INTRODUCTION
Cancer is a collective term used to define a set of diseases characterized by uncontrolled, dysregulated cell proliferation and metastasis at a later stage to invade and affect other organ systems of the body. Despite extensive efforts in the past decades to check cancer, it remains a leading cause of morbidity and mortality in humans[1]. Oral cancer is the sixth most typical malignancy globally and majorly affects Southeast Asia. Smoking, consumption of alcohol, and chewing betel quid are some of the risk factors that can cause oral cancer[2]. In spite of the advancements in cancer diagnosis and treatment, the oral cancer 5-year survival rate for patients is still only 50%.
Herbal medicine with its natural active ingredients presents a docile and continuing way for the restoration and is therefore regarded as a potential source of upcoming therapy. The discovery, development, and production of a novel drug usually has several challenges. However, conventional remediation is far more affordable with low levels of side effects, easily reachable, and rationally familiar [3–6]. Most plants have medicinal properties and are found to be an affluent source of compounds that can be used further for the development and synthesis of novel drugs. However, presentence use of these medicinal agents may lead to drug resistance and other adverse effects in patients [7–9]. World Health Organization proposed that conventional medicines are exploited for healthcare by nearly 80% of people in developing countries [10].
Traditional acquaintance with the therapeutics plants has, however, constantly directed the exploration of new drugs[11]. Furthermore, traditional therapeutic plants are easily available, less expensive, and noticeably useable, is Quercus infectoria is among the precious traditional plants found in Malaysia, commonly known as “Manjakani,” It is a small tree indigenous to Asia Minor, Greece, and Iran [12] has been regarded as popular as the oak tree. Galls are found on their fragile growing branches due to the damage caused by the gall wasp (Adleriagallae tinctoria). Traditionally, in oriental traditional medicine, it is used to treat inflammatory disease.
Certain pharmacological analyses have established that the galls of Q. infectoria had possess antidiabetic, anti-Parkinsonism, antiviral [13], local anesthesia [14], antifungal [15], anti-bacterial [16], and larvicidal properties [17]. It has been investigated that the crucial constituents of Q. infectoria found to have moderate amounts of ellagic acid and free gallic acid and some number of tannins nearly 50%–70% [18–20]. The idea of traditional knowledge-driven sophisticated drug discovery can help in phytochemicals to be explored as a novel drug molecule discovery approach is a new avenue with a lot of possibilities [21]. Chemotherapy involving killing the cancer cells has been the common therapeutic approach to treat various types of cancers. However, it was reported that chemotherapeutic agents may eventually result in resistance to cancer cells and a plethora of undesired health effects in patients [7]. Therefore, it is imperative and immediate need to develop chemotherapeutic compounds with minimal side effects and maximum efficacy [22].
TGF-β, a multifaceted signaling molecule, exerts significant influence on diverse physiological processes encompassing cell growth, specialization, demise, and movement. Molecules down-regulating transforming growth factor (TGF) beta expression may have strong anti-cancerous properties [23]. One of the aims of this study is to study the regulation of TGF beta expression in oral cancer cell lines post-treatment with extracts of the Q. infectoria. Oral squamous cell carcinomas (OSCCs) add up to over 90% of dangerous tumors of oral depression and constitute the most harmful tumors of the head and neck [24]. The cell of origin of OSCC is the oral keratinocyte. OSCC, as some other growths, is caused by deoxyribonucleic acid (DNA) change, regularly unconstrained yet expanded by introduction to any of a scope of mutagenschemical, physical, or microbial. The different changes in the DNA can advance from an ordinary keratinocyte to a precancerous or a conceivably threatening keratinocyte that is portrayed by a capacity to multiply in a less controlled manner than typical [25]. The cells end up self-ruling and a genuine tumor comes about, described by intrusion over the epithelial cellular film and at last metastasis to lymph nodes, bone, brain, liver, and different locales [26]. As in different parts of the upper aerodigestive tract, there is a solid and synergistic relationship between oral malignancy with tobacco utilization and alcohol abuse. Oral cancer, particularly in the Indian subcontinent, ranks among the most prevalent malignancies due to widespread tobacco use [27].
Previous studies conducted in the Kingdom of Saudi Arabia revealed a connection between, their patient’s oral cancer and their history of shamma use [28]. Oral growth is principally assembled under tobacco-related malignancies. About 33% of the worldwide weight of oral disease is prevalently ascribed to the high commonness of tobacco utilization inside India. Around 57% of all men and 11% of all ladies in India, in the vicinity of 15 and 49 years old utilize tobacco in some shape or other. A current report demonstrated that in excess of 33% of Indians utilize tobacco through smoking, biting, application to gums and oral cavities, or blowing into the nose. Among these tobacco consumers, 21% devoured just tobacco without smoke, 9% used tobacco with smoke, and 5.3% utilized smokeless tobacco and smoked too [29]. The blend of paan likewise contains nitrosoanabasine and 3-(methylnitrosamino) propionic corrosive as added substances. Another normal practice with tobacco use is liquor utilization which is likewise credited to oral tumour.
The majority of existing tumor markers are generally helpful in settling on a clinical choice after initial suspicion of cancer or its behavior which has been as of now raised by more conventional means [30]. Tumor markers identified in circulating body fluids and in the tissue such as Vascular endothelial development factor, Platelet-derived endothelial cell development factor, Fibroblast development factor, Bcl2, Cathepsin-D, CD44, CD80, CD105, endoglin, cytokeratins, CEA, CA-19-9, CA-125, SCC-Ag, calretinin, Cerb2, Cyclin, MIB and p53 [31]. Bcl-2 is the regulatory protein that coordinates the downfall of living cells (apoptosis). During tumorigenesis in oral malignancies, there exists a loss of p53 function with a concomittent bcl-2 expression from apoptosis and, thus helping the cancerous cells to escape apoptosis, and also leads to additional genetic alterations [32]. Thus Bcl-2 can be taken as an important early tool in suggesting cancer of the oral cavity. Cancer Antigen19-9, SCC-Ag and carcinoembryonic antigen present in saliva were in addition expanded with no measurable criticalness [33].
Quercus infectoria Olivier also called biji manjakani in Malaysia is an ancient and medicinal tree found in Asia [34]. Quercus infectoria has been proven to be effective in curing bowel disease owing to its presence of a higher concentration of tannins. Other phytochemicals such as gallic acid, and ellagic acid are also reported to appear in the extract [35,36]. The chemopreventive role of acetone extracts of Q. infectoria against 1,2, dimethyl-hydrazine induced colonic cancer was tested. DMH induced aberrant crypts and was associated with higher mortality and loss of body weight. Quercus infectoria extract was found to be efficient in decreasing oxidative stress by improving the concentration of antioxidants. Thus, Q. infectoria extract possesses anticancer potential against colon cancer [37]. The primary objective of the current research work is to evaluate the anti-oral cancer activity of Q. infectoria (Manjakani) galls crude extract by means of appropriate preliminary phytochemical screening and in-vitro assays of cell proliferation, cell cycle analysis, apoptosis, TGF beta expression, and mitochondrial membrane potential.
MATERIALS AND METHODS
Chemicals and reagents
The required chemicals were procured from commercial sources such as Hi media, Fischer and SRL companies, Bangalore, India. The chemicals and reagents include “Dulbecco’s phosphate;buffered saline, dimethyl sulfoxide; (DMSO), 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT reagent), Fluorescent Activated Cell Sorter; (FACS) Calibur; (BD Biosciences, USA);Microplate reader; GC-MS, Absolute ethanol, double distilled water; Dulbecco’s Modified-Eagle’s-Medium (DMEM)fetal bovine serum; (Himedia,Cat no. RM10432,); D-PBS (Cat no-TL1006, Himedia); propidium iodide (BD biosciences,Cat 556463), paclitexol (Sigma ,Cat No-T7191,); Mito-Screen Kit JC-1 (BD biosciences,Cat No- 551302);” human Caspase 3 ELISA kit (Thermo,Cat No. BMS2012INST); human Caspase 9 ELISA kit (Cat No. BMS2025, Thermo); PE mouse anti-human TGF-β1 (BD biosciences,Cat No. 562339).
Methodology
The galls from Q. infectoria were collected and macerated to prepare ethanolic extract. Phytochemical qualitative test, high-performance thin-layer chromatography, and GC-MS profiles were carried out to prove the presence of tannins in ethanolic extract of Q. infectoria. A molecular docking study was conducted to predict the binding affinity of bioactive compounds (tannins) on target oral cancer cells to show their protection. The cytotoxic potential of tannins extracted from Q. infectoria was tested by MTT, PI assay, BrDU assay, apoptosis cell cycle analysis, flow cytometry and morphology of KB cell lines to be viewed upon treatment with the extract. The specific protein (Bcl-2) and the gene expression in the KERATIN-HeLa cells (KB) cell line upon tannin treatment were detected by western blot and RT-PCR technique. The Caspase 3 and 9 enzymatic activity were carried out using the ELISA method. Statistics results were statistically analyzed. Standard deviation was calculated.
Collection of plant material
Quercus infectoria was obtained from the vicinity of Bangalore, Karnataka (Southern India). The specimen was confirmed by Dr. Rama Rao, Research Officer, from the Department of Botany, Regional Ayurveda, Research Institute for Metabolic-Disorders, Bangalore.
Ethanolic extraction of Q. infectoria
The Q. infectoria plant galls parts were collected, dried completely under shade, and ground to fine powder with an electric grinder and extracted exhaustively using Soxhlet apparatus with 70% ethanol solvent and retained at room temperature for 24 hours. Following filtering, the mixture was concentrated by using the rotary evaporator at 40°C, while under vacuum pressure. After obtaining the crude extract fractions with varying polarities, they were refrigerated until needed again.
Phytochemical analysis
To identify the existence of several phytoconstituents, the qualitative analysis of metabolites(secondary) from Q. infectoria, including its flavones, saponins, phenolics, tannins and glycosides, alkaloids, was carried out with standard biochemical processes [37].
Cell line and cell culture
American Type Culture Collection (ATCC), located in Virginia, US, is where the KB cell line was obtained. “The cultures of KB cells were kept at 37°C in a humid incubator with DMEM supplemented in 10% fetal bovine serum, 100 gml-1 of streptomycin, and 100 UIml-1 of penicillin.”
Maintenance of cell line
KB cell lines were bought from ATCC (CCL −17) and kept in the water bath at 37°C. It was spun until only a single ice crystal was left. A 15 ml centrifuge tube was used to transfer the entire contents. It was then centrifuged at room temperature for 10 minutes at 200× g. The remaining DMSO was then eliminated by discarding the supernatant. After reaching 80% confluency, the cells are subcultivated at a 1:4 ratio while still being kept in the T25 flask at the prescribed density.
MTT assay for cell viability
The MTT assay is a colorimetric method that measures cell cytotoxicity by assessing the reduction of MTT, a yellow dye, to purple formazan crystals [38]. After being sown in a 96-well plate, KB cells were incubated for the entire night. The spent media was taken out once the cell was attached. The cells were incubated for 24, 48, and 72 hours at a temperature of 37°C and 5% CO2 with the required quantities of plant extract (25–400 μg/ml). After treatment, the cells were reincubated for 2 hours at 37°C with the addition of 0.5 mg/ml of MTT reagent. A few hours later, 20μl of DMSO was added and the medium was disposed of. Next, using a microplate reader, the absorbance was determined at 570 nm [39].
The following formula was used to compute the cell viability, assuming untreated cells as a 100% viable population.
Cell cycle analysis
Initially, a 6-well plate was seeded with KB cells (1 × 106 cells/well) and left for 12 hours and then exposed to 10 M of Paclitaxel for positive control and IC25 (36.56 μg/ml)IC50 (73.12μg/ml) IC75 (109.68 μg/ml) of Q. infectoria for test samples and the solvent for negative control, respectively. Control cells and the treated cells were cleaned with 1× PBS after 72 hours. of incubation; and then fixed in 70% ethanol (−20°C) for 30 minutes. PBS was used to wash these fixed cells and added with 50μl of RNase A solution. To this pellet of cells in RNase A solution, 400μl of propidium iodide (Cat 556463, BD biosciences) solution per million cells was added directly, mixed well, and kept for 5–10 minutes at room temperature. Using the CellQuest program from BD Biosciences, flow cytometry (BD FACS Callibur) was used to ascertain the cell cycle distribution of 10,000 cells.
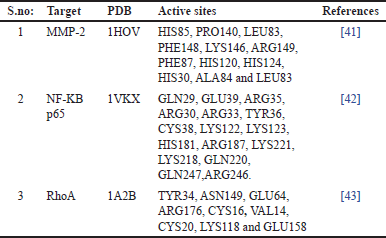 | Table 1. Preparation of the target, active sites of the target proteins. [Click here to view] |
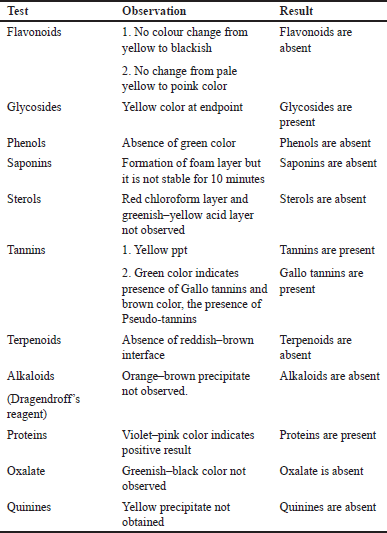 | Table 2. Phytochemical screening of ethanolic extract of Q. infectoria. [Click here to view] |
RT-PCR and Western blot studies
KB cells were treated with the IC50 concentration of the extract and harvested at 24, 48, and 72 hours. To prepare lysates, cells were resuspended in a lysis buffer containing 1% Triton X-100, 0.1% SDS, 10 mM Tris (pH 7.4), 100 mM NaCl, 10% glycerol, and 0.5% deoxycholate, incubated on ice for 30 minutes with intermittent vortexing, and clarified by centrifugation at 13,000 rpm for 10 minutes at 4°C. Equal amounts of protein (40–50 μg) were then loaded onto sodium dodecyl-sulfate polyacrylamide gel electrophoresis gels and transferred to nitrocellulose or PVDF membranes. All these procedures were conducted following the method described [40].
TGF-beta expression studies
KB cells (1 × 106 cells per well) were seeded in the 6-well plate and incubated for 12 hours. After incubation, the cells were cultured in a medium (10 uM of Paclitaxel) as a positive control and IC25 (36.56 μg/ml), IC50 (73.12μg/ml), IC75 (109.68 μg/ml) of Q. infectoria) for another 72 hours. Following PBS washes, cells were trypsinized and collected via centrifugation, using 70% ice-cold ethanol cells were stained and incubated for 30 minutes at −20°C. Cells were cleaned twice with 1× PBS and incubated with 50μl of Mouse anti-human TGF-β1- PE (Cat No. 562339, BD biosciences) antibody in the dark for 30 minutes. After incubation, 450μl of PBS was added to the cell pellet and mixed thoroughly before analyzing by FACS.
Caspase 3 expression studies by ELISA
The manufacturer’s protocol was followed for the assay (Catalog Number BMS2012INST, Thermo). In brief, the protocol can be summarized as the following; KB cells (1 × 106 cells per well) were packed in a 6-well plate. After an incubation period of 12 hours, cultured medium for the cell containing 10 uM of Paclitaxel (positive control) and IC25 (36.56 μg/ml), IC50 (73.12μg/ml), IC75 (109.68 μg/ml) of Q. infectoria) for 72 hours. Cells were then washed once with PBS, trypsinized, and harvested by gentle centrifugation. Cell pellets were resuspended in 200 ul of lysis buffer (provided in the kit) and incubated for 1 hour at room temperature with shaking. The cell extracts were transferred to micro-centrifuge tubes and centrifuged at (1,000 × g) for 15 minutes.
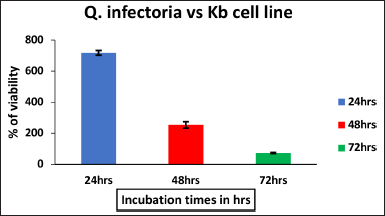 | Figure 1. The effect of ethanol extract of Q.infectoria on KB cell line against Positive control with different hours of incubation. The data are represented by mean ± SD. [Click here to view] |
The cleared lysate was aliquoted to cleanse microfuge tubes. All assay was carried out in duplicate. From seven Caspase 3 dilutions, a standard calibration curve was prepared. From the curve, the concentration of the sample was calculated. After incubation, washing was done to remove unbound antihuman detection antibody (Caspase-3) and IgG-HRP (anti-rabbit). A colored product was formed depending on the presence of Caspase 3 in the sample. The reaction was ended by adding acid and by measuring the absorbance at 450 nm.
Caspase 9 expression studies by ELISA
The assay was performed as per instructions of the manufacturer (Cat No. BMS2025, Thermo). Briefly, the protocol can be summarized as the following; KB cells (1 × 106 cells per well) were packed in a 6-well plate and incubated (12 hours at 37°C) in a humidified incubator. Post-adherence for 12 hours, the cells were cultured in a medium containing 10uM of Paclitaxel and IC25 (36.56 μg/ml), IC50 (73.12μg/ml), IC75 (109.68 μg/ml) of Q. infectoria for 72 hours. Cells were then washed once with PBS, trypsinized, and harvested by gentle centrifugation. The pellet of the cells was re-suspended in 200 ul of lysis buffer (provided in the kit) and incubated for 1 hour with gentle shaking at room temperature. After being put into microcentrifuge tubes, the cell extracts were centrifuged for 15 minutes at 1,000 × g. An aliquot of the cleansed lysate was placed into fresh microfuge tubes. All assays were carried out in duplicate.
The sample’s Human Caspase-9 binds to the antibodies present in the microwells. The first antibody captures human Caspase-9, which is bound by the polyclonal discovering antibody (rabbit). A colored product was in both the standard and sample, depending on the quantity of human Caspase 9. Acid is added to halt the process, and absorbance is gauged (450 nm). A calibration curve was created using a standard dilution of 7 human Caspase 9, and the calibration curve allowed for the determination of the sample’s Caspase 9 concentration.
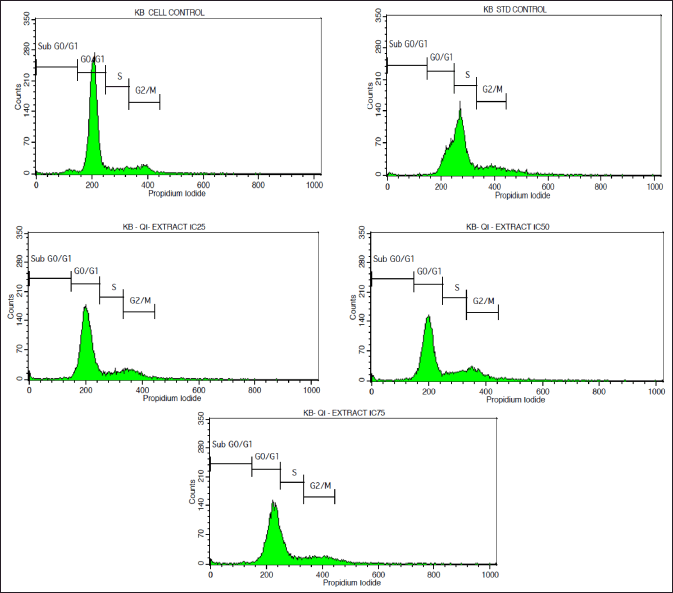 | Figure 2. FACS Histograms displaying the cell cycle distribution phases in the KB cell line treated with various doses of Q. infectoria extracts. p-values < 0.05 will be considered significant. [Click here to view] |
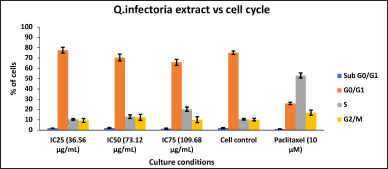 | Figure 3. Bar Plots of the phases of cell cycle of KB cells, treated with various concentrations of extracts of Q. infectoria. [Click here to view] |
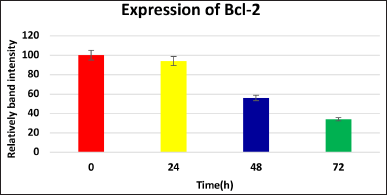 | Figure 4. Levels of Bcl-2, after treatment with IC50 value of the extract for 24, 48, and 72 hours(Western blot method). [Click here to view] |
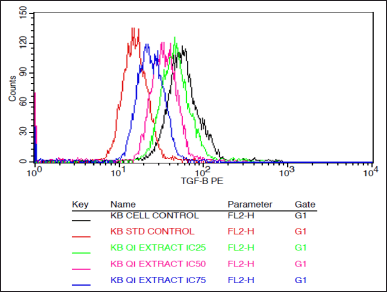 | Figure 5. Histogram depicting the TGF beta expression in KB cells treated with various concentrations of the extract of Q. infectoria and positive control paclitaxel. [Click here to view |
Evaluation of mitochondrial membrane potential (ΔΨ m)
The KB cells (1 × 106 cells per well) were sowed in a 6-well plate. After incubation, the cells of KB were cultured in a medium containing 10 uM of Paclitaxel and IC25 (36.56 μg/ml), IC50 (73.12μg/ml), IC75 (109.68 μg/ml) of Q. infectoria) for 72 hours. With PBS solution, cells were washed, trypsinized, and harvested by centrifugation. Using 70% ice-cold ethanol cells were stained and incubated at (−20°C) for 30 minutes. Freshly prepared (0.5 ml )JC-1 (BD Biosciences, Catalog No. 551302). Each pellet received a working solution, and they were then placed in a CO2 incubator and incubated for 10–15 minutes(37°C). Re-suspended in 0.5 ml of assay buffer after using 1 ml of assay buffer solution. Using BD FACS Calibur cells were analysed for mitochondrial membrane potential.
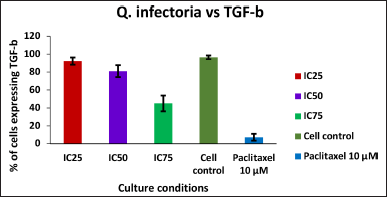 | Figure 6. Graph depicting the TGF beta expression levels in KB cells treated with various concentrations of the extract of Q. infectoria and positive control paclitaxel. [Click here to view] |
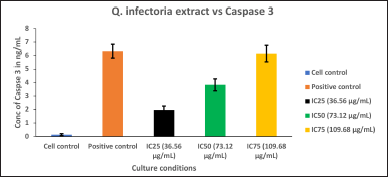 | Figure 7. Graph depicting the Caspase 3, quantification in KB cells treated with various concentrations of the extract of Q. infectoria and positive control (paclitaxel). [Click here to view] |
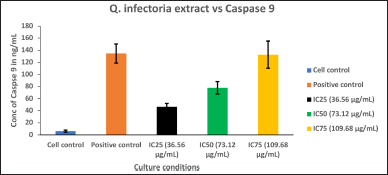 | Figure 8. Graph depicting the Caspase 9 quantification in KB cells treated with various concentrations of extract of Q. infectoria and positive control (paclitaxel). [Click here to view] |
Insilico molecular docking analysis of Q. infectoria
To analyze the activity of Q. infectoria against oral cancer, the phytocompounds isolated from Q. infectoria were screened against the targets matrix metalloproteinase-2 (MMP-2), NF-kB, and RhoA. Here, the MMP-2 plays an important role in the invasion and migration of cancer cells. This NF-kB is the key factor in the proliferation and cancer cells of survival. Moreover, RhoA inhibition results in tumor cell death and the reduction of metastasis.
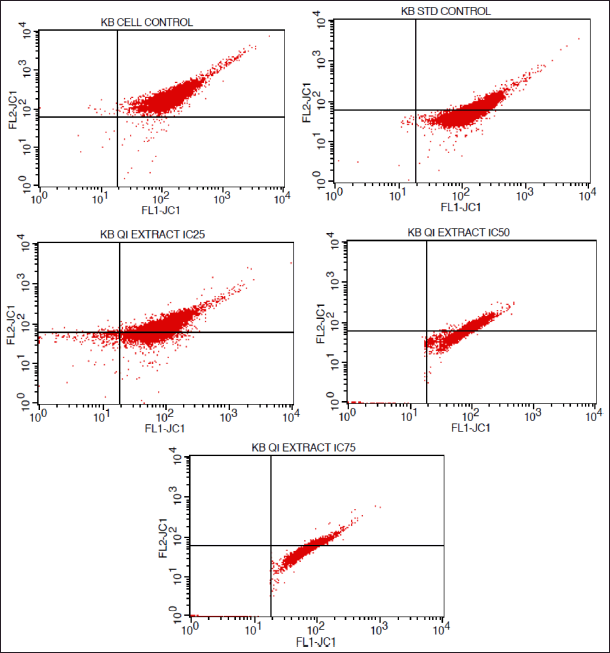 | Figure 9. FACS histograms depicting the MMP of KB cells treated with various concentrations of the extract of Q. infectoria. [Click here to view] |
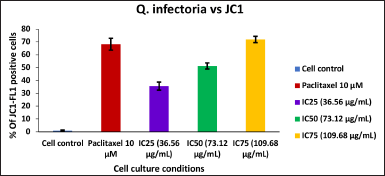 | Figure 10. Bar graph represent the MMP of KB cells treated with various concentrations of the extract of Q. infectoria. [Click here to view] |
 | Table 3. +ve phytochemical constituents showing presence of secondary metabolites. [Click here to view] |
The three dimensional (3D) structures of the target proteins namely MMP-2, RhoA, and NF-kB p65 were acquired from PDB, the protein data bank. PDB IDs: 1HOV, 1VKX (chain: A), and 1A2B, respectively. The non-standard amino acids and water molecules connected with the coordinate structure were eliminated. The MMP-2 has one Zn2+ ion, which was not removed from the structure. The binding site information of each target was retrieved from the literature (Table 1).
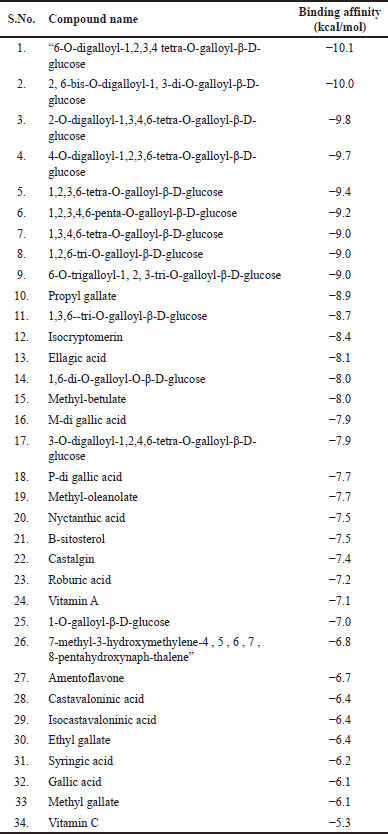 | Table 4. PyRx (Autodock Vina) virtual screening results for phytocompounds isolated from Q. infectoria against MMP-2. [Click here to view] |
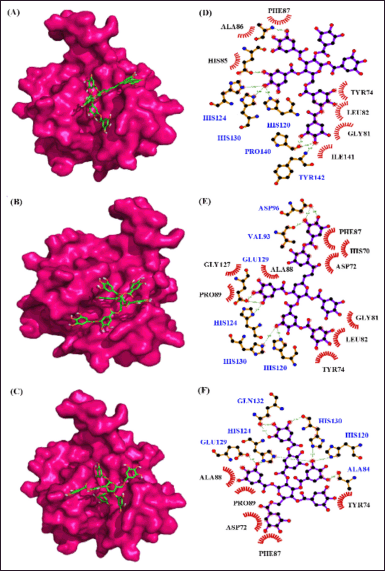 | Figure 11. Interaction of MMP–2 with phytocompounds of Q. infectoria. Binding mode of MMP–2 with (A) “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (B) 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose and (C) 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose. Hydrogen bond and hydrophobic interaction of MMP–2 with (D) 6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (E) 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose and (F) 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose.” [Click here to view] |
Ligand preparation
A total of 35 phytocompounds isolated from Q. infectoria were identified from the literature [44]. Among the 35 compounds, the 3D structures of a few compounds were acquired from the NCBI database [Pubchem] in the SDF format and the remaining structures were illustrated with the help of ACD/ChemSketch software [45]. All the ligands were transformed into PDB files through the Open babel tool [46].
Virtual screening
Python Prescription Virtual Screening Tool (PyRx)–screening of phytocompounds
The docking-based virtual screening (DBVS) was carried out through the PyRx. It includes AutoDock, AutoDock Vina, and Open Babel to carry out DBVS and import ligands. First, the target structures were converted into PDBQT format. All the ligands were energy minimized through the universal field force and conjugated gradient algorithm. The control grid box was set to cover the protein residues mediating target protein activity. In structure-based virtual screening, the binding energy was calculated through PyRx AutoDock Vina. Finally, the PyRx resulted from high-binding energy compounds subjected to AutoDock 4.2.6 [47].
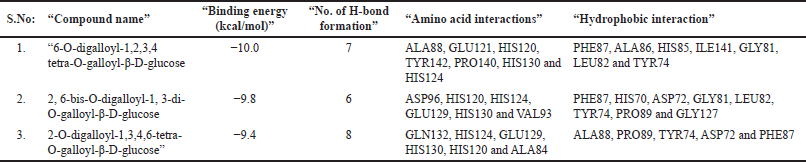 | Table 5. AutoDock results for MMP-2 and phytocompounds isolated from Q. infectoria. [Click here to view] |
AutoDock–molecular docking
Molecular docking was carried out, to determine the binding orientation and affinity of the protein-ligand complex. Here, we have employed AutoDock 4.2.6 for docking, which utilizes the Lamarckian-Genetic-Algorithm (LGA). The protein and ligand structures were optimized through AutoDock Tool, and it was converted into PDBQT format. The control grid box was fixed to cover the active site of the target protein. The AutoGrid generates a grid and desolvation map. The algorithm was set to 150 population, energy evaluation of 2,500,000, and a maximum of 27,000 generations. The mutation rate and crossover rate were fixed at 0.02–0.8, respectively. The AutoDock run was generated, and the protein-ligand complex was analyzed for binding affinity, hydrogen bond, and hydrophobic interactions. The LGA utilizes empirical scoring functions. The binding energy was expressed in k-cal/mol.
Statistical analysis
Results were shown as mean ± SD. Oneway analysis of variance and post hoc least-significant difference test were done to determine statistical significance. p values less than 0.05 will be considered as significant.
RESULTS
Preliminary phytochemical screening
Analysis of the Q. infectoria revealed the presence of distinct metabolic compounds such as tannins, phenols, and several other phytoconstituents represented below (Tables 2 and 3)
In comparison with other metabolites, Q. infectoria displayed a distinctive presence of tannins especially gallo tannins and glycosides which are its main constituents.
The cytotoxicity effect of Q.infectoria on KB cell line
The cytotoxic effect of Q. infectoria extract on KB cells, was measured through the thiazol tetrazolium (MTT) assay. The percentage of cell viability at five different concentrations (25–400 μg per ml) of the extract was studied. With the increase in incubation time, Q. infectoria extracts significantly prohibit the KB cell proliferation (Fig. 1) in a dose-dependent manner after 72 hours of the treatment. The IC50 value of Q. infectoria ethanolic extract was determined to be 76.82 μg/ml.
Cell cycle analysis of KB cells
As depicted in Figure 2, KB cells cycle analysis demonstrated that the, KB cells treated with the concentrations of IC25 (36.56 μg/ml), IC50 (73.12μg/ml), and IC75 (109.68 μg/ml) of Q. infectoria have high cells in a dose-dependent manner at G0/G1 phase. The positive control, paclitaxel (10 uM) showed a low number of cells (G0/G1 stage) and a high number of cells (S phase). There is no significant change detected among all the groups during the G2/M phase and the sub G0/G1 phase. (Figs 2 and 3)
RT-PCR and western blot analysis
Treatment with the extract differentially affected the expression of key regulatory proteins in KB cells. Bcl-2, an anti-apoptotic protein, was significantly down-regulated after 48–72 hours of treatment (Fig. 4), while remaining unchanged at 24 hours. This selective effect suggests the extract may target the Bcl-2 pathway to promote cell death, potentially offering a novel therapeutic mechanism.
TGF beta expression analysis of KB cells
As shown in Figures 5 and 6, TGF beta expression was downregulated in a dose-dependent manner, IC75 concentration was shown to be the most effective in the down-regulation of the TGF beta. Paclitaxel-treated cells (positive control) showed the lowest expression, while there was a high expression in solvent-treated cells (negative control). This data indicates that TGF beta, which plays a crucial role in the proliferation and metastasis of cancer cells, was down-regulated by the extract of Q. infectoria.
 | Table 6. PyRx (Autodock Vina) virtual screening results for phytocompounds isolated from Q. infectoria against NF-Kb p65. [Click here to view] |
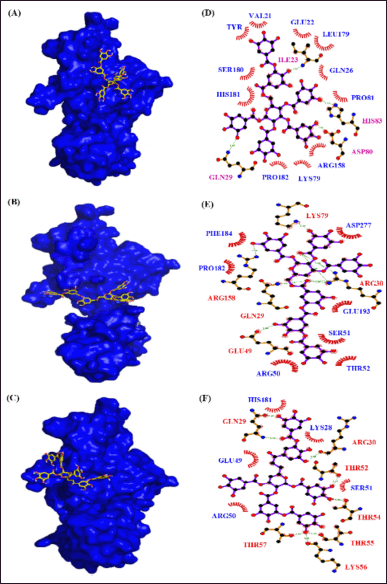 | Figure 12. Interaction of NF-KB p65 with phytocompounds of Q. infectoria. Binding mode of NF-KB p65 with (A) “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (B) 6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose and (C) 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose. Hydrogen bond and hydrophobic interaction of NF-KB p65 with (D) 6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (E) 6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose and (F) 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose.” [Click here to view] |
Caspase 3 induction
As shown in Figure 7, Caspase 3 was up-regulated in a dose-dependent manner, and IC75 concentration was shown to be the most effective in the highest induction of Caspase 3. The cells treated with paclitaxel (positive control) exhibited the maximum induction, whereas the cells treated with solvent (negative control) showed the lowest expression. This data indicates that cancer cell proliferation was inhibited through the induction of Caspase 3 by the extract of Q. infectoria.
 | Table 7. AutoDock results for NF-KB p65 and phytocompounds isolated from Q. infectoria. [Click here to view] |
Caspase 9 induction
Caspase 9 was upregulated in a dose-dependent manner, IC75 concentration was shown to be the most effective in the highest induction of Caspase 9, as shown in Figure 7. Cells treated with paclitaxel (positive control) exhibited the maximum induction, whereas the cells treated with solvent (negative control) showed the lowest expression (Fig. 8). This data demonstrated that there was a significant induction of Caspase 9, an indicator of cancer cell death by the extract of Q. infectoria.
Evaluation of mitochondrial-membrane-potential (ΔΨ m)
As shown in Figures 9 and 10, mitochondrial membrane potential was up-regulated in a dose-dependent manner, and IC75 concentration was shown to be the most effective in the highest induction of potential. It was interesting to note that paclitaxel-treated positive control cells, showed high induction, while there was a low induction of mitochondrial potential in solvent-treated cells (negative control). This data demonstrated that there was an induction of matrix metalloproteinase (MMP) an indicator of cancer cell death by the extract of Q. infectoria.
Virtual screening
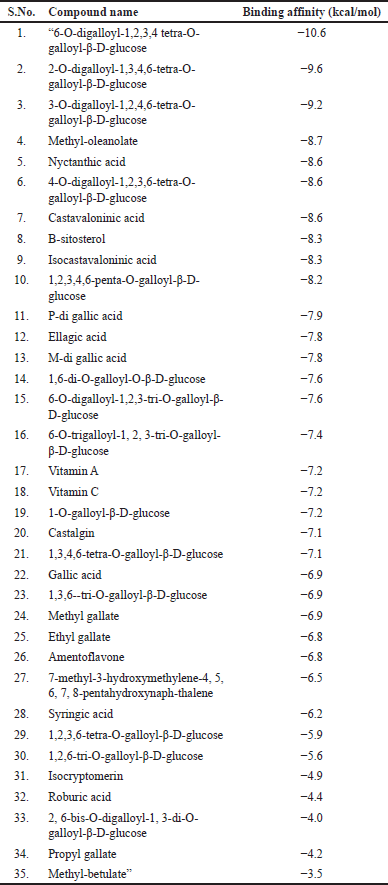
The virtual screening of 35 phytocompounds isolated from Q. infectoria against MMP−2, NF-Kb p65, and RhoA targets resulted in the binding affinity of protein-ligand-complex. Based on the binding affinity, the best interacting protein-ligand complexes were selected. Here, the DBVS of MMP−2 and 35 phytocompounds resulted in the high binding affinity of the tannin compounds. The tannins, “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose, 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose and 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose” are having the best binding affinity of −10.1 k-cal per mol, −10.0 k-cal per mol and −9.8 k-cal per mol, respectively. The other tannins “such as 6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose, 1,2,6-tri-O-galloyl-β-D-glucose, 1,3,4,6-tetra-O-galloyl-β-D-glucose, 1,2,3,4,6-penta-O-galloyl-β-D-glucose, 1,2,3,6-tetra-O-galloyl-β-D-glucose and 4-O-digalloyl-1,2,3,6-tetra-O-galloyl-β-D-glucose” have the binding affinity ranges from −9.0 to −9.7 kcal/mol. The other compounds namely methyl-betulate, “1,6-di-O-galloyl-O-β D-glucose,” ellagic acid, isocryptomerin, “1,3,6-tri-O-galloyl-β-D glucose” and propyl gallate the binding affinity falls within the range of −8.0 to −8.9 kcal per mol. The binding affinity of the remaining 19 compounds falls within the range of −5.3 to −7.9 kcal per mol (Table 4).
From the virtual screening results, the 10 best binding affinity compounds were chosen and subjected to molecular docking using Auto-Dock 4.2.6. It reveals “6-O-digalloyl-1,2,3,4 tetra O-galloyl-β-D-glucose” with the high binding affinity of −10.0 kcal per mol, 7 H-bond formations (ALA88, GLU121, HIS120, TYR142, PRO140, HIS130, and HIS124) and seven hydrophobic interactions (PHE87, ALA86, HIS85, ILE141, GLY81, LEU82, and TYR74) (Fig. 11). Here, the polar amino acids are contributing more in the H-bond formation and non-polar amino acids are highly involved in the hydrophobic interactions. Similarly, MMP-“2-2, 6-bis O-digalloyl-1, 3 di-O-galloyl β-D glucose” complex revealed a binding affinity of −9.8 kcal/mol. It has six H-bond formations (ASP96, HIS120, HIS124, GLU129, HIS130, and VAL93) and eight hydrophobic interactions (PHE87, HIS70, ASP72, GLY81, LEU82, TYR74, PRO89, and GLY127). The polar and non-polar amino acids contribute more to the H-bond and hydrophobic interactions, respectively. Also, “MMP-2—2-O-digalloyl 1,3,4,6-tetra-O galloyl-β D-glucose’ complex has a binding affinity of −9.4 kcal per mol. It has eight H-bond formations (GLN132, HIS124, GLU129, HIS130, HIS120, and ALA84) and five hydrophobic interactions (ALA88, PRO89, TYR74, ASP72, and PHE87) (Table 5).
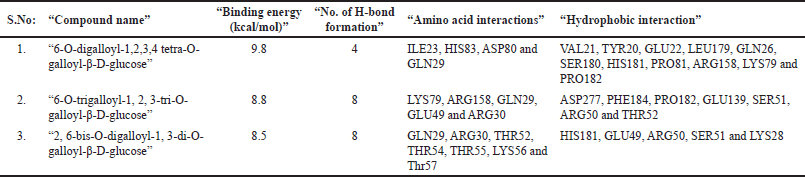
The DBVS of NF-kB p65 against 35 phytocompounds reveals that tannins, “such as 6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose, 6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose and 2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose” have the high binding affinity of −10.2 k-cal/mol, −9.2 k-cal per mol and −9.0 k-cal per mol, respectively (Table 6). The AutoDock results of the 10 best binding affinity compounds reveal the same tannin compounds with the binding energy of 9.8 kcal/ per mol, 8.8 kcal/ per mol, and 8.5 kcal per mol. The NF-kB “p65-6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose” complex had 4 H-bond interactions (ILE23, HIS83, ASP80, and GLN29) and 11 hydrophobic interactions (VAL21, TYR20, GLU22, LEU179, GLN26, SER180, HIS181, PRO81, ARG158, LYS79, and PRO182) (Fig. 12). For, NF-kB “p65-6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose” complex the binding affinity was found to be −9.8 kcal/mol with four H-bonds (GLU64, VAL14, SER88, and CYS20) and nine hydrophobic interactions (VAL21, TYR20, GLU22, LEU179, GLN26, SER180, HIS181, PRO81, ARG158, LYS79, and PRO182). The NF-kB “p65-6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose” complex results with −8.8 kcal/mol with five H-bond interaction (LYS79, ARG158, GLN29, GLU49, and ARG30) and seven hydrophobic interactions (ASP277, PHE184, PRO182, GLU139, SER51, ARG50, and THR52). Similarly, NF-kB “p65-2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose” complex has −8.5 kcal/mol with eight H-bond interaction (GLN29, ARG30, THR52, THR54, THR55, LYS56, and THR57) and five Hydrophobic interactions (HIS181, GLU49, ARG50, SER51, and LYS28) (Table 7).
Here, though the NF-kB p65 has eight H-bond interactions with “2, 6-bis-O-digalloyl-1, 3-di-O-galloyl-β-D-glucose” than the compounds “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose” and “6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose,” it has low binding energy than the other two tannins. In case of NF-kB “p65-6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose” complex and NF-kB “p65-6-O-trigalloyl-1, 2, 3-tri-O-galloyl-β-D-glucose” complex, though it has low H-bond interaction (4 and 5), the hydrophobic interaction has contributed more to the binding-affinity of the protein-ligand-complex.
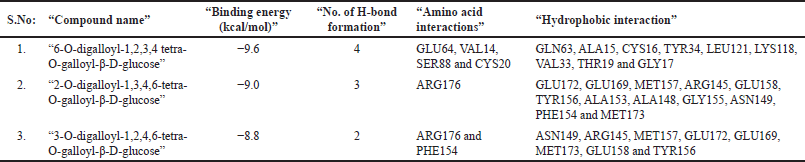
In another DBVS of RhoA and 35 phytocompounds, the best binding affinity was observed for the tannins “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose., 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose and 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose” with the binding energy of −10.6 k-cal/mol, −9.6 k-cal per mol and −9.2 k-cal per mol (Table 8). The AutoDock results of the 10 best compounds reveal the high affinity for the tannins, “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (−9.6 kcal/mol), 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose (−9.0 kcal/mol) and 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose (−8.8 kcal/mol). Here, the RhoA-6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose” complex has 4 H bond interaction (GLU64, VAL14, SER88, and CYS20) and nine hydrophobic interactions (GLN63, ALA15, CYS16, TYR34, LEU121, LYS118, VAL33, THR19, and GLY17) (Fig. 13). Also, the RhoA “2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose” complex was found to have three H-bond formation and 12 hydrophobic interactions (GLU172, GLU169, MET157, ARG145, GLU158, TYR156, ALA153, ALA148, GLY155, ASN149, PHE154, and MET173). Similarly, the ‘RhoA-3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose’ complex has two H-bond formations (ARG176 and PHE154) and eight hydrophobic interaction (ASN149, ARG145, MET157, GLU172, GLU169, MET173, GLU158, and TYR156) (Table 9).
 | Table 8. PyRx (Autodock Vina) virtual screening results for phytocompounds isolated from Q. infectoria against RhoA. [Click here to view] |
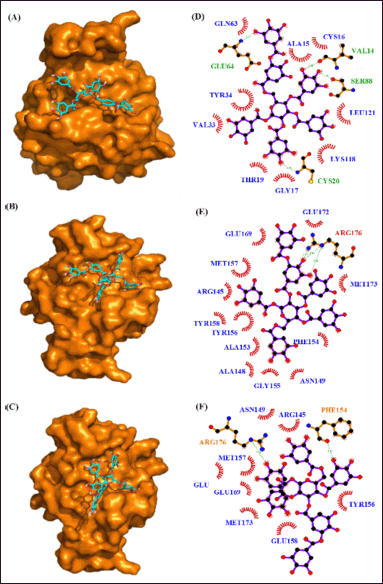 | Figure 13. Interaction of RhoA with phytocompounds of Q. infectoria. Binding mode of RhoA with (A) “6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (B) 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose and (C) 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose. Hydrogen bond and hydrophobic interaction of RhoA with (D) 6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose (E) 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose and (F) 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose.” [Click here to view] |
 | Table 9. AutoDock results for RhoA and phytocompounds isolated from Q. infectoria. [Click here to view] |
DISCUSSION
Identifying natural compounds to develop novel chemotherapeutics to treat oral cancer is a crucial need. Since ancient times, plant products have been used to treat several ailments. Certain plant phytochemicals have been used to develop drugs to treat several diseases including cancer. Phytoconstituents of medicinal plants still remain an important source for developing and designing novel anti-cancer drugs. Various research has demonstrated that natural compounds from medicinal plants are safe and have the potential to prevent the growth of various types of cancers [48]. Based on the literature that Q. infectoria possessed various medicinal properties and that it was especially useful in treating cancers, we used the MTT method to measure its IC50 (50% growth inhibition) by evaluating the cytotoxicity toward KB cells at different doses [49]. We noticed a concentration-dependent suppression of cell proliferation in KB cell lines exposed to different doses of Q. infectoria’s ethanolic extract. This suggested that the extract of Q. infectoria hindered the multiplication of KB cells. Paciltaxel was used as the +ve (positive) control to validate our findings. This study therefore paves the way for further functional studies to identify the mechanism by which, the proliferation of oral cancer cells is inhibited. These studies include cell-cycle analysis, apoptosis-induction, and mechanistic studies including TGF beta and mitochondrial membrane potential.
The unearthing development of the latest chemotherapeutics’, that is effective for the management of oral cancer is crucially required. Ever since ancient times, the usage of natural compounds has still essential to identify novel anti-cancer drugs. Various research has exhibited and assured evidently that naturally occurring medicinal plants possess the safe potential to inhibit the progress of different cancers [50]. On the contrary, based on the literature, Q. infectoria has various therapeutic properties and is specially used in the treatment of oral cancer. We have assessed its antiproliferative activity on KB cells at various concentrations to identify its IC50 (‘50% growth inhibition’) by MTT assay [51]. Treatment with various concentrations of the ethanolic extract results in dose-dependent cell proliferation inhibition, signifying that the ethanolic extract hindered the proliferation of the KB cells, when compared with paclitaxel as a positive control. IC75 (109.68 μg/ml) of Q. infectoria is high at the G0/G1 phase in the dose-dependent manner. The positive control, paclitaxel (10 uM) showed the lower number of cells (G0/G1 stage) and the high number of cells at the S phase. Upregulation of MMP corresponding with the induction of “Caspase 3” and “Caspase 9” levels in the cells treated with IC75 indicates the cytotoxicity and apoptosis pathway activation. The downregulation of TGF beta levels, the cells treated with IC75, and paclitaxel-treated cells signify the inhibition of TGF beta-induced cancer cell proliferation and metastasis. This finding is exciting and can be further explored to confirm metastasis inhibition studies. It was also observed that treatment with Q. infectoria extract led to cell cycle arrest and subsequent mitochondrial apoptosis due to Bcl-2 reduction [52].
Our data revealed a substantial rise in the G0/G1 population of KB cells treated with QI, pointing toward G0/G1 cell cycle arrest. This aligns with the established role of tannins in triggering cell cycle arrest across diverse cancer cell lines [53,54]. Notably, the anti-proliferative and pro-apoptotic effects of QI tannins surpassed those of cisplatin, the reference drug. This observation echoes evidence from other studies highlighting the potency of natural products against cancer cells, often exhibiting a more favorable side effect profile compared to conventional chemotherapeutics [55,56].
The results of the current study indicate that secondary bioactive compounds like tannins and glycoside present in the ethanolic extract, which induce apoptosis in Caspase 3 and Caspase 9-dependent manner and down-regulated TGF beta while causing mitochondrial membrane potential in KB cells. A recent study shed light on the previously unknown molecular pathways through which an alkaloid inhibits DMH/DSS-induced colon cancer [57]. The observed bioactive compounds were revealed to be responsible for the new source of the latest anti-cancer drugs. Our current study sheds light on the mechanisms by which tannins exert their anti-proliferative and pro-apoptotic effects, potentially through targeting critical signaling pathways in cancer cells. A recent similar study suggests that tannin extract from Avicennia marina, a mangrove plant, exhibits potential in targeting cancer cell growth and survival [58,59].
The virtual screening and molecular docking of 35 phytocompounds isolated from Q. infectoria against the target proteins MMP-2, NF-kB p65, and RhoA reveals that among the 35 phytocompounds, tannins have a favorable binding affinity against these important cancer targets. The comparison of all these docking results reveals that the ‘6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose’ compound was found to have high binding energy with all three cancer targets. The protein-ligand complex is stabilized through the H-bond and hydrophobic interactions. If the hydrophobic interaction is more, there will be less H-bond interaction and vice versa. To further confirm the binding affinity of these complexes, molecular dynamics studies were carried out.
Our findings on Q. infectoria’s antitumorigenic activity echo recent studies reporting its efficacy against breast and colon cancer cells [60,61]. Similarly, we confirm its pro-apoptotic effect in KB cells, likely via ROS generation and cell cycle arrest–mechanisms also linked to tannins from other sources [62,63]. This reinforces the broad antitumor potential of Q. infectoria tannin extracts and warrants further investigation into its specific mechanisms.
CONCLUSION
In conclusion, we have observed that the ethanolic extract of Q. infectoria inhibits the proliferation of oral cancer KB cell line in vitro. Therefore, it could be a potential source of anticancer agents that target oral cancer cells. However, further molecular studies pertaining to the mechanism of action of the extracts are crucial to establish the anti-cancer effect of Q. infectoria. To the best of our knowledge, this is the first research report in which Q. infectoria ethanolic extracts were explored for anticancer activity on oral cancer cell lines by in vitro assays. Hence, the current study revealed that the ethanolic extract possesses a wide range of anti-cancer activity in human cancer cells by apoptosis induction and arresting cell cycle via intrinsic pathway. In contrast, ethanolic extract displays minimal toxicity to healthy cells, so it was revealed to be a selective agent for inducing apoptosis between cancer and healthy cells. Consequently, ethanolic extract obtained from Q. infectoria exhibited anticancer properties and has some potential lead molecules for drug development. The comparison of all these docking results reveals that the ‘6-O-digalloyl-1,2,3,4 tetra-O-galloyl-β-D-glucose’ compound was found to have high binding energy with all three cancer targets. The protein-ligand complex is stabilized through the H-bond and hydrophobic interactions. If the hydrophobic interaction is more, there will be less H-bond interaction and vice versa. To further confirm the binding affinity of these complexes, molecular dynamics studies were carried out.
ACKNOWLEDGMENT
Authors acknowledge the College of Applied Medical Sciences in Jubail, Imam Abdulrahman Bin Faisal University for their support to carry out this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was primarily supported by grants (Grant No. 2019-165-AMSJ), from the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University Dammam, Saudi Arabia.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Nagai H, Kim YH. Cancer prevention from the perspective of global cancer burden patterns. J Thorac Dis. 2017;9(3):448–51. CrossRef
2. Gupta PC, Murti PR, Bhonsle RB, Mehta FS, Pindborg JJ. Effect of cessation of tobacco use on the incidence of oral mucosal lesions in a 10-yr follow-up study of 12,212 users. Oral Dis.1995;1:54–8. CrossRef
3. John J, Nagappan N. Antimicrobial efficacy of herbal and chlorhexidine mouth rinse a systemic review. J Dent Med.2013;2:5–10. CrossRef
4. Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: an integrated approach. Ayu.2012;33:9–10. CrossRef
5. Vickers A, Zollman C, Lee R. Herbal medicine. West J Med.2001;175:125–8. CrossRef
6. Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6(11):1–5. CrossRef
7. Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull.2014;4(5):421–7. CrossRef
8. Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther.2010;17(8):523–31. CrossRef
9. Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: pathogenesis, prevention and treatment. Toxicol Appl Pharmacol. 2007;224(3):326–36. CrossRef
10. Asase A.Ghana’s herbal medicine industry: prospects, challenges and ways forward from a developing country perspective.Front Pharmacol.2023;14:1267398. CrossRef
11. Buenz EJ, Schnepple DJ, Bauer BA, Elkin PL, Riddle JM, Motley TJ. Techniques: bioprospecting historical herbal texts by hunting for new leads in old tomes. Trends Pharmacol Sci. 2004;25(9):494–8. CrossRef
12. Basri DF, and Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol.2005;37:26–69. CrossRef
13. Fatima S, Farooqi AHA, Kumar R, Kumar TS, and Khanuja SPS. Antibacterial activity of possessed by medicinal plants used in tooth powders. J Med Aromat Plant Sci.2002;22:187–9.
14. Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000;14(7):510–6. CrossRef
15. Redwane A, Lazrek HB, Bouallam S, Markouk M, Amarouch H, Jana M. Larvicidal activity of extracts from Quercus lusitania var. infectoria galls (Oliv.). J Ethnopharmacol. 2002;79(2):261–3. CrossRef
16. Bhalodia NR, Shukla VJ. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2(2):104–9 CrossRef
17. Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90(2-3):285–92. CrossRef
18. Dar MS, Ikram M. Studies on Quercus infectoria; isolation of syringic acid and determination of its central depressive activity. Planta Med. 1979;35(2):156–61. CrossRef
19. Reena Gupta and Jitendra Gupta. Practical manual of pharmacognosy. 1st ed. Agra, UP: Narain Publishers & Distributors; 2022.
20. Kokate CK. Practical pharmacognosy.5th ed. New Delhi, India: Vallabh prakashan; 2014.
21. Umachigi SP, Jayaveera KN, Ashok Kumar CK, Kumar GS, Vrushabendra swamy BM, Kishore Kumar DV, et al. Studies on wound healing properties of Quercus infectoria. Trop J Pharm Res. 2008;7(1):913–9. CrossRef
22. Umthong S, Phuwapraisirisan P, Puthong S, Chanchao C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement Altern Med. 2011;11:37. CrossRef
23. Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25(3):435–57. CrossRef
24. Roselló-Díez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;27;332(6033):1086–8. CrossRef
25. Green H, Tseng H. Basonuclin: a zinc finger protein of epithelial cells and reproductive germ cells. In: Iuchi S, Kuldell N. editors. Zinc finger proteins. Molecular biology intelligence unit. Boston, MA: Springer; 2005. CrossRef
26. Zhou J, Wu SG, Sun JY, Li FY, Lin HX, Chen QH, et al. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol. 2017;143(1):115–22. CrossRef
27. Jiang X, Wu J, Wang J, Huang R. Tobacco and oral squamous cell carcinoma: a review of carcinogenic pathways. Tob Induc Dis. 2019;17:29. CrossRef
28. Allard WF, DeVol EB, Te OB. Smokeless tobacco (shamma) and oral cancer in Saudi Arabia. Community Dent Oral Epidemiol. 1999;27(6):398–405. CrossRef
29. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923–30. CrossRef
30. Nagpal M, Singh S, Singh P, Chauhan P, Zaidi MA. Tumor markers: a diagnostic tool. Natl J Maxillofac Surg. 2016;7(1):17–20. CrossRef
31. Grismaldo A, Sobrevia L, Morales L. Role of platelet-derived growth factor c on endothelial dysfunction in cardiovascular diseases. Biochim Biophys Acta Gen Subj. 2022;1866(10):130188. CrossRef
32. Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25(1):104–13 CrossRef
33. Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19-9—tumor marker: past, present, and future. World J Gastrointest Surg. 2020;12(12):468–90. CrossRef
34. Zin NNINM, Rahimi WNAWM, Bakar NA. A review of Quercus infectoria (Olivier) galls as a resource for anti-parasitic agents: In Vitro and In Vivo studies. Malays J Med Sci. 2019;26(6):19–34. CrossRef
35. Ahmed AA, Salih FA. Quercus infectoria gall extracts reduce quorum sensing-controlled virulence factors production and biofilm formation in Pseudomonas aeruginosa recovered from burn wounds. BMC Complement Altern Med. 2019;19(1):177. CrossRef
36. Burlacu E, Nisca A, Tanase C. A comprehensive review of phytochemistry and biological activities of Quercus Species. Forests. 2020;11:904. CrossRef
37. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel). 2017;6(4):42. CrossRef
38. Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018;2018(6):pdb-rot095505. CrossRef
39. Gomez-Flores R, Verástegui-Rodríguez L, Quintanilla-Licea R, Tamez-Guerra P, Monreal-Cuevas E, Tamez-Guerra R, et al. Antitumor properties of Gymnosperma glutinosum leaf extracts. Cancer Invest. 2009;27(2):149–55. CrossRef
40. Ghasemian M, Mahdavi M, Zare P, Ali Hosseinpour Feizi M. Spiroquinazolinone-induced cytotoxicity and apoptosis in K562 human leukemia cells: alteration in expression levels of Bcl-2 and Bax. J Toxicol Sci. 2015;40(1):115–26. CrossRef
41. Bhattacharyya S, Ghosh H, Covarrubias-Zambrano O, Jain K, Swamy KV, Kasi A, et al. Anticancer activity of novel difluorinated curcumin analog and its inclusion complex with 2-Hydroxypropyl-β-Cyclodextrin against pancreatic cancer. Int J Mol Sci. 2023;24(7):6336. CrossRef
42. Riedlinger T, Liefke R, Meier-Soelch J, Jurida L, Nist A, Stiewe T, et al. NF-κB p65 dimerization and DNA-binding is important for inflammatory gene expression. FASEB J. 2019;33(3):4188–202. CrossRef
43. Bao H, Li F, Wang C, Wang N, Jiang Y, Tang Y, et al. Structural basis for the specific recognition of RhoA by the dual GTPase-activating protein ARAP3. J Biol Chem. 2016;291(32):16709–19. CrossRef
44. Elham A, Arken M, Kalimanjan G, Arkin A, Iminjan M. A review of the phytochemical, pharmacological, pharmacokinetic, and toxicological evaluation of Quercus infectoria galls. J Ethnopharmacol. 2021;273:113592. CrossRef
45. Osterberg T, Norinder U. Prediction of drug transport processes using simple parameters and PLS statistics. The use of ACD/logP and ACD/ChemSketch descriptors. Eur J Pharm Sci. 2001;12(3):327–37. CrossRef
46. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open babel: an open chemical toolbox. J Cheminform. 2011;3:33. CrossRef
47. Trott O and Olson AJ. “Software news and update autoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading.” J Comput Chem.2009;31(2):455–61. CrossRef
48. Choi ES, Kim JS, Kwon KH, Kim HS, Cho NP, Cho SD. Methanol extract of Sanguisorba officinalis L. with cytotoxic activity against PC3 human prostate cancer cells. Mol Med Rep. 2012;6(3):670–4. CrossRef
49. Seneme EF, Dos Santos DC, Silva EMR, Franco YEM, Longato GB. Pharmacological and therapeutic potential of myristicin: a literature review. Molecules. 2021;26(19):5914. CrossRef
50. Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9(7):581–91 CrossRef
51. He Y, Zhu Q, Chen M, Huang Q, Wang W, Li Q, et al. The changing 50% inhibitory concentration (IC50) of cisplatin: a pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget. 2016;7(43):70803–21. CrossRef
52. Kumar S, Agnihotri N. Piperlongumine, a piper alkaloid targets Ras/PI3K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomed Pharmacother. 2019;109:1462–77. CrossRef
53. Koopaie M, Karimi H, Sohrabi M, Norouzi H. Cytotoxic, anti-proliferative, and apoptotic evaluation of Ramalina sinensis (Ascomycota, Lecanoromycetes), lichenized fungus on oral squamous cell carcinoma cell line; in-vitro study. BMC Complement Med Ther. 2023;23(1):296. CrossRef
54. Yao J, Xiao J, Wei X, Lu Y. Chaetominine induces cell cycle arrest in human leukemia K562 and colon cancer SW1116 cells. Oncol Lett. 2018;16(4):4671–8. CrossRef
55. Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–94. CrossRef
56. Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60(1):52–60. CrossRef. PMID: 9014353.
57. Kumar S, Agnihotri N. Piperlongumine targets NF-κB and its downstream signaling pathways to suppress tumor growth and metastatic potential in experimental colon cancer. Mol Cell Biochem. 2021;476(4):1765–81. CrossRef
58. Melo LFM, Aquino-Martins VGQ, Silva APD, Oliveira Rocha HA, Scortecci KC. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023;414:135645. CrossRef
59. Albinhassan TH, Saleh KA, Barhoumi Z, Alshehri MA, Al-Ghazzawi AM. Anticancer, anti-proliferative activity of Avicennia marina plant extracts. J Cancer Res Ther. 2021;17(4):879–86. CrossRef
60. Kamarudin NA, Nik Salleh NNH, Tan SC. Gallotannin-enriched fraction from Quercus infectoria galls as an antioxidant and inhibitory agent against human glioblastoma Multiforme. Plants (Basel). 2021;10(12):2581. CrossRef
61. Yusof WNSW, Abdullah H. Phytochemicals and Cytotoxicity of Quercus infectoria Ethyl Acetate Extracts on Human Cancer Cells. Trop Life Sci Res. 2020;31(1):69-84. CrossRef
62. Kuczler MD, Olseen AM, Pienta KJ, Amend SR. ROS-induced cell cycle arrest as a mechanism of resistance in polyaneuploid cancer cells (PACCs). Prog Biophys Mol Biol.2021;165:3–7. CrossRef
63. Wang J, Xiao H, Zhu Y, Liu S, Yuan Z, Wu J, et al. Tannic acid induces the mitochondrial pathway of apoptosis and S phase arrest in porcine intestinal IPEC-J2 Cells. Toxins (Basel). 2019;11(7):397. CrossRef