cascade (namely, factor II or thrombin, factor IXa, and factor Xa), thereby providing a counter mechanism to clot formation. According to the results obtained (Table 1), there were slight differences between both the venom-treated groups of rats and the control group regarding the mean of antithrombin activity (p < 0.05). On the other hand, the activity of heparin was significantly elevated in both venom-injected groups compared to control (p < 0.05).
It is important to consider several possible mechanisms for the appearance of FDPs in the plasma of rats following Viperidae venom injection. First, among the venom components might be active proteases, which can directly cleave the fibrinogen molecule into low-molecular fragments. Second, FDPs could be released into the bloodstream due to the lysis of fibrin clots by components of the fibrinolytic system in venom-treated animals. Therefore, investigating the state of the fibrinolytic system in rats under the influence of Viperidae venom was deemed necessary.
The key component of the fibrinolytic system is plasminogen, an inactive proenzyme that can be converted into the active enzyme plasmin, which facilitates the degradation of fibrin into soluble FDPs. The regulation and control of the fibrinolytic system are mediated by specific molecules, including plasminogen activators and inhibitors [24]. The results of our study indicated that plasmin activity was slightly higher in the Vbb group compared to the control values (p = 0.042), whereas in the rats of the Vbn group, this parameter was increased by 25% (p = 0.005). These findings may be attributed to the presence of plasminogen activators in Viperidae venom, which have also been identified among other snake toxins [25]. These plasminogen activators may convert plasminogen to active enzyme plasmin, facilitating clot lysis.
Since α2-antiplasmin (also known as α2-plasmin inhibitor) is the main physiological inhibitor of the fibrinolytic enzyme plasmin, we also studied its activity in venom-treated rats. Our results showed that in the period of 24 hours after venom injection, the activity of α2-antiplasmin was reduced by 20% in rats of the Vbb group (p = 0.023) and by 25% in the Vbn group (p = 0.015) when compared to control values. Thus, our study’s findings revealed that Viperidae envenoming may activate fibrinolysis. Such an effect could be associated with enhanced plasma profibrinolytic potential due to both increased plasmin activity and decreased α-2-antiplasmin activity. We think direct effectors of α-2-antiplasmin may exist among the viper toxins; they can be the reason for a low level of α-2-antiplasmin activity, making plasmin activity grow up.
Our findings align with previous reports highlighting the complex effects of Viperidae venom on hemostasis system, which may be attributed to differences in venom composition between subspecies, geographic populations, and experimental conditions [8,26]. Different studies revealed that certain venom components frequently have contradictory effects on the hemostatic parameters, influencing both coagulation and fibrinolysis in different ways, leading to varied clinical presentations [1]. Indeed, specific venom proteases (e.g., serine proteases and metalloproteinases) that may either activate or degrade fibrinolytic factors may be a reason for a venom pro-fibrinolytic effect as well as an inhibitory effect on fibrinolysis [27,28]. Our results further support the need for additional investigations into the interplay between venom-induced fibrinolysis and potential pro-thrombotic effects, particularly in envenomed individuals with pre-existing coagulopathies.
The present study has some limitations, which should be acknowledged. Only a single dose of each venom was tested. Specifically, rats received a single intraperitoneal injection of V. b. berus venom at a dose of 1.576 μg/g of animal weight and V. b. nikolskii venom at a dose of 0.972 μg/g of animal weight. These doses were previously documented as LD50 [29] and have been widely tested on various biological effects [11,30,31]. The use of an acute LD50 for each venom allowed us to standardize the comparison between the two venoms. On the other hand, a dose-response study would provide deeper insights into the severity and progression of venom-induced coagulation disturbances. Thus, in the further research, we are going to explore a broader range of venom concentrations to better understand the dose-dependent effects and refine our understanding of venom toxicity and its systemic consequences. The other limitation, which should be mentioned is a single time-point measurement. All measurements were taken only at 24 hours post-injection, which may have missed potential early-phase (e.g., immediate coagulation changes) or late-phase (e.g., delayed fibrinolytic responses) effects. A time-course study, with multiple sampling points, would help elucidate the dynamics of venom-induced alterations in hemostasis. Finally, while we evaluated biochemical coagulation parameters, this study did not include direct in vivo assessments of bleeding tendency or thrombotic events, such as bleeding time, clot formation assays, or histopathological examination of microthrombi in organs. These analyses would provide more comprehensive insights into the pathophysiological consequences of envenomation. To summarize, future studies should address these limitations by incorporating a broader range of venom doses, multiple time points, and additional in vivo functional assays to provide a more complete understanding of venom-induced coagulation disorders.
CONCLUSION
Thus, the alterations in rats’ hemostasis system under either V. b. berus or V. b. nikolskii envenomation were characterized by declining the content of fibrinogen and rising the levels of fibrin/FDPs as well as FIPFs. In addition, plasmin activity, which is considered to be the key regulator of fibrinolytic process, was increased in rats of both venom-treated groups, while α-2-antiplasmin activity was decreased after envenomation. It should be noted that the detected changes were maximally pronounced in rats after V. b. nikolskii venom injection.
Our study demonstrated that there were no strong differences between the effects of both venoms on the hemostasis in experimental rats. Thus, V. b. berus and V. b. nikolskii venom toxins can interfere with normal blood coagulation by similar mechanisms. The relatively similar composition of different Viperidae venoms suggests that hypocoagulation and enhanced fibrinolysis should not be considered distinct pathologies but rather two interconnected aspects of the same pathophysiological process. Unfortunately, the exact mechanisms underlying coagulopathy induced by viper envenomation remain incompletely understood. Identifying these mechanisms would provide a deeper understanding of the hemostatic disturbances caused by viper venoms and contribute to the development of improved guidelines for the management and treatment of patients suffering from viper bites.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Biomedical Ethics Committee of the ESC “Institute of Biology and Medicine” of Taras Shevchenko National University of Kyiv (protocol No. 9 dated September 4, 2023).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing the manuscript, and no images were manipulated using AI.
REFERENCES
1. Oliveira AL, Viegas MF, da Silva SL, Soares AM, Ramos MJ, Fernandes PA. The chemistry of snake venom and its medicinal potential. Nat Rev Chem. 2022;6(7):451–69. CrossRef
2. Casewell NR, Jackson TNW, Laustsen AH, Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020;41(8):570–81. CrossRef
3. Ferraz CR, Arrahman A, Xie C, Casewell NR, Lewis RJ, Kool J, et al. Multifunctional toxins in snake venoms and therapeutic implications: from pain to hemorrhage and necrosis. Front Ecol Evol. 2019;7:218. CrossRef
4. Kang TS, Georgieva D, Genov N, Murakami MT, Sinha M, Kumar RP, et al. Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J. 2011;278(23):4544–76. CrossRef
5. Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:17063. CrossRef
6. Alvarez-Flores MP, Faria F, de Andrade SA, Chudzinski-Tavassi AM. Snake venom components affecting the coagulation system. In: Gopalakrishnakone P, Inagaki H, Mukherjee A, Rahmy T, Vogel CW, editors. Snake venoms. Toxinology. Dordrecht, The Netherlands: Springer; 2016. CrossRef
7. Di Nicola MR, Pontara A, Kass GEN, Kramer NI, Avella I, Pampena R, et al. Vipers of major clinical relevance in Europe: taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology. 2021;453:152724. CrossRef
8. Bocian A, Urbanik M, Hus K, ?yskowski A, Petrilla V, Andrej?áková Z, et al. Proteome and peptidome of Vipera berus berus venom. Molecules. 2016;21(10):1398. CrossRef
9. Serrano SM, Shannon JD, Wang D, Camargo AC, Fox JW. A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: an approach to understanding venom proteomics. Proteomics. 2005;5(2):501–10. CrossRef
10. Munawar A, Trusch M, Georgieva D, Spencer P, Frochaux V, Harder S, et al. Venom peptide analysis of Vipera ammodytes meridionalis (Viperinae) and Bothrops jararacussu (Crotalinae) demonstrates subfamily-specificity of the peptidome in the family Viperidae. Mol Biosyst. 2011;7(12):3298–307. CrossRef
11. Palamarchuk M, Niyazmetov T, Halenova T, Raksha N, Maievskyi O, Dzevulska I, et al. Effect of Vipera berus berus and Vipera berus nikolskii Venom on proteolytic balance in the tissue of the adrenal glands and testicles of rats. Biomed Biotechnol Res J. 2022;6(4):543–9. CrossRef
12. Rachkovska A, Krenytska D, Karbovskyy V, Halenova T, Raksha N, Vovk T, et al. Characteristics of products of fibrinogen origin in the presence of anti- SARS-CoV-2 IgG in the bloodstream. Rev Recent Clin Trials. 2023;18(1):69–75. CrossRef
13. Matkivska R, Shchypanskyi S, Raksha N, Vovk T, Halenova T, Maievskyi O, et al. Venom-induced consumption coagulopathy in rats following Leiurus macroctenus (Scorpiones: Buthidae) envenomation. Curr Top Pept Protein Res. 2023;24:57064.
14. Rachkovska A, Krenytska D, Karbovskyy V, Raksha N, Halenova T, Vovk T, et al. A study of fibrinolytic system components in donor groups depending on various titers of circulating anti-SARS-CoV-2 IgG in the bloodstream. Blood Coagul Fibrin. 2023;34(7):439–45. CrossRef
15. Strubchevska K, Rachkovska A, Krenytska D, Karbovskyy V, Kozyk M, Secor B, et al. Coagulation parameters in post-covid-19 condition in relation to various titers of anti-SARS-CoV-2 IgG in blood plasma. Int J Gen Med. 2023;16:6127–35. CrossRef
16. Mackie I, Casini A, Pieters M, Pruthi R, Reilly-Stitt C, Suzuki A. International council forstandardisation in haematology recommendations onfibrinogen assays, thrombin clotting time and related tests inthe investigation of bleeding disorders. Int J Lab Hematol. 2024;46(1):20–32. CrossRef
17. Dang XT, Xuan Nguyen T, Nguyen TTH, Ha HT. Coagulopathy after Viper Snakebite in Vietnam and relationship with time of admission. J Multidiscip Healthc. 2021;14:1259–65. CrossRef
18. Isbister GK, Maduwage K, Scorgie FE, Shahmy S, Mohamed F, Abeysinghe C, et al. Venom concentrations and clotting factor levels in a prospective cohort of Russell’s Viper bites with coagulopathy. PLoS Negl Trop Dis. 2015;9(8):e0003968. CrossRef
19. Storozhuk OB, Shevchuk SV, Storozhuk LO, Dovgalyuk TV, Storozhuk BG. Relationship between pre- and post-thrombosis factors in patients with stage VD CKD treated by long-term hemodialysis. Wiadomo?ci Lekarskie.2021;74(3):471–4.
20. Dineshkumar T, Dhanapriya J, Sakthirajan R, Thirumalvalavan K, Kurien AA, Balasubramaniyan T, et al. Thrombotic microangiopathy due to Viperidae bite: two case reports. Indian J Nephrol. 2017;27(2):161–4. CrossRef
21. Noutsos T, Currie BJ, Isbister GK. Snakebite associated thrombotic microangiopathy: a protocol for the systematic review of clinical features, outcomes, and role of interventions. Syst Rev. 2019;8:212. CrossRef
22. Kini RM. Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J. 2006;397(3):377–87. CrossRef
23. Hutton RA, Warrell DA. Action of snake venom components on the haemostatic system. Blood Rev. 1993;7:176–89.
24. Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7(1):4–13. CrossRef
25. Sanchez EF, Flores-Ortiz RJ, Alvarenga VG, Eble JA. Direct fibrinolytic snake venom metalloproteinases affecting hemostasis: structural, biochemical features and therapeutic potential. Toxins (Basel). 2017;9(12):392. CrossRef
26. Kini RM, Koh CY. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: definition and nomenclature of interaction Sites. Toxins. 2016;8(10):284. CrossRef
27. Calderón L, Lomonte B, Gutiérrez JM, Tarkowski A, Hanson LA. Biological and biochemical activities of Vipera berus (European viper) venom. Toxicon. 1993;31(6):743–53. CrossRef
28. Czajka U, Wiatrzyk A, Luty?ska A. Mechanism of Vipera berus venom activity and the principles of antivenom administration in treatment. Przegl Epidemiol. 2013;67(4):641–6.
29. Shitikov V, Malenyov A, Gorelov R, Bakiev A. “Dose-response” models with mixed parameters by the example of venom toxicity estimation of the common European adder Vipera berus // Principy èkologii. 2018;7(2):150160. CrossRef
30. Palamarchuk M, Bobr A, Mudrak A, Gunas I, Maievskyi O, Samborska I, et al. Proteolytic homeostasis in the tissue of the spleen and the heart of rats injected with the venom of Vipera berus berus and Vipera berus nikolskii. Curr Appl Sci Technol. 2023;23(6):19. CrossRef
31. Raksha N, Vovk T, Halenova T, Mudrak A, Slyeptsova I, Mudrak H, et al. Influence of Vipera berus berus and Vipera berus nikolskii venom on protein-peptide profile in the liver, kidneys, and small intestine of rats. Curr Top Peptide Protein Res. 2022;23:63–72.