INTRODUCTION
A biphasic system known as an emulsion occurs when one phase is uniformly dispersed in the other phase as tiny droplets of diameters between 0.1 and 100 mm. Emulsions with this particle size distribution are thermodynamically unstable. Nanoemulsions have globule sizes that range from 20 to 600 nm, which makes them more stable and effective compared to traditional emulsions. Water-in-oil (W/O), oil-in-water (O/W), and complex emulsions such as water-in-oil-in-water (W/O/W) are the three main forms of emulsions [1]. A multiple emulsion is another name for a complex emulsion. Figure 1 depicts the three emulsion types. The emulsion is typically O/W if the oil is the dispersed phase, and W/O if the aqueous medium is the dispersed phase. The multiple emulsions, on the other hand, are intricate systems [2].
The current emulsification method commonly used in the pharmaceutical and cosmetic industry is the hot emulsification process, where the aqueous phase and oil phases are added at elevated temperatures in the presence of an emulsifier under continuous homogenization, such methods might not be suitable for the pharmaceutical actives which are thermolabile and sometimes the impurity profile of actives increases while increasing the process temperature. The cold emulsification method for nanoemulsions, on the other hand, may improve the product’s stability and therapeutic efficacy while reducing side effects and harmful reactions [3].
Cold emulsification techniques such as ultra-sonication [4] and micro fluidization [5] are widely used as cold processes for nanoemulsion in liquid form, challenges with these methods is the stabilization and difficulties for scaling up the process to commercial scale as these methods need complex manufacturing setup. Cold emulsification through inline homogenizer with varying the rotar and stator configurations has proven the concept that the process is a continuous process and can be easily scaled up. This has been widely used in the cosmetic industry along with hot emulsification but not evaluated much in the pharmaceutical industry. For pharmaceutical use, the same inline homogenizer can be used with the varying configuration of rotar and stator to achieve the desired globule size. To further stabilize the nanoemulsion the liquid emulsion can be incorporated into a self emulsified pH independent gel base through a cold incorporation process. Cold emulsification process may help in controlling the impurity profiling of active pharmaceutical ingredients which are sensitive to heat process and may help in increasing the shelf life of the product. Nanoemulsion of thermolabile drugs through cold emulsification may also help in better penetration of active ingredients when applied topically.
A topical formulation can be improved, if it satisfies the target product profile with no adverse and toxic effects, and is chemically and physically stabilized with advanced ingredients and processes to ensure the desired shelf life of the product. When required for the target indication, the release of the drug is easy for its penetration inside the epidermis from the formulation and has better spreadability and consumer or patient compliance. It should contain safe ingredients at optimal dosages prescribed in respective monographs or with good safety data. It should be a sustainable and environment-friendly method of manufacturing [6].
Nanoemulsions offer various advantages over conventional emulsions such as improved bioavailability of entrapped drugs, chemical protection of labile drugs, ease in manufacturing when compared to bio-polymeric nanoparticles, wider options for selecting base materials such as lipids, similar raw materials as used in emulsions, and offers great long-term stability and efficacy. Nanoemulsions are a great choice for application in personal care, health care, and cosmetic products for various reasons [7]. Active compounds can be successfully delivered via the skin using nanoemulsions. Rapid drug release is made possible by the emulsion technologies used that provide huge surface area. The tiny droplet size enables homogeneous deposition on substrates. The system’s low surface tension as well as the low interfacial tension between the two phases may help in the spreading, wetting, and penetration of cosmetic formulation. The elasticity of the small droplets keeps them from clumping and stops surface fluctuations. Because the gravitational force is greatly reduced by the extremely small droplet size, Brownian motion might be sufficient to overcome gravity. As a result, there will not be any sedimentation or creaming when the product is stored. The small size of the droplets also avoids flocculation. Liposomes and vesicles, which are far less stable, can be replaced by nanoemulsions. In some instances, lamellar liquid crystalline phases can also form surrounding the nanoemulsion droplets. The system may have a pleasant aesthetic aspect and skin feel because of its clarity, fluidity (at moderate oil concentrations), and lack of any thickeners. Fragrances, which are often used in personal care products, can be transported by nanoemulsions. In addition, this might be used in alcohol-free perfumes. Nanoemulsions can be produced with a low surfactant concentration, in contrast to microemulsions, which frequently require a surfactant concentration of 20% or higher [8].
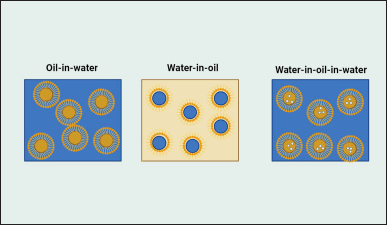 | Figure 1. Different types of nanoemulsions explaining majorly three types. [Click here to view] |
METHOD OF PREPARATION OF NANOEMULSION
The high-pressure homogenization method, ultrasonication method, spontaneous emulsification method, micro fluidization method, and phase inversion method are just a few of the methods that have been used to create nanoemulsions. The double emulsion-solvent evaporation method is frequently used to create multiple emulsions. These nano-sized emulsions, which were exploited as drug delivery systems, have been studied using a range of approaches. Figure 2 explains the three major techniques to create nanoemulsions which are explained in detail in the next section.
Cold emulsification or high-pressure homogenization
The formation of nanoemulsions, where the amount of mechanical energy required surpasses the interfacial energy by many orders of magnitude, requires a high-energy mechanism, and a variety of emulsifiers. An application of high-magnitude energy is required to produce submicron droplets. The high-energy method produces substantial interfacial areas to produce nanoscale emulsions by applying mechanical force. The fluid pressure causes bigger droplets to break apart into smaller ones by breaking the surface tension between the two entirely immiscible phases. As a result, a high total droplet surface area per volume is attained. The huge droplets are split and deformed into smaller droplets by the high-energy process, and surfactant is then absorbed at the interface to achieve high steric stability. High-energy cold emulsification techniques enable high-shear mixing with a rotor/stator system. The globule size of the nanoemulsion can be varied by altering the rotar and stator configuration of the inline homogenizer. The high shear mixing of the aqueous phase and oil phase leads to the nanosizing globules of the bi-phasic system and the nano globules size makes the nanoemulsion more stable and superior in sensory than conventional emulsion base products where the globules size in micro meters. With this technique of emulsification, there should be no challenge in scaling up the product to any commercial level as high-pressure homogenization is a continuous process and there is sufficient evidence published on the reproducibility and scalability of the homogenization process. Such an emulsion formulation precludes the production of any related chemicals when a thermolabile medication is included in it [9].
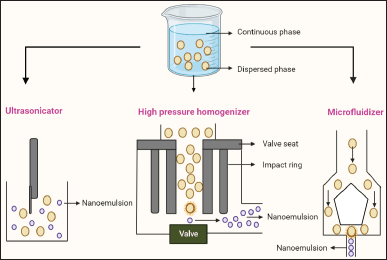 | Figure 2. Different methods of preparation of nanoemulsions are mainly three common methods such as ultrasonication, high-pressure homogenization, and microfluidization. [Click here to view] |
Ultrasonication
The ultrasonication technique involves a sonicator probe, known as a sonotrode, which provides the power. The mechanical vibration caused by the probe’s contact with the biphasic system leads to cavitation, which causes the liquid vapour cavities to collapse [10]. The oil droplet disruption can be accelerated by the physical effects of ultrasound caused by acoustic cavitation, resulting in stable oil in water emulsions with smaller droplet sizes. Using ultrasonic homogenizers in an industrial setting has several advantages, including the potential for aseptic processing, low energy usage, small droplet formation, and affordable operating expenses. Due to the cavitation forces, high-intensity ultrasonic waves cause liquids to rapidly vibrate before exploding. The powerful disruptive forces that result from this cause smaller emulsion droplets to form. Depending on the purpose, nanoemulsions can be produced utilizing ultrasonic homogenizers on a small scale [11].
Microfluidization
The high-energy procedure called microfluidization is primarily concerned with the dynamics of the specifically designed microchannels [12]. The turbulence and force are created to open the barrier and let the lipid carrier flow through the columns. To construct dependable nanoscale delivery systems, a compressed air-powered pump is required to combine lipids and active ingredients at extremely fast rates in the intended microchannels. The microfluidization method makes it simpler to produce a stable nano-sized emulsion with particles smaller than 160 nm [13].
The process involves creating nanoemulsion and using a pneumatic pump to force the coarse emulsion through the tight spaces. Different passes result in different sizes [14]. It is also a rapid and effective technique to develop a number of nano-delivery devices. Given the increased bioactive chemical stability, uniformity, and reproducibility of recently developed nanosystems, food-grade delivery techniques can be developed for more widespread use in the production of functional foods [15].
Numerous studies have shown that emulsification by microfluidization is superior to other traditional homogenization techniques. In comparison to traditional homogenization, a microfluidizer appears to create thinner and smaller particle size distributions [16]. It has also been claimed that the microfluidizer produces stable O/W submicron-sized emulsions more effectively than the homogenizer does at low stabilizer concentrations. Some researchers have considered utilizing this apparatus to homogenize dairy model emulsions [17]. In addition, submicron emulsions created by microfluidization displayed a slower rate of droplet diameter development over time [18].
Phase inversion temperature (PIT)
Transitional inversion is brought on by hydrophilic lipophilic balance variations in the system as a result of temperature changes which is essential for the formation of nanoemulsions via the PIT method. The preparation method has a considerable impact on the emulsification process, which can lead to a variety of droplet size distributions and directly impact the stability of the final product [19].
Lower temperatures make temperature-sensitive surfactants water soluble, and a positive surfactant layer curvature is apparent at the droplet interface. On the other hand, high temperatures make the surfactants oil-soluble and make the surfactant layer curvature at the droplet interface evident [20].
As the surfactants have an equal amount of attraction for both the water and oil phases at a medium temperature, the instant surfactant layer curvature becomes zero at the droplet interface [21].
CHARACTERIZATION OF NANOEMULSIONS
Nanoemulsion characterization in current days typically is not restricted to optical microscopy (where the size elucidation used to happen in micrometer). More sophisticated and precise equipment is now in place which can analyze the structure and behavior of nanoemulsions. These techniques include X-ray or neutron scattering, dynamic light scattering, atomic force microscopy, and cryo-electron microscopy. Nanoemulsions and traditional microscale emulsions exhibit interesting physical variations. For instance, the considerable multiple scattering of visible light causes microscale emulsions to typically appear white. Multiple scattering occurs when there is a difference in the refractive indices between the continuous and dispersed phases and when the light is repeatedly bent between plateau edges, droplets, and films. Photons entering the emulsion are several times dispersed by microscale structures before they leave the emulsion without optical absorption [22].
Because the features in nanoemulsions are much smaller than when compared with visible wavelengths, the majority of them continue to be optically transparent even at extremely high phase volume ratios and for extremely wide fluctuations in refractive indices. Nanoemulsions may eventually become opaque due to an increase in droplet size over time. Nanoemulsions commonly exhibit droplet deformation phenomena with larger elastic moduli than regular emulsions because their surface area-to-volume ratios are significantly more than those of traditional emulsions. Due to this, stabilizing nanoemulsions requires a higher concentration of surfactant than microscale emulsions, but typically less than lyotropic microemulsion phases. The following sections go over several aspects of nanoemulsion characterization [23].
Morphology
With the availability of precise and validated equipment infrastructure today, it is very convenient to examine the morphology of the nanoemulsion the prominent techniques currently being used are scanning electron microscopy, and transmission electron microscopy, these equipment are easy to operate and high performance liquid chromatography (HPLC) based and the globules can be viewed in three dimensions using scanning electron microscopy (SEM) [24]. To modify and develop intelligent nanoemulsion-based systems, it is crucial to visualize the interface morphology and measure the interfacial contact forces of nanoemulsion droplets. At various magnifications and an adequate accelerating voltage, usually 20 kV, the samples are examined. The surface morphology of the system’s dispersion phase can be thoroughly analyzed with SEM. It is possible to use image analysis software, such as that from Leica Imaging Systems, Cambridge, United Kingdom, to automatically examine surface morphology. The best photographs acquired with a transmission electron microscopy (TEM) show the dispersion phase. Using a copper grid covered with PioloformTM with a 200 m mesh size, a small carbon-coated grid, or a drop of 2% uranyl acetate solution applied with a micropipette, the sample is thoroughly stained. The substance is next investigated using a transmission electron microscope set to an 80 kV voltage, such as the Joel 1230, Tokyo, Japan. To assess the varied diameters and size distributions of TEM micrographs, a digital image processing program should be used [25].
Polydispersity
Polydispersibility is used to describe the degree of “non-uniformity” in a distribution matrix. A low polydispersity index ensures a complete solubilization of the dispersed phase in a bicontinuous nanoemulsion evidently, the minimum size droplets and polydispersity are influenced by the method chosen for this preparation. Increasing the number of cycles of the emulsion in a homogenizer ensures that all droplets experience the peak shear rate generated by a flow-producing device during emulsification. This is a complementary condition to obtain low polydispersity nanoemulsion to a certain point [8]. A particle size analyzer can be used by photon correlation spectroscopy (PCS) to examine the size, polydispersity, and zeta potential of nanoemulsion droplets. This instrument even quantitates the polydispersity index, which is a measurement of the width of the size distribution taken from the cumulative analysis of dynamic light scattering. Information on the quality or homogeneity of the dispersion is provided by both the PCS and the polydispersity index. The laser diffraction method is an additional technique for measuring particle size [26].
Viscosity
The proportions of water, oil, and surfactant in a nanoemulsion determine its viscosity which is measured by a viscometer. Lowering the content of cosurfactants and surfactants raises viscosity by raising the interfacial tension between the water and oil, whereas raising the water content lowers viscosity. For drugs to discharge effectively and steadily, viscosity is essential. Since they are essentially O/W type, nanoemulsion formulations frequently have lower apparent viscosities and are less greasy than W/O formulations. They are easier to wash off after application to the skin and ought to release their active ingredients more quickly. Numerous methods and technologies can be used to assess the rheological properties of nanoemulsion carriers [27].
In vitro skin permeation studies
The Franz diffusion cell is used to ascertain the drug release profile while designing a nanoemulsion approach for transdermal delivery. The depth to which the drug enters the skin is determined using confocal scanning laser microscopy. It is possible to observe how the drug responds in the receiving medium, which is often phosphate buffer solution, by adding a minute amount of the preparation to the donor compartment of a Franz cell with a membrane acting as a barrier and stirring on a magnetic stirrer at 37°C (pH 7.4) [28]. At regular intervals, the medium is sampled for dispersion, and the medium is then replaced with a volume matching the volume of the medium. To release the drug, the sample must first be withheld before being filtered through a 0.22 to 50 m filter. The substance is next examined by the drug’s highest absorption wavelength using UV-visible spectroscopy. The use of a diffusion cell is an alternate and well-liked technique for ex-vivo release experiments. The lipids and underlying cartilage are carefully removed, together with the skin from the ear or the abdomen. The diffusion cell that has been previously loaded with receptor solution is covered by skin that has been cut to the appropriate size. Samples are taken out of the receptor medium and replaced with equal volumes of the medium at intervals of up to 24 hours. The removed samples are then analyzed using HPLC or UV spectroscopy to see if the drug has migrated. For in vitro release investigations, a semi-permeable membrane, such as a regenerated cellulose membrane, could be utilized in place of skin [29]. A whole live animal may receive administration or application of the preparation during an in vivo release study, sometimes known as dermatopharmacokinetics. Blood samples are frequently taken, and the drug concentration of the plasma is then analyzed by HPLC following centrifugation. To calculate the pharmaceutical formulation’s bioavailability, more evidence on in vivo and in vitro tests is required [30].
CURRENT CHALLENGES AND SOLUTIONS FOR FABRICATION OF NANOEMULSIONS
Although low-energy production methods exist, they are not used for large-scale production of nanoemulsions because they frequently require high surfactant concentrations and fail to produce stable nano-sized emulsions apart from this cold process for manufacturing nanoemulsion such as microfluidization and ultrasonication are complicated equipment and difficult for scaling up of the process at large scale. In fact, at the laboratory scale. This equipment (ultrasonication and microfluidization) is difficult to maintain and expensive. Nanoemulsion production requires a significant amount of energy input. To make nanoemulsions on a large scale, the high-energy method, which employs mechanical tools such as high-pressure homogenizers, along with suitable rotars and stators needs to be used. This barrier explains why the commercialization of nano-sized emulsion compositions is proceeding slowly. For thermolabile drugs, a hot emulsification process is not at all recommended as that may lead to an increase in impurities and hence may reduce the shelf life of the overall formulation. To fabricate nanoemulsion through a cold process for a thermolabile drug, we need to define all the critical quality attributes for finished goods and all the critical process parameters need to be understood thoroughly. The production of submicron droplets, the roles of surfactants, the chemistry at the interfaces, and the cosurfactants required to produce nanoemulsions should be well known.
In current scenarios, most of the industries are operating with conventional homogenizer setup and they are using conventional emulsifiers and co-emulsifiers. Because of a lack of proper infrastructure and capability, most of the thermolabile active ingredients are not being considered for topical micro and nanoemulsion dosage forms. Inline homogenizers with proper rotar and stator configuration along with self-emulsifying polymers will open the path of development of innovative nanoemulsion gel for thermolabile drugs. Since thermolabile drugs are prone to convert into impurities and hence toxicity, cold emulsification processes must be adopted in the near future to differentiate the diffusion profiles and increase the efficacy of the formulations.
APPLICATIONS
Small droplet size, better sensorial, better penetration, tunable rheology, and excellent stability are only a few of the special characteristics of nanoemulsions. Due to these characteristics, nanoemulsions are a desirable candidate for use in food, cosmetics, pharmaceutical, and other industries as well as in drug delivery applications as depicted in Figure 3. Moreover, they can act as the fundamental components of technologically advanced materials with distinctive features.
Brain drug delivery
Oral drugs for issues related to the central nervous system have problems that are frequently related to the composition and physical characteristics of the drug substance. For a variety of reasons, intranasal nanoemulsion may be a suitable alternative to oral delivery for such medicines [31]. These beneficial results can be explained by brain endothelial cell mechanisms including transcytosis and endocytosis for the nanodroplets. For increased nasal delivery of rivastigmine to the brain, Haider et al. [32] created a nanoemulsion of the drug rivastigmine hydrochloride and proposed the same for improved brain delivery of neurodegenerative drugs because it helps in preventing hydrolysis through the enzymes such as acetylcholinesterase and butyrylcholinesterase and subsequently boosts central cholinergic function and acetylcholine bioavailability. In vivo studies revealed that compared to the nasal solution or intravenous nanoemulsion treatment, nasal nanoemulsion administration produced significantly higher drug concentrations in the brain. Another application for nose-to-brain nanoemulsions is the treatment of migraine [33], the most common neurovascular headache disorder by intense and throbbing pain throughout the head [34]. When compared to other alternatives, the zolmitriptan mucoadhesive nanoemulsion for the treatment of migraine headaches is a promising drug delivery method [35].
Sumatriptan nasal spray is a different intranasal medication delivery technique that is used to treat migraines. For the treatment of basilar migraine, the drug’s administration demonstrates a quick start to action by depositing in the olfactory region of the brain and subsequently going through the nasal pathway directly entering the brain [36].
When given as an O/W nanoemulsion, paclitaxel and C(6)-ceramide were combined and tested on human glioblastoma cells. The prepared nanoemulsion had oil droplets that were around 200 nm in diameter. Compared to single drugs, the administration of U-118 cells resulted in a considerable rise in cytotoxicity. Due to the combination of paclitaxel and ceramide, the cytotoxic effect was increased. In tumor models such as glioblastoma, the study offered a superior substitute for the formulation of O/W nanoemulsions for combination-type therapy of brain tumor cells [37].
 | Figure 3. Various applications of nanoemulsions in different fields such as cosmetics, foods, pharmaceuticals, drug delivery, and gene delivery. [Click here to view] |
In a different study, sertraline hydrochloride nanoemulsion enhanced the drug’s solubility from 0.5 to 94.28 mg/ml to produce a quick onset of action and boost bioavailability. The system’s in vitro permeability was discovered to be 62.8% and 0.56%. Thus, intranasal administration may have benefits over currently available oral tablets and other oral drugs for the treatment of depression and other mental illnesses [38].
Nanoemulsions for the treatment of neurodegenerative diseases
For the treatment of neurodegenerative illnesses, cutting-edge technology such as nanoemulsions can be used to achieve targeted delivery. One of the most prevalent neurodegenerative disorders is Alzheimer’s disease (AD), followed by Parkinson’s disease (PD), Prion’s disease, and other conditions. Mustafa et al. [39] created a nanoemulsion loaded with ropinirole for the treatment of PD. The physicochemical properties of the formulation, such as viscosity and particle size, were then evaluated. The Wistar rat brain was subjected to ex vivo, in vitro, and in vivo tests. The researchers concluded that nanoemulsion-loaded ropinirole holds promise for the treatment of PD and intranasal drug delivery. For the treatment of PD, a resveratrol-rich, vitamin E-infused nanoemulsion was formed. The researchers mixed vitamin E with propylene glycol mono-caprylic ester to make an O/W nanoemulsion that was kinetically stable. Resveratrol nanoemulsions showed noticeably robust ex vivo mucosal flow through the porcine nasal mucosa in the Franz diffusion cell assembly. Wistar rats utilized in brain-targeting studies revealed elevated drug levels in the brain after receiving resveratrol nanoemulsions intranasally. Furthermore, histological examinations proved that intranasally delivered resveratrol nanoemulsion led to slight degenerative changes in the brain [40].
In a study, the mucoadhesive strength of hyaluronic acid-based nanoemulsions for AD was compared to that of non-mucoadhesive nanoemulsions. With fluxes through the membrane, two polyphenols were certainly diffused over the nasal mucosa for up to 6 hours, which was then compared to oral preparations [41]. According to Sood et al., curcumin-donepezil nanoemulsion was given for targeted delivery in AD. In vivo pharmacokinetic investigations in rats with streptozotocin-induced AD showed that drug localization in the brain is higher when supplied intravenously than when administered orally [42]. In the pharmacodynamic analysis of behavioral tests in rats, the test group given nanoemulsions displayed superior memory and learning compared to the drug treatment alone. A biochemical examination reported that the group that received nanoemulsion treatment had noticeably higher levels of acetylcholine in the brain. The degree of oxidative stress was dramatically lowered in animals undergoing combination therapy. Intranasal administration of an acetylcholinesterase inhibitor along with a neuroprotective and anti-amyloid drug appears to be a feasible strategy for controlling AD [43].
Michal Mizrahi et al. examined the potential of pomegranate seed oil (PSO) in a nanoemulsion form to delay the clinical progression and neurodegenerative pathological signs of Prion’s disease in rats [44]. It was noted that the treatment of PSO in the animal model delayed the onset of action of the drug. It delayed the development of the illness in comparison to doses of natural PSO. A review of brain samples demonstrated that there were no negative effects and mice-treated brains showed a significant neuroprotective effect [45].
Anti-aging properties of nanoemulsions
Research on disorders linked to aging that aims to postpone, prevent, or reverse the aging process is extremely required for both healthy and delayed aging and scientific advancement. Nanoemulsions as anti-aging systems improve drug transport to the site of action, show prolonged effects, and reduce unfavorable reactions and side effects. Some lipids such as palm and olive oil, are used to create some nanoemulsions as they have emollient characteristics that allow phytoconstituents to penetrate the skin more effectively. Gamma-aminobutyric acid or hyaluronic acid may also be used as possible permeation enhancers due to their high water-absorption abilities, which hydrate both the stratum corneum layer and the dermis layer [46]. Nanoemulsions containing curcumin, zataria multiflora, and soybean isoflavone aglycones have shown considerable potential in wound healing [47]. Oleanane acid and ursolic acid-loaded nanoemulsions have also demonstrated a high capacity for penetration and powerful anti-inflammatory action [48].
A sunscreen with a nanoemulsion base that uses micelles of avocado oil droplets as an active component was created by Silva et al. [49]. To increase the sun protection factor of the stable formulation to 3, titanium dioxide and octyl methoxycinnamate, two chemical and physical sunscreen ingredients, were added. The in vitro release demonstrated the delayed and sustained release of the nanoemulsion. According to the findings, the nanoemulsion method improved the UV filter characteristics of protective ingredients, resulting in a sunscreen with a higher sun protection factor.
Using rice bran oil, a popular cosmetic raw material for anti-aging and sunscreen applications, Bernardi et al. [50] created a nanoemulsion system. An in vitro skin irritation investigation conducted as part of the formulation’s efficacy study revealed that the ideal formulation had a low potential for irritation. The in vivo tests, meantime, showed that using the formulation on the skin improved the moisture content of the skin and allowed for the maintenance of a normal skin pH level. The study concluded that the nanoemulsion would function well as a cosmetic delivery system for a variety of natural ingredients.
Bioactive encapsulated nanoemulsions
Nanoemulsions have been used to encapsulate bioactive vitamins, acidulants, preservatives, lipids, colorings, antioxidants, flavorings, and other ingredients and additives for food. Hydrophobic medicines and other bioactive molecules, such as phytochemicals, have been delivered using them frequently. Nanoemulsions, which are employed as efficient delivery vehicles for raising the beneficial lipid content of meat products, have been used to encapsulate alpha-linoleic acid-rich flaxseed oil, a substantial dietary source of omega-3 fatty acids from plants [51].
When the level of tocopherols encapsulated in nanoemulsions was compared to that in emulsion and bulk oil in an in vivo animal investigation, it was discovered that the release was much faster in the nanoemulsion. The outcomes confirmed an excellent in vitro-in vivo correlation [52].
To improve their bioactivity, dispersibility, and stability, various oil-soluble vitamin types have been encapsulated in nanoemulsions considering how vitamin A (retinol) is encapsulated in nanoemulsions made from various oils and emulsifiers [53].
Excipient nanoemulsions could serve as the foundation for a number of products aimed at the food or supplement industries, such as dressings for salads, sauces for steamed vegetables, rich creams for fruits, and beverages combined with dietary supplements. Nanoemulsions are preferably suitable for this purpose as they may be created to include tiny oil droplets that are swiftly digested in the human stomach. The hydrophobic components can be transported and solubilized by these droplets, which soon generate mixed micelles [54].
O/W nanoemulsions encapsulated with beta-carotene were made by emulsifying whey protein isolates (WPIs) with dextran. The nanoemulsions stabilized with WPI-dextran conjugate demonstrated the highest beta-carotene retention rate after a 1-month storage period because of the relatively high scavenging activity of diphenyl-1-picryl-hydrazine [55].
The effectiveness of these formulations needs to be evaluated in the future using research on human nutrition, presuming they are first shown to be safe for oral ingestion [55].
Gene delivery by nanoemulsions
In a study by Schuh et al. [56] the delivery of donor oligonucleotides to cells utilizing the clustered regularly interspaced short palindromic repeat/Cas9 (CRISPR/Cas9) genome editing approach was evaluated. The activity of the α-L-iduronidase gene was increased to an average level of 2%–4% of the normal activity when nanoemulsions were added to the cell culture. Confocal microscopy analysis of fibroblasts demonstrated a decrease in lysosome concentration in the cells, which was later validated by flow cytometry.
In a different study, the authors encapsulated plasmid DNA in lipid nanoemulsions and employed pegylated nanoemulsions for adsorption. The protein produced by the α-L-iduronidase gene was present in every tissue sampled for analysis. Furthermore, the authors have shown that pegylation makes nanoemulsions more stable. This enables evaluations from a larger perspective than cell culture [57].
According to the dosage and timing of the drug’s activity, Fraga et al. [58] investigated the effectiveness of nanoemulsion implantation with a plasmid harboring the α-L-iduronidase gene in a distinct investigation. Using a comparable cationic nanoemulsion and DNA adsorption and encapsulation, they investigated the MPS I mouse model and found that the α-L-iduronidase activity was boosted, with the impact being better with a higher dose.
These investigations showed that using nanoemulsions as a nucleic acid carrier may have a dose and time-dependent effect that can enhance the transport of genetic material in gene therapy [59].
In a different study, charge-converting nanoemulsions that are alkaline phosphatase-responsive were used to transport drugs to retinal cells and transfect them with plasmid DNA, with promising results for both drug and gene delivery and transfection efficiency. In this study, the functional layer of polyphosphate on the positively charged poly-L-lysine-oleylamine conjugate/triphosphate (PLOA/TPP) nanoemulsions helped the nanocarriers move through the vitreous humor with less risk of encountering the vitreous humor’s negatively charged constituents and made it easier for cell membrane-bound alkaline phosphatase to access the nanocarriers. In addition, PLOA/TPP nanoemulsions were loaded with green fluorescent protein plasmid complexes that contained a hydrophobic ion pair, leading to strong protein expression with a transduction rate of about 50%. These systems face many challenges during formulation and stability as depicted in Figure 4; however, they may be effective delivery systems for retinal drugs and genes owing to their low cytotoxicity [60].
PATENTS AND CLINICAL TRIALS
There are various patents submitted and accepted which include nanoemulsions for medical and cosmetic purposes. Many trials of formulating nanoemulsions for skin conditions, cosmetics, and other purposes using photodynamic therapy, gel formation, and other techniques are added to patents in Table 1 including botulinum toxin nanoparticle compositions, which were synthesized using high shear forces by the process of microfluidization and high-pressure homogenization eliminated the use of abrasive agents in the composition to achieve transdermal delivery of the toxin and achieved a better effect on the skin without significant irritation. In another study of nanoemulsion formulation, the anti-cancer drug dacarbazine shows higher anti-cancer efficacy in solid tumors, when compared to the free solution of the drug. The formulation is prepared by microfluidization and the drug is encapsulated in the nanoemulsion. This improves the targeted delivery of the drug and reduces tumor progression at the particular site. Ultrasonic radiation is also used for treating a tumor by delivering the anti-cancer drug encapsulated in a nanoemulsion. Furthermore, the tumor is exposed to about 1 to 5 MHz of ultrasonic radiation using direct intratumoral injection. Combining drug delivery through ultrasound-triggered drug release within the tumor using nanoemulsion conversion to microbubbles may result in an effective double-targeting chemotherapeutic approach for solid tumors. Various nanoemulsions are used to reduce and accelerate the healing process of the burn wound. Synergism is also achieved in one study where a combination of tocopherol, phosphatidylcholine, and sodium pyruvate encapsulated and delivered as a nanoemulsion showed better results in treating cancer when compared to the free solutions.
 | Figure 4. Challenges for delivering genetic material using nanoemulsions. [Click here to view] |
 | Table 1. Patents on various nanoemulsions. [Click here to view] |
INDUSTRIAL AND FUTURE PERSPECTIVES
Nanoemulsion has proven to be a flexible and effective new medication delivery technology. The ability of nanoemulsions to solubilize non-polar active chemicals has led to several applications as a drug delivery approach in the pharmaceutical industry. Future applications of nanoemulsion in various therapeutic disciplines or in the development of cosmetics for transdermal and topical formulation are quite bright. One of the many uses for nano-sized emulsions is in the field of drug delivery, where they serve as effective carriers for medicines and bioactive agents, enabling administration via a variety of routes. For meeting nutritional needs, targeted drug release, distribution of vaccines, and targeting of drugs to specific locations, their parenteral administration is necessary. There are many benefits and uses for oral drug administration using these vehicles, where the size of the droplets affects how well they are absorbed in the gastrointestinal region. Currently, most of the drug molecules, which are heat sensitive, are not being considered for emulsification as the industry has the option of only hot emulsification which cannot be used for the emulsification of thermolabile drugs. Hence, the cost-effective and sustainable cold emulsification process should open the path for the development of topical formulations of some actives that have a good bioavailability profile while using topically. We should soon be adopting a cold emulsification process as advanced emulsifiers and new generation cost-effective high-pressure homogenizers are making a way for this to happen soon.
CONCLUSION
The innovative fast absorption technology of nanoemulsion must be explored for scaling up versatile pharmaceutical products for the betterment of society. Nanoemulsions provide effective drug distribution and control. As per the thorough literature studies on nanoemulsion, researchers must work on the cold process of emulsification on thermolabile drugs with high-shear homogenizers as this method can be widely scaled up in a commercial scale setup by using better rotar and stator configuration. This method is very cost-effective industry-friendly, sustainable, and has very good scalability. The preservation of the encapsulated drug and its components and improved distribution compared to most traditional dosage forms are both supported by the current research data. Researchers and industry professionals may be able to use the benefits of nanoemulsion carriers to get over odd drug delivery issues such as permeability and in vivo stability. There is a need for further investigation to check the usefulness of emulsion nanotechnology for the administration of herbal and small-molecule medications. New characterization techniques for nanoemulsions are making it easy to formulate optimum formulations with a reduced number of trials. Nanoemulsions have the potential to be the future of pharmaceuticals by making formulation development easy using cold emulsification techniques and creating a wide variety of products in every domain.
LIST OF ABBREVIATIONS
AD, Alzheimer’s disease; O/W, Oil-in-water; PCS, Photon correlation spectroscopy; PD, Parkinson’s disease; PIT, Phase inversion temperature; PLOA/TPP, Poly-L-lysine-oleylamine conjugate/triphosphate; PSO, Pomegranate seed oil; SEM, Scanning electron microscopy; TEM, Transmission electron microscope; W/O, Water-in-oil; WPI, Whey protein isolates.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–27. CrossRef
2. Patidar A, Thakur DS, Kumar P, Verma J. A review on novel lipid based nanocarriers. Int J Pharm Pharm Sci. 2010;2(4):30–5.
3. Lin TJ. Low energy emulsification. J Soc Chem. 1978;125:117–25. CrossRef
4. Modarres-Gheisari SM, Gavagsaz-Ghoachani R, Malaki M, Safarpour P, Zandi M. Ultrasonic nano-emulsification—a review. Ultrason Sonochem. 2019;52:88–105. CrossRef
5. Ganesan P, Karthivashan G, Park SY, Kim J, Choi DK. Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments. Int J Nanomed. 2018;13:6109–21. CrossRef
6. Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–9:303–18. CrossRef
7. Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Control Release. 2008;128(3):185–99. CrossRef
8. Fryd MM, Mason TG. Advanced nanoemulsions. Annu Rev Phys Chem. 2012;63:493–518. CrossRef
9. Schork FJ, Luo Y, Smulders W, Russum JP, Butté A, Fontenot K. Miniemulsion polymerization. In: Okubo M (Eds.). Polymer particles. Advances in polymer science. Berlin, Heidelberg, Germany: Springer; 2005. Vol. 175, pp 129–255. CrossRef
10. Awad TS, Moharram HA, Shaltout OE, Asker DY, Youssef MM. Applications of ultrasound in analysis, processing and quality control of food: a review. Food Res Int. 2012;48(2):410–27. CrossRef
11. Uluata S, Decker EA, McClements DJ. Optimization of nanoemulsion fabrication using microfluidization: role of surfactant concentration on formation and stability. Food Biophysics. 2016;11(1):52–9. CrossRef
12. Jing S, Li Q, Zheng L, Yue L, Fan S, Tao G, et al. Dynamic high pressure microfluidization-assisted extraction and bioactivities of Cyperus esculentus (C. esculentus L.) leaves flavonoids. Food Chem. 2016;192:319–27. CrossRef
13. Bai L, McClements DJ. Development of microfluidization methods for efficient production of concentrated nanoemulsions: comparison of single-and dual-channel microfluidizers. J Colloid Interface Sci. 2016;466:206–12. CrossRef
14. Ricaurte L, de Jesús Perea-Flores MJ, Martinez A, Quintanilla-Carvajal MX. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov Food Sci Emerg Technol. 2016;35:75–85. CrossRef
15. Liu CM, Liang RH, Dai TT, Ye JP, Zeng ZC, Luo SJ, et al. Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocoll. 2016;57:55–61. CrossRef
16. Liu F, Zhu Z, Ma C, Luo X, Bai L, Decker EA, et al. Fabrication of concentrated fish oil emulsions using dual-channel microfluidization: impact of droplet concentration on physical properties and lipid oxidation. J Agric Food Chem. 2016;64(50):9532–41. CrossRef
17. Robin O, Remillard N, Paquin P. Influence of major process and formulation parameters on microfluidized fat globule size distribution and example of a practical consequences. Colloids Surf Physicochem Eng Asp. 1993;80(2–3):211–22. CrossRef
18. Pinnamaneni S, Das NG, Das SK. Comparison of oil-in-water emulsions manufactured by microfluidization and homogenization. Pharmazie. 2003;58(8):554–8.
19. Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci. 2012;17(5):246–54. CrossRef
20. Spernath L, Regev O, Levi-Kalisman Y, Magdassi S. Phase transitions in O/W lauryl acrylate emulsions during phase inversion, studied by light microscopy and cryo-TEM. Colloids Surf Physicochem Eng Asp. 2009;332(1):19–25. CrossRef
21. Morales D, Solans C, Gutiérrez JM, Garcia-Celma MJ, Olsson U. Oil/water droplet formation by temperature change in the water/C16E6/mineral oil system. Langmuir. 2006;22(7):3014–20. CrossRef
22. Mason TG, Graves SM, Wilking JN, Lin MY. Extreme emulsification: formation and structure of nanoemulsions. Condens Matter Phys. 2006. CrossRef
23. Wei X. Disperse systems. 2017. Vol. 2.
24. Samah NA, Williams N, Heard CM. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int J Pharm. 2010;401(1–2):72–8. CrossRef
25. Saint Ruth H, Attwood D, Ktistis G, Taylor CJ. Phase studies and particle size analysis of oil-in-water phospholipid microemulsions. Int J Pharm. 1995;116(2):253–61. CrossRef
26. Li X, Anton N, Ta TM, Zhao M, Messaddeq N, Vandamme TF. Microencapsulation of nanoemulsions: novel Trojan particles for bioactive lipid molecule delivery. Int J Nanomed. 2011:1313–25. CrossRef
27. Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech. 2007;8:249–57. CrossRef
28. McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8(6):1719–29. CrossRef
29. Amselem S, Friedman D. Submicron emulsions as drug carriers for topical administration. Submicron emulsions in drug targeting and delivery. Boca Raton, FL: CRC Press; 1998. Vol. 9, pp 153–73. CrossRef
30. Silva HD, Cerqueira MÂ, Vicente AA. Nanoemulsions for food applications: development and characterization. Food Bioprocess Technol. 2012;5:854–67. CrossRef
31. Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(05):626. CrossRef
32. Haider MF, Khan S, Gaba B, Alam T, Baboota S, Ali J, et al. Optimization of rivastigmine nanoemulsion for enhanced brain delivery: in-vivo and toxicity evaluation. J Mol Liq. 2018;255:384–96. CrossRef
33. Chatterjee B, Gorain B, Mohananaidu K, Sengupta P, Mandal UK, Choudhury H. Targeted drug delivery to the brain via intranasal nanoemulsion: available proof of concept and existing challenges. Int J Pharm. 2019;565:258–68. CrossRef
34. Panda N, Reddy AV, Reddy GS, Panda KC. Formulation design and in vitro evaluation of zolmitriptan immediate release tablets using primojel and AC-Di-Sol. J Pharm Sci Res. 2015;7(8):545–53.
35. Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G, et al. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11(2):84. CrossRef
36. Tepper SJ, Cady RK, Silberstein S, Messina J, Mahmoud RA, Djupesland PG, et al. AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder vs 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS study): a comparative randomized clinical trial across multiple attacks. Headache. 2015;55(5):621–35. CrossRef
37. Desai A, Vyas T, Amiji M. Cytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulations. J Pharm Sci. 2008;97(7):2745–56. CrossRef
38. Mishra DK, Kumar A, Raj R, Chaturvedi A. Capmul MCM based nanoemulsion for intranasal delivery of an antidepressant. Bull Pharm Res. 2013;3(1):34–9.
39. Mustafa G, Baboota S, Ahuja A, Ali J. Formulation development of chitosan coated intra nasal ropinirole nanoemulsion for better management option of Parkinson: an in vitro ex vivo evaluation. Curr Nanosci. 2012;8(3):348–60. CrossRef
40. Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology. 2014;25(48):485102. CrossRef
41. Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016;23(4):1444–52. CrossRef
42. Sood S, Jain K, Gowthamarajan K. Curcumin-donepezil–loaded nanostructured lipid carriers for intranasal delivery in an Alzheimer’s disease model. Alzheimers Dement 2013;9(4):299. CrossRef
43. Espinoza LC, Silva-Abreu M, Clares B, Rodríguez-Lagunas MJ, Halbaut L, Cañas MA, et al. Formulation strategies to improve nose-to-brain delivery of donepezil. Pharmaceutics. 2019;11(2):64. CrossRef
44. Mizrahi M, Friedman-Levi Y, Larush L, Frid K, Binyamin O, Dori D, Fainstein N, Ovadia H, Ben-Hur T, Magdassi S, Gabizon R. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: the case of genetic CJD. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10(6):1353-63. CrossRef
45. Friedman-Levi Y, Meiner Z, Canello T, Frid K, Kovacs GG, Budka H, et al. Fatal prion disease in a mouse model of genetic E200K Creutzfeldt-Jakob disease. PLoS Pathog. 2011;7(11):e1002350. CrossRef
46. Tou KA, Rehman K, Ishak WM, Zulfakar MH. Influence of omega fatty acids on skin permeation of a coenzyme Q10 nanoemulsion cream formulation: characterization, in silico and ex vivo determination. Drug Dev Ind Pharm. 2019;45(9):1451–8. CrossRef
47. Farahani H, Barati A, Arjomandzadegan M, Vatankhah E. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int J Biol Macromol. 2020;162:762–73. CrossRef
48. Xue F, Li X, Qin L, Liu X, Li C, Adhikari B. Anti-aging properties of phytoconstituents and phyto-nanoemulsions and their application in managing aging-related diseases. Adv Drug Deliv Rev. 2021;176:113886. CrossRef
49. Silva FF, Ricci-Júnior E, Mansur CR. Nanoemulsions containing octyl methoxycinnamate and solid particles of TiO2: preparation, characterization and in vitro evaluation of the solar protection factor. Drug Dev Ind Pharm. 2013;39(9):1378–88. CrossRef
50. Bernardi DS, Pereira TA, Maciel NR, Bortoloto J, Viera GS, Oliveira GC, et al. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnol. 2011;9(1):1–9. CrossRef
51. Abbasi F, Samadi F, Jafari SM, Ramezanpour S, Shargh MS. Ultrasound-assisted preparation of flaxseed oil nanoemulsions coated with alginate-whey protein for targeted delivery of omega-3 fatty acids into the lower sections of gastrointestinal tract to enrich broiler meat. Ultrason Sonochem. 2019;50:208–17. CrossRef
52. Cheong AM, Tan CP, Nyam KL. In vitro evaluation of the structural and bioaccessibility of kenaf seed oil nanoemulsions stabilised by binary emulsifiers and β-cyclodextrin complexes. J Food Eng. 2016;189:90–8. CrossRef
53. Park H, Mun S, Kim YR. UV and storage stability of retinol contained in oil-in-water nanoemulsions. Food Chem. 2019;272:404–10. CrossRef
54. Chen X, McClements DJ, Zhu Y, Chen Y, Zou L, Liu W, et al. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food Res Int. 2018;114:30–7. CrossRef
55. Fan Y, Yi J, Zhang Y, Wen Z, Zhao L. Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017;63:256–64. CrossRef
56. Schuh RS, de Carvalho TG, Giugliani R, Matte U, Baldo G, Teixeira HF. Gene editing of MPS I human fibroblasts by co-delivery of a CRISPR/Cas9 plasmid and a donor oligonucleotide using nanoemulsions as nonviral carriers. Eur J Pharm Biopharm. 2018;122:158–66. CrossRef
57. Fraga M, Bruxel F, Diel D, de Carvalho TG, Perez CA, Magalhães-Paniago R, et al. PEGylated cationic nanoemulsions can efficiently bind and transfect pIDUA in a mucopolysaccharidosis type I murine model. J Control Release. 2015;209:37–46. CrossRef
58. Fraga M, de Carvalho TG, Bidone J, Schuh RS, Matte U, Teixeira HF. Factors influencing transfection efficiency of pIDUA/nanoemulsion complexes in a mucopolysaccharidosis type I murine model. Int J Nanomed. 2017:2061–7. CrossRef
59. Fraga M, Schuh RS, Poletto E, de Carvalho TG, França RT, Pinheiro CV, et al. Gene therapy of mucopolysaccharidosis type I mice: repeated administrations and safety assessment of pIDUA/Nanoemulsion complexes. Curr Gene Ther. 2021;21(5):464–71. CrossRef
60. Nguyen Le NM, Zsák S, Le-Vinh B, Friedl JD, Kali G, Knoll P, et al. Charge-converting nanoemulsions as promising retinal drug and gene delivery systems. ACS Appl Mater Interfaces. 2022;14(39):44981–91. CrossRef