INTRODUCTION
Millions of individuals worldwide suffer from peptic ulcer disease (PUD), a gastrointestinal ailment, at some point in their lives [1,2]. It occurs when the effects of defensive factors such as prostaglandins, gastric mucus and bicarbonate secretion, and mucosal blood flow are weaker than those of the aggressive factors such as gastric acid, pepsin, some medicines, e.g., nonsteroidal anti-inflammatory drugs, and Helicobacter pylori, which can cause damage to the gastrointestinal mucosa [1]. Although conventional drugs such as proton pump inhibitors are effective in treating PUD, they have several adverse effects and drug interactions, which limit their use. As a result, herbal medicines have gained worldwide acceptance as alternative treatments. Natural products can be valuable, effective, and safer anti-ulcer agents [3,4]. Early investigations in animal models of several plant extracts and plant-derived compounds led to the development of anti-ulcer drugs after demonstrating promising results in clinical trials. An example is the development of the anti-ulcer drug carbenoxolone from the root of licorice (Glycyrrhiza glabra) [4,5].
The Zingiberaceae family is a large group of plants commonly found in Thailand, and many medicinal plants within this family are distributed throughout the country. Zingiber is a moderately large genus of this family includes 100–150 species, approximately 26 of which are found in Thailand [6]. The rhizomes of many Zingiber species exhibit anti-ulcer activities [7–11], including Zingiber simaoense (Z. simaoense) [12], which has been traditionally used in Thai medicine to treat conditions such as flatulence, bloating, and abdominal pain [13]. A recent study has shown that the ethanol extract of Z. simaoense rhizome (ZSE) can protect against gastric ulcers in rats induced by various ulcer-inducing agents. One possible mechanism of ZSE is primarily related to its ability to increase gastric wall mucus production. Regarding its safety, ZSE has no acute toxicity effect at a 2,000 mg/kg dose in rats [14]. Furthermore, ZSE has also demonstrated a gastric ulcer healing effect in rats [15]. Therefore, owing to its potential to prevent and treat gastric ulcers, Z. simaoense rhizome could be a promising candidate for drug investigation and development. Exploring the sub-fraction of ZSE with the most potent anti-gastric ulcer activity is the crucial next step, as it could lead to the identification of active compounds and the development of alternative anti-ulcer drugs derived from natural resources. This study aimed to identify the sub-fraction of ZSE with the highest anti-gastric ulcer activity using a rat model of acidified ethanol (EtOH/HCl)-induced gastric ulcer.
MATERIAL AND METHODS
Plant material
The rhizomes of Z. simaoense (family Zingiberaceae) were cultivated in Chiang Rai, Thailand. The approximate GPS coordinates of the location are 20°04′13.7″N 99°53′27.7″E, and the harvesting took place during 1–15 February 2016. The identified voucher specimen of the plant (no. 145–2) is at Mae Fah Luang University, Chiang Rai, Thailand.
Extraction and fractionation
A total of 610 g of dried rhizomes from Z. simaoense were ground before being soaked in 95% ethanol (EtOH) at room temperature for three nights (3 × 20 l each night). The resulting extract was filtered and concentrated using vacuum concentration and lyophilization, resulting in the ethanol extract of Z. simaoense rhizome (ZSE). The ZSE was identified through gas chromatography/mass spectrometry (GC/MS). The remaining ZSE (39.12 g) was then dissolved in 1 l of water and partitioned with n-hexane, dichloromethane, ethyl acetate, n-butanol, and water. All fractions were evaporated in vacuo and lyophilized. The solvents were chosen according to their increasing polarity index, which allowed for a systematic fractionation of nonpolar to highly polar constituents. Those with marked anti-gastric ulcer activity were combined and subjected to silica gel column chromatography (70–230 mesh, Merck, Darmstadt, Germany) eluted with stepwise gradient elution using n-hexane:dichloromethane (100:0 to 0:100, v/v), dichloromethane:ethyl acetate (100:0 to 0:100, v/v), ethyl acetate:n-butanol (100:0 to 0:100, v/v), and n-butanol:water (100:0 to 0:100, v/v). Silica gel pre-coated aluminum plates (70–230 mesh, Merck, Darmstadt, Germany) were used to monitor the column through thin layer chromatography (TLC). TLC dots were analyzed at 254 and 365 nm under UV light. The fractions with similar TLC spots were pooled and dried to obtain three promising sub-fractions of increasing polarity (SbFr-1 to SbFr-3). The sub-fractions were assessed for their anti-gastric ulcer activity, and the most active sub-fraction was then subjected to fingerprint analysis using GC/MS.
GC/MS analysis
GC/MS was used for fingerprint analyses of ZSE as well as for identifying the most active sub-fraction. The procedures and conditions used in the study were as described by Baiubon et al. [14]. The analysis was performed using a GC 7890 System (Agilent Technologies, Inc., Santa Clara, CA), equipped with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 µm film thickness). The temperature of the GC oven was programmed to start at 50°C for 5 minutes. Subsequently, it was increased to 200°C at a rate of 10°C/minute, then to 250°C at 5°C/minute, and continued at that temperature for 10 minutes. The injection temperature was set at 250°C. The carrier gas (helium) flow rate was 1.5 ml/minute with a 1:25 split ratio. The GC was connected to a mass-selective detector (Agilent HP 5973). The parameters of the MS operating system were 70 eV of ionization voltage and 230°C ion source temperature. To determine the components in the eluent, the retention times and mass spectra were compared with those in the NIST05a.L Database (Agilent Technologies Inc., Santa Clara, CA).
Experimental animals
The experimental animals in this study were male (200–250 g) and female (160–180 g) Sprague–Dawley rats from the National Laboratory Animal Center (Mahidol University, Salaya, Nakorn Pathom, Thailand). For at least 1 week before starting the experiments, the animals were kept in a room under controlled conditions (24°C ± 1°C, 50% ± 10% relative humidity, and 12-hour light-12-hour dark cycle) with free access to drinking water and standard pelleted food. The Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (protocol number 16/2559) approved all protocols involving animals in the study.
Acidified ethanol (EtOH/HCl)-induced gastric ulcer
To explore the sub-fraction of ZSE that exhibited the most potent anti-gastric ulcer activity, separation of components of ZSE using solvent partitioning and column chromatography was performed. Each fraction and sub-fraction was tested for anti-gastric ulcer activity using the EtOH/HCl-induced gastric ulcer in a rat model [16] with slight modifications. Forty-two of the 48-hour-fasted rats with free access to water, which was withdrawn 1 hour before the beginning of the experiments, were divided into seven groups. Each group received one of the following: vehicle (5% Tween 80, control group), standard drug (0.10 mg/kg misoprostol, reference group), or one of the fractions from solvent partitioning (240 mg/kg of each fraction), administered 1 hour before gastric ulcer induction. The rats were then orally administered 1 ml of acidified ethanol solution (60:40 v/v of absolute EtOH and HCl) to induce gastric ulcers. One hour later, the rats were sacrificed, and the stomachs were excised to assess gastric ulcers. The gastric lesions were quantified by measuring each lesion’s length (in millimeters) under a dissecting microscope (10×). The ulcer index of each group was obtained from the combined lesion length of the rats in that group divided by the total number of rats in that group. Using the following formula, the percentage of gastric ulcer inhibition was calculated: % Inhibition = [(Ulcer index of the control group - Ulcer index of the tested group) × 100]/Ulcer index of the control group [17]. The statistical comparison of ulcer indexes among groups in each study was then evaluated.
Fractions HX-Fr and DC-Fr were selected for further fractionation by silica gel column chromatography due to their significant percent inhibition of gastric ulcers. Five groups of six rats each received vehicle (5% Tween 80, control group), standard drug (0.10 mg/kg misoprostol, reference group), and sub-fractions from column chromatography (60 mg/kg of each sub-fraction). They were then evaluated for anti-gastric ulcer activity using an EtOH/HCl-induced gastric ulcer in a rat model, following a procedure similar to the one described above.
Sub-fraction 1 was selected for further analysis of the dose-response relationship due to its significant percentage inhibition of gastric ulcers. Seven groups, each containing six rats, received vehicle (5% Tween 80, control group), standard drug (0.10 mg/kg misoprostol, reference group), and sub-fraction 1 (at 1.88, 3.75, 7.50, 30, or 60 mg/kg) after which anti-gastric ulcer activity testing was performed using EtOH/HCl-induced gastric ulcer in a rat model like the one described above.
Acute toxicity study
The study was conducted following the Organization of Economic Cooperation and Development guideline for the testing of chemicals (Test No. 420) with minor modifications [18]. Ten adult female Sprague Dawley rats (7 weeks, 160–180 g) were randomly assigned to one of two groups. The rats in both groups were fasted for 16–18 hours (with access to water but not food) before receiving the test substances. Rats in the vehicle control group received 5% Tween 80, while the test group was administered the most potent sub-fraction (sub-fraction 1) by oral gavage at a dose of 2,000 mg/kg. Following this administration, signs, symptoms, and any changes in the appearance of the eyes, skin, fur, and mucous membranes were observed and noted at 1, 2, 4, and 6 hours, then once a day for 14 days. On the 15th day, surviving rats were sacrificed to identify gross pathological changes in the internal organs. Any abnormalities in the internal organs in the experimental group were noted and compared to the control group.
Statistical analysis
The mean ± standard error of the mean (SEM) was used to express the experiment’s data. One-way analysis of variance (ANOVA) followed by the post hoc least-significant difference (LSD) test and the Kruskal–Wallis’s test followed by Dunn’s test was used for the statistical comparisons between groups of parametric and nonparametric data, respectively. The dose-response relationship was modeled using four-parameter logistic regression (4PL). The parameters were estimated through nonlinear regression using a curve-fitting algorithm. The model’s goodness-of-fit was assessed by calculating the R-squared (R²) value, while the statistical significance of the fit was evaluated using the F-test. Significant p values were those with a value of less than 0.05.
RESULTS
Extraction and fractionation
The 95% EtOH extract of Z. simaoense rhizome yielded 7.40% (w/w) of dried rhizome powder. A portion of the ZSE was subjected to fingerprint analysis using GC/MS. The remaining 39.12 g was successively partitioned to obtain n-hexane (HX-Fr), dichloromethane (DC-Fr), ethyl acetate (EA-Fr), n-butanol (BT-Fr), and aqueous (AQ-Fr) fractions, with yields of 32.85%, 24.23%, 0.66%, 1.89%, and 6.36% yield (w/w), respectively. Based on their positive anti-gastric ulcer activity and the available quantities, the HX-Fr (12.18 g) and DC-Fr (8.91 g) were combined and subjected to column chromatography, eluted with gradient concentrations of n-hexane, ethyl acetate, methanol, and water to yield sub-fractions 1–3 with 11.24%, 14.41%, and 3.37% yield (w/w), respectively. The overview of the extraction of Z. simaoense rhizomes, the fractionation process, and the amounts of all sub-fractions are shown in Figure 1.
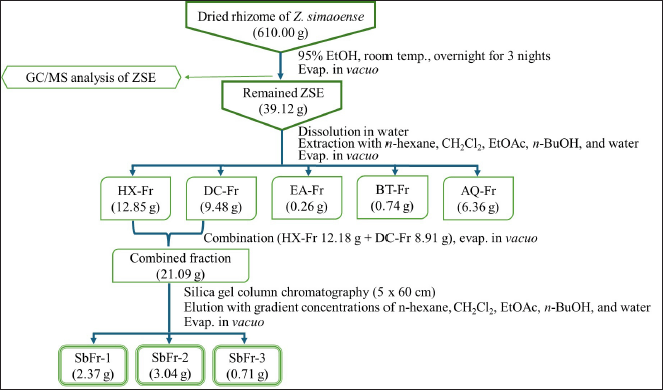 | Figure 1. Extraction of Z. simaoense rhizomes and fractionation of Z. simaoense ethanol extract (ZSE). Evap. = evaporation, HX-Fr = hexane fraction, DC-Fr = dichloromethane fraction, EA-Fr = ethyl acetate fraction, BT-Fr = butanol fraction, AQ-Fr = aqueous fraction, SbFr = sub-fraction. [Click here to view] |
GC/MS analysis
Sixteen of thirty peaks of the GC/MS chromatogram of ZSE were identified using the National Institute of Standards and Technology (NIST) Research Library (Table 1). The identified compounds included one monoterpene, ten sesquiterpenes, four fatty acids, and a 1,6-naphthyridine analog. The major components of ZSE were α-eudesmol (17.02%), 2-naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-α,α,4a,8-tetramethyl-, (2R-cis)- (10.47%), n-hexadecanoic acid (8.43%), and elemol (6.59%) (Fig. 2). In addition, the GC/MS chromatogram of ZSE sub-fraction 1 displayed 30 peaks, of which 19 were identified using the NIST library (Table 2). The identified compounds included ten sesquiterpenes, one triterpene, six fatty acids, a dicarboxylic phenolic compound, and a chromene analog. The major components of ZSE sub-fraction 1 were α-eudesmol (20.64%), (23S)-ethylcholest-5-en-3-β-ol (19.13%), elemol (7.39%), n-hexadecanoic acid (6.46%), and β-cadinene (6.08%).
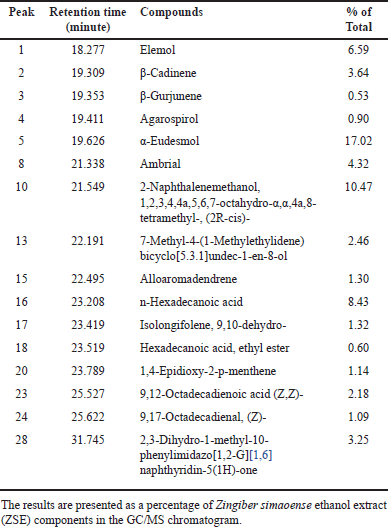 | Table 1. Chemical constituents of ZSE identified by GC/MS. [Click here to view] |
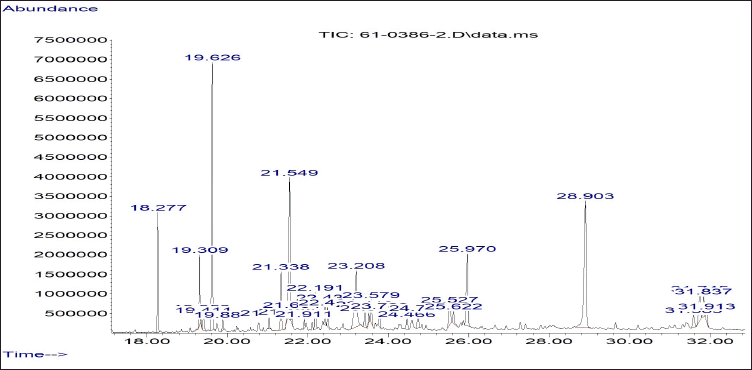 | Figure 2. The GC/MS chromatogram of Zingiber simaoense ethanol extract (ZSE). The major components of ZSE are α-eudesmol (RT 19.626 minutes), 2-naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-α,α,4a,8-tetramethyl-, (2R-cis)- (RT 21.549 minutes), n-hexadecanoic acid (RT 23.208 minutes), and elemol (RT 18.277 minutes). [Click here to view] |
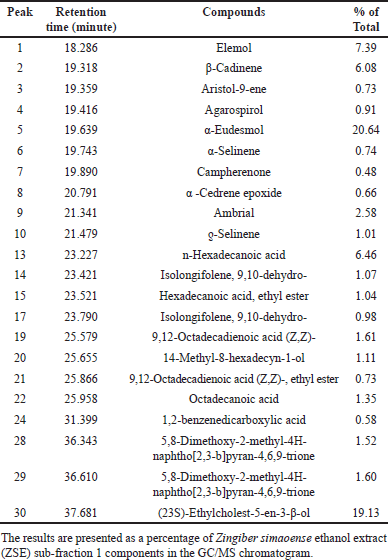 | Table 2. Chemical constituents of ZSE sub-fraction 1 identified by GC/MS. [Click here to view] |
Investigation of anti-gastric ulcer activity
Figure 3 shows the anti-gastric ulcer activities in rats of various fractions of ZSE at the same dose of 240 mg/kg. The ulcer indexes of all fractions (HX-Fr, DC-Fr, EA-Fr, and BT-Fr groups), with the exception of the AQ-Fr group, significantly reduced ulcer inhibition compared to the control group (Table 3).
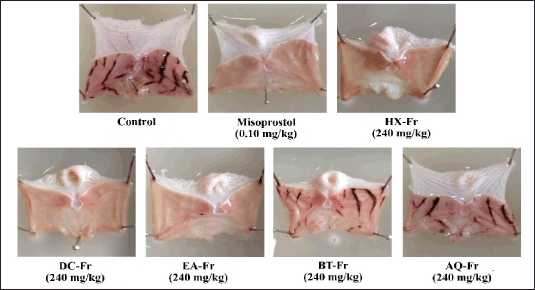 | Figure 3. Illustrations of gastric ulcer lesions of rats after gastric ulcer induction with HCl/EtOH in the control group (received 5% Tween 80), reference drug group (received 0.10 mg/kg of misoprostol), and test groups (received 240 mg/kg of each fraction from solvent partitioning of ZSE). HX-Fr = n-hexane fraction, DC-Fr = dichloromethane fraction, EA-Fr = ethyl acetate fraction, BT-Fr = n-butanol fraction, AQ-Fr = aqueous fraction. [Click here to view] |
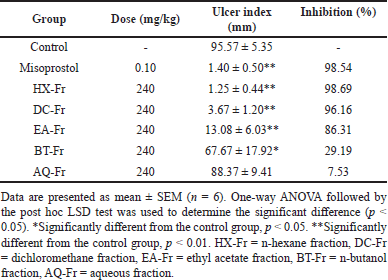 | Table 3. Effects of various fractions of ZSE at the dose of 240 mg/kg on gastric ulcers induced by acidified ethanol in rats. [Click here to view] |
HX-Fr and DC-Fr exhibited the highest ulcer inhibition and were therefore combined and subjected to column chromatography separation, as described in the plant extraction and fractionation section. The eluents were pooled into three sub-fractions based on their thin-layer chromatography profiles. The anti-ulcer activities of these sub-fractions were evaluated in the rats at a dose of 60 mg/kg, and the results are shown in Table 4. Misoprostol and sub-fraction 1 markedly decreased gastric ulcer formation with comparable efficacy, achieving 96.82% and 96.87% ulcer inhibition, respectively (Fig. 4). Sub-fraction 1 was further tested at various doses to evaluate its dose-dependent anti-ulcer activity. While the lowest dose (1.88 mg/kg) did not show a significant difference compared to the control group, the anti-gastric ulceration of this sub-fraction appeared to be dose-dependent (Table 5 and Fig. 5). The dose-response curve followed a sigmoidal shape, with the fitted 4PL model yielding a minimum response (A) of −0.51, a maximum response (B) of 95.37, an EC50 (C) of 3.72 mg/kg, and a Hill slope (D) of 4.31 (Fig. 6). The model demonstrated an excellent fit, with an R² value of 0.9991. Additionally, the F-test resulted in a p-value of 0.0458, confirming the statistical significance of the dose-response relationship.
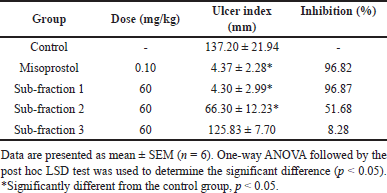 | Table 4. Effects of various sub-fractions of ZSE at the dose of 60 mg/kg on gastric ulcers induced by acidified ethanol in rats. [Click here to view] |
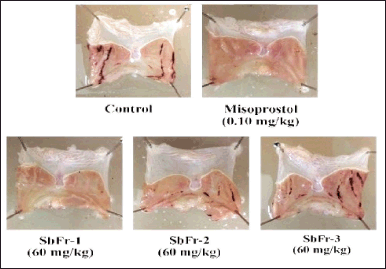 | Figure 4. Illustrations of gastric ulcer lesions of rats after gastric ulcer induction with HCl/EtOH in the control group (received 5% Tween 80), reference drug group (received 0.10 mg/kg of misoprostol), and test groups (received 60 mg/kg of each sub-fraction from column chromatography of ZSE). SbFr = sub-fraction. [Click here to view] |
 | Table 5. Effects of ZSE sub-fraction 1 at various doses on gastric ulcers induced by acidified ethanol in rats. [Click here to view] |
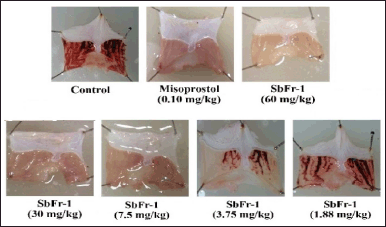 | Figure 5. Illustrations of gastric ulcer lesions of rats after gastric ulcer induction with HCl/EtOH in the control group (received 5% Tween 80), reference drug group (received 0.10 mg/kg of misoprostol), and test groups (received ZSE sub-fraction 1 at various doses). SbFr = sub-fraction. [Click here to view] |
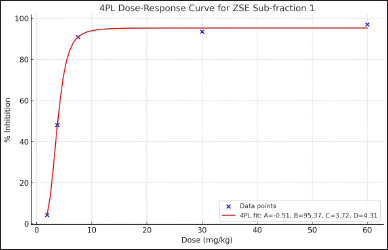 | Figure 6. The 4PL dose-response curve for ZSE Sub-fraction 1 demonstrates the relationship between dose (mg/kg) and % inhibition. The fitted 4PL model shows a sigmoidal shape, with the following parameters: a (minimum response) = −0.51, B (maximum response) = 95.37, C (EC50) = 3.72 mg/kg, and D (Hill slope) = 4.31. [Click here to view] |
Acute toxicity study
Throughout the study period, no indication of acute toxicity was shown in any of the rats administered the control vehicle and ZSE sub-fraction 1, the fraction with the most potent anti-gastric ulcer activity, at a dose of 2,000 mg/kg body weight.
DISCUSSION
The rhizome of Z. simaoense Y. Y. Qian, a Thai medicinal material known as “Khing Krang” or “Khing Klang,” is mentioned in a traditional Thai medicine book titled “The Book of Osot-phra-na-rai” [13]. Traditionally, the rhizome has been used similarly to “Khing” (Zingiber officinale) for treating gastrointestinal disorders such as abdominal pain, flatulence, and bloating, which are clinical manifestations of gastric ulcers. Although studies have shown the gastroprotective and gastric ulcer healing activities of ZSE [14,15], its chemical standardization for use has yet to be investigated. Bioassay-guided fractionation is a technique for screening plant extracts by chromatographically extracting and fractionating them until almost pure biologically active compounds are obtained. This approach reduces the time-consuming nature of drug development and effectively screens out harmful compounds from crude extracts, enabling chemical standardization and quality control [19,20].
In this study, the extract was systematically partitioned into fractions and sub-fractions based on polarity to isolate the most active sub-fraction. The solvents n-hexane, dichloromethane, ethyl acetate, n-butanol, and water were chosen according to their increasing polarity, facilitating the separation of nonpolar to highly polar constituents. n-Hexane, a nonpolar solvent, was employed to extract nonpolar compounds such as hydrocarbons and terpenoids. In contrast, highly polar solvents like water and n-butanol were used to extract polar compounds such as glycosides, sugars, and tannins. Solvents with medium polarity, such as dichloromethane and ethyl acetate, were utilized to target compounds of intermediate polarity, including sesquiterpenes, nonpolar phenolics, and flavonoids [21]. This systematic partitioning is expected to aid in the future development of health products from Z. simaoense. The fractions of n-hexane and dichloromethane from crude ZSE displayed promising activity, whereas the ethyl acetate, n-butanol, and aqueous fractions were less active. This finding might suggest that the best anti-ulcer activity from ZSE came from the nonpolar chemical constituents, which prefer to penetrate the lipid bilayer sites of action [22]. It may also account for the diminished anti-ulcer efficacy observed in the more polar fractions, particularly the aqueous fraction (AQ-Fr), which possesses the highest polarity.
Upon sub-fractionation by column chromatography, sub-fraction 1, the most nonpolar of all the sub-fractions, showed potent dose-dependent anti-gastric ulcer activity, remaining effective even at a reduced dose of 3.75 mg/kg. This sub-fraction was mainly produced by eluting the combined n-hexane and dichloromethane fractions using 100% n-hexane. The sub-fraction helped to identify the chemical constituents in ZSE. GC/MS analysis of the components of sub-fraction 1 indicated that terpenoid compounds, including α-eudesmol and elemol, were the most abundant, accounting for 20.64% and 7.39% of this sub-fraction, respectively. Similarly, these two compounds were also found in large proportions (17.02% and 6.59%, respectively) in ZSE. This finding suggests that ZSE and ZSE sub-fraction 1 share the same major constituents, indicating that these compounds are likely responsible for the key anti-gastric ulcer activity of this plant material.
Elemol, a sesquiterpene, has been reported as to be an important terpenoid compound with insecticidal and anti-termite properties [23]. There is only one report that this bioactive compound, isolated from the leaves of Cryptomeria japonica, displayed moderate anti-gastric ulcer activity caused by HCl/EtOH [24]. Eudesmols are sesquiterpenoid alcohols that occur in many natural products. There are three main isomers of eudesmol including α-, β-, and γ-eudesmol [25]. These isomers have been reported for their biological activities, such as antihypertensive [26], anticonvulsant [27], antitumor [28], antimicrobial [29], antimigraine [30], insecticidal [31], and anti-inflammatory properties [32]. In mice, induction with atropine, dopamine, and serotonin (5-HT) exhibits some pharmacological effects of β-eudesmol. The β-eudesmol at the 50 or 100 mg/kg dosage could reverse the inhibitory effects of gastrointestinal motility induced by atropine, dopamine, and 5-HT [33]. However, despite the lack of supporting data for the anti-ulcer activity of the specific α-eudesmol, evaluating this terpenoid in further exploration would be interesting.
Despite the finding that α-eudesmol and elemol were present in significant proportions in the most active anti-gastric ulcer sub-fraction, it is premature to conclude that these terpenoids are solely responsible for the observed activity for two reasons. First, neither compound was isolated and tested for anti-gastric ulcer activity. Second, the range of chemicals scanned by the GC/MS may not encompass all the existing compounds in the extract or the sub-fractions [34]. It is also possible that other active secondary metabolites that were not detected could have contributed to the anti-gastric ulcer activity. These compounds may exert the anti-gastric ulcer activity alone or may synergize with those in the active sub-fraction 1. Therefore, further in-depth studies of the anti-gastric ulcer activity of these terpenes are necessary. Additionally, the use of HPLC with standard calibration would improve the quantification and reproducibility of active compounds in herbal formulations.
In the acute toxicity evaluation, no signs of toxicity or mortality were observed in either the control group or the ZSE sub-fraction 1 group throughout the 2-week experiment. The oral LD50 of ZSE sub-fraction 1 was determined to be greater than 2,000 mg/kg. This finding, in part, supports the safety of using this medicinal plant. However, sub-chronic or chronic toxicity studies are needed to evaluate long-term effects and potential side effects before conducting pharmaceutical pre-formulation and clinical studies in humans.
CONCLUSION
The study has shown that sub-fraction 1, eluted from the combined n-hexane and dichloromethane fractions of Z. simaoense rhizome ethanol extract, exhibits the highest anti-gastric ulcer activity. α-Eudesmol and elemol, the most abundant compounds in sub-fraction 1, may be responsible for this activity. Further in-depth studies of the anti-gastric ulcer activity of these terpenes should be conducted. The oral LD50 of sub-fraction 1 in rats is greater than 2,000 mg/kg, which partially confirms its safety. These findings will be beneficial for the future development of herbal health products derived from Z. simaoense rhizomes.
ACKNOWLEDGMENT
The authors sincerely appreciate Asst. Prof. Chaiyong Rujjanawate from the School of Medicine, Mae Fah Luang University, Chiang Rai, for providing the plant materials and valuable advice for the GC/MS analysis.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by the Faculty of Medicine, Chiang Mai University, grant number 031-2560.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals details are given in the ‘Material and Method’ section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Yuan Y, Padol IT, Hunt RH. Peptic ulcer disease today. Nat Clin Pract Gastroenterol Hepatol. 2006;3(2):80–9. CrossRef
2. Georges MH. Peptic ulcer disease and Helicobacter pylori. In: Kohlstadt I, editor. Advancing medicine with food and nutrients. 2nd ed. New York, NY: CRC Press; 2012. p. 229.
3. Schmeda-Hirschmann G, Yesilada E. Traditional medicine and gastroprotective crude drugs. J Ethnopharmacol. 2005;100(1-2):61–6. CrossRef
4. Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14(8):581–91. CrossRef
5. Engqvist A, von Feilitzen F, Pyk E, Reichard H. Double-blind trial of deglycyrrhizinated liquorice in gastric ulcer. Gut. 1973;14(9):711–5. CrossRef
6. Theilade I. A synopsis of the genus Zingiber (Zingiberaceae) in Thailand. Nord J Bot. 1999;19(4):389–410. CrossRef
7. Al-Amin M, Sultana GN, Hossain CF. Antiulcer principle from Zingiber montanum. J Ethnopharmacol. 2012;141(1):57–60. CrossRef
8. Siddaraju MN, Dharmesh SM. Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res. 2007;51(3):324–32. CrossRef
9. al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS, Tariq M. Gastroprotective activity of ginger Zingiber officinale rosc., in albino rats. Am J Chin Med. 1989;17(1-2):51–6. CrossRef
10. Yamahara J, Mochizuki M, Rong HQ, Matsuda H, Fujimura H. The anti-ulcer effect in rats of ginger constituents. J Ethnopharmacol. 1988;23(2-3):299–304. CrossRef
11. Yamahara J, Hatakeyama S, Taniguchi K, Kawamura M, Yoshikawa M. Stomachic principles in ginger. II. Pungent and anti-ulcer effects of low polar constituents isolated from ginger, the dried rhizoma of Zingiber officinale Roscoe cultivated in Taiwan. The absolute stereostructure of a new diarylheptanoid. Yakugaku Zasshi. 1992;112(9):645–55. CrossRef
12. Qian YY. Zingiber simaoense. Bull Bot Res, Harbin. 1998;18:284–6.
13. Picheansoonthon C, Chaowalit M, Jirawong W. The explanation of traditional recipes: Osot?phra?na?rai (Title in Thai). Bangkok, Thailand: Amarin Printing and Publishing; 2005. 275 p (in Thai).
14. Baiubon P, Kunanusorn P, Khonsung P, Chiranthanut N, Panthong A, Rujjanawate C. Gastroprotective activity of the rhizome ethanol extract of Zingiber simaoense Y. Y. Qian in rats. J Ethnopharmacol. 2016;194:571–6. CrossRef
15. Kunanusorn P, Laprasert C, Panthong A, Khonsung P, Chiranthanut N, Rujjanawate C. Gastric ulcer healing activity against acidified ethanol-induced gastric ulcer and gastroprotective mechanisms of Zingiber simaoense rhizome ethanol extract in rats. Pharmacogn Mag. 2020;16(68):152–60. CrossRef
16. Mizui T, Doteuchi M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1983;33(5):939–45. CrossRef
17. Cho CH, Ogle CW. A correlative study of the antiulcer effects of zinc sulphate in stressed rats. Eur J Pharmacol. 1978;48(1):97–105. CrossRef
18. OECD. Guidelines for the testing of chemicals. Section 4-Health Effects (No 420 Acute Oral Toxicity-Fixed Dose Procedure). Paris, France: Organization for Economic Cooperation and Development; 2002.
19. Cannell RJP. How to approach the isolation of a natural product. In: Cannell RJP, editor. Natural products isolation, methods in biotechnology. Totowa, NJ: Humana Press Inc.; 1998. 1–51 pp.
20. Liang YZ, Xie P, Chan K. Quality control of herbal medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1-2):53–70. CrossRef
21. Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, et al. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf. 2013;2:5–23. CrossRef
22. Orsi M, Essex JW. Passive permeation across lipid bilayers: a literature review. In: Sansom MSP, Biggin PC, editors. Molecular simulations and biomembranes. London, UK: The Royal Society of Chemistry; 2010, pp. 76–90.
23. Carroll JF, Paluch G, Coats J, Kramer M. Elemol and amyris oil repel the ticks Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in laboratory bioassays. Exp Appl Acarol. 2010;51(4):383–92. CrossRef
24. Matsunaga T, Hasegawa C, Kawasuji T, Suzuki H, Saito H, Sagioka T, et al. Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol Pharm Bull. 2000;23(5):595–8. CrossRef
25. Bomfim DS, Ferraz RP, Carvalho NC, Soares MB, Pinheiro ML, Costa EV, et al. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic Clin Pharmacol Toxicol. 2013;113(5):300–6. CrossRef
26. Arora CK, Arora RB, Mesta CK, Shanbag SN, Seshari R, Maheshwari ML, et al. Hypotensive activity of beta-eudesmol and some related sesquiterpenes. Indian J Med Res. 1967;55(5):463–72.
27. Chiou LC, Ling JY, Chang CC. Chinese herb constituent beta-eudesmol alleviated the electroshock seizures in mice and electrographic seizures in rat hippocampal slices. Neurosci Lett. 1997;231(3):171–4. CrossRef
28. Ma EL, Li YC, Tsuneki H, Xiao JF, Xia MY, Wang MW, et al. Beta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J Asian Nat Prod Res. 2008;10(1-2):159–67. CrossRef
29. Costa EV, Teixeira SD, Marques FA, Duarte MC, Delarmelina C, Pinheiro ML, et al. Chemical composition and antimicrobial activity of the essential oils of the Amazon Guatteriopsis species. Phytochemistry. 2008;69(9):1895–9. CrossRef
30. Horak S, Koschak A, Stuppner H, Striessnig J. Use-dependent block of voltage-gated Cav2.1 Ca2+ channels by petasins and eudesmol isomers. J Pharmacol Exp Ther. 2009;330(1):220–6. CrossRef
31. Chu SS, Jiang GH, Liu ZL. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag Sci. 2011;67(10):1253–7. CrossRef
32. Seo MJ, Kim SJ, Kang TH, Rim HK, Jeong HJ, Um JY, et al. The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharmacol Immunotoxicol. 2011;33(1):178–85. doi: 10.3109/08923973.2010.491082
33. Kimura Y, Sumiyoshi M. Effects of an Atractylodes lancea rhizome extract and a volatile component β-eudesmol on gastrointestinal motility in mice. J Ethnopharmacol. 2012;141(1):530–6. CrossRef
34. Rosenthal D. Theoretical limitations of gas chromatographic/mass spectrometric identification of multicomponent mixtures. Anal Chem. 1982;54(1):63–6. CrossRef