INTRODUCTION
Postoperative pain management is crucial for promoting recovery, reducing the risk of complications, decreasing discomfort, and increasing patient satisfaction [1]. There are several approaches primarily aimed at achieving optimal analgesia while reducing the incidence of adverse events. These include the use of various adjunctive therapies such as nefopam, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and ketamine, which aim to achieve adequate analgesia, minimize side effects and complications, and improve overall patient recovery [2,3]. Although opioids are the most used postoperative analgesics, they increase the risk of persistent postoperative pain [3] and the occurrence of adverse effects [4–7].
One of the approaches to achieve balanced analgesia is the use of nefopam alone or in combination with other opioids. Nefopam is a drug of choice for the management of moderate pain, especially postoperative pain and pain resulting from nerve damage [8]. It is a benzoxazocine synthesized from O-Benzoylbenzoic acid and has unique pharmacologic properties unlike other analgesics [9]. Additionally, nefopam is chemically distinct and pharmacologically unrelated to currently known analgesics. It does not affect platelet function and shows no anti-inflammatory effect when administered to patients [10–12].
Previous studies, including Girard et al. [8], Martinez et al. [13], Barazanchi et al. [14], Evans et al. [5], and Zhao et al. [15] examined the effects of nefopam on opioid administration in postoperative patients [5,8,13–15]. Girard et al. [8] reviewed 10 studies that investigated the role of nefopam in multimodal analgesia. The results in 8 of the 10 studies indicated that the combination of nefopam with other opioid analgesics resulted in pain reduction in patients [8]. Martinez et al. [13] evaluated the analgesic efficacy and safety of nefopam for postoperative pain relief. They found that nefopam provided analgesia and was non-inferior to other analgesics, including opioids [13]. In another systematic review, Barazanchi et al. [14] also concluded that the administration of nefopam, together with paracetamol, provided effective pain relief in postoperative patients [14].
Evans et al. [5] conducted a meta-analysis of the efficacy and safety of nefopam compared with other analgesics in the management of postoperative pain. The study showed that nefopam was as effective as other analgesics, including opioids and NSAIDs, in controlling postoperative pain. However, several side effects were also mentioned [5]. The study also indicated that nefopam was associated with an increased risk of adverse events such as nausea, vomiting, and sweating [5]. Finally, Zhao et al. [15] examined the safety and efficacy of nefopam for pain management in laparoscopic cholecystectomy in a meta-analysis. Significant differences were found between the treatment (nefopam) and placebo groups, with the former showing lower pain scores and adverse effects compared to the latter [15].
Although previous reviews have indicated the pain-reducing effect of nefopam, there is no quantitative measure of opioid reduction in postoperative patients. Additionally, there is still an unclear explanation of the association between nefopam use and reduction in opioid consumption and the incidence of adverse events in postoperative patients. Therefore, this study builds on the limitations of previous studies and attempts to fill the existing gap by providing a comprehensive summary of the available literature on the potential role of nefopam in reducing opioid consumption for pain management in postoperative patients. It also examines the level of patient satisfaction with nefopam treatment and the potential occurrence of adverse events during postoperative use.
METHODS
The MEDLINE®, EMBASE, Google Scholar, and Cochrane Library databases were searched for studies that had evaluated the efficacy and safety of nefopam in relation to opioid consumption in postoperative patients. Open web sources were also searched. Database searches were conducted between January 2023 and March 2023.
Inclusion criteria
Studies involving postoperative adult patients who had been given nefopam and compared with a control group were included for review. Only studies published in peer-reviewed journals were integrated. The review also used studies published between 2000 and 2023 since nefopam use has increased in the last two decades.
Exclusion criteria
Studies that either did not involve postoperative adult patients or had not been published in peer-reviewed journals were excluded from the current review. Additionally, studies involving animals and those that did not compare nefopam to a control group were excluded.
Search strategy
A literature search was conducted using precise keywords based on a Problem/Patient, Intervention, Comparative, and Results technique, as well as topic titles and Boolean Operators. The phrases “opioid,” “postoperative,” and “nefopam” were used as keywords. Search terms used included postoperative pain management, nefopam, analgesia, and opioid consumption.
- Impact: (“impact” OR “impactful” OR “impacting” OR “impacts” OR “impacted”) [All Fields] OR “impacted” [MeSH Terms].
- Nefopam: “nefopam” [MeSH Terms] OR “nefopam” [All Fields].
- Opioid: (“analgesics, opioid” [Pharmacological Action] OR “analgesics, opioid” [MeSH Terms] OR (“analgesics” [All Fields] AND “opioid” [All Fields]) OR “opioid analgesics” [All Fields] OR “opioid” [All Fields] OR “opioids” [All Fields] OR “opioid’s” [All Fields]).
- Consumption: “consumptions” [All Fields] OR “economics” [MeSH Terms] OR “economics” [All Fields] OR “consumption” [All Fields].
- Postoperative: “postoperative period” [MeSH Terms] OR (“postoperative” [All Fields] AND “period” [All Fields]) OR “postoperative period” [All Fields] OR “postop” [All Fields] OR “postoperative” [All Fields] OR “postoperatively” [All Fields] OR “postoperatives” [All Fields].
- Patients: (“patient’s” [All Fields] OR “patients” [MeSH Terms] OR “patients” [All Fields] OR “patient” [All Fields] OR “patients’” [All Fields]).
Based on the research topic and search strategy, a PICOS question was formulated as follows: Population: patients undergoing postoperative treatment.
Intervention: administration of nefopam.
Comparison: use of opioid analgesics.
Outcome: impact on patients’ consumption and occurrence of adverse effects.
PICOS question: In adult postoperative patients, does nefopam administration result in a significant reduction in opioid consumption and occurrence of adverse effects compared to opioid analgesics without nefopam?
After screening and selection of studies for systematic review, a risk of bias (ROB) assessment was performed using the Cochrane ROB-2 tool. ROB-2 is a recently updated tool that researchers can use to assess articles against six main criteria [16].
DistillerSR was used for data extraction and subsequent visualization using descriptive statistics. This software streamlines the collection, screening, and analysis of literature using automation techniques [17]. A standardized data extraction form was used by three reviewers to extract and review the data. Each reviewer assessed all the studies and extracted data using the template, followed by a joint critical evaluation to harmonize potential discrepancies [18]. Through discussion and consensus, emerging discrepancies were addressed and solved.
The most important information from the studies in question was extracted in a separate table. The following information was requested during data extraction: author and year of publication; information on the participants—age, gender, duration of the postoperative phase; response rate; study groups and interventions; study duration; study country; type of publication (Table 1).
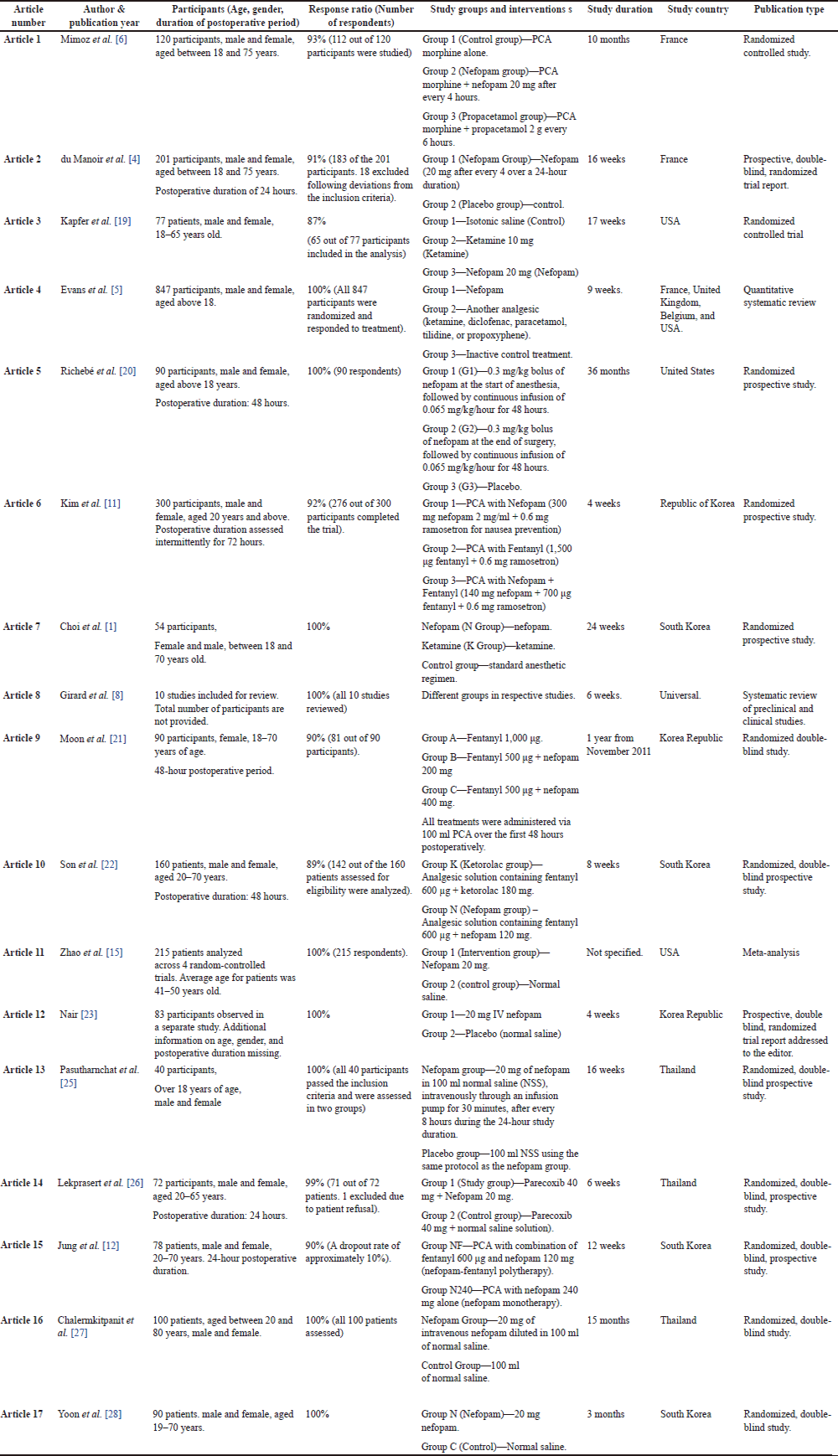 | Table 1. Data extraction summary for included studies. [Click here to view] |
Finally, the results were summarized in another separate table. The information obtained from the eligible studies included the following: Study groups and interventions, indicators of postoperative pain, indicators of opioid consumption, impact on opioid consumption, the percentage reduction in opioid consumption, patient satisfaction with treatment, safety, and incidence of adverse events (Table 3).
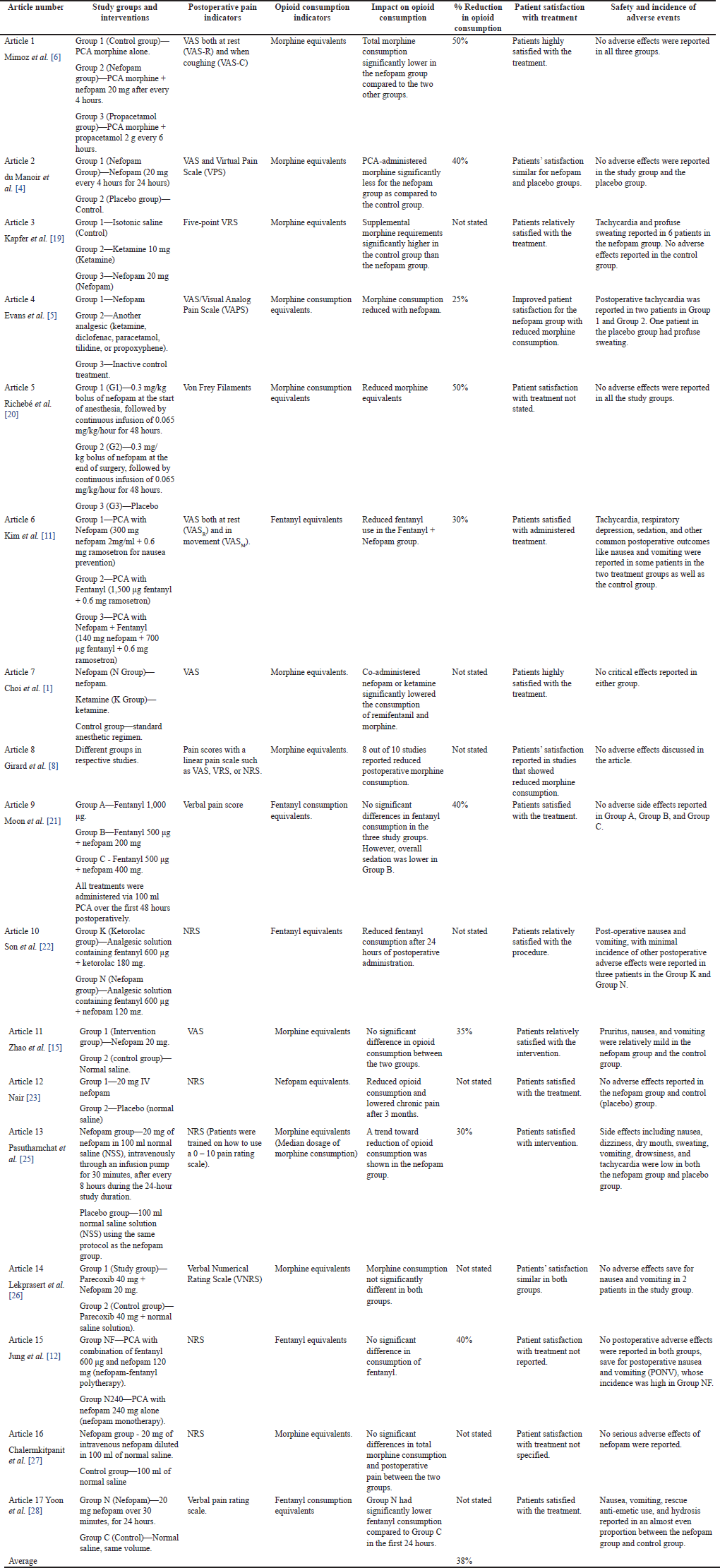 | Table 3. Results summary for included studies. [Click here to view] |
PROSPERO REGISTRATION
This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42022364446. Details of the protocol can be accessed at: https://www.crd.york.ac.uk/prospero/.
RESULTS
Characteristics and summary of the eligible studies
The study selection process was conducted under strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A total of 454 studies were identified through initial database searches, with another four identified from web sources. Eight studies were removed as duplicates, with the remaining 450 studies screened. 401 studies that did not meet the inclusion criteria were excluded upon screening. Only 49 studies were evaluated for eligibility, with 32 being excluded for overlapping data and involvement of adults without postoperative pain. The remaining 17 studies were included for analysis. Figure 1 shows the PRISMA flow diagram of the systematic review strategy.
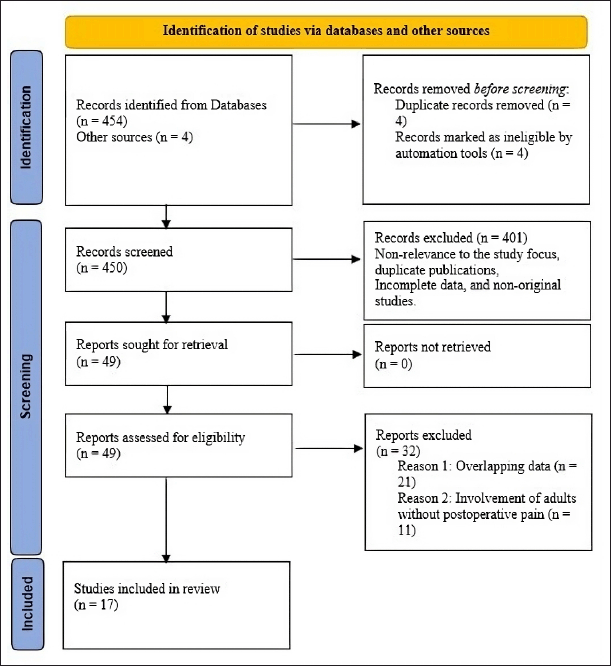 | Figure 1. PRISMA flow diagram of the systematic review strategy. [Click here to view] |
The low number of eligible studies (n = 17) reflects the stringent inclusion criteria applied to ensure the selection of high-quality and relevant data. Several studies were excluded due to limited methodological rigor, small sample sizes, or non-alignment with the research objective. This careful selection was essential to ensure robust findings and minimize bias, despite the limited pool of suitable studies.
Most studies (n = 4) were conducted in South Korea, whereas others were conducted in the United States (n = 3), the Republic of Korea (n = 3), Thailand (n = 3), and France [2]. Two studies were wider than one country. The remaining two studies were designated as “global” because they were conducted in different regions. A total of 2,617 adults were included in 16 studies. However, in one of the systematic reviews, the exact number of participants in the integrated studies was not provided [8]. All participants underwent postoperative pain management. Table 1 lists all study characteristics of the included articles.
Mimoz et al. [6] found that pain relief and reduced opioid consumption were higher when nefopam was administered together with other analgesics, especially paracetamol. Adverse effects such as nausea and dizziness also occurred [6]. du Manoir et al. [4] found that significantly less morphine was administered via patient-controlled analgesia (PCA) in the nefopam group [4]. On the other hand, Kapfer et al. [19] concluded that tachycardia and excessive sweating were more common in patients receiving nefopam than in the other two groups (isotonic saline and ketamine). Pain relief was faster in the nefopam and ketamine groups than in the control group after the additional morphine infusion was initiated [19]. Evans et al. [5] showed that opioid consumption and pain intensity decreased significantly in the nefopam group [5].
Richebé et al. [20] found significant changes in opioid consumption between the treatment and control groups. No adverse effects were reported [20]. Kim and Abdi [10] found a significant decrease in opioid consumption in the treatment groups receiving either nefopam alone or nefopam and fentanyl. Choi et al. [1] also showed that concomitant administration of nefopam or ketamine significantly decreased consumption of remifentanil and morphine [1]. Girard et al. [8] showed lower morphine consumption, with no adverse effects reported in the nefopam group [8]. Moon et al. [21] found no difference in satisfaction between patients receiving fentanyl and those receiving fentanyl with nefopam [21].
Son et al. [22] reported side effects such as postoperative nausea and vomiting [22]. Likewise, Zhao et al. [15] reported opioid-related side effects such as nausea, vomiting, and pruritus [15]. According to Nair [23] and Na et al. [24], there was lower opioid consumption and lower levels of chronic pain after nefopam use [23,24]. Pasutharnchat et al. [25] reported that patients in the nefopam group experienced significant pain reduction. Adverse events such as dizziness, drowsiness, sweating, dry mouth, and nausea occurred in both groups [25]. In a separate study, Lekprasert et al. [26] found that the nefopam group had similar postoperative pain scores compared to the control group [26]. According to Jung et al. [12], there was no statistically significant difference in numeric rating scale (NRS) scores between groups throughout the postoperative period [12].
Chalermkitpanit et al. [27] showed that there was no significant difference in morphine consumption between the nefopam group and the control group. There was also no significant difference in postoperative pain scores between the two groups. However, morphine consumption was slightly lower in the nefopam group [27]. On the other hand, Yoon et al. [28] found that the nefopam group had significantly lower fentanyl consumption compared with the control group. The nefopam group also had a significantly lower pain score. However, there were no significant differences in the occurrence of side effects, quality of recovery, and length of hospital stay [28].
Overall, this systematic review showed that nefopam generally reduced opioid consumption in postoperative patients. No adverse effects were reported in 9 of the 17 studies. In the remaining seven studies, various adverse effects were reported, mostly in the treatment groups, but with a relatively low frequency. The results of the individual studies are summarized in Table 3.
Quality assessment and ROB
Cochrane’s ROB-2 tool was used to assess the ROB in individual studies. Based on the ROB assessment, the overall quality of the included articles was good. Only one article [23] had a high risk. The majority of the remaining 16 articles had low risk. A summary of the assessment performed is presented in Table 4 for each study, based on the average of the authors’ ratings.
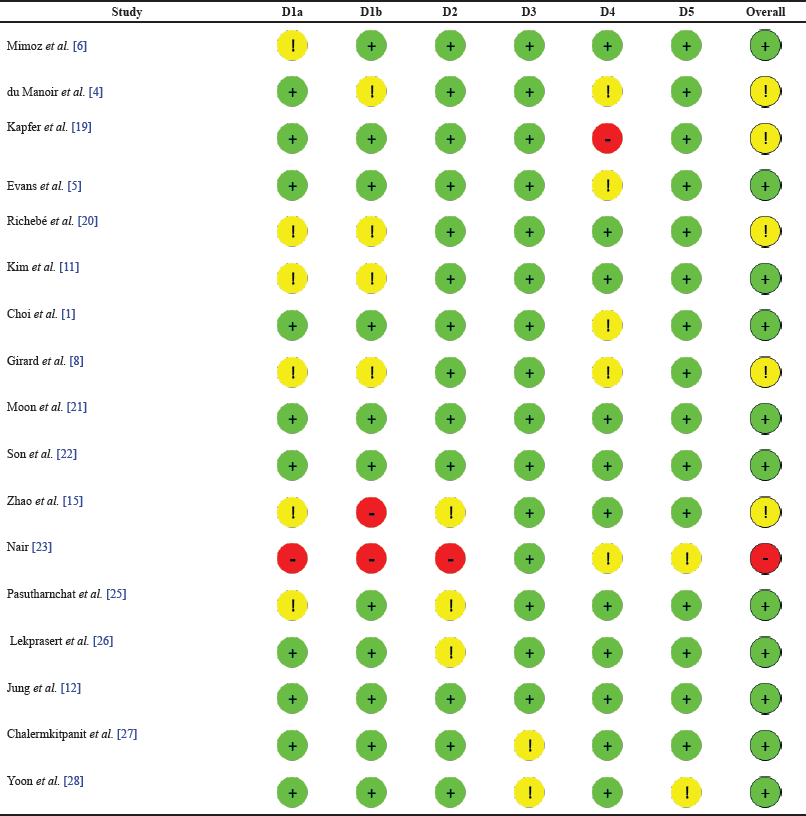 | Table 4. ROB assessment. [Click here to view] |
A high ROB was reported in the report by Nair [23] because the summative report had no explicit research methods included. However, the report was important in the current study as it also contributed knowledge on the use of nefopam in postoperative pain management. The overall goal of the current study was to find literature surrounding the topic and evaluate evidence in line with the set objectives. Therefore, while some of the domains included in Cochrane’s ROB-2 tool revealed some issues creating the bias, it was still included as a relevant source of literature given that it was a report of a previous study.
Impact of nefopam on opioid consumption
This review found a significant decrease in opioid consumption in 12 of the 17 studies, whereas the remaining 5 studies found no significant difference in opioid consumption between the nefopam and control groups. Of the 12 studies that reported reductions in opioid consumption, 9 reported specific percentage reduction values as follows: Mimoz et al. [6]—50%, du Manoir et al. [4]—40%, Evans et al. [5]—25%, Richebé et al. [20]—50%, Kim et al. [11]—30%, Moon et al. [21]—40%, Zhao et al. [15]—35%, Jung et al. [12]—40%, Pasutharnchat et al. [25]—30%. Based on the above values from studies reporting specific values, the summary of the calculation of the percentage reduction in opioid use is: (50+40+25+50+30+40+35+40+30)/9 = 37.77 ≈ 38%. The average reduction in opioid consumption due to the postoperative use of nefopam was, therefore, 38%.
The reported percentage decrease in opioid consumption ranged from 25% to 50% across the studies. This variation could be attributed to differences in study populations, interventions, and methodologies. Some studies had confidence intervals (CIs) that included zero or negative values, suggesting that the observed effect may not be statistically significant or may favor the control group as shown in Table 2. The overall range of CIs from all the readings was (−1.62–2.743). The forest plot in Figure 2 also shares findings of the calculated CIs. While most studies reported a positive effect (reduction in opioid consumption), some studies, such as Evans et al. [5] and Richebé et al. [20], had CIs that included negative values, suggesting the possibility of increased opioid consumption or no effect. More recent studies such as Moon et al. [21], Zhao et al. [15]), Pasutharnchat et al. [25], and Jung et al. [12], generally showed a consistent trend of decreased opioid consumption, with narrower CIs, indicating more precise estimates of the effect. Overall, the results indicate that the treatments or interventions evaluated in these studies may reduce opioid consumption, but the magnitude of the effect varies across studies, as shown in the forest plot in Figure 2.
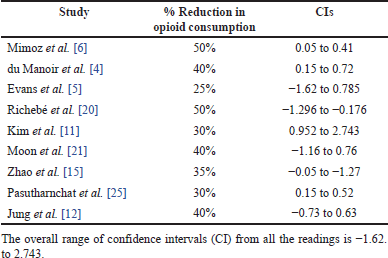 | Table 2. Summary of average reduction in opioid consumption and CIs. [Click here to view] |
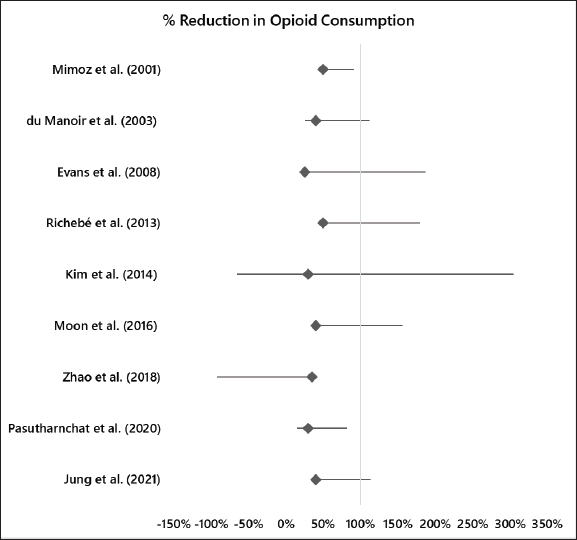 | Figure 2. Forest plot for reduction in opioid consumption. [Click here to view] |
The study also showed that nefopam administration was associated with pain reduction in most patients. While the included studies used different pain scores, including Verbal analogue scale (VAS), Verbal Rating Scale (VRS), NRS, and Verbal analogue scale at rest (VAS-R), most of the studies reported that patients had better pain relief when postoperative nefopam was administered. Using NRS, Jung et al. [12], Moon et al. [21], and Son et al. [22] all reported scores below 5, showing significant pain reduction in the nefopam groups [27,29]. Both Choi et al. [1] and Zhao et al. [15] also recorded reduced pain scores using the VAS [22,26]. However, Kapfer et al. [19] indicated that there were no major differences between the nefopam group and the control group when pain scores were measured using VRS.
Patient satisfaction with treatment
Most patients were satisfied with the treatment administered. In 13 out of the 17 studies included in the review, patient satisfaction was reported, ranging from moderate to significantly high levels of satisfaction with the use of nefopam in postoperative pain management. Two studies did not report the eventual level of satisfaction, while the remaining two studies reported similar satisfaction levels between the treatment group and the control group. As such, the researcher concluded that nefopam administration in postoperative pain management is positively correlated with patient satisfaction.
Safety and incidence of adverse effects
No adverse effects were reported in 9 out of the 17 studies. In the remaining seven studies, various adverse effects were reported, mostly in the treatment groups but with a relatively low incidence. The most common adverse effects included tachycardia, sweating, nausea, and drowsiness. Two pruritus cases were also reported in one of the studies, with one case of respiratory depression also reported. Hypoventilation, malaise, and general cutaneous allergy also occurred in three patients in the treatment group of one of the randomized controlled studies. In cases where nausea, vomiting, and sweating were reported, the cases were relatively mild. However, there were cases of profuse sweating in two participants.
DISCUSSION
Nefopam use in postoperative pain management
Although opioids have long been used in postoperative pain management, the prevalence of associated side effects has led to increased research into non-opioid analgesics. According to Stephan and Parsa [30], opioid analgesics relieve postoperative pain but also have distressing negative effects on the body. Opioids not only impair the release of μ opioid receptors but also prevent the release of beta-endorphin, which is crucial for pain management. In addition, Li et al. [31] noted that opioids complicate pain management by causing adverse effects such as postoperative nausea/vomiting, pruritus, respiratory depression, and urinary retention. Because of the adverse side effects of opioids, multimodal analgesia is increasingly being considered for postoperative pain management, including various approaches such as preemptive analgesia, PCA, neuraxial anesthesia, and non-opioid medications.
Another important problem in the postoperative use of opioids is neuroadaptation. According to Lavand’homme and Steyaert [29], while opioids are the most effective drugs currently used to treat chronic pain, they have limited ability to provide long-term analgesia due to neuroadaptation. Further evidence suggests that neuroadaptation occurs mainly through opioid-induced hyperalgesia and tolerance [29]. In some cases, opioids also have opposite effects, such as enhancing postoperative pain. To mitigate the potential occurrence of neuroadaptation and opposite effects, different opioids, dose limitations, and non-opioid analgesics can be used [32].
Nefopam exerts its analgesic effect through various pharmacological mechanisms. Its central analgesic effect helps to modulate pain perception by acting centrally in the brain and spinal cord [8]. Some of the key attributes include preventing the reuptake of neurotransmitters such as norepinephrine, dopamine, and serotonin, increasing their concentration in the synaptic cleft, thereby enhancing various descending inhibitory pain pathways [8]. Although nefopam’s analgesic effect is primarily centrally mediated, it also has a peripheral analgesic effect by inhibiting the release of inflammatory mediators and prostaglandins. Nefopam also exhibits N-Methyl-D-aspartate (NMDA) receptor antagonism, which modulates pain perception and reduces the transmission of pain signals by blocking NMDA receptors [31]. Overall, the above pharmacological mechanisms enhance nefopam’s pain relief abilities while reducing opioid consumption, as revealed by findings of the current study involving postoperative patients.
The current study found that nefopam is a viable non-opioid analgesic for postoperative pain management. Recent studies on the efficacy of nefopam in postoperative pain management suggest that patients’ pain is reduced due to the morphine-sparing effect of nefopam [28]. In this systematic review, nefopam significantly reduced opioid consumption in postoperative patients, justifying its morphine-sparing effect. The mean decrease in opioid consumption across studies was 38%. Overall, the attained average figure suggests that patients receiving nefopam to supplement an analgesic regimen with opioids are likely to experience almost as much reduction in pain intensity as patients receiving high doses of opioids. Thirteen studies reported lower opioid consumption when nefopam was administered postoperatively. In the studies that reported pain reduction, there was a strong statistical correlation between nefopam use, reduced morphine consumption, and pain reduction in participating patients. In contrast, five studies reported no significant difference in opioid consumption and postoperative pain management after nefopam administration [12,15,21,26,27].
In addition, the overall analgesic effect of nefopam was found to be superior to that of opioids alone. Greater pain relief was reported in cases where nefopam was co-administered with other analgesic adjuvants such as acetaminophen [6] and fentanyl [22,29], as well as ketamine [1]. Because the doses of nefopam administered varied, researchers were unable to identify specific doses associated with nefopam’s morphine-sparing effects. In the past, the role of nefopam in the management of postoperative pain has been questioned because of the limited characterization of the regimens and doses used, particularly in combination with other analgesic adjuvants [2].
Previous studies examining the analgesic effects of nefopam have shown that it has nearly similar efficacy compared with opioid alternatives. According to Tramoni et al. [33], 20 mg of nefopam shows efficacy equivalent to that of 6–12 mg of morphine. Yoon et al. [28] also indicated that 20 mg of nefopam is equivalent to about 7.5 mg of ketorolac and morphine in postoperative pain control [28]. In sum, the results of the current study are consistent with the existing literature on analgesic efficacy. For example, du Manoir et al. [4] and Evans et al. [5] found that the analgesic efficacy of nefopam was comparable to that of other opioids [4,5]. However, opioids were more likely to cause adverse effects in patients than non-opioid analgesics.
A study conducted by Tramoni et al. [33] indicated that nefopam reduced opioid consumption by up to 50%, but the average reduction was about 25%. The findings are consistent with the results of most of the studies included in the analysis. For example, du Manoir et al. [4] reported a 40% reduction in opioid consumption when 20 mg of nefopam was infused every 4 hours [4]. Evans et al. [5] found that infusion of nefopam resulted in a 25% reduction in opioid consumption compared with the control group, which had no change in opioid consumption [5]. According to Richebé et al. [20], continuous nefopam infusion resulted in a 50% reduction in opioid consumption in the nefopam group. Based on the results of the current analysis, it can be concluded that nefopam could reduce opioid consumption by up to 50%, depending on the amount administered to patients.
Safety and incidence of adverse effects
Based on the observed adverse effects, this systematic review found that co-administration of nefopam is generally well tolerated by patients. The overall trend of adverse effects was generally low. In studies that reported adverse effects, nausea, sweating, and postoperative tachycardia were common. However, the outcomes were mostly classified as uncomfortable and not significant medical problems. These findings are consistent with existing literature. According to Charoenpol et al. [2], co-administration of nefopam in the management of moderate pain is often associated with a minimal incidence of adverse effects.
The common adverse effects of nefopam that have been document in previous research include nausea, vomiting, hypotension, tachycardia, sedation, sweating, respiratory depression, itching, urinary retention, and dry mouth [2]. Compared to opioids used in postoperative pain management, the tendency of adverse effects following nefopam administration is generally lower. This is partly attributed to its central analgesic effect that is associated with minimal unwanted reactions in other parts of the body [2]. As revealed in the current study, the occurrence of adverse effects for most treatment groups given nefopam was generally lower as compared to groups given opioids and other analgesics, as shown in Table 5.
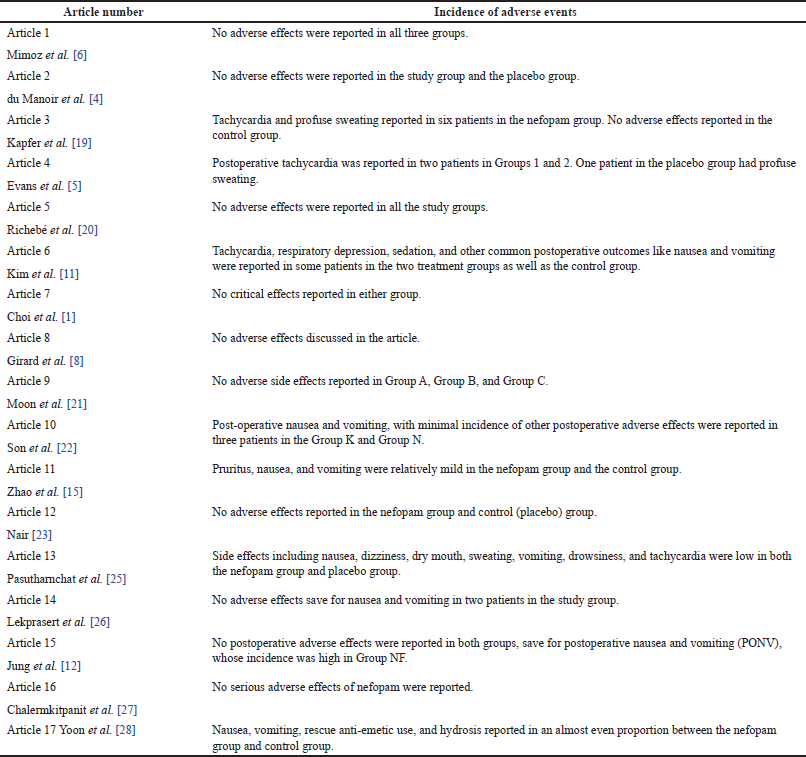 | Table 5. Incidence of adverse effects. [Click here to view] |
Chalermkitpanit et al. [27] believe that nefopam is increasingly used in the management of postoperative pain because it has minimal adverse effects on patients, with postoperative nausea and vomiting, sweating, and tachycardia being the most observed adverse effects [27]. In the current review, the studies by Jung et al. [12] and Lekprasert et al. [26] showed that most patients had few adverse effects in their respective treatment groups [12,26]. However, postoperative tachycardia can be dangerous in patients with impaired cardiac function [34].
Patient satisfaction with the treatment
This review found that concomitant administration of nefopam resulted in pain relief in most patients. A variety of pain scales were used in the included studies, including the VAS, the VRS, the NRS, and the VAS-R. Most studies indicated that patients experienced better pain relief when nefopam was co-administered postoperatively with other analgesics. Jung et al. [12], Moon et al. [21], and Son et al. [22] reported NRS scores below five, which showed significant pain reduction in the nefopam groups [12,21,22]. Both Choi et al. [1] and Zhao et al. [15] also recorded reduced pain scores using the VAS [1,15]. However, Kapfer et al. [19] pointed out that there were no significant differences between the nefopam group and the control group when pain scores were measured using the VRS [19]. The current study found that most patients were satisfied with the treatment. Finally, the low incidence of adverse effects of nefopam also resulted in a high level of satisfaction among most patients.
LIMITATIONS
At the study level, this systematic review did not assess outcomes for patients receiving combination therapy of nefopam and different types of opioids and also excluded studies of patients receiving long-term postoperative pain therapy, which could provide valuable insights into the effects of nefopam on opioid consumption in postoperative patients.
At the review level, adverse effects were not discussed in detail in the included studies. Factors such as the type of surgery, dosage and timing of nefopam administration, and the corresponding influences on opioid consumption may have been incompletely investigated.
CONCLUSION AND FUTURE IMPLICATIONS
This study offers a general interpretation of the results in the context of existing evidence and provides implications for future research. The systematic review demonstrated that nefopam is effective in postoperative pain management, as evidenced by lower pain scores and lower morphine consumption in postoperative patients. Specifically, the results suggest that nefopam significantly reduces opioid consumption with a relatively low incidence of side effects. Future research is needed to further investigate the potential of nefopam as an alternative to opioids to reduce opioid consumption in postoperative patients, as well as the potential influence of factors such as type of surgery, dosage, and timing of administration on the effect of nefopam on opioid consumption. Future studies could also examine comparative effectiveness in long-term postoperative pain management, particularly examining pain intensity and the duration of analgesic effect. Additionally, combination therapy studies could be conducted to evaluate the synergic effects of nefopam with other analgesics. Finally, there is still a significant literature gap regarding the pharmacokinetic and pharmacodynamic properties of nefopam. Profiling nefopam based on these properties could avail further understanding of its mechanisms of action as well as the overall influence on reduction in opioid consumption when used postoperatively.
LIST OF ABBREVIATIONS
NRS, Numeric Rating Scale; NSAIDs, Non-steroidal anti-inflammatory drugs; PCA, Patient-controlled analgesia; RCTs, Randomized-controlled trials; VAS, Verbal analogue scale; VAS-R, Verbal analogue scale at rest; VRS, Verbal rating scale.
AUTHOR’S CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
LIST OF VARIABLES
The variables for which data were sought are listed and defined below:
Nefopam administration—The main aim of the study was to assess the role of nefopam in reducing opioid consumption in postoperative patients. Therefore, postoperative nefopam administration, either on a singular method or in combination with other analgesics was examined.
Opioid consumption—Traditional approach for treatment of acute postoperative pain, involving administration of various opioid analgesics such as morphine and fentanyl.
Occurrence of adverse effects—outcomes such as vomiting, sweating, and tachycardia, associated with postoperative administration of nefopam and alike analgesics.
Overall patient satisfaction with treatment—assessment of the extent of satisfaction based on pain reduction, opioid consumption, and the occurrence of side effects.
REFERENCES
1. Choi SK, Yoon MH, Choi JI, Kim WM, Heo BH, Park KS, et al. Comparison of effects of intraoperative nefopam and ketamine infusion on managing postoperative pain after laparoscopic cholecystectomy administered remifentanil. Korean J Anesthesiol. 2016;69(5):480–6.
2. Charoenpol FN, Khampitak N, Aimnang C, Pachirat K, Sirithanaphol W, Rompsaithong U, et al. Single-dose intravenous nefopam on postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of prostate: a randomized, double-blind placebo-controlled trial. J Anesth. 2023;37(1):72–8.
3. Franzese A, Borrelli O, Corrado G, Rea P, Di Nardo G, Grandinetti AL, et al. Domperidone is more effective than cisapride in children with diabetic gastroparesis. Aliment Pharmacol Ther. 2002;16:951–7. doi: https://doi.org/10.1046/j.1365-2036.2002.01240.x
4. du Manoir B, Aubrun F, Langlois M, le Guern ME, Alquier C, Chauvin M, et al. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br J Anaesth [Internet]. 2003 [cited 2023 Jan 5];91(6):836–41. Available from: https://pubmed.ncbi.nlm.nih.gov/14633755/
5. Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008;101(5):610–7.
6. Mimoz O, Incagnoli P, Josse C, Gillon MC, Kuhlman L, Mirand A, et al. Analgesic efficacy and safety of nefopam vs. propacetamol following hepatic resection. Anaesthesia. 2001;56(6):520–5.
7. Mitra S, Carlyle D, Kodumudi G, Kodumudi V, Vadivelu N. New advances in acute postoperative pain management. Curr Pain Headache Rep. 2018;22:1.
8. Girard P, Chauvin M, Verleye M. Nefopam analgesia and its role in multimodal analgesia: a review of preclinical and clinical studies. Clin Exp Pharmacol Physiol. 2016;43(1):3–12.
9. Hariton E, Locascio JJ. Randomised controlled trials—the gold standard for effectiveness research. BJOG. 2018;125(13):1716.
10. Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain [Internet]. 2014 [cited 2023 Jan 5];27(2):103. Available from: /pmc/articles/PMC3990817/
11. Kim K, Kim WJ, Choi DK, Lee YK, Choi IC, Sim JY. The analgesic efficacy and safety of nefopam in patient-controlled analgesia after cardiac surgery: a randomized, double-blind, prospective study. J Int Med Res [Internet]. 2014 [cited 2023 Jan 5];42(3):684–92. Available from: http://www.uk.sagepub.com/aboutus/openaccess.htm
12. Jung KT, So KY, Kim SC, Kim SH. Effect of nefopam-based patient-controlled analgesia with and without fentanyl on postoperative pain intensity in patients following laparoscopic cholecystectomy: a prospective, randomized, controlled, double-blind non-inferiority trial. Medicina [Internet]. 2021 [cited 2023 Jan 5];57(4):316. Available from: https://www.mdpi.com/1648-9144/57/4/316
13. Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth. 2017;118(1):22–31.
14. Barazanchi AWH, MacFater WS, Rahiri JL, Tutone S, Hill AG, Joshi GP, et al. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth. 2018;121(4):787–803.
15. Zhao T, Shen Z, Sheng S. The efficacy and safety of nefopam for pain relief during laparoscopic cholecystectomy: a meta-analysis. Medicine. 2018;97(10):e0089.
16. Higgins JP, Savovi? J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Li MCT, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley-Blackwell; 2019 Sep 23. pp. 205–28.
17. DistillerSR.com. Literature review software. Available from: https://www.distillersr.com/products/distillersr-systematic-review-software
18. Stoll CRT, Izadi S, Fowler S, Green P, Suls J, Colditz GA. The value of a second reviewer for study selection in systematic reviews. Res Synth Methods. 2019;10(4):539–45.
19. Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg [Internet]. 2005 Jan [cited 2023 Jan 5];100(1):169. Available from: /pmc/articles/PMC1283103/
20. Richebé P, Picard W, Rivat C, Jelacic S, Branchard O, Leproust S, et al. Effects of nefopam on early postoperative hyperalgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(3):427–35.
21. Moon JY, Choi SS, Lee SY, Lee MK, Kim JE, Lee JE, et al. The effect of nefopam on postoperative fentanyl consumption: a randomized, double-blind study. Korean J Pain. 2016;29(2):110–8.
22. Son JS, Doo A, Kwon YJ, Han YJ, Ko S. A comparison between ketorolac and nefopam as adjuvant analgesics for postoperative patient-controlled analgesia: a randomized, double-blind, prospective study. Korean J Anesthesiol. 2017;70(6):612–8.
23. Nair A. Nefopam: another pragmatic analgesic in managing chronic neuropathic pain. Indian J Palliat Care [Internet]. 2019 Jul 1 [cited 2023 Jan 5];25(3):482. Available from: /pmc/articles/PMC6659541/
24. Na HS, Oh AY, Koo BW, Lim DJ, Ryu JH, Han JW. Preventive analgesic efficacy of nefopam in acute and chronic pain after breast cancer surgery: a prospective, double-blind, and randomized trial. Medicine. 2016;95(20):e3705.
25. Pasutharnchat K, Wichachai W, Buachai R, Yuen Ho K, Hadi Mohamed A. Analgesic efficacy of nefopam for cancer pain: a randomized controlled study. F1000Res. 2020 [cited 2023 Jan 5];9:378. Available from: https://doi.org/10.12688/f1000research.23455.1
26. Lekprasert V, Yapanan L, Ittichaikulthol W, Buachai R, Soisod P, Sophonsritsuk A. Perioperative intravenous patient-controlled analgesic efficacy of morphine with combined nefopam and parecoxib versus parecoxib in gynecologic surgery: a randomized, double-blind study. Anesthesiol Res Pract. 2021;2021:5461890.
27. Chalermkitpanit P, Limthongkul W, Yingsakmongkol W, Thepsoparn M, Pannangpetch P, Tangchitcharoen N, et al. Analgesic effect of intravenous nefopam for postoperative pain in minimally invasive spine surgery: a randomized prospective study. Asian Spine J. 2022;16(5):651.
28. Yoon S, Lee HB, Na KJ, Park S, Bahk J, Lee HJ. Effect of continuous infusion of intravenous nefopam on postoperative opioid consumption after video-assisted thoracic surgery: a double-blind randomized controlled trial. Pain Physician. 2022;25(6):491–500.
29. Lavand’homme P, Steyaert A. Opioid-free anesthesia opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. 2017;31(4):487–98.
30. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63–7.
31. Li Y, Dong H, Tan S, Qian Y, Jin W. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: a single-center, randomized controlled trial. Medicine. 2019;98(7):e14362.
32. Weber L, Yeomans DC, Tzabazis A. Opioid-induced hyperalgesia in clinical anesthesia practice: what has remained from theoretical concepts and experimental studies?. Curr Opin Anaesthesiol. 2017;30(4):458–65.
33. Tramoni G, Viale JP, Cazals C, Bhageerutty K. Morphine-sparing effect of nefopam by continuous intravenous injection after abdominal surgery by laparotomy. Eur J Anaesthesiol. 2003;20(12):990–2.
34. Moussa MK, Lefevre N, Valentin E, Meyer A, Grimaud O, Bohu Y, et al. Dynamic intermittent compression cryotherapy with intravenous nefopam results in faster pain recovery than static compression cryotherapy with oral nefopam: post-anterior cruciate ligament reconstruction. J Exp Orthop. 2023;10(1):72.