INTRODUCTION
Heterocyclic substances such as 2-aminothiophenes are crucial to the synthesis of numerous medicinally important substances. Based on Gewald’s investigation, the chemistry of these molecules gained more attention by researchers [1]. The ability of the 2-aminothiophene skeleton to function as a precursor for the creation of physiologically active compounds is considered due to various biological activities, which include anti-inflammatory [2], antimicrobial [3], antioxidant and antibacterial [4], antifungal [5], antiproliferative [6], anti-leishmanial [7], anticonvulsant [8], and antitumor activities [9]. Many medicinally important drugs such as tinoridine, tiaprofenic acid, and tenidap contain the thiophene ring in their structural frame work and they were used as anti-inflammatory medications. The anti-inflammatory action of all these commercially available medications is due to their capacity to scavenge free radicals [10–12].
3,4-Dimethoxy cinnamic acid is naturally found in coffee beans [13] and is chemically 3-(3,4-dimethoxyphenyl)propenoic acid. 3,4-Dimethoxy cinnamamide derivatives have gained significant attention in the field of medicinal chemistry due to various biological activities including anti-inflammatory and antioxidant [14], anticancer [15,16], fungicidal [17], and neuroprotective activities [18]. It was reported that novel 3,4-dimethoxy cinnamic acid derivatives having N-benzyl pyridinium showed higher cholinesterase inhibitory activity than unsubstituted cinnamic acid derivatives [19]. The presence of methoxy groups in 3,4-dimethoxy cinnamamides enhances their biological activities because they participate in various interactions, which are crucial for their binding affinity and selectivity towards specific biological targets.
Tranilast, N-(3′,4′-dimethoxycinnamoyl)anthranilic acid, a marketed drug commonly prescribed for the treatment of bronchial asthma, allergic rhinitis, dermatitis, and other allergic conditions. In addition, it has been investigated for its potential use in the treatment of various inflammatory disorders such as keloids and ocular inflammation. Various biological activities of Tranilast reported [20] are depicted in Figure 1. Based on the above observations in the present work new chemical entities were designed to synthesize using molecular hybridization technique. Two biologically active moieties 3,4-dimethoxy cinnamic acid and substituted 2-aminothiophenes are coupled to get novel amide derivatives. The present study aimed to modify the structure of Tranilast by replacing anthranilic acid with substituted 2-aminothiophenes. The study also aimed to evaluate in-vitro antioxidant, in-vitro anti-inflammatory activities of synthesized compounds.
MATERIALS AND METHODS
Reagents and instruments
All the chemicals (reagents and solvents) were purchased from commercial suppliers (Merck and Avra chemicals) and they were used as received without further purification. The progress of the reactions was monitored by thin-layer chromatography (TLC) on TLC Silica gel 60 F254 plates. Spots were visualized by ultraviolet light. Melting points were determined by an electrical melting point apparatus (Temp-SM1056) and were uncorrected. FT-IR spectra for all the compounds were recorded on Bruker analyzer FT-IR spectrophotometer (KBr pressed pellet technique). 1H and 13C NMR spectra were recorded on Bruker AMX-400 MHz and 100 MHz spectrometers (chemical shifts in ppm) using tetramethylsilane as an internal standard. The mass spectra of the compounds were recorded on Agilent 6120 single quadrupole LC-MS with ES-APCI.
Synthesis of substituted 2-aminothiophenes
Ketones like 2-butanone, cyclopentanone, cyclohexanone, and cycloheptanone (0.01 mol), activated nitriles such as ethyl cyanoacetate, cyanoacetamide, and malononitrile (0.01 mol), and sulfur (0.01 mol, 0.32 g) in 20 ml of ethanol were stirred at 50°C. To this mixture morpholine (0.025 mol, 2.16 ml) was added dropwise, and stirring was continued for another 3 hours at ambient temperature. Furthermore, the resultant solution was left overnight in the refrigerator and the obtained crude compound was filtered, dried, and recrystallized from absolute ethanol. Synthesized substituted 2-aminothiophenes (1-12) were characterized by reported reference melting points [21-23].
 | Figure 1. Various biological activities of tranilast. [Click here to view] |
Synthesis of (E)-3-(3,4-dimethoxyphenyl)acryloyl chloride
3,4-Dimethoxy cinnamic acid (0.01 mol, 2.26 g) and thionyl chloride (0.04 mol, 3 ml) were refluxed at 80°C–90°C for about 4 hours [24]. The unreacted thionyl chloride was distilled off and the resultant precipitate was used for the next step without any purification.
General procedure for the synthesis of novel 3,4-dimethoxy cinnamamide derivatives containing substituted 2-aminothiophenes (1A-12A)
(E)-3-(3,4-Dimethoxyphenyl)acryloyl chloride in 10 ml acetone was added dropwise to a solution containing substituted 2-aminothiophene (0.01 mol) and pyridine in 10 ml acetone, keeping the temperature at 0°C [25]. The reaction mixture was stirred at room temperature for half an hour until the completion of the reaction, monitored by TLC. The obtained precipitate was filtered, dried, and recrystallized by using absolute ethanol. Furthermore, all the synthesized compounds were characterized by IR, 1H-NMR, 13C-NMR, and Mass spectra.
Ethyl 2-((E)-3-(3,4-dimethoxyphenyl)acrylamido)-4,5-dimethylthiophene-3-carboxylate (1A)
Light brown color solid. IR [KBr film] cm-1: 3232.77 (NH, str), 2840.09 (CH, str), 1782.41 and 1683.57 (C = O, str), 1266.13 (asymmetric C-O-C, str), 1024.59 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.35–1.38 (t, 3H, O-CH2CH3), 1.66 (s, 3H, CH3), 2.31 (s, 3H, CH3), 3.94 (s, 6H, OCH3), 4.27–4.32 (q, 2H, O-CH2CH3), 6.32–6.36 (d, 1H, Ar-H), 6.89–6.91 (d, 1H, CO-CH = CH), 7.100–7.104 (d, 1H, Ar-H), 7.15 (s, 1H, Ar-H), 7.73–7.77 (d, 1H, CO-CH = CH), 11.56 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 12.3, 14.0, 14.8, 55.9, 56.0, 60.7, 103.2, 109.8, 116.4, 118.0, 119.9, 128.2, 129.4, 130.0, 143.3, 148.6, 148.7, 160.1, 162.1, 166.4. ES + APCI MS: m/z 390 (M + H) +.
2-((E)-3-(3,4-Dimethoxyphenyl)acrylamido)-4,5-dimethylthiophene-3-carboxamide (2A)
Brown solid. IR [KBr film] cm-1: 3329.57 and 3247.93 (NH, str), 2937.54, 2837.91 (CH, str), 1685.95 (C = O, str), 1267.89 (asymmetric C-O-C, str), 1023.34 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.64 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.94 (s, 6H, OCH3), 6.33-6.36 (d, 1H, Ar-H), 6.89–6.91 (d, 1H, CO-CH = CH), 7.10 (s, 1H, Ar-H), 7.15–7.17 (d, 1H, Ar-H), 7.72–7.76 (d, 1H, CO-CH = CH), 10.10 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 12.2, 14.5, 56.0, 56.1, 103.1, 109.7, 116.2, 117.8, 119.7, 128.0, 129.2, 129.9, 143.1, 148.5, 148.6, 160.1, 161.9, 166.3. ES + APCI MS: m/z 361 (M + H) +.
(E)-N-(3-Cyano-4,5-dimethylthiophen-2-yl)-3-(3,4-dimethoxyphenyl) acrylamide (3A)
Brown solid. IR [KBr film] cm-1: 3365.78 (NH, str), 2933.01, 2843.72 (CH, str), 2194.24 (CN str), 1682.98 (C = O, str), 1262.36 (asymmetric C-O-C, str), 1024.46 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.64 (s, 3H, CH3), 2.31 (s, 3H, CH3), 3.95 (s, 6H, OCH3), 6.33-6.37 (d, 1H, Ar-H), 6.90–6.92 (d, 1H, CO-CH = CH), 7.10 (s, 1H, Ar-H), 7.15–7.17 (d, 1H, Ar-H), 7.74–7.78 (d, 1H, CO-CH = CH), 10.61 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 12.3, 14.7, 56.2, 56.9, 102.2, 109.7, 112.4, 118.5, 123.1, 128.4, 128.8, 129.8, 130.7, 143.8, 148.4, 148.5, 162.1, 166.3. ES + APCI MS: m/z 343 (M + H) +.
Ethyl 2-((E)-3-(3,4-dimethoxyphenylacrylamido)-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate (4A)
Light brown solid. IR [KBr film] cm-1: 3179.01 (NH, str), 2955.59 (CH, str), 1745.29 and 1654.33 (C = O, str), 1205.63 (asymmetric C-O-C, str), 1047.43 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.40–1.44 (t, 3H, O-CH2CH3), 2.35–2.37 (t, 2H, CH2), 2.57–2.72 (t, 2H, CH2), 2.74–2.83 (m, 2H, CH2), 3.83 (d, 6H, OCH3), 4.35–4.59 (q, 2H, O-CH2CH3), 6.53–6.59 (d, 1H, Ar-H), 7.10–7.11 (d, 1H, CO-CH = CH), 7.41 (s, 1H, Ar-H), 7.58–7.59 (d, 1H, Ar-H), 7.76–7.80 (d, 1H, CO-CH = CH), 11.75 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 14.8, 27.8, 27.8, 29.9, 55.2, 55.7, 60.1, 108.9, 109.0, 110.7, 119.9, 126.3, 127.7, 127.9, 129.5, 139.9, 147.8, 148.1, 159.9, 161.3, 165.7. ES + APCI MS: m/z 402 (M + H) +.
2-((E)-3-(3,4-Dimethoxyphenyl)acrylamido)-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxamide (5A)
Brown solid. IR [KBr film] cm-1: 3454.15 and 3374.65 (NH, str), 2944.69 (CH, str), 1689.05 (C = O, str), 1270.93 (asymmetric C-O-C, str), 1028.41 (symmetric C-O-C, str). 1H-NMR [400 MHz, DMSO] δ: 1.99–2.00 (t, 2H, CH2), 2.34–2.38 (t, 2H, CH2), 2.45–2.48 (m, 2H, CH2), 3.79–3.80 (d, 6H, OCH3), 6.41–6.45 (d, 1H, Ar-H), 6.97–6.99 (d, 1H, CO-CH = CH), 7.19–7.21 (d, 1H, Ar-H), 7.30 (s, 1H, Ar-H), 7.50–7.54 (d, 1H, CO-CH = CH), 11.60 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 27.7, 27.7, 29.8, 55.5, 55.9, 109.2, 109.3, 110.9, 120.2, 126.5, 127.9, 128.1, 129.8, 140.2, 148.0, 148.4, 160.1, 161.5, 166.0. ES + APCI MS: m/z 373 (M + H)+.
(E)-N-(3-Cyano-5,6-dihydro-4H-cyclopenta[b]thiophen-2-yl)-3-(3,4-dimethoxyphenyl)acrylamide (6A)
Brown solid. IR [KBr film] cm-1: 3322.99 (NH, str), 2935.17, 2840.19 (CH, str), 2124.12 (CN, str), 1682.46 (C = O, str), 1262.10 (asymmetric C-O-C, str), 1024.12 (symmetric C-O-C, str). 1H-NMR [400 MHz, DMSO] δ: 1.96–2.00 (t, 2H, CH2), 2.33–2.37 (t, 2H, CH2), 2.44–2.47 (m, 2H, CH2), 3.79–3.80 (d, 6H, OCH3), 6.42–6.46 (d, 1H, Ar-H), 6.97–6.99 (d, 1H, CO-CH = CH), 7.19–7.21 (d, 1H, Ar-H), 7.31 (s, 1H, Ar-H), 7.50–7.54 (d, 1H, CO-CH = CH), 10.79 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 27.7, 27.7, 29.8, 56.2, 56.3, 95.3, 112.9, 115.3, 116.6, 118.7, 119.9, 128.8, 128.9, 139.7, 143.8, 146.5, 146.7, 161.6, 166.5. ES + APCI MS: m/z 355 (M + H) +.
Ethyl 2-((E)-3-(3,4-dimethoxyphenyl)acrylamido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (7A)
Light brown solid. IR [KBr film] cm-1: 3298.72 (NH, str), 2939.08, 2854.81 (CH, str), 1742.36 and 1648.33 (C = O, str), 1205.86 (asymmetric C-O-C, str), 1026.15 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.33–1.37 (t, 3H, O-CH2CH3), 1.58–1.59 (m, 4H, CH2-CH2), 2.39–2.41 (m, 4H, CH2-CH2), 3.94 (s, 6H, OCH3), 4.35–4.40 (q, 2H, O-CH2CH3), 6.32–6.36 (d, 1H, Ar-H), 6.89–6.91 (d, 1H, CO-CH = CH), 7.095–7.099 (d, 1H, Ar-H), 7.14 (s, 1H, Ar-H), 7.72–7.76 (d, 1H, CO-CH = CH), 11.56 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 14.3, 22.5, 23.4, 24.2, 26.2, 56.3, 56.8, 60.5, 109.3, 109.5, 111.7, 119.5, 127.3, 128.1, 128.9, 130.1, 142.3, 148.4, 149.1, 160.1, 162.3, 166.7. ES + APCI MS: m/z 416 (M + H) +.
2-((E)-3-(3,4-Dimethoxyphenyl)acrylamido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide (8A)
Brown solid. IR [KBr film] cm-1: 3405.61 and 3341.67 (NH, str), 2940.87, 2841.67 (CH, str), 1683.89 (C = O, str), 1264.01 (asymmetric C-O-C, str), 1025.19 (symmetric C-O-C, str). 1H-NMR [400 MHz, DMSO] δ: 1.57–1.58 (m, 4H, CH2-CH2), 2.49–2.51 (m, 4H, CH2-CH2), 3.79–3.80 (d, 6H, OCH3), 6.42–6.46 (d, 1H, Ar-H), 6.97–6.99 (d, 1H, CO-CH = CH), 7.19–7.21 (d, 1H, Ar-H), 7.31 (s, 1H, Ar-H), 7.50–7.54 (d, 1H, CO-CH = CH), 11.60 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 22.6, 23.5, 25.0, 27.1, 56.1, 56.9, 109.9, 110.0, 112.7, 120.1, 126.9, 128.5, 129.9, 130.1, 142.3, 148.4, 149.2, 160.1, 162.3, 167.0. ES + APCI MS: m/z 387 (M + H) +.
(E)-N-(3-Cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-3-(3,4-dimethoxy phenyl)acrylamide (9A)
Brown solid. IR [KBr film] cm-1: 3155.52 (NH, str), 2954.79 (CH, str), 2214.71 (CN, str), 1651.39 (C = O, str), 1267.01 (asymmetric C-O-C, str), 1027.57 (symmetric C-O-C, str). 1H-NMR [400 MHz, DMSO] δ: 1.54–1.58 (m, 4H, CH2-CH2), 2.69 (s, 2H, CH2), 2.97 (s, 2H, CH2), 3.82 (s, 6H, OCH3), 6.45–6.48 (d, 1H, Ar-H), 6.96–6.99 (d, 1H, CO-CH = CH), 7.22–7.24 (d, 1H, Ar-H), 7.35 (s, 1H, Ar-H), 7.54–7.57 (d, 1H, CO-CH = CH), 10.99 (s, 1H, NH). 13C-NMR [100 MHz, DMSO] δ: 27.1, 27.9, 28.0, 28.3, 56.0, 56.9, 99.1, 110.5, 113.9, 118.1, 123.5, 127.6, 131.4, 136.6, 143.1, 144.0, 149.4, 151.3, 163.1, 165.6. ES + APCI MS: m/z 369 (M + H) +.
Ethyl 2-((E)-3-(3,4-dimethoxyphenyl)acrylamido)-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate (10A)
Light brown solid. IR [KBr film] cm-1: 3170.11 (NH, str), 2928.51 (CH, str), 1762.68 and 1637.39 (C = O, str), 1232.05 (asymmetric C-O-C, str), 1046.56 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.41–1.45 (t, 3H, O-CH2CH3), 1.63–1.69 (m, 4H, CH2-CH2), 1.81–1.87 (qu, 2H, CH2), 2.57–2.60 (t, 2H, CH2), 2.75–2.77 (t, 2H, CH2), 3.96 (s, 6H, OCH3), 4.36–4.42 (q, 2H, O-CH2CH3), 6.48–6.52 (d, 1H, Ar-H), 6.88–6.90 (d, 1H, CO-CH=CH), 7.09–7.11 (d, 1H, Ar-H), 7.15 (s, 1H, Ar-H), 7.71–7.74 (d, 1H, CO-CH = CH), 11.42 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 14.3, 27.5, 28.0, 28.9, 29.2, 30.9, 57.3, 57.8, 61.9, 108.1, 109.4, 111.5, 119.4, 127.2, 128.0, 128.8, 129.9, 142.2, 148.3, 149.0, 160.0, 162.2, 166.6. ES + APCI MS: m/z 430 (M + H) +.
2-((E)-3-(3,4-Dimethoxyphenyl)acrylamido)-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxamide (11A)
Brown solid. IR [KBr film] cm-1: 3242.15 and 3168.09 (NH, str), 2930.63, 2819.55 (CH, str), 1634.21 (C = O, str), 1242.85 (asymmetric C-O-C, str), 1025.84 (symmetric C-O-C, str). 1H-NMR [400 MHz, CDCl3] δ: 1.69–1.72 (m, 4H, CH2-CH2), 1.86–1.91 (qu, 2H, CH2), 2.72–2.79 (m, 4H, CH2-CH2), 3.76 (s, 6H, OCH3), 6.40–6.44 (d, 1H, Ar-H), 6.96–6.97 (d, 1H, CO-CH = CH), 7.18–7.20 (d, 1H, Ar-H), 7.29 (s, 1H, Ar-H), 7.49–7.53 (d, 1H, CO-CH = CH), 11.12 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 27.4, 27.9, 28.8, 29.2, 30.9, 56.4, 56.7, 108.2, 109.4, 112.1, 120.0, 125.2, 128.1, 128.6, 130.0, 142.2, 148.3, 149.1, 160.0, 162.3, 166.7. ES + APCI MS: m/z 401 (M + H) +.
(E)-N-(3-Cyano-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophen-2-yl)-3-(3,4-dimethoxyphenyl)acrylamide (12A)
Brown solid. IR [KBr film] cm-1: 3259.27 (NH, str), 2936.83, 2859.38 (CH, str), 2257.58 (CN, str), 1652.71 (C = O, str), 1210.75 (asymmetric C-O-C, str), 1051.49 (symmetric C-O-C, str). 1H-NMR [400 MHz, DMSO] δ: 1.62–1.68 (m, 4H, CH2-CH2), 1.81–1.86 (m, 2H, CH2), 2.56–2.57 (t, 2H, CH2), 2.73–2.75 (t, 2H, CH2), 3.73 (s, 6H, OCH3), 6.95–6.98 (d, 1H, CO-CH = CH), 7.21–7.24 (d, 1H, Ar-H), 7.45–7.48 (d, 1H, Ar -H), 7.69 (s, 1H, Ar-H), 7.95–7.97 (d, 1H, CO-CH = CH), 10.94 (s, 1H, NH). 13C-NMR [100 MHz, CDCl3] δ: 27.4, 27.9, 28.8, 29.2, 30.8, 56.4, 56.7, 99.7, 108.1, 109.3, 112.0, 119.9, 125.3, 128.1, 128.8, 129.9, 142.0, 148.1, 149.6, 161.2, 165.3. ES + APCI MS: m/z 383 (M + H) +.
Biological evaluation
In-vitro antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay [26] and hydroxyl radical scavenging activity [27] were carried out to assess the in-vitro antioxidant activity as per the reference procedures for all the synthesized compounds (1A-12A) in comparison with reference standard Ascorbic acid.
DPPH free radical scavenging assay
DPPH free radical scavenging ability of the synthesized compounds (1A-12A) was carried out according to the reference procedure. Solutions of synthesized compounds at 100 μM concentration were added to 100 μM DPPH in 95% ethanol. The resultant solution was kept at an ambient temperature for 20 minutes and absorbance was measured at 517 nm. Ascorbic acid (100 μM) was used as a reference. The percentage of DPPH free radical scavenging activity was calculated as per the following formula:
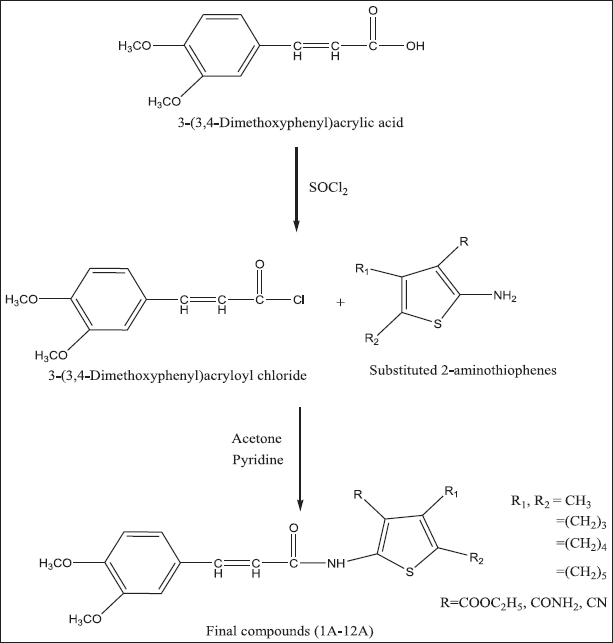 | Scheme 1. Schematic representation for the synthesis of 3-(3,4-dimethoxyphenyl)acryl amido derivatives (1A-12A). [Click here to view] |
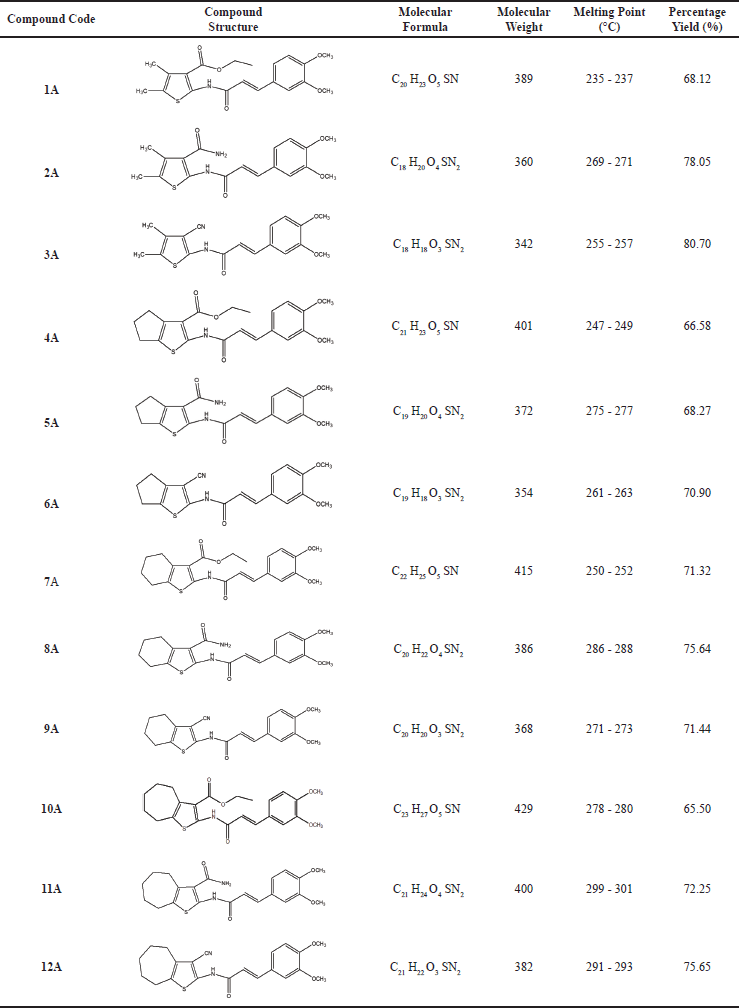 | Table 1. Physical data of novel 3,4-dimethoxy cinnamamide derivatives containing substituted 2-aminothiophenes (1A-12A). [Click here to view] |
Percentage of DPPH free radical scavenging = [(Control-Test)/Control] × 100
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging ability of the samples was determined according to the reference procedure. Briefly, individual test compounds (1 ml) at 100 μM concentration were added to the reagent containing 1 ml FeSO4 (1.5 mM), 0.7 ml H2O2 (6 mM) and 0.3 ml sodium salicylate (20 mM) and incubated for 1 hour at 37°C.The absorbance of the resulting solution was measured at 562 nm. Ascorbic acid was used as standard. The percentage scavenging was calculated according to the following formula:
% Scavenging activity = [1 - (A1-A2)/A0] × 100
where A0 is the control solution absorbance (containing all reagents except the test compound), A1 is the test compound absorbance with sodium salicylate and A2 is absorbance without sodium salicylate.
In-vitro anti-inflammatory activity
In-vitro anti-inflammatory efficacy of the synthesized compounds 1A-12A was performed by using the Protein denaturation method [28] and human red blood cells (HRBC) Membrane stabilization method [29,30] in comparison with Ibuprofen as the reference standard.
Protein denaturation method
The protein denaturation method was carried out by preparing the reaction mixture (0.5 ml) consisting of 0.45 ml of bovine serum albumin (5 % w/v aqueous solution) and 0.05 ml of test samples of different concentrations (10, 25, 50, and 100 μg/ml). For the control solution, 0.05 ml distilled water was used instead of the test sample, while the product control solution lacked bovine serum albumin. The pH of the solutions was adjusted to 6.3 using 1 N hydrochloric acid. The above solutions were incubated at 37°C for 20 minutes and the temperature was increased to 57°C for 3 minutes. After incubation, solutions were allowed to cool and 2.5 ml of phosphate buffer saline was added. Ibuprofen was used as the standard. The absorbance was measured using a UV-Visible Spectrophotometer at 416 nm. The percentage inhibition of protein denaturation was calculated as per the following formula:
% Inhibition of protein denaturation = 100 – [(Optical density of test solution – Optical density of product control)/Optical density of test control × 100]
HRBC membrane stabilization method
In the HRBC membrane stabilization method, HRBC suspension was prepared by collecting human blood from the volunteers and mixed with an equal volume of sterilized Alsever solution (2 % Dextrose, 0.8 % Sodium citrate, 0.05 % Citric acid, and 0.42 % Sodium chloride in water). The blood was centrifuged at 3,000 rpm for 10 minutes and packed blood cells were rinsed thoroughly using an isosaline solution thrice. The volume of blood cells was measured and reconstituted as 10 % v/v suspension with isosaline. The reaction mixture consists of 1 ml of phosphate buffer [pH 7.4, 0.15 M], 2 ml of hyposaline [0.36 %], 0.5 ml of HRBC suspension [10 % v/v], and 1 ml of test samples of various concentrations (10, 25, 50, and 100 μg/ml). Ibuprofen was used as standard. Instead of hyposaline, 2 ml of distilled water was used as a control to achieve 100 % hemolysis. All the test solutions were incubated at 37°C for 30 minutes and centrifuged at 3,000 rpm for 20 minutes. The optical density of incubated solutions was measured at 560 nm. The percentage of HRBC membrane stabilization was calculated using the following formula:
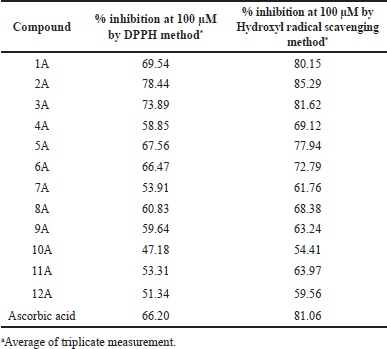 | Table 2. In-vitro antioxidant activity of 1A-12A against DPPH and Hydroxyl free radical scavenging assays. [Click here to view] |
% Protection = 100 – [(Optical density of test sample/Optical density of control) × 100]
RESULTS AND DISCUSSION
Chemistry
In present work, 3,4-dimethoxy cinnamic acid was converted into (E)-3-(3,4-dimethoxy phenyl)acryloyl chloride using thionyl chloride and condensed with substituted 2-aminothiophenes (1-12) in the presence of acetone and pyridine as illustrated in Scheme 1 to get various novel 3,4-dimethoxy cinnamamide derivatives (1A–12A) in good yield. The physical data is depicted in Table 1. Structures of all synthesized compounds (1A–12A) were deduced from FT-IR, 1H-NMR, 13C-NMR, and Mass spectral analysis. The FT-IR spectra of compounds revealed the absence of amine and carboxylic acid peaks. The appearance of the amide peak confirms the formation of the amide compounds. The absorption bands due to NH stretching and carbonyl stretching of the amide functional group appeared in the range of 3454–3155 cm-1 and 1689–1634 cm−1, respectively. The absorption band at 1782–1742 cm−1 in compounds 1A, 4A, 7A, and 10A confirmed the presence of carbonyl carbon in the ester functional group. A sharp band at 2257–2124 cm−1 in compounds 3A, 6A, 9A, and 12A confirmed the presence of a nitrile functional group. Proton NMR spectra of synthesized compounds revealed the presence of a 3,4-dimethoxyphenyl acrylamido group connecting with substituted thiophene moiety. In 1H-NMR spectra, protons of 3,4-dimethoxyphenyl acrylamido group appear in the following ranges, NH protons appear as a singlet at δ 10.10–11.79 and protons of CH = CH of acryloyl groups shows doublet at δ 7.49–7.97 and δ 6.88–6.99. Two aromatic hydrogens appeared as two doublets at δ 6.32–7.24 and 7.09–7.48; one aromatic hydrogen shows a singlet at δ 7.10–7.69 and protons of two methoxy groups appears as a singlet at δ 3.73–3.95; The protons of substituted thiophene ring appears in the following ranges, CH3 of compounds 1A, 2A, and 3A appears as singlet at δ 1.64–2.35; CH2 of fused cyclic rings in compounds 4A-12A appears as multiplet between δ 1.54 and 2.97; Methyl groups in ethyl ester of compounds 1A, 4A, 7A, and 10A shows triplet at δ 1.33–1.45 and the adjacent CH2 shows quartet at δ 4.27–4.42.
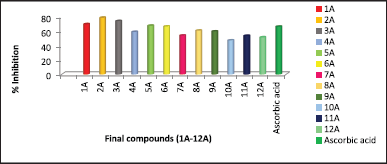 | Figure 2. Comparative percentage inhibition values of 1A–12A by DPPH free radical scavenging assay. [Click here to view] |
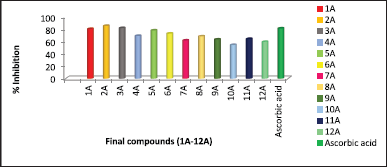 | Figure 3. Comparative percentage inhibition values of 1A-12A by Hydroxyl radical scavenging activity. [Click here to view] |
In 13C-NMR spectra, amide carbonyl carbon signal appears between δ 160.0 and 160.1, acryloyl carbon signal appears at δ 118.1–144.0, and aromatic carbons appeared in the range of δ 109.2–151.3. Methoxy group carbons appear between δ 55.2 and 57.8. Thiophene ring carbons appear in the range of δ 95.3–167.0, whereas ester carbonyl carbon shows the signal at δ 159.9–160.1 and nitrile carbon signal varies between δ 108.1 and 113.9. Aliphatic carbon signals appeared in the range of δ 14.0–61.9. Mass spectra of all the synthesized compounds showed [M + H]+ peak equivalent to their molecular weight.
Biological evaluation
In-vitro antioxidant activity
The DPPH free radical scavenging assay of the synthesized compounds (1A-12A) was carried out at a concentration of 100 μM and the results are presented in Table 2. All the compounds exhibited good antioxidant activity, among all 2A, 3A, 1A, 5A, and 6A exhibited the highest percentage inhibition of DPPH free radical, greater than standard drug Ascorbic acid. The substituent group at the third position of the thiophene ring of synthesized compounds played a greater effect on the DPPH scavenging ability. On observation of data, modification of 4,5-dimethyl groups on thiophene to cyclic analogs resulted in a reduction of antioxidant activity. An increase in the ring size of cyclic derivatives decreased antioxidant activity. The Comparative percentage inhibition at 100 μM concentration of synthesized compounds and the standard was depicted in Figure 2.
Hydroxyl radical scavenging activity results revealed that all the evaluated compounds showed scavenging activity towards hydroxyl radical, the data presented in Table 2 and the comparative results depicted in Figure 3. All the compounds showed better antioxidant activity in this model. Among all, 2A and 3A compounds exhibited greater activity and 1A compound showed equal scavenging activity towards hydroxyl radical when compared with standard Ascorbic acid. The effect of substituent groups over the thiophene ring followed a similar pattern of antioxidant activity when compared with the results of DPPH free radical scavenging.
In-vitro anti-inflammatory activity
Protein denaturation is a well-documented method for this analysis. Percentage inhibition by protein denaturation was measured for all the synthesized compounds (1A-12A) and the data is given in Table 3. The comparative results are presented in Figure 4. The results revealed that all the compounds showed good activity, among all the compounds 2A, 3A, and 1A were more active at all concentrations tested and noticed the highest inhibition percentages 86.51, 85.34, and 83.87, respectively, at 100 μg/ml. The activity of these compounds is comparable with the known standard Ibuprofen (85.04 % at 100 μg/ml). In the HRBC membrane stabilization method percentage protection was measured for all the synthesized compounds, and the data is given in Table 4 and the comparative results are depicted in Figure 5. The evaluation of results revealed that compounds 2A and 3A were more active and showed 82.23 and 80.28 percentage protections respectively at 100 μg/ml. In general, the percentage protection increased with increasing concentration of test compound. The data also revealed the importance of amide and nitrile substitution over thiophene ring. 4,5-dimethyl substitution on thiophene ring increases in-vitro anti-inflammatory activity and modification of methyl groups to cyclic analogues reduced the activity.
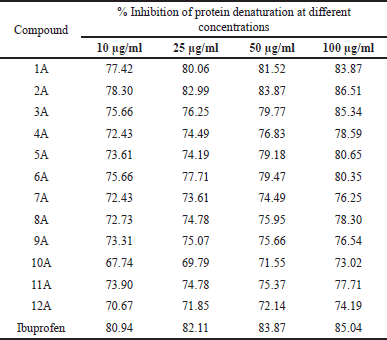 | Table 3. In-vitro anti-inflammatory activity of 1A-12A by protein denaturation method. [Click here to view] |
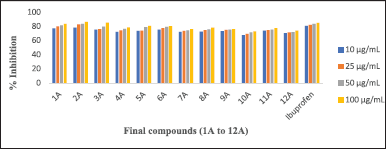 | Figure 4. Comparative percentage inhibition values of 1A-12A by protein denaturation method. [Click here to view] |
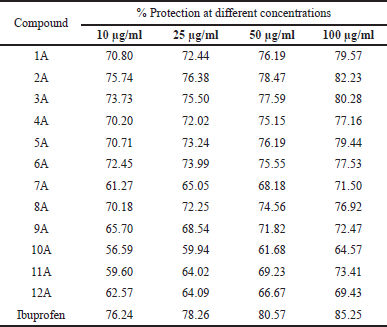 | Table 4. In-vitro anti-inflammatory activity of 1A-12A by HRBC membrane stabilization method. [Click here to view] |
 | Figure 5. Comparative In-vitro anti-inflammatory activity of 1A–12A by HRBC membrane stabilization method. [Click here to view] |
CONCLUSION
A series of thiophene analogs of Tranilast was prepared with slight modification by replacing anthranilic acid with substituted 2-aminothiophenes. Investigation of in-vitro antioxidant and anti-inflammatory evaluation data revealed that all the synthesized compounds, 3-(3,4-dimethoxyphenyl)acryl amido derivatives of substituted thiophenes, showed good scavenging ability against DPPH and hydroxyl radicals. The compounds 2A and 3A exhibited significant in-vitro anti-inflammatory activity by protein denaturation and HRBC membrane stabilization methods. This is due to the presence of amide or nitrile functionality at the third position and methyl groups at the fourth and fifth position on thiophene ring. A similar pattern of activity was also observed for cyclic compounds having amide or nitrile functionality. The present research concludes that the in-vitro anti-inflammatory activity indicated the importance of structural components of evaluated compounds and offers a fresh perspective for the development of potential lead. Furthermore, in-vivo anti-inflammatory activity and the safety of tested compounds needed to be determined by the carrageenan rat paw edema method and acute toxicity studies.
LIST OF ABBREVIATIONS
1H-NMR, Proton nuclear magnetic resonance; 13C-NMR, Carbon-13 nuclear magnetic resonance; HRBC, Human red blood cells; IR, Infrared; TLC, Thin layer chromatography.
ACKNOWLEDGMENTS
One of the authors, Satya Sree B, is thankful to the V. V. Institute of Pharmaceutical Sciences for providing the necessary facilities to carry out this research work. The author is also thankful to the Hindu College of Pharmacy for providing IR spectra and to Laila Implex Research Center, Vijayawada, Andhra Pradesh, for providing 1H, 13C NMR, and Mass spectra.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gewald K, Schinke E, Bottcher H. 2-Amino-thiophene aus methylenaktiven Nitrilen, Carbonylverbindungen und Schwefel. ChemBer. 1966;(99):94–100. CrossRef
2. Priyanka SC, Sohan SC, Rabindra K. Molecular docking, synthesis and anti-inflammatory activity of some tetrasubstituted thiophene derivatives. Int Res J Pharm. 2018;9(6):163–9. CrossRef
3. Mahdavi B, Hosseyni-Tabar SM, Rezaei-Seresh E, Rezaei-Seresht H, Falanji F. Synthesis and biological evaluation of novel hybrid compounds derived from gallic acid and the 2-aminothiophene derivatives. J Iran Chem Soc. 2020;(17):809–15. CrossRef
4. Madhavi K, Ramanamma KV. Synthesis and evaluation of ethyl 2-(2-cyano-3-(substituted phenyl)acrylamido)-4, 5, 6, 7-tetrahydrobenzo[b]thiophene-3-carboxylates for antioxidant and antibacterial activities. Int J Curr Microbiol Appl Sci. 2016;5(1):364–75. CrossRef
5. Asiri YI, Muhsinah AB, Alsayari A, Venkatesan K, Al-Ghorbani M, Mabkhot YN. Design, synthesis and antimicrobial activity of novel 2-aminothiophene containing cyclic and heterocyclic moieties. Bioorganic Med Chem Lett. 2021;(44):128117–22. CrossRef
6. Thomas J, Jecic A, Vanstreels E, Berckelaer LV, Romagnoli R, Dehaen W, Liekens S, Balzarini J. Pronounced anti-proliferative activity and tumor cell selectivity of 5-alkyl-2-amino-3-methylcarboxylate thiophenes. Eur J Med Chem. 2017;(132):219–35. CrossRef
7. Rodrigues KADF, Dias CNDS, Neris PLDN, Rocha JDC, Scotti MT. 2-Amino thiophene derivatives present antileishmanial activity mediated by apoptosis and immunomodulation in vitro. Eur J Med Chem. 2015;(106):1–14. CrossRef
8. Kunda PK, Rao JV, Mukkanti K, Induri M, Reddy GD. Synthesis, anticonvulsant activity and in-silico studies of schiff bases of 2-aminothiophenes via guanidine-catalyzed Gewald reaction. Trop J Pharm Res. 2013;12(4):566–76. CrossRef
9. Ibrahim BA, Mohareb RM. Uses of ethyl benzoyl acetate for the synthesis of thiophene, pyran, and pyridine derivatives with antitumor activities. J Heterocycl Chem. 2020;(57):4023–35. CrossRef
10. Shimada O, Yasuda H. Hydroxyl radical scavenging action of tinoridine. Agents Actions. 1986;19(3-4):208–14. CrossRef
11. Tuzun S, Uzun H, Aydin S, Dinc A, Sipahi S, Topcuoglu MA et al. Effect of flubiprofen and tiaprofenic acid on oxidative stress markers in osteoarthritis: a prospective, randomized, open label, active and placebo-controlled trial. Curr Therap Res. 2005;66(5):335–44. CrossRef
12. Lau CS, Belch JJF. The in vitro free radical scavenging activity of tenidap, a new dual cyclo-oxygenase and 5-1ipoxygenase inhibitor. Mediators Inflamm. 1992;(1):141–3. CrossRef
13. Andrade PB, Leitao R, Seabra RM, Oliveira MB, Ferreira MA. 3,4-Dimethoxycinnamic acid levels as a tool for differentiation of Coffea canephora var. robusta and Coffea arabica. Food Chem. 1998;61(4):511–4. CrossRef
14. Nobelos PT, Papagiouvannis G and Rekka EA. Ferulic, sinapic, 3,4-dimethoxycinnamic acid and indomethacin derivatives with antioxidant, anti-inflammatory and hypolipidemic functionality. Antioxidants. 2023;12(7):1436–51. doi: https://doi.org/10.3390/antiox12071436.
15. De P, Baltas M, Belval FB. Cinnamic acid derivatives as anticancer agents-a review. Curr Med Chem. 2011;18(11):1672–703. CrossRef
16. Ling Y, Gao WJ, Ling C, Liu J, Meng C, Qian J, et al. β-Carboline and N-hydroxy cinnamamide hybrids as anticancer agents for drug-resistant hepatocellular carcinoma. Eur J Med Chem. 2019;(168):515–26. CrossRef
17. Chen L, Zhao B, Fan Z, Hu M, Li Q, Hu W, et al. Discovery of novel isothiazole, 1,2,3-thiadiazole, and thiazole-based cinnamamides as fungicidal Candidates. J Agric Food Chem. 2019;67(45):12357–65. CrossRef
18. Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 2012;12(8):749–67. CrossRef
19. Lan JS, Hou JW, Liu Y, Ding Y, Zhang Y, Li L, et al. Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J Enzyme Inhib Med Chem. 2017;32(1):776–8. CrossRef
20. Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res. 2015;(91):15–28. CrossRef
21. Josue SBF, Erika MC, Joel JJ, Flavia MS. A new protocol for the synthesis of 2-aminothiophenes through the Gewald reaction in solvent-free conditions. Heterocycl Let. 2011;1(1):61–7. CrossRef
22. Haswani NG, Sanjaykumar BB. Synthesis and antimicrobial activity of novel 2-(pyridin -2-yl)thieno[2,3-d]pyrimidin-4 (3H)-ones. Turk J Chem. 2011;35(6):915–24. CrossRef
23. Durgareddy GANK, Ravikumar R, Ravi S, Srinivas RA. A CaO catalyzed facile one pot synthesis of 2-aminothiophenes using Gewald reaction. Der Pharma Chemica. 2013;5(6):294–8. CrossRef
24. Gangadhara S, Prasad Ch, Venkateswarlu P. Synthesis, antimicrobial and antioxidant activities of novel series of cinnamamide derivatives having morpholine moiety. Med Chem. 2014;4(12):778–83. CrossRef
25. Jitareanu A, Tataringa G, Zbancioc AM, Tuchilus C, Balan M, Stanescu U. Cinnamic acid derivatives and 4-aminoantipyrine amides–synthesis and evaluation of biological properties. Res J Chem Sci. 2013;3(3):9–13. Available from: http://www.isca.in
26. Madhavi K, Swathi K, Anitha B, Sree GRU, Sravanthi G, Ashwini G. Synthesis and evaluation of novel α-cyano-N-(4-hydroxyphenyl)cinnamamides for antioxidant, anti-inflammatory activities: in-silico prediction of drug likeness properties. Int J Pharm Sci Res. 2019;10(1):203–13. CrossRef
27. Gopalakrishnan VK, Rajamanikandan S, Sindhu T, Durgapriya D, Sophia D, Ragavendran P. Radical scavenging and antioxidant activity of ethanolic extract of Mollugo nudicaulis by in vitro assays. Ind J Pharm Edu Res. 2011;45(4):310–6.
28. Kar B, Kumar RS, Karmakar I, Dola N, Bala A, Mazumder UK, et al. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac J Trop Biomed. 2012;(2):S976–80. CrossRef
29. Kumar V, Bhat ZA, Kumar D, Puja B, Sheela S. In-vitro anti-inflammatory activity of leaf extracts of Basella Alba linn. Var. Alba. Int J Drug Dev Res. 2011;3(2):176–9. Available from: http://www.ijddr.in.
30. Shahnaj PM, Nandita D, Nusrat J, Afia AM, Nahar L, Islam EM. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res Notes. 2015;8:412–8. CrossRef