INTRODUCTION
The market of Halal Food and Pharmaceuticals expanded globally, and it is recently estimated that the market size has increased due to the fast growing demand of halal products and halal awareness among Muslim’s consumers. In line with the increased markets of halal products, the authenticity and the halal status of product components in the home-made industry, local markets and supermarkets have also been asked by Muslim communities, who have countered against the use of nonhalal components in the products either intentionally or unintentionally [1]. To the followers of Muslim and Jews, the halal and kosher status of products assures that the products do not contain nonhalal components including pig derivatives such as pork, lard, and porcine gelatins (PG) as well as from prohibited ingredients which are not allowed according to Islamic law. For Muslim consumers, halal products confirm that the consumed products have been made and produced using equipment that is dedicated to halal products. Parallel to Muslims, Jewish kosher are also not allowed to consume pork and other pig derivatives. For this reason, the confirmation that the products are free from nonhalal components such as PG is very essential for Kosher and halal authentication analysis [2].
Gelatin is one of food, cosmetics, and pharmaceutical components found in industrial and home-made products. Gelatin is a water-soluble protein obtained from the results of thermal partial hydrolysis of collagen in skins, bones, and connective tissues of animals [3]. Gelatin is water-soluble fibrous collagen hydrolysates with a molecular weight of 97–250 kDa. In the products, gelatin can function as a good ingredient to enhance the elasticity, thickness, stabilizing, and emulsification agents. The most important physical properties of gelatin are Bloom value and viscosity. The Bloom value measures the strength of gelatin, displaying its level of firmness or softness. Higher values directly correlate with stronger gelatin. Gelatin with a Bloom value of >220–300 g is considered as high strength, >150–220 g (medium strength), and ≤150 g (low strength). Gelatin extracted from land animals (bovine and porcine) is ideal to be used in certain products, including food, cosmetics, and pharmaceuticals, but some consumers reject gelatins obtained from mammalian due to some reasons, including social, cultural, religious (halal and nonhalal related issues), or health-related concerns especially the risk of contracting prion diseases (in particular, spongiform encephalopathy). [4]. There are two types of gelatins existed, namely type A and Type B-gelatins according to the production processes of gelatins from their sources. Gelatins obtained from the extraction of collagen in acidic conditions are considered as Type A. In addition, Type B-gelatin is obtained during the extraction of collagen in basic or alkali conditions. Type-A and Type-B gelatins revealed some bit differences in terms of physical and chemical characteristics [5].
The issue arising from the use of gelatin in certain products is related to its source, which is that most gelatin sources come from bovine and porcine. PG is the preferable type of gelatin in non-Muslim countries because PG is cheaper than other gelatin types and has been widely used as a substitute for beef gelatin (BG) to prevent mad cow disease contraction [6]. For the Muslim community, the use of BG is not problematic since BG is a halal component according to Sariah (Islamic law), provided that bovine is slaughtered according to Sariah principles. PG is nonhalal and non-Kosher components; therefore, Muslims and Jews are prohibited from consuming any products containing PG. In addition, there are some concerns related to BG because BG has the potential to carry certain diseases, such as BSE or bovine spongiform encephalopathy [5]. Furthermore, some people have allergenic reactions due to the exposure of certain gelatins-derived products capable of inducing the responses of immune systems. Thus, the availability of standard methods for the analysis of gelatins is urgently needed to support halal certification.
The detection of gelatin in food and pharmaceutical products can be carried out by several methods either using chemical or biological methods. Several chemical or biological methods for the detection of gelatin include: Fourier Transform Infrared Spectroscopy (FTIR): This approach analyses the infrared spectrum of gelatin to discover particular functional groups that are unique to each species. For example, FTIR was employed in conjunction with chemometrics [7]. HPLC can separate and detect the peptides found in gelatin, which can be utilized to determine the species of origin. For example, Halal Gelatin Analysis for Detection in Confectionery and Food Supplements [8]. Polymerase chain reaction (PCR) is a molecular biology technique for amplifying specific DNA sequences. It is frequently used in concert with other procedures to determine the presence of gelatin in specific animal sources. PCR or real-time PCR for analysis of DNAs present in gelatins [9] and Liquid Chromatography-Mass Spectrometry (LC-MS/MS): This technique combines the separation ability of liquid chromatography with the detection sensitivity of mass spectrometry. LC-MS/MS by identifying peptide markers that are specific for gelatin types are taken into account as standard methods for the identification of gelatin sources [10]. The similar physical appearance between BG and PG causes difficulty in their detection without the application of instrumental analysis such as chromatograph and spectrophotometer [11]. Instrumental analyses involving molecular spectroscopy (MS) and chromatography resulted huge number of data (big data analysis), so the application of multivariate data analysis (MDA) or chemometrics is unavoidable. Previous literature has addressed identifying and detecting PG, such as a short overview of Halal and Kosher gelatin with special emphasis on the present situation and progress of authentication of gelatin products [3]. Some analytical methods and advantages and disadvantages for differentiating gelatines are intended for halal authentication studies [12]. Determining gelatin sources using FTIR spectroscopy combined with chemometrics fuzzy autocatalytic set (c-FACS) to distinguish between bovine, porcine, and fish gelatins (FG) [13]; however, the review focusing on physico-chemical methods based on molecular spectroscopic and chromatographic methods in combination with chemometrics for analysis of gelatins is very limited. Therefore, the present review comprehensively discussed the application of MS and chromatographic-based methods in combination with chemometrics for the identification of gelatin sources and the authentication of gelatins in food and pharmaceutical products.
METHODS
During performing this review, some databases including Scopus, PubMed, and DOAJ along with publisher databases such as ScienceDirect, Springer, Wiley, ACS, and Taylor & Francis were explored using some keywords of Halal gelatin, Kosher gelatin, PG, spectroscopic and gelatin, chromatography and gelatin, and chemometrics. The relevant articles were downloaded and stored in the reference management software of Mendeley.
Analytical techniques for the identification of gelatin sources
The detection and identification of gelatin sources are very challenging tasks due to the close similarities among gelatins from different origins, especially in their amino acid sequences and compositions. Some biochemical markers are exploited for the identification of gelatins such as amino acid composition by liquid chromatographic techniques combined with pattern recognition of principal component analysis (PCA) [14], the identification of specific functional groups present in gelatins using Fourier transform infrared spectroscopy combined with PCA and fuzzy graph method [15,16], and Raman spectroscopy assisted by Soft independent modeling of class analogy (SIMCA) and Partial least square-discriminant analysis (PLS-DA) [17]. However, these methods are suitable for gelatins in pure form, while the detection of gelatins in complex matrices need a specific method, since the chemical compositions (amino acids, functional groups) are similar. For this reason, more specific methods are needed for the identification of gelatins in complex products including LC-MS/MS through analysis of peptide markers present in different gelatin sources.
Molecular spectroscopy
MS is the study concerning with the interaction between electromagnetic radiation (EMR) with samples, objects, or specific analytes in the molecular levels. The different compositions of components in food and pharmaceutical preparations contribute to the different profiles of spectra (intensities, wavelengths in Ultraviolet-visible (UV-Vis) spectra, or wavenumbers in IR) [18]. Combined with MDA or chemometrics, MS is widely applied in fats and oils analysis. MS provides some advantages including not destructive, rapid, minimum sample preparation, and is considered as green analytical method due to the minimum use of chemical reagents and solvents [19]. Ultraviolet-visible, Infrared, Raman, and nuclear magnetic resonance (NMR) are normally used in the analysis of food and pharmaceutical products [20].
Molecular spectroscopic-based methods including UV-Vis, Raman, Infrared, and NMR provide a huge number of data, even from the single spectral scanning, therefore, the use of chemometrics is essential because of its sophisticated statistical software [21]. The International Organization named with ICS or International Chemometrics Society provide the definition of chemometrics as the science that correlates the chemical measurements (chemical responses) made on a chemical system to the property of interest (such as concentration) through the application of mathematical or statistical methods [22]. Numerous software related to chemometrics and statistics are available either commercially or open sources such as Unscrambler®, Minitab®, MATLAB®PLS_Toolbox, R program for Statistics, and SIMCA®. Some reviews on the applicability of each chemometrics software [23,24]. The chemometrics applications widely applied for treating big data include: (1) multivariate calibrations such as Stepwise multiple linear regression (SMLR), PCR, and Partial least square (PLS); (2) exploratory data analysis such as PCA, cluster analysis, Linear discriminant analysis (LDA), and SIMCA; and (3) pre-processing spectra such as mean centering, smoothing, derivatization, and standard normal variate [25,26].
Ultraviolet-visible spectra and chemometrics
The use of the UV-Vis spectrum at a wavelength of 200–400 nm from PG and BG has been carried out [27]. Both gelatins were subjected to pepsin hydrolysis to provide peptides with specific patterns that can be differentiated using UV-Vis spectra. The spectra of BG and PG exhibited some differences in absorbance profiles in two fragments of gelatins especially in the wavelength range of 210–240 nm. Hydrolysis of gelatins by pepsin had contributed to some changes in the location and number of peptide carbonyl either in PG or in BG. This result was in agreement with that reported in which the changes in amino acid composition especially glycine, proline, and arginine contributed to the differentiation of PG and BG through investigating the UV-Vis spectra. The primary distinctions among amino acids in gelatins’ spectra are observed in the ranges of 3,290–3,280 cm−1 and 1,660–1,200 cm−1 within the whole infrared spectra range of 4,000–650 cm−1 [28].
The application of UV-Vis spectra combined with the chemometrics of PCA for the identification of gelatin sources was studied. In this study, the Maillard reaction between the gelatins (PG and BG) and numerous reducing sugars such as D-(+)-glucose, D-(+)-galactose, D-(+)-xylose, D-(+)-mannose, D-(-)-fructose, D-(+)-maltose monohydrate, L-rhamnose, and L-arabinose which resulted the different degree of browning at 420 nm. PG revealed the highest browning value with D-(+)-xylose compared to other reducing sugars. This result was confirmed by PCA results in which D-(+)-xylose provides the higher loading to differentiation of PG and BG [29]. The score plots of the first principle component (PC1) and the second component (PC2) using a variable of browning values at wavelength 420 nm were capable of classifying and discriminating the gelatin sources (porcine, bovine, and fish). The browning values for fish gelatine with D-(+)-xylose, bovine gelatine (BG) with D-(+)-xylose, and PG with D-(+)-xylose are 0.248, 0.420, and 0.280, respectively [29]. Previously, the authors optimized the reaction conditions during Maillard reaction of gelatins from different sources (BG, PG, and FG) with reducing sugars using experimental design of response surface methodology by investigating some parameters (temperature, time, and the presence of metal ion Cu2+). The reaction of gelatins with sugars (Maillard reaction) was obtained using the temperature of 95°C for 9 hours with a concentration of Cu2+ of 5 mM as evaluated by absorbance values of browning solution at 420 nm. The results of Maillard reactions between gelatins and sugars could be extended for differentiation of BG, PG, and FG based on the UV spectra at 420 nm [30].
Fourier transform infrared spectroscopy and chemometrics
Among the molecular spectroscopic techniques, FTIR spectroscopy in mid IR region (corresponding to wavenumbers of 4,000–400 cm-1) is the most reported ones for analysis of PG in food products and pharmaceutical samples such as candy, soft capsules, and hard capsule shells. FTIR spectra provides the reliable tools for the characterization of gelatins from different sources by investigating the fingerprinting profile of gelatins, especially those that are related to amide bonds. Absolute peak intensities were used to compare gelatins and their conformational changes in structure. The Amide I band (between 1,600 and 1,700 cm-1) proved the most effective for analyzing protein structure using infrared red spectroscopy [31,32]. Equipped with user friendly sampling technique of attenuated total reflectance (ATR) and chemometrics techniques, FTIR spectroscopy has emerged as a reliable method for the differentiation of halal and nonhalal gelatins [20]. Table 1 compiled the combination of FTIR spectra and chemometrics for analysis of PG in several matrixes of food such as candy and pharmaceutical products (dental materials, capsule shells, and so on). Differentiation of PG, BG, and cold FG can be carried out using FTIR spectra by comparing the ratio of absorbance values at certain peaks in which the intensities of peaks could be identified and correlated to gelatin types. Hierarchical clustering and PCA were used to clearly distinguish and classify all of the gelatin sources tested (bovine, porcine, and fish). The intensities of peaks Amide-I (1,700–1,600 cm−1) and Amide-II (1,565–1,520 cm−1) spectral bands were used in a chemometric method [33].
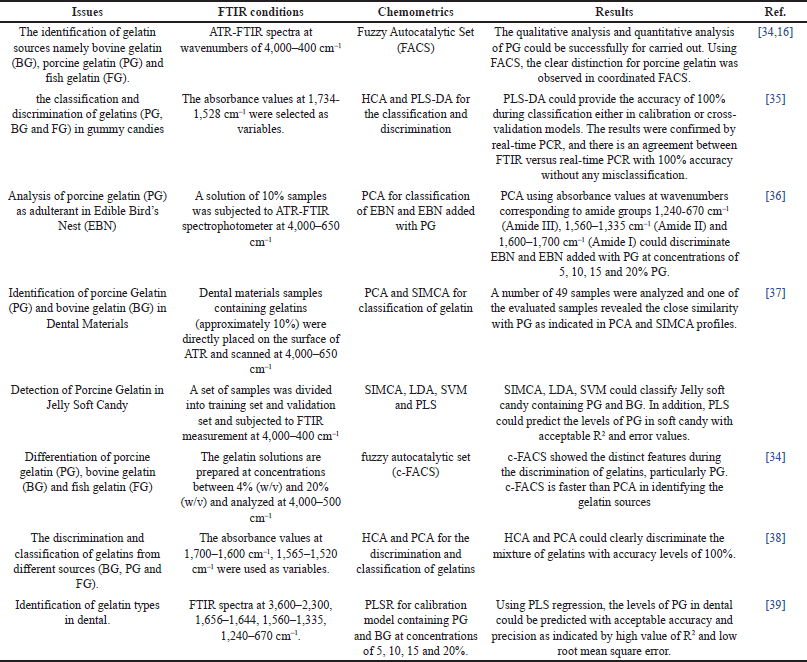 | Table 1. The use of FTIR spectroscopy and chemometrics for identification of gelatins in food and pharmaceutical products. [Click here to view] |
A new chemometrics approach based on a Fuzzy Autocatalytic Set (FACS) combined with FTIR spectra of gelatins was applied to identify gelatin sources, namely PG, BG, and FG. The concept of FACS was the combination result between a Fuzzy graph and an Autocatalytic Set. FACS was used to explore the peak regions in FTIR spectra contributing to gelatins from different sources (bovine, porcine, and fish). The peaks related to Amide II and Amide III contributed to such differentiation. Based on FACS results exploring Amide II peaks, BG spectra has unique peaks at wavenumbers of 1,480–1,474 cm-1 while PG and FG revealed unique peaks at wavenumbers of 1,448–1,441 and 1,496–1,490 cm-1, respectively. For Amide III, the unique peaks were observed at combined wavenumbers regions of 1,252–1,249 and 1,232–1,228 cm-1 BG, 680–678 cm-1 PG, and the combined wavenumbers of 1,303–1,302 and 1,283–1,280 cm-1 FG. The wavenumbers found suggest that each gelatin has its character and features. Coordinated FACS revealed that nonhalal PG has a distinct appearance and clusters far apart from bovine and fish nodes. This information could be expanded for qualitative and quantitative PG analysis using suitable chemometrics [40]. The chemometrics of FACS using a variable of absorbance values at 1,600–1,000 cm−1 was also compared with other chemometrics techniques namely PCA and LDA for the differentiation of PG, BG, and FG. Using FACS, the clear distinction for PG was observed in coordinated FACS. The authors concluded that FACS method is rigorous and faster than PCA and LDA in discriminating the gelatin sources [16].
FTIR-ATR spectra in combination with chemometrics of Hierarchical cluster analysis HCA and PLS-DA for the classification and discrimination of gummy candies containing gelatin with different sources (PG, BG, and FG) [35]. After selecting the best wavenumber regions capable of discriminating gelatins according to their sources, finally, the absorbance values at wavenumbers of 1,734 and 1,528 cm−1 were selected as variables during HCA and PLS-DA. Gummy candy samples were classified using normal to reprolevel and Ward’s method with first-derivative spectrum preprocessing. This region comes from the vibration of Amide I (1,700–1,600 cm−1) and Amide II (1,565–1,520 cm−1). Amide I is responsible for the stretching vibrations of carbonyl group (C=O) and the stretching vibration of C-N from the peptide linkages. Using this condition, HCA is a cluster analysis technique that aims to create a hierarchy of clusters, PCA, is a dimensionality reduction method that is commonly used to reduce the dimensionality of big data sets. It works by reducing a large collection of variables into a smaller one that retains the majority of the information from the larger set. PLS-DA is important in metabolomics studies because it provides a sophisticated statistical framework for determining complex correlations within high-dimensional datasets. PLS-DA excels in simultaneous dimensionality reduction and classification, enabling researchers to identify patterns associated with various experimental circumstances or sample classes. A could perfectly differentiate gummy candy samples containing different gelatins. PLS-DA could provide an accuracy of 100% during classification either in calibration or cross-validation models. Class predictions of gummy candies were performed with 100% correct classification. The results obtained using ATR-FTIR spectra were confirmed by real-time PCR, and there is an agreement with 100% accuracy for both analytical techniques (FTIR versus real-time PCR) without any misclassification or false prediction. A similar approach, comparing the results of gelatin identification using FTIR spectroscopy and real-time PCR, has also been taken [15]. In this study, the authors used absorbance values at combined wavenumbers of 1,450 cm−1–1,300 cm−1, 1,543 cm−1, and 2,800–3,000 cm-1 as variables during PCA. The PC1, PC2, and PC3 contributed to variances of 39%, 31%, and 14.5%, respectively, with a cumulative variance of 84.5%. There are no jelly candy samples in the pork gelatin sample quadrant. Hence, the gelatin used in the jelly candy samples is BG rather than pork gelatin. There is an agreement among results obtained using FTIR spectra and polymerase chain reaction in which all jelly candy samples did not contain PG.
Near infrared spectroscopy (NIRS)
As mid infrared spectroscopy, NIRS also offers some advantages during the analysis of gelatin sources including nondestructive, low-cost analysis and in some cases without sample pretreatment. NIRS combined with MDA is also suitable for online applications [41]. NIR spectra consisted of EMR from the visible region to mid-infrared region corresponding to wavelengths of 800–2,500 nm (wavenumbers of 12,821–4,000 cm−1). NIR spectra originate mainly from vibrations of functional groups of –CH, –OH, –SH, and –NH as the results of band combinations or overtones or fundamental mid-infrared bands [42]. Another drawback of NIR spectra is the fact that NIR spectra contain background interference from the noise and overlapping bands and low sensitivity for quantitative analysis [43]. The combination of NIRS and chemometrics has proven their effectiveness for qualitative and quantitative analyses of nonhalal components [20].
The combination of NIRS and selected chemometrics techniques of PCA and PLS-DA has been employed fruitfully for the characterization of nonaged and aged skin PG coming from three different production sites designated with A, B, and C in relation to dissolution profiles of PG [44]. Skin PG from C exhibited no variability in dissolution rate, while PGs from A and B dissolved more slowly after aging. PLS-DA applying the absorbance values at 400–2,400 nm as a variable could classify PGs according to its sites with accuracy levels of 97%–100%, while PCA using the same variable used in PLS-DA revealed that PCA allowed the separations of gelatins based one PC1 and PC2. Type B-skin PG are mostly located in the positive parts of PC1 and PC2 score plots, while types A and C-skin PG gelatin are in the negative parts of PC1 and PC2 score plots. B-type skin PG was clearly separated from A and C types, but the separation of type A-skin PG gelatin from C type is less evident [44].
The combination of NIRS and chemometrics of supervised pattern recognition methods [LDA, SIMCA, back propagation neural network (BPNN), and SVM] has been developed for the authentication of edible gelatin from the adulteration practice. NIR spectra of 144 samples consisting of six kinds of adulterated gelatin gels with different mixture ratios were subjected to some spectral pre-processing treatments, including multiplicative scatter correction, smoothing, and min-max normalization [45]. PCA could not separate six edible gelatin types clearly, therefore, supervised pattern recognition techniques were applied. LDA, SIMCA, BPNN, and SVM using a variable of absorbance values at wavelength 800–2,200 nm could provide clear discrimination among authentic and adulterated gelatins with a total accuracy rate of 97.44%, 100%, 97.44%, and 100%, respectively. SIMCA and SVM offered accuracy levels of 100%. However, due to the possible clustering overlap between different classes when using the SIMCA model, the SVM model shows the preferable ability to recognize edible gelatin adulteration. Sample overlapping was observed using SIMCA at a significance level (α) of 0.05. The results showed that NIR spectroscopy combined with SVM can accurately identify the adulteration practice of edible gelatins. This provides tools for detecting industrial gelatin illegally added to food and pharmaceutical products [45].
Data fusion of NIRS with other spectroscopic data in combination with MDA is a synergistic strategy for the authentication analysis of products [46]. Data fusion of NIRS with fluorescence spectroscopy, and laser induced breakdown spectroscopy (LIBS) has been studied for improving the accuracy of gelatin identification from different sources (skin PG, bone PG, skin BG, bone BG, and skin FG). The spectral regions at a wavelength of 1,100–2,200 nm (NIRS), 350–950 nm (fluorescence spectra), and LIBS at 200–1,000 nm corresponding to nonmetallic and metallic elements of carbon, nitrogen, oxygen, sodium, potassium, and so on, combined with chemometrics for classification of five gelatins [46]. The variables were extracted using a competitive adaptive reweighted sampling method and a random forest model (RFM) was used for classification technique. From RFM results, the data fusion provides better classification modeling than the individual spectral data in which the accuracy levels of classification precision were 100%, 100%, 100%, 100%, 100%, and 93.33% for skin PG, bone PG, skin BG, bone BG, and skin FG, respectively with average accuracy levels of 98.67%. The results confirmed that the data fusion based on different spectral data of NIRS, fluorescence, and LIBS combined with chemometrics of RFM could provide the rapid and accurate identification of gelatin sources [47].
Chromatographic-based methods
Due to its capability to provide good separation of gelatin components (peptides or amino acids), liquid chromatography with several detectors has been widely employed for the differentiation of gelatin from different sources.
High-performance liquid chromatography
HPLC is an analytical method for identifying halal and nonhalal gelatins based on sugars and amino acids [48]. The HPLC method successfully determined the amino acid profiles in 7 soft candy samples containing gelatin, which were selectively and accurately separated [49]. The chromatogram results showed that peak heights could be used to distinguish between PG and BG. PCA was performed to facilitate the visualization of halal and nonhalal gelatin. PCA was performed by extracting significant variables from the peak height for each amino acid to provide the classification of gelatins from different sources [50]. All gelatins can be differentiated by their marker peptides in the gelatin mixtures. However, the number of detectable marker peptides in each gelatin decreased with a reduced concentration of target gelatin in their mixtures. HPLC has successfully identified PG and BG using different extraction temperatures, and PG and BG have different peptide profiles when extracted at different temperatures. HPLC-LTQ/Orbitrap high-resolution mass spectrometry could be a promising technology for accurately identifying bovine, porcine, and donkey hide gelatin and controlling food products’ quality [51]. This indicates that HPLC is an analytical method that can be used to analyze the halalness of gelatin in food and pharmaceutical products [52,53].
Analysis of gelatin in pharmaceutical products is also carried out based on amino acid composition [54]. HPLC could accurately distinguish the type of gelatins in the chewable tablet formulation based on amino acid analysis. Chewable tablet formulations containing gelatin from different sources showed good stability with extended shelf life and are within reach for several active pharmaceutical ingredients [55]. HPLC combined with PCA can sensitively detect the gelatin sources in capsule shells and successfully separate PG and BG based on the score plot of the first principal components (PC1) and second principal components (PC2), porcine and BG in the capsule could be distinguished [56]. HPLC was also able to characterize gelatin in the nanoparticle dosage forms. This confirmed that HPLC is an analytical method that can be used to analyze the halal status of gelatins in pharmaceutical products [57]. DA used a variable of amino acid compositions (Hydroxyproline, Histidine, Serine, Arginine, Glycin, Aspartic acid, Glutamic acid, Threonine, Alanine, Proline, Cysteine, Lysine, Tyrosine, Methionine, Valine, Isoleucine, Leucine, and Phenylalanine) to successfully classify 100% of FG, BG, and PG, while PCA could identify the dominant amino acid in each gelatin source [58]. HPLC combined with chemometrics has successfully differentiated PG and BG based on amino acid components. Twenty peaks were applied as variables (peak height, peak area, the percentages of area, and width). Using these variables, multivariate statistical methods such as PCA can also be employed as a tool for good classification between BG and PG [59].
LC-MS/MS
LC-MS/MS is one of the most powerful analytical instruments to differentiate gelatin [10]. LC-MS/MS could analyze up to the peptide level of gelatin. The peptides were obtained from protein digestion using proteolytic enzymes such as trypsin, which has become the most common proteolytic enzyme used for protein digestion. The obtained peptides are separated through the LC proteomic column and detected using the mass spectrometer detector [5]. LC-MS/MS has a superior capability among other techniques for gelatin speciation, such as good sensitivity, reliability, and specificity. In addition, LC-MS/MS has been widely used in molecular weight analysis of biomolecules including proteins and peptides. These techniques have shown their ability to identify gelatin’s origin in food samples containing gelatin as an additive. LC-MS/MS also allows for the identification of peptide biomarkers of gelatin from different species, which benefits gelatin authentication [49]. The presence of PG in several dairy products such as cheese, ice cream, and yogurt were successfully identified using a nanoUPLC-MSE workflow [60]. The method was performed in two main steps. In the first step, gelatin was extracted from cheese, ice cream, and yogurt. In the second step, the extracted gelatin was digested using proteomic grade trypsin, then the digested peptides were separated through a nano UPLC and analyzed using electrospray ionization quadrupole time of flight mass spectrometry. The functioning of data-independent mode became the main novelty of this developed method. In addition, this method enabled accurate mass acquisition of digested peptides. Results revealed that peptide markers from porcine and BG were successfully detected using this nanoUPLC-MSE workflow. A number of unique peptides were found in PG, for instance, peptides with sequences of GETGPAGPAGPVGPVGAR, GFP*GSP*GNVGPAGK, and IGQPGAVGPAGIR. It implies that this method could be used for analysis of PG in dairy products for authentication purposes [60].
Gelatin from three different sources, namely bovine, donkey, and porcine has been successfully differentiated using LC-MS/MS-based proteomics [61]. Trypsin digestion was performed to obtain the peptide sequence from each gelatin. Eleven peptide markers were selected to discriminate bovine, donkey, and PG. The biomarkers could be detected in food products with three different matrices to detect PG in food products. Multiple reaction monitoring using a label-free quantitative method was applied to determine the level of PG using the identified peptide markers. Among those 11 peptide markers, two peptide markers of PG resulted in good linearity. This method was also successfully applied to detect PG in candy and dairy products [61].
The differentiation between bovine and PG was successfully carried out using LC-MS/MS-based proteomics and chemometrics [62]. Protein extraction was conducted on gelatin samples, followed by trypsin digestion to yield peptides. The species-specific peptide markers were identified for both porcine and BG. The initial stage in distinguishing between pig gelatin and BG using liquid chromatography-mass spectrometry tandem mass spectrometry (LC-MS/MS) involved digesting the gelatins with the proteolytic enzyme trypsin. Trypsin hydrolyzes the peptide bond connecting the carboxyl group of arginine (R) or the carboxyl group of lysine (K) with the amino group of the neighboring amino acids. The obtained peptides would be separated based on an interaction between peptides and the stationary phase, as indicated with certain retention times (RTs). Chemometrics of PCA using the variables of Rt and calculated mass (m/z) were built to classify porcine and BG. The PCA score plot revealed that BG could be successfully differentiated from PG by using the variables of RT and calculated m/z [62].
The differentiation of gelatin from three mammalian gelatin, including bovine-hide, porcine-hide, and donkey-hide gelatin, was successfully performed using liquid chromatography connected to linear ion trap /Orbitrap high-resolution mass spectrometry [63]. There were 28, 27, and 14 detected distinct peaks in bovine-hide, porcine-hide, and donkey-hide gelatin, respectively. For instance, peptides of 1066GETGPAGPAGPIGPVGAR1083, 1052GETGPAGPAGPVGPVGAR1069, and 1066GEAGPAGPAGPIGPVGAR1083 were found as the unique peptides from bovine-hide, porcine-hide, and donkey-hide gelatin. The method was also tested in the gelatin mixtures with various concentration levels. Results demonstrated that all gelatins could be differentiated using the detected peptide markers. However, when the concentration of gelatin target was reduced in the mixtures, the number of detected peptide markers also decreased. A number of 11, 15, and 5 peptide markers of bovine-hide, porcine-hide, and donkey-hide gelatine were detected in the presence of a gelatin target under 10%. For instance, peptides of 791GEAGPSGPAGPTGAR795, 907GPRGETGPAGRPGEVG*P*PG*PPGPAGEK933, and 656GETGLRGDIGS*PGRDGAR673 were found as the peptide marker from bovine-hide, porcine-hide, and donkey-hide gelatin It can be concluded that the LC-LTQ/Orbitrap HRMS could be a promising analytical technique for analysis of gelatin from different sources further for detecting PG adulteration in other gelatin for halal authentication purposes [34].
CONCLUSION
The concerns regarding the use of gelatin in food and pharmaceutical goods revolve around the source of gelatin, specifically whether it is derived from swine (nonhalal according to certain madzhab) or bovine (halal or permissible) sources. Gelatin is mostly derived from cows and pigs, specifically known as bovine and PG, respectively. The development of analytical techniques for PG identification is quite necessary. Molecular spectroscopy provides valuable information that can be used as a rapid and reliable analytical technique for the analysis of gelatins in food and pharmaceutical products, especially in combination with MDA or chemometrics. Porcine and BG are successfully classified and differentiated by applying molecular spectra responses as variables combined with chemometrics of classification, such as PCA and LDA. Furthermore, the levels of gelatins in the products are also fruitfully predicted using MS employing multivariate calibrations of SMLR, PCR, and PLSR. Some statistical parameters are used during multivariate calibrations.
ACKNOWLEDGMENT
The authors thank Universitas Gadjah Mada for financial support during this study through Riset Kolaborasi Indonesia 2024 program contract number 1910/UN1/DITLIT/PT.01.03/2024.
LIST OF ABBREVIATIONS
BG Bovine gelatin
LC-MS/MS liquid chromatography tandem mass spectrometry
MDA Multivariate data analysis
MSC Multiplicative scatter correction
NMR Nuclear magnetic resonance
PCA Principal component analysis
PG Porcine gelatin
PLS partial least square
PLS-DA Partial least square-discriminant analysis
PCR Principal component regression
SIMCA Soft independent modelling of class analogy
SMLR Stepwise multiple linear regression
SNV Standard normal variate
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Lever J, Miele M. The growth of halal meat markets in Europe: an exploration of the supply side theory of religion. J Rural Stud. 2012;28(4):528–37.
2. Norazmi MN, Lim LS. Halal pharmaceutical industry: opportunities and challenges. Trends Pharmacol Sci. 2015;36(8):496–7.
3. Uddin SMK, Hossain MAM, Sagadevan S, Al Amin M, Johan MR. Halal and Kosher gelatin: applications as well as detection approaches with challenges and prospects. Food Biosci. 2021;44(PA):101422.
4. Ahmed MA, Al-Kahtani HA, Jaswir I, AbuTarboush H, Ismail EA. Extraction and characterization of gelatin from camel skin (potential halal gelatin) and production of gelatin nanoparticles. Saudi J Biol Sci. 2020;27(6):1596–601.
5. Huang Y, Li T, Deng G, Guo S, Zaman F. Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: a mini review. Rev Anal Chem. 2020;39(1):260–71.
6. Lubis HN, Mohd-Naim NF, Alizul NN, Ahmed MU. From market to food plate: current trusted technology and innovations in halal food analysis. Trends Food Sci Technol. 2016;58:55–68.
7. Rohman A, Che Man YB. Analysis of pig derivatives for halal authentication studies. Food Rev Int. 2012;28(1):97–112.
8. Eyupoglu OE. Halal gelatin analysis using developed on-line RP-HPLC-DAD-UV henna protein detection in confectionery and food supplements. EC Emerg Med Crit Care [Internet]. 2020;4(10):79–87. Available from: https://www.researchgate.net/publication/344445801
9. Kang SSN, Lee HG, Kim H. Development and comparison of a porcine gelatin detection system targeting mitochondrial markers for Halal authentication. LWT. 2018 Nov;97:697–702.
10. Jannat B, Ghorbani K, Shafieyan H, Kouchaki S, Behfar A, Sadeghi N, et al. Gelatin speciation using real-time PCR and analysis of mass spectrometry-based proteomics datasets. Food Control. 2018 May;87:79–87.
11. Pradini D, Juwono H, Madurani KA, Kurniawan F. A preliminary study of identification halal gelatin using Quartz Crystal Microbalance (QCM) sensor. Malaysian J Fundam Appl Sci. 2018;14(3):325–30.
12. Rohman A, Windarsih A, Erwanto Y, Zakaria Z. Review on analytical methods for analysis of porcine gelatine in food and pharmaceutical products for halal authentication. Trends Food Sci Technol [Internet]. 2020;101:122–32. Available from: https://doi.org/10.1016/j.tifs.2020.05.008
13. Hassan N, Ahmad T, Zain NM. Chemical and chemometric methods for halal authentication of gelatin: an overview. J Food Sci. 2018;83(12):2903–11.
14. Azilawati MI, Hashim DM, Jamilah B, Amin I. RP-HPLC method using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate incorporated with normalization technique in principal component analysis to differentiate the bovine, porcine and fish gelatins. Food Chem. 2015;172:368–76.
15. Jariyah, Yulistiani R, Afdilah SW, Mas’udah KW. Detection of pork gelatin in jelly candy using fourier transform infrared (FTIR) and polymerase chain reaction (PCR). E3S Web Conf. 2021;328:0–7.
16. Hassan N, Ahmad T, Zain NM, Awang SR. Identification of bovine, porcine and fish gelatin signatures using chemometrics fuzzy graph method. Sci Rep. 2021;11(1):1–10.
17. Forooghi E, Vali Zade S, Sahebi H, Abdollahi H, Sadeghi N, Jannat B. Authentication and discrimination of tissue origin of bovine gelatin using combined supervised pattern recognition strategies. Microchem J. 2023;187(December 2022):108417.
18. Kumar Y, Chandrakant Karne S. Spectral analysis: a rapid tool for species detection in meat products. Trends Food Sci Technol. 2017;62:59–67.
19. Cozzolino D. An overview of the use of infrared spectroscopy and chemometrics in authenticity and traceability of cereals. Food Res Int. 2014;60:262–5.
20. Rohman A, Windarsih A. The application of molecular spectroscopy in combination with chemometrics for halal authentication analysis: a review. Int J Mol Sci. 2020;21(14):1–18.
21. Jamwal R, Amit, Kumari S, Sharma S, Kelly S, Cannavan A, et al. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib Spectrosc. 2021;113(April 2020):103222.
22. Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. 6. ed. Harlow, UK: Prentice Hall; 2010. p. 278.
23. Brereton RG, Jansen J, Lopes J, Marini F, Pomerantsev A, Rodionova O, et al. Chemometrics in analytical chemistry—part II: modeling, validation, and applications. Anal Bioanal Chem. 2018 Oct;410(26):6691–704.
24. Irnawati, Riyanto S, Rohman A. Adulteration of Gabus (Channa striata) fish oil with corn oil and palm oil: the use of FTIR spectra and chemometrics. Food Res. 2021;5(2):184–90.
25. Moros J, Garrigues S, Guardia M de la. Vibrational spectroscopy provides a green tool for multi-component analysis. TrAC Trends Anal Chem. 2010 Jul;29(7):578–91.
26. Rohman A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: a review. Int J Food Prop. 2017;20(7):1447–56.
27. Hermanto S, Sumarlin LO, Fatimah W. Differentiation of bovine and porcine gelatin based on spectroscopic and electrophoretic analysis. J Food Pharm Sci. 2013;1(3):301–6.
28. Nhari RMHR, Ismail A, Che Man YB. Analytical methods for gelatin differentiation from bovine and porcine origins and food products. J Food Sci. 2012;77(1):R42–6.
29. Hamid AH, Nurrulhidayah AF, Sani MSA, Muhammad NWF, Othman R, Rohman A. Discrimination of porcine and bovine gelatines based on reducing sugar types on maillard reaction. Food Res. 2020;4(2):301–6.
30. Hamid AH, Elgharbawy AA, Rohman A, Rashidi O, Hammed H, Nurrulhidayah AF. Optimisation of browning index of maillard reaction in gelatine powder by response surface methodology (RSM) for halal authentication. Food Res. 2019;3(5):525–9.
31. Al-Saidi GS, Al-Alawi A, Rahman MS, Guizani N. Fourier transform infrared (FTIR) spectroscopic study of extracted gelatin from shaari (Lithrinus microdon) skin: effects of extraction conditions. Int Food Res J. 2012;19(3):1167–73.
32. Andakke JN, Rumengan IFM, Nainggolan HHY, Parapat LRME, Pandey E, Suptijah P, et al. Molecular structure of gelatin extracted from parrot (Scarus sp) fish scales. J Pesisir Dan Laut Trop. 2020;8(1):15.
33. Abbas LM, Ahmed. GA-R. ATR-FTIR Spectroscopy for discrimination of gelatin from different sources: a comparative study. Res J Pharm Biol Chem Sci. 2020;11(4):135–44.
34. Hassan N, Ahmad T, Zain NM, Awang SR. A fuzzy graph based chemometrics method for gelatin authentication. Mathematics. 2020;8(11):1–18.
35. Cebi N, Dogan CE, Mese AE, Ozdemir D, Arici M, Sagdic O. A rapid ATR-FTIR spectroscopic method for classification of gelatin gummy candies in relation to the gelatin source. Food Chem [Internet]. 2019;277:373–81. Available from: https://doi.org/10.1016/j.foodchem.2018.10.125
36. Jamalludin NH, Tukiran NA. Analysis of gelatin adulteration in edible bird’s nest using Fourier transform infrared (FTIR) spectroscopy. Int J Adv Sci Eng Inf Technol. 2018;8(6):2355–9.
37. Roswiem AP, Mustaqimah DN. Identification of gelatin source in toothpaste products using combination of attenuated total reflection-fourier transform infrared ( ATR-FTIR ) spectroscopy and chemometrics. Int J Halal Res. 2020;2(1):30–9.
38. Cebi N, Durak MZ, Toker OS, Sagdic O, Arici M. An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chem. 2016;190:1109–15.
39. Irfanita N, Jaswir I, Mirghani MES, Sukmasari S, Ardini YD, Lestari W. Rapid detection of gelatin in dental materials using attenuated total reflection fourier transform infrared spectroscopy (ATR-FTIR). J Phys Conf Ser. 2017;884(1):012090.
40. Hassan N, Ahmad T, Zain NM, Ashaari A. A novel chemometrics method for halalauthentication of gelatin in food products. Sains Malaysiana. 2020;49(9):2083–9.
41. Li X, Zhang L, Zhang Y, Wang D, Wang X, Yu L, et al. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci Technol. 2020;101(May):172–81.
42. Rohman A, Windarsih A, Hossain MAM, Johan MR, Ali ME, Fadzilah NA. Application of near- and mid-infrared spectroscopy combined with chemometrics for discrimination and authentication of herbal products: a review. J Appl Pharm Sci. 2019;9:137–47.
43. Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44(3 SPEC. ISS.):683–700.
44. Duconseille A, Andueza D, Picard F, Santé-Lhoutellier V, Astruc T. Variability in pig skin gelatin properties related to production site: a near infrared and fluorescence spectroscopy study. Food Hydrocoll. 2017;63:108–19.
45. Zhang H, Sun H, Wang L, Wang S, Zhang W, Hu J. Near infrared spectroscopy based on supervised pattern recognition methods for rapid identification of adulterated edible gelatin. J Spectrosc. 2018;2018:1–9.
46. Li Y, Zhang JY, Wang YZ. FT-MIR and NIR spectral data fusion: a synergetic strategy for the geographical traceability of Panax notoginseng. Anal Bioanal Chem. 2018;410(1):91–103.
47. Zhang H, Liu Z, Zhang J, Zhang L, Wang S, Wang L, et al. Identification of edible gelatin origins by data fusion of NIRS, fluorescence spectroscopy, and LIBS. Food Anal Methods. 2021;14(3):525–36.
48. Velasco-Bejarano B, Gómez-Tagle A, Noguez-Córdova MO, Zambrano-Zaragoza ML, Miranda-Molina A, Bautista J, et al. Determination of clenbuterol at trace levels in raw gelatin powder and jellies using ultra-high-performance liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. 2022 Feb;370:131261.
49. Ishaq A, Rahman U ur, Sahar A, Perveen R, Deering AJ, Khalil AA, et al. Potentiality of analytical approaches to determine gelatin authenticity in food systems: a review. LWT. 2020 Mar;121:108968.
50. Amira Aqilah S, Nur Azira T, Haizatul Hadirah G, Nurul A, Syahirah SN. Analytical methods for gelatin differentiation. J Halal Ind Serv [Internet]. 2019;(1):a0000048. Available from: http://www.journals.hh-publisher.com/index.php/JHIS/article/view/57
51. Sha XM, Hu ZZ, Ye YH, Xu H, Tu ZC. Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocoll. 2019 Jul;92:163–72.
52. Ali E, Sultana S, Hamid SBA, Hossain M, Yehya WA, Kader A, et al. Gelatin controversies in food, pharmaceuticals, and personal care products: authentication methods, current status, and future challenges. Crit Rev Food Sci Nutr. 2018 Jun;58(9):1495–511.
53. Ismail AM, Sani MSA, Azid A, Zaki NNM, Arshad S, Tukiran NA, et al. Food forensics on gelatine source via ultra-high-performance liquid chromatography diode-array detector and principal component analysis. SN Appl Sci. 2021 Jan;3(1):1–19.
54. Arsyanti L, Erwanto, Rohman A, Pranoto Y. Chemical composition and characterization of skin gelatin from buffalo (Bubalus bubalis). Int Food Res J. 2018;25(3):1095–9.
55. Dille MJ, Hattrem MN, Draget KI. Soft, chewable gelatin-based pharmaceutical oral formulations: a technical approach. Pharm Dev Technol. 2018 May;23(5):504–11.
56. Widyaninggar A, Triyana K, Rohman A. Differentiation between porcine and bovine gelatin in capsule shells based on amino acid profiles and principal component analysis. Indones J Pharm. 2012;23(2):104–9.
57. Farrugia CA, Groves MJ. Gelatin behaviour in dilute aqueous solution: designing a nanoparticulate formulation. J Pharm Pharmacol. 2010;51(6):643–9.
58. Abdullah Sani MS, Ismail AM, Azid A, Samsudin MS. Establishing forensic food models for authentication and quantification of porcine adulterant in gelatine and marshmallow. Food Control. 2021 Dec;130:108350.
59. Nemati M, Oveisi MR, Abdollahi H, Sabzevari O. Differentiation of bovine and porcine gelatins using principal component analysis. J Pharm Biomed Anal. 2004;34(3):485–92.
60. Yilmaz MT, Kesmen Z, Baykal B, Sagdic O, Kacar O, Yetim H, et al. A novel method to differentiate bovine and porcine gelatins in food products: nanoUPLC-ESI-Q-TOF-MSE based data independent acquisition technique to detect marker peptides in gelatin. Food Chem. 2013;141(3):2450–8.
61. Zhu X, Gu S, Guo D, Huang X, Chen N, Niu B, et al. Determination of porcine derived components in gelatin and gelatin-containing foods by high performance liquid chromatography-tandem mass spectrometry. Food Hydrocoll. 2023 Jan;134:107978.
62. Salamah N, Erwanto Y, Martono S, Maulana I, Rohman A. Differentiation of bovine and porcine gelatines using lc-ms/ms and chemometrics. Int J Appl Pharm. 2019;11(4):159–63.
63. Sha XM, Zhang LJ, Tu ZC, Zhang LZ, Hu ZZ, Li Z, et al. The identification of three mammalian gelatins by liquid chromatography-high resolution mass spectrometry. LWT—Food Sci Technol. 2018;89:74–86.