INTRODUCTION
Vascular aging is an important risk factor for cardiovascular diseases (CVDs) which is associated with endothelial dysfunction. One process that has been connected to aging and vascular pathologies is cellular senescence. Senescence is an arrest of cellular growth induced by stress stimuli such as inflammation, hyperglycemia, and oxidative stress. Excessive production of reactive oxygen species (ROS) stimulates vascular senescence, leading to decreased nitric oxide (NO) production, resulting in attenuated NO bioavailability and enhanced ROS. In addition, senescent cells can secrete chemokines and pro-inflammatory cytokines [1]. Several studies also demonstrated that ROS-induced senescence is involved in mitochondrial dysfunction, leading to ROS production [2,3]. Mitochondrial ROS production also activates inflammation and suppresses NO bioavailability. ROS stimulates senescence through the activation of p53/p21, p16, and suppression of Sirt1 [4–6]. Moreover, several studies have shown that p38 mitogen -activated protein kinase (MAPK) is involved in endothelial senescence [7–9].
α-Mangostin is the most xanthone substance that is extracted from mangosteen (Garcinia mangostana L.). It has been shown to have anti-oxidant properties [10,11], anti-tumour effects [12–14], anti-inflammatory effects [15,16], anti-apoptosis effects [13,17], and anti-angiogenesis effects [18–21]. The study demonstrated that α-mangostin suppressed ultraviolet B-induced apoptosis and senescence in keratinocytes [22]. Recently, a study showed mangosteen peel extracts have anti-oxidant, anti-elastase, and anti-collagenase activities [23]. The molecular effect of α-mangostin on H2O2-induced endothelial senescence has not been widely studied. The aim of this study is to determine the effect of α-mangostin on H2O2-induced endothelial senescence.
MATERIALS AND METHODS
Reagents
Fetal bovine serum (FBS), penicillin and streptomycin, Dulbecco’s Modified Eagle’s Medium (DMEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin-ethylenediaminetetraacetic acid, were purchased from Gibco-Invitrogen (Grand Island, NY, USA). 5,5’, 6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimi-dazoylcarbocyanine iodide (JC-1), Griess and nitrite were obtained from Merck Millipore (Darmstadt, Germany). 2’, 7’ dichlorodihydrofluorescein diacetate (DCF-DA) was purchased from Sigma-Aldrich (St. Louis, MO, USA), and the senescence assay kit was purchased from Biovision (Milpitas, California, USA). Sirt1(catalog No. 2310), p21(catalog No. 2947), acetylated p53 (catalog No. 2570), p53 (catalog No. 2524), p16 (catalog No. 80772), MnSOD (catalog No. 13194), and β-actin (catalog No. 3700) were obtained from Cell Signaling Technology (Danvers, MA, USA). Phospho-p38 (catalog No. Sc17852) and p38 MAPK (catalog No. Sc535) were purchased from Santa Cruz Biotechnology (CA, USA). Secondary antibodies against mouse (catalog No. AP124P) or rabbit (catalog No. AP132P) and enhanced chemiluminescence were purchased from Merck Millipore.
Plant material and extraction
α-Mangostin was kindly provided by Prof. Primchanien Moongkarndi, Faculty of Pharmacy, Mahidol University, Thailand. The extraction of α-mangostin was performed as in previous studies [24,25]. α-Mangostin was dissolved with dimethylsulfoxide (DMSO).
Cell culture
EA.hy926 (ATCC number CRL-2922), human endothelial cell lines, were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin at 37°C in 5% CO2. At 70% confluence, the cells were treated with a low serum medium (LSM) (DMEM supplemented with 1% FBS, 10 U/ml penicillin, and 10 U/ml streptomycin) for at least 6 hours before subsequent experiments.
The dose and time course of H2O2-induced cell senescence signaling were determined using Western blot. Cells were treated with 200 μM H2O2 for 60 minutes, and then the medium was replaced with LSM for 5, 15, 30, and 60 minutes 3, 6, 12, 24, and 48 hours. Also, H2O2 was used at doses of 25, 50, 100, and 200 μM for 60 minutes, and the medium was then replaced with LSM for the times stated above to detect p21 and Sirt1 signaling.
Cytotoxicity assay
The concentration of α-mangostin was determined using an MTT assay. Cells were treated with 0.1, 1, 2, and 4 μM α-mangostin for 24 hours. After treatment, the cells were incubated with MTT for 2 hours. Finally, the formazan crystal was dissolved by adding DMSO. The optical density was determined using a microplate reader (SpectraMax iD3, Molecular Devices) at 540 nm.
Senescence associated β-galactosidase assay
Cells were pretreated with α-mangostin or Vitamin C (Vit C) for 30 minutes and then treated with 50 μM H2O2 for 60 minutes. The medium was then replaced with LSM for 24 hours. Senescent cells were detected using the β-galactosidase assay according to the manufacturer’s protocol. Four images were randomized using a microscope with 200× magnification.
Intracellular ROS assay
Cells were pretreated with 0.1 and 1 μM of α-mangostin or Vit C for 30 minutes and then incubated with 20 μM DCF-DA for 20 minutes. Consequently, cells were incubated with 50 μM H2O2 for 30 minutes. Quantification of the fluorescence intensity was immediately detected by a microplate reader at 504 nm excitation and 529 nm emission.
Proliferation assay
Cells were cultured and then pretreated with 0.1 and 1 μM α-mangostin at or Vit C for 30 minutes. After that, the cells were treated with 50 μM H2O2 for 60 minutes. Consequently, cells were incubated in LSM for 24 hours. MTT assay was performed as the protocol stated above.
NO production assay
After treatment, the medium was replaced with LSM without phenol red for 24 hours. The media were determined nitrite concentration by Griess reagent. Optical density (OD) was evaluated using a microplate reader at a wavelength of 540 nm. The nitrite concentration was determined according to the nitrite standard.
Mitochondrial membrane potential assay
The fluorescent probe JC-1 was used for monitoring the mitochondrial membrane potential to determine mitochondrial function. The lipophilic, cationic JC-1 dye can enter into the mitochondria and then accumulate, forming reversible complexes called J-aggregates. Thus, healthy cells stain with JC-1 and then spontaneously form red fluorescent J-aggregates. By contrast, in senescent cells, JC-1 can enter the mitochondria but cannot form J-aggregates. After treatment, the medium was replaced with LSM for 24 hours. Cells were incubated with 20 μM JC-1 for 30 minutes. The four images were randomly taken using a fluorescent microscope with 200× magnification (Zeiss Axio observer). The fluorescent intensity was analyzed with ImageJ software. The red/green fluorescent intensity ratio was then calculated.
Western blot analysis
After treatment, the medium was replaced with LSM for an indicated time. Cytosolic protein was extracted using radioimmunoprecipitation assay buffer and separated using gel electrophoresis. Subsequently, separated proteins on the gel were transferred to a polyvinylidine difluoride membrane. The membrane was incubated with primary antibodies (1:1,000) at 4°C overnight and incubated with secondary antibodies (1:2,000) at room temperature for 60 minutes. The bands of protein were detected by using enhanced chemiluminescence and then placed in the Gel Doc XR+ system. Quantification of the band density was analyzed with ImageJ software.
Statistical analysis
All data are expressed as mean ± SD. Comparisons between the groups were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey test for comparisons of multiple groups. The statistically significant was considered if the p-value was less than 0.05.
RESULTS
H2O2-induced senescence
Blotting was used to determine the time and concentration of H2O2-induced cellular senescent signaling, therefore 200 μM H2O2 increased acetylated p53/p53 and p21 at 24 hours and p16 expression at 48 hours and decreased Sirt1 expression at 3 hours. p21 expression was the highest, and Sirt1 expression was the lowest in H2O2 treatment at 50 and 100 μM (Fig. 1). Hence, 50 μM H2O2 was used in further experiments.
Cytotoxicity of α-mangostin
MTT assay was used to determine the non-toxic concentrations of α-mangostin. α-Mangostin concentrations greater than 2 μM significantly decreased the number of viable cells. The surviving cells treated with 0.1–1 μM of α-mangostin for 24 hours were not significantly changed (Fig. 2). Therefore, 0.1 and 1 μM α-mangostin were used in the following experiments.
α-Mangostin reduces H2O2-induced cell senescence
β-galactosidase is a marker of cell senescence. After treatment of 50 μM H2O2 for 1 hour and then replaced with LSM for 24 hours, cells were significantly increased in β-galactosidase stain positive about 50%. Both α-mangostin + H2O2 and Vit C + H2O2 groups were significantly attenuated in β-galactosidase stain positive (Fig. 3).
α-Mangostin attenuates H2O2-induced intracellular ROS production
Next, we determined the effect of α-mangostin on H2O2-induced intracellular ROS formation by DCF-DA staining assay. α-Mangostin and Vit C significantly reduced H2O2-induced ROS. α-Mangostin at 0.1 and 1 μM and Vit C 200 μM reduced H2O2-induced intracellular ROS production by 6, 8, and 8%, respectively (Fig. 4).
α-Mangostin increases cell proliferation
Cell growth arrest is a common marker of cellular senescence. We determined cell proliferation using an MTT assay. H2O2 decreased endothelial proliferation significantly. Only 1 μM α-mangostin significantly increased H2O2-suppressed proliferation (Fig. 5).
 | Figure 1. Effects of H2O2 on cell senescent signaling. (A) Cells were treated with 200 μM H2O2 at various times to determine p21, p53, Sirt1, and p16. (B) Cells were treated with various concentrations of H2O2 for the indicated times of 24 hours to determine p21 and 3 hours for Sirt1. [Click here to view] |
 | Figure 2. The effect of α-mangostin on cell viability. EA.hy926 cells were treated with various α-mangostin concentrations and assessed cell viability using MTT assay (n = 3, **p < 0.01, ***p < 0.001 versus control). [Click here to view] |
 | Figure 3. α-Mangostin suppressed H2O2-induced cell senescence. (A) Cells were pretreated α-mangostin and then treated with H2O2. Cell senescence was detected by β-galactosidase staining. (B) β-galactosidase associated cells were counted manually (n = 3, #p < 0.05 versus vehicle, *p < 0.05 versus vehicle + H2O2). [Click here to view] |
α-Mangostin increases NO production
Basically, healthy endothelial cells release NO, primary endothelium-derived autacoids, to regulate vascular walls in a quiescent state. We determined the effect of α-mangostin on NO production by using Griess assay. Cells treated with H2O2 significantly decreased in NO formation by 10%. Both α-mangostin + H2O2 and Vit C + H2O2 groups enhanced NO production. Only 1 μM α-mangostin and 200 μM Vit C significantly increased H2O2-suppressed NO production (Fig. 6).
α-Mangostin prevents mitochondrial dysfunction
Next, we determined the effect of α-mangostin on H2O2-induced mitochondrial dysfunction by JC-1 staining. Cells treated with H2O2 significantly decreased mitochondrial membrane potential. α-Mangostin at 0.1 and 1 μM and Vit C 200 μM significantly increased mitochondrial membrane potential by 57%, 65%, and 78%, respectively (Fig. 7).
 | Figure 4. α-Mangostin reduced H2O2-induced ROS production. Cells were pretreated with α-mangostin and then treated with H2O2. ROS production was determined by DCF-DA (n = 3, #p < 0.05 versus vehicle, *p < 0.05, **p < 0.01 versus vehicle + H2O2). [Click here to view] |
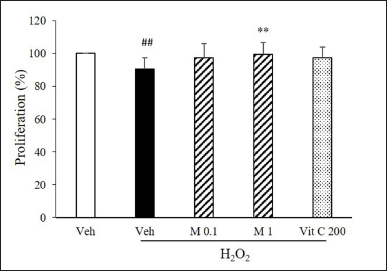 | Figure 5. α-Mangostin restored H2O2-suppressed cell proliferation. Cells were pretreated with α-mangostin and then treated with H2O2. Cell proliferation was determined using MTT assay (n = 5, ##p < 0.01 versus vehicle, **p < 0.01 versus vehicle + H2O2). [Click here to view] |
 | Figure 6. α-Mangostin inhibited H2O2-suppressed NO production. Cells were pretreated with α-mangostin and then treated with H2O2. NO production was determined by Griess assay (n = 3, ##p < 0.01 versus vehicle, *p < 0.05, **p < 0.01 versus vehicle + H2O2). [Click here to view] |
 | Figure 7. α-Mangostin increased mitochondrial membrane potential. (A) Cells were pretreated with α-mangostin and then treated with H2O2. The mitochondrial membrane potential was detected by JC-1 staining. (B) Quantification of the fluorescence intensity was determined by ImageJ software (n = 3, ##p < 0.01 versus vehicle, ***p < 0.001 versus vehicle + H2O2). [Click here to view] |
α-Mangostin suppresses H2O2-induced senescence through p38 MAPK, Sirt1, and MnSOD
The effect of α-mangostin on the downstream signaling of cell senescence was determined. α-Mangostin significantly decreased phosphorylation of p38 MAPK. α-Mangostin also significantly increased Sirt1 and MnSOD expression (Fig. 8).
 | Figure 8. α-Mangostin inhibited H2O2-induced senescence through p38 MAPK, Sirt1, and MnSOD. Cells were pretreated with α-mangostin and then treated with H2O2. (A) The expressions of p38, Sirt1, and MnSOD were detected by Western blot. (B) Quantification of band intensity was determined by ImageJ software (n = 3, #p < 0.05 versus vehicle, *p < 0.05, **p < 0.01 versus vehicle + H2O2). [Click here to view] |
DISCUSSION
The main finding of this study is the protective effect of α-mangostin on endothelial senescence induced by oxidative stress. We have shown that (1) exogenous ROS-induced intracellular ROS production is inhibited by α-mangostin; (2) H2O2-induced senescence resulting in decreased cell proliferation and NO bioavailability is inhibited by α-mangostin; (3) H2O2 induced a reduction in the mitochondrial membrane potential is restored by α-mangostin; and (4) H2O2-induced phosphorylation of p38 MAPK and suppressed expression of Sirt1 and MnSOD is reversed by α-mangostin.
Age-related degeneration in function occurs in normal physiology and pathological diseases, including hypertension and CVDs. Vascular disease is associated with endothelial dysfunction, impaired angiogenesis, and arterial stiffness and remodeling. Recently, several studies have been shown that endothelial cell senescence associated with age-related vascular pathologies. Senescence is a process whereby the irreversible arrest of cell growth in response to various stresses, including oxidative stress, occurs. Oxidative stress-induced cell senescence damages the DNA and mitochondria and stimulates cytosolic stress response kinases such as p38 and JNK MAPK. ROS induces endothelial senescence through stimulation of p53/p21 and p16 expression and suppression of Sirt1 [26]. It has been shown that Sirt1 suppresses oxidative stress-induced cell senescence by the activation of MnSOD [27].
α-Mangostin, a polyphenolic xanthone derivative, is a potent anti-oxidant property [28]. Our previous studies showed that α-mangostin attenuated ROS formation by hypoxia induced in Bovine retinal endothelial cells [18] and high glucose induced in human umbilical vein endothelial cell (HUVEC) [17]. This study showed α-mangostin inhibited H2O2-induced endothelial senescence and reduced intracellular ROS production. We also demonstrated that α-mangostin significantly reversed the reduction of cell proliferation and NO production induced by H2O2. These results indicated that α-mangostin ameliorates cell senescence and consequently increases cell proliferation and NO generation. In addition, our study found that α-mangostin inhibited p38 MAPK and increased Sirt1 and MnSOD; however, α-mangostin did not change the expression of p53/p21 and p16 (data not shown). Recently, a study by Tousian et al. [29], demonstrated that α-mangostin decreased high glucose-induced endothelial senescence via inhibition of p21 and p53 and increase of Sirt1 expression. It is probable that different stimulations may activate distinct downstream signaling.
Mitochondria is the major source of adenosine triphosphate (ATP) production in cardiac myocytes and other cells. By contrast, endothelial mitochondria play a pivotal role in cellular signaling [30]. Excessive ROS activates mitochondrial dysfunction, resulting in increased mitochondrial ROS production. Several studies have shown that H2O2 stimulates cell senescence by inducing mitochondrial dysfunction, including loss of mitochondrial membrane potential [3,31]. This study found that α-mangostin significantly restored the reduction in mitochondrial membrane potential. Previously, it has been shown that α-mangostin reduced rotenone-induced mitochondrial dysfunction in neuroblastoma cells [32]. Other study also demonstrated that α-mangostin attenuated isoproterenol-induced cardiac mitochondrial dysfunction in rats [33]. These results indicated that α-mangostin can protect against mitochondrial dysfunction.
CONCLUSION
This study has demonstrated that α-mangostin has anti-oxidant and anti-senescent actions on endothelial cells through p38 MAPK, Sirt1, and MnSOD pathways. α-Mangostin may be used as a natural substance to prevent CVDs.
ACKNOWLEDGMENTS
This study was supported by Naresuan University (NU), and National Science, Research and Innovation Fund (NSRF) (Grant No. R2565B089). The authors would like to thank Prof. Primchanien Moongkandee for procuring α-mangostin, Assoc. Prof. Wisuda Suvitayavat for advice and the Naresuan University Writing Clinic (DIALD) for their editing assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68. CrossRef
2. Moiseeva O, Bourdeau V, Roux A, Deschênes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29(16):4495–507. CrossRef
3. Martini H, Passos JF. Cellular senescence: all roads lead to mitochondria. FEBS J. 2023;290(5):1186–202. CrossRef
4. Campisi J, D’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. CrossRef
5. Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S, et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30(11):2205–11. CrossRef
6. Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127. CrossRef
7. Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30(8):1536–48. CrossRef
8. Wu Z, Yu Y, Liu C, Xiong Y, Montani JP, Yang Z, et al. Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway. Aging (Albany NY). 2015;7(1):70–81. CrossRef
9. Hongo A, Okumura N, Nakahara M, Kay EP, Koizumi N. The effect of a p38 mitogen-activated protein kinase inhibitor on cellular senescence of cultivated human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2017;58(9):3325–34. CrossRef
10. Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54(6):2077–82. CrossRef
11. Kosem N, Han YH, Moongkarndi P. Antioxidant and cytoprotective activities of methanolic extract from Garcinia mangostana Hulls. Sci Asia. 2007;33:283–92. CrossRef
12. Kosem N, Ichikawa K, Utsumi H, Moongkarndi P. In vivo toxicity and antitumor activity of mangosteen extract. J Nat Med. 2013;67:255–63. CrossRef
13. Lee HN, Jang HY, Kim HJ, Shin SA, Choo GS, Park YS, et al. Antitumor and apoptosis-inducing effects of alpha-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int J Mol Med. 2016;37(4):939–48. CrossRef
14. Zhu X, Li J, Ning H, Yuan Z, Zhong Y, Wu S, et al. α-Mangostin induces apoptosis and inhibits metastasis of breast cancer cells via regulating RXRα-Akt signaling pathway. Front Pharmacol. 2021;12:739658. CrossRef
15. Mohan S, Syam S, Abdelwahab SI, Thangavel N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food Funct. 2018;9(7):3860–71. CrossRef
16. Herrera-Aco DR, Medina-Campos ON, Pedraza-Chaverri J, Sciutto-Conde E, Rosas-Salgado G, Fragoso-González G. Alpha-mangostin: anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem Toxicol. 2019;124:300–15. CrossRef
17. Jittiporn K, Moongkarndi P, Samer J, Suvitayavat W. Protective effect of α-mangostin on high glucose induced endothelial cell apoptosis. Walailak J Sci Tech. 2018;15(8):579–87. CrossRef
18. Jittiporn K, Suwanpradid J, Patel C, Rojas M, Thirawarapan S, Moongkarndi P, et al. Anti-angiogenic actions of the mangosteen polyphenolic xanthone derivative α-mangostin. Microvasc Res. 2014;93:72–9. CrossRef
19. Wihastuti TA, Sargowo D, Tjokroprawiro A, Permatasari N, Widodo MA, Soeharto S. Vasa vasorum anti-angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition by mangosteen pericarp ethanolic extract (Garcinia mangostana Linn) in hypercholesterol-diet-given Rattus norvegicus Wistar strain. Vasc Health Risk Manag. 2014;10:523–31. CrossRef
20. Lee JS, Shukla S, Kim JA, Kim M. Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential. PLoS One. 2015;10(2):e0118552. CrossRef
21. Jiang TT, Ji CF, Cheng XP, Gu SF, Wang R, Li Y, et al. α-Mangostin alleviated HIF-1α-mediated angiogenesis in rats with adjuvant-induced arthritis by suppressing aerobic glycolysis. Front Pharmacol. 2021;12:785586. CrossRef
22. Ko JM. Protective Effects of α-mangostin on UVB-induced oxidative stress and cellular senescence. Asian J Beauty Cosmetol. 2015;13(6):813–8.
23. Widowati W, Ginting CN, Lister INE, Girsang E, Amalia A, Wibowo SHB, et al. Anti-aging effects of mangosteen peel extract and its phytochemical compounds: antioxidant activity, enzyme inhibition and molecular docking simulation. Trop Life Sci Res. 2020;31(3):127–44. CrossRef
24. Moongkarndi P, Jaisupa N, Samer J, Kosem N, Konlata J, Rodpai E, et al. Comparison of the biological activity of two different isolates from mangosteen. J Pharm Pharmacol. 2014;66(8):1171–9. CrossRef
25. Moongkarndi P, Srisawat C, Saetun P, Jantaravinid J, Peerapittayamongkol C, Soi-ampornkul R, et al. Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells. J Proteome Res. 2010;9(5):2076–86. CrossRef
26. Shakeri H, Lemmens K, Gevaert AB, Meyer GRYD, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315(3):H448–62. CrossRef
27. Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. 2017;2017:7543973. CrossRef
28. Ibrahim MY, Hashim NM, Mariod AA, Mohan S, Abdulla MA, Abdelwahab SI, et al. α-Mangostin from Garcinia mangostana Linn: an updated review of its pharmacological properties. Arab J Chem. 2016;9(3):317–29. CrossRef
29. Tousian H, Razavi BM, Hosseinzadeh H. Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. Daru. 2020;28(1):45–55. CrossRef
30. Park J, Lee J, Choi C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS One. 2011;6(8):e23211. CrossRef
31. Salazar G. NADPH oxidases and mitochondria in vascular senescence. Int J Mol Sci. 2018;19(5):1327. CrossRef
32. Hao XM, Li LD, Duan CL, Li YJ. Neuroprotective effect of α-mangostin on mitochondrial dysfunction and α-synuclein aggregation in rotenone-induced model of Parkinson’s disease in differentiated SH-SY5Y cells. J Asian Nat Prod Res. 2017;19(8):833–45. CrossRef
33. Sampath PD, Kannan V. Mitigation of mitochondrial dysfunction and regulation of eNOS expression during experimental myocardial necrosis by alpha-mangostin, a xanthonic derivative from Garcinia mangostana. Drug Chem Toxicol. 2009;32(4):344–52. CrossRef