INTRODUCTION
Free radicals are produced within our body through a variety of chemical reactions, such as lipid peroxidation, aging, membrane damage, and carcinogenic reactions, and antioxidants, that can intercept the propagation and termination of the chain reaction by scavenging the free radicals (Fang et al., 2002; Hanasaki et al., 1994; Li et al., 2015; Peng et al., 2014). Antioxidants are a class of compounds present in medicinal herbs, fruit, vegetables, and spices that can act as free radical scavengers due to the presence of poly-hydroxyl groups containing compounds in their chemical constituents, such as polyphenolic acids, flavonols, flavones, isoflavones, and flavonoid glycosides (Hanasaki et al., 1994; Korkina and Afanasev, 1997). The health benefits of herbal supplements are widespread due to the presence of antioxidant components; however, the materials used for the preparation of herbal supplements can affect the safety of the finished products as they not only have health-beneficial components but also contain a number of nonessential and toxic metals (Ansari et al., 2015; Chambial et al., 2017; Saper et al., 2008).
The human body needs sodium, potassium, calcium, magnesium, and phosphorous as major essential elements and iron, zinc, selenium, chromium, manganese and iodine, copper, and fluoride as trace essential elements from the diet for maintaining good health (Hermann, 2017; Sobolev et al., 2021). Humans usually consume nonessential metals from natural sources, pharmaceutical products, and dietary supplements (Khan et al., 2021; Korfali et al., 2013; Nessa et al., 2016). Plants and vegetables that grow in polluted environments contain not only essential elements but also nonessential toxic metals, such as aluminum, cadmium, lead, mercury, and arsenic. Aluminum (Al) has no beneficial role in the human body; historically, it has been used in foil and alloys. It is one of the most abundant metal presents in the earth’s crust (8%) and is a highly reactive metal. Humans can ingest Al through drinking water, supplements, antacids, and foods (EFSA, 2008; Klotz et al., 2017; WHO, 2011a). The excessive ingestion of Al may cause neurotoxicity, anemia, encephalopathy with dementia, and metabolic bone disease (Bondy, 2014; Klotz et al., 2017; Yeh et al., 2016). Lead (Pb) and cadmium (Cd) are toxic elements, and they have no health-beneficial effects in mammals (Chambial et al., 2017). Historically, Pb has been used in paint, pigment, cable water pipes, lead-based batteries, and leaded gasoline. The presence of Pb in herbal supplements is mostly related to the global supply chain (Saper et al., 2008). Overexposure to Pb leads to Pb poisoning that affects a number of internal organs of the human body, such as the nervous system, reproductive system, heart, kidney, and intestine (Araujo et al., 2004; WHO, 2011b), and leads to anemia, encephalopathy, delirium, headache, seizures, and short-term memory loss (Patrick, 2006; Wani et al., 2015). Naturally, Cd is present in soil and water, which can contaminate the medicinal herbs and spices used in the preparation of herbal supplements. Long-term oral exposure to Cd may lead to many toxicological effects on the internal organs of mammals and leads to cancer in the kidney, liver, and prostate (Bernard, 2008; WHO, 2011b). The atomic weight of Ni is five times more in comparison with the specific gravity of water; hence, Ni is considered a heavy metal (Duffus, 2002). Ni is widely distributed in nature, with approximately 2 g/l present in freshwater, which can leach to surface water through rains, and approximately 3–1,000 mg/kg present in agricultural soils, which can also lead to the presence of Ni in medicinal plants, herbs, and spices grown on contaminated soil (Nickel, 1991). Historically, Ni has been used in batteries, coins, and armor. Overexposure to Ni may lead to a number of toxic effects on the human body, such as dermatitis, asthma, and lung cancer (Costa et al., 1994; Sunderman Jr, 1993).
A unique property of the element is that it can accumulate in the body with the time of exposure and produces adverse effects if the tolerable level is exceeded. Hence, different regulatory bodies (WHO, 2011a, 2011b; USP-NF, 2019) set the permissible level of daily, weekly, and monthly intake for toxic elements. There is no harm in consuming herbal supplements; this belief drives us to use herbal supplements at one time in our lifetime. Most herbal supplements are instructed to be taken for more than 4 weeks in order to achieve the desired effect; hence, long-term consumption of herbal supplements can be an additive for exceeding the limit of nonessential trace toxic metals. Hence, this work aims to evaluate the quality and safety of herbal supplements in terms of their antioxidant status and the presence of nonessential trace toxic metals within the product. In this aspect, this study covered the estimation of Al, Cd, Ni, and Pb from 22 herbal supplements that were commercially available in the United Arab Emirates (UAE) pharmaceutical markets.
MATERIALS AND METHODS
Herbal sample description
Twenty-two herbal supplement samples were purchased from the Pharmacies of the UAE in September 2021. All the products were manufactured in the USA. The studied samples were coded as S1–S22; among them, two products (S5 and S14) were in tablet dosage form and the remaining were in a capsule dosage form. The common name, botanical sources, daily recommended doses, and the uses of each product are tabulated in Table 1.
Chemicals
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) disodium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH?), potassium persulfate, Folin–Ciocalteu reagent, L-ascorbic acid (AA), and quercetin (QU) were purchased from Sigma-Aldrich Co. (USA). Inductively coupled plasma (ICP) multielement standard solution IV (23 elements 1,000 mg/l, Merck, Darmstadt, Germany), hydrogen peroxide (30% H2O2, Alpha Chemika, India) and nitric acid (HNO3, 65%, Sigma-Aldrich, Germany), and analytical and spectroscopic grade methanol (Honeywell, France) were also purchased. Milli-Q Ultrapure water (Type 1, Millipore, Bedford, MA) was used for sample, standard, and reagent preparation. Pyrex-grade glassware and polypropylene bottles (Azlon, UK) were used in this preparation.
Sample preparation for nonessential trace toxic metal analysis
The samples were prepared using the microwave acid digestion technique with slight modification (Mustafa et al., 2004). The mean weight of 20 capsules or tablets was recorded; in the case of capsules, the mean weight of powdered contents was determined. Three portions of 0.5 g of sample powder of each product were transferred to the digestion tube, and 2 ml HNO3, 1 ml H2O2, and 5 ml Milli-Q water were added. The samples were digested using the Ethos Easy Advanced microwave digestion system (Italy) at 1,000 W. The digestion was conducted at 100°C, with a ramp time of 10 minutes, a holding time of 5 minutes, and a cooling time of 10 minutes. A reagent blank was also prepared and digested along with the sample solutions. The digested samples were transferred into the volumetric flask (50 ml) and made up the volume with acidified Milli-Q water (2% HNO3). Before metal analysis, the sample solutions were double filtered using Whatman qualitative No. 1 filter paper (diameter 150 mm) and hydrophilic Polytetrafluoroethylene (PTFE ) 0.45 μm syringe filter (Millipore, Millex-LCR).
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) instrument parameters and standard preparations for metal analysis
Shimadzu ICP-9820 (ICP-AES; Software ICPE-9800) was used for trace metal analysis. Plasma conditions for measurement of elements were as follows: radio frequency power, 1.20 kW; plasma gas flow, 10 l/minute; auxiliary gas flow, 0.60 l/minute; carrier gas flow, 0.70 l/minute. The pressure for Argon gas was 463.64 (Pa). ICP multielement standard solution IV was used to prepare different concentrations of working standard solutions. Then, 2% nitric acid with Milli-Q water was used as a diluent and blank solution. Four metals such as Al (aluminum), Ni (nickel), Cd (cadmium), and Pb (lead) were determined and selected as the top most recommended wavelengths from the machine for acquiring the intensity related to the concentration of the metals and the selected wavelengths were verified through checking the best-fit curves options. Six-point calibration curves were generated using 0.01, 0.3, 0.5, 1, 3, to 5 μg/ml metal standard solutions. The wavelengths used in this measurement were 396.153 nm (Al), 226.502 nm (Cd), 341.476 nm (Ni), and 220.353 nm (Pb), respectively.
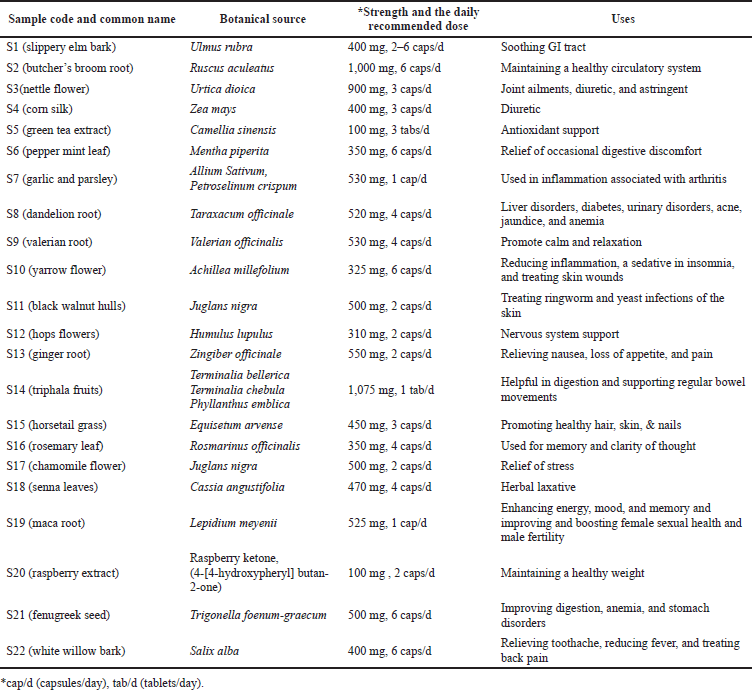 | Table 1. Description of herbal supplements studied for their antioxidant and nonessential trace toxic metal contents. [Click here to view] |
Analytical recovery and precision of ICP-AES metal analysis
The accuracy of the analytical method applied for sample preparation and analysis of their metal contents was determined using analytical recovery studies (ICH, 2005). In this determination, 10, 25, and 50 μg of the equivalent ICP multielement standard solution IV were added to S10 (Yarrow flower), and the control samples were prepared without added standards. The three replicates of S10 were digested using a microwave digestion system. The sample was prepared using the same procedure described in the “sample preparation for non-essential trace toxic metal analysis” section. The samples were analyzed for their Cd, Pb, Al, and Ni contents with the ICP-AES instrument. The metal recovery from the added standards was obtained by subtracting it from their corresponding controls. For evaluation of the within-day precision of the analytical method, three levels, such as 0.2, 0.5, and 1.0 μg/ml, of the ICP multielement standard solution IV were analyzed at three different hours within the day. For interday precision, the standards were analyzed for five consecutive days. The variation in the concentrations of Cd, Pb, Al, and Ni was checked with the five-point calibration curves constructed during each analysis.
Sample preparation for radical scavenging activities and polyphenol determination
The mean weight of 20 capsules (without shell) and tablets were determined, and the tablets were ground into fine powder for further use. Three replicates of 0.5 g powder of each product were mixed with 10 ml spectroscopic grade methanol into the screw-capped test tubes and sonicated for 10 minutes, and later the solution was left standing overnight at room temperature (25°C). The decanted extract solution was then filtered using a 0.45 μm syringe filter and refrigerated in the screw-capped vial for further analysis.
Polyphenol content determination
The polyphenol contents of studied herbal products were estimated using the method described by Nessa et al. (2021). In this determination, QU was used as a standard polyphenol, and 1–100 μg/ml of QU solution in spectroscopic grade methanol was utilized to construct a calibration curve. Then, 100 μl of standards and extract solutions were mixed with 2 ml Folin–Ciocalteu reagent (1:10 diluted) and 2 ml of 7.5% anhydrous sodium carbonate solution in the screw-capped test tubes and the solutions stood in a dark place for 90 minutes for the development of dark-blue color. At the end of the defined time, the absorbance of the colored solution was measured at 760 nm using a Shimadzu-1900 UV-VIS spectrophotometer (Japan) against the blank solution prepared without added samples. The experiment was repeated three times for each product and standard concentration. The standard curve with the regression equation y = 0.0042× −0.0306 (R² = 0.9978) was used to calculate the mg QU equivalent polyphenol present per g of powder contents (mgQEq/g) of each product.
DPPH? scavenging activity
The DPPH? scavenging activity of 22 herbal supplements was determined using the method described by Nessa et al. (2021). At first, the DPPH? solution in methanol (1 μg/ml) was prepared daily, and the absorbance was adjusted with methanol to 0.750 ± 0.03 at 517 nm against methanol as a blank using a Shimadzu-1900 UV-VIS spectrophotometer before commencing the analysis. In this measurement, 100 μl of previously prepared capsule/tablet extract solution (three replicates) was mixed with 2 ml of DPPH? solution and recorded the decrease in absorbance at every 2-minute interval for 30 minutes at 517 nm against the methanol. The changes in absorbance of 2 ml of DPPH? without added samples where the sample was replaced with 100 μl of methanol were also determined for 30-minute intervals and considered as blank. The percent scavenging of DPPH? at 30-minute intervals was calculated using the following formula: [{(ABlank − ASample)/ABlank} × 100], where ABlank is the absorbance of DPPH? without added sample and ASample is the absorbance of DPPH? with the sample. Finally, 100 μM of AA (in water) and QU (9:1, methanol:water) were used as reference compounds during this measurement.
ABTS?+ radical cation scavenging activity
The ABTS?+ scavenging activities of herbal supplements were determined using the method described in (Nessa et al., 2021). In this method, 7 mM of ABTS disodium salt and 2.5 mM potassium persulphate were prepared with Milli-Q water, and then 100 ml of each solution was transferred to the amber-colored reagent bottle and stored in the dark place for about 20 hours to facilitate the generation of ABTS?+. In this measurement, 2 ml of ABTS?+ solution (absorbance was adjusted to 0.750 ± 0.05 at 734 nm with ethanol) was mixed with 100 μl of extract solution, and the absorbance was read at 734 nm at 1-minute intervals for 7 minutes using Shimadzu-1900 UV-VIS spectrophotometer against ethanol. A blank (ABTS?+) was run in the same manner where a sample was replaced with 100 μl water. The percent scavenging activities of ABTS?+ by the herbal supplements at 7 minutes were calculated using the following formula: [{(ABlank − ASample)/ABlank} × 100]. AA (in water) acid and QU (9:1, methanol:water) were used as standards in this measurement.
Statistical analysis
The mean values of DPPH? and ABTS?+ scavenging activities of 22 herbal supplements were compared using a one-way analysis of variance. The software SPSS Statistics (IBM, version 23) and Tukey’s test (p < 0.05) were performed for comparison between mean values.
RESULTS AND DISCUSSION
Products weight variation
Among the studied herbal supplements, 20 products were supplied as hard capsules, and two products (S5 and S14) were in the tablet dosage form. The weight of 20 capsules/tablets of each product was recorded for the hard capsules; the weight variation was recorded with and without capsule shells. All the products contained a single herb except S7 (two herbs) and S14 (three fruits). The product weight varies from 0.306 (S20) to 1.204 g (S14). Individual weight variations of studied hard capsules were less than 5%, which complies with the USP-NF recommended limits (±10%) (USP-NF, 2019). For tablet products, the recorded average weight of S5 and S14 was 0.241 and 1.204 g, respectively, which also complied with the USP-approved limits. According to USP-NF (2019), a 7.5% weight variation is allowed if the average weight of dietary supplements (tablets) ranges from 0.130 to 0.325 g, and a 5% weight variation is allowed if the weight is more than 0.324 g. The percent relative standard division (%RSD) for individual weight variation for studied products was within 5%; hence, all the studied products passed the weight variation test. The recorded average weights for the 22 products are shown in Table 2.
Polyphenol contents and free radical scavenging activity
The health-beneficial effects of herbs, fruits, and spices are related to their polyphenolic contents and their antioxidant status. Hence, the polyphenol contents and free radical scavenging activities of the studied products were conducted (Nessa et al., 2021). The polyphenol contents of the studied herbs ranged from 0.20 to 2.84 mg QEq/g of powder. The minimum polyphenol contents were recorded in S20, which contained 0.259 g raspberry extract, and it scavenges about 20.05% DPPH? and 39.60% ABTS?+, and the highest level was recorded in S5 (green tea tablet), which scavenges about 89.11% DPPH? and 99.48% ABTS?+. Hence, there was a clear correlation between the polyphenol contents of herbal supplements with their free radical scavenging activities.
The %DPPH? scavenging activity at 30-minute intervals of the products S1, S5, S8, S9, S10, S13, S14, S16, and S17 exhibited ≈ 80%–89% radical scavenging activities, whereas ≈60% to 69% radical scavenging activity was observed from the products S2, S6, and S18, respectively. The products S4, S7, S12, S22, and QU showed about 50%–59% DPPH? scavenging activities. The lowest (<50%) DPPH? scavenging activity was observed from the products S3 (35.67%), S11 (43.32%), S15 (48.49%), S19 (47.89%), S20 (20.05%), S21 (43.54%), and AA (45.40%), respectively. The reaction rate of DPPH? and antioxidants was gradual, and the DPPH? concentration decreased with time. The products S1, S6, S10, and S16 reacted very fast and stabilized the reaction within 2 minutes, S17 at 10 minutes, and other products within 15–20 minutes. Hence, selection for 30 minutes was appropriate for the completion of the reaction. The changes in DPPH? concentration with time in the presence of antioxidants are shown in Figure 1. In comparison, in terms of the DPPH radical scavenging activities among the studied products, there were no statistically (p < 0.05) significant differences in activities between S11 and S21; S15 and S19; and S7, S12, and S22, respectively. Furthermore, products such as S1, S5, S8, S9, S10, S13, S14, S16, and S17 showed to have statistically (p < 0.05) similar DPPH? scavenging activities.
 | Table 2. Weight variation, polyphenolic content of studied herbal supplements, and their free radical scavenging activities. [Click here to view] |
The percent ABTS?+ radical scavenging activity of the herbal supplements was higher than their corresponding DPPH? radical scavenging activity. In comparison, DPPH? is a stable radical, and the media of reaction was methanol. However, ABTS?+ was produced by an immediate chemical reaction, and the media of the reaction was water. Hence, the affinity of reaction between extracts solution towards ABTS?+ and DPPH? might be different, and differences in scavenging activity were revealed. Nine products such as S1, S5, S8, S9, S10, S13, S14, S16, and S17 scavenged ABTS?+ ≥90%, whereas the highest DPPH? scavenging activity was ≤89%. The overall ranking based on the ABTS?+ radical scavenging activity of studied herbal supplements was decreased in the following order: S5 ≈ S10 > S8 ≈ S9 ≈ S16 > S14 ≈ S17 >S1 ≈ S13 > S6 ≈ S18 > S4 > S2 > S12 > S22 > QU > S7 > S11 > S19 > S15 > AA> S3 > S21 > S20. QU and AA were used as reference compounds. Based on the free radical scavenging reaction, ABTS?+ reacted faster with the extracts as the reaction interval was only 7 minutes, and within this time interval, seven products scavenged >97% ABTS?+, indicating reaction time was sufficient for scavenging enough ABTS?+. The decreases of ABTS?+ with time in the presence of 22 herbal supplement extract solutions are presented in Figure 2. Statistically, the products S3 and S11, S15, and S19 showed no significant (p < 0.05) difference in ABTS?+ scavenging activity. In addition, other products such as S5, S8–S10, S14, S16, and S17; S1, S6, S13, and S18 also had statistically (p < 0.05) similar activities in respect of ABTS?+ scavenging activity.
 | Figure 1. Hydrogen donating abilities of 22 herbal supplements (5% methanol extract solution) as depicted with scavenging of DPPH? at 517 nm. [Click here to view] |
 | Figure 2. Hydrogen donating abilities of 22 herbal supplements (5% methanol extract solution) as depicted with scavenging of ABTS?+ radical cation at 734 nm. [Click here to view] |
Nonessential trace toxic metal contents of herbal supplements
The presence of trace levels of Al, Cd, Ni, and Pb in the herbal supplements was determined using the ICP spectrometer. The detector linearity of the machine was checked by constructing calibration curves using 0.01–5 μg/ml metal standard solutions, and the linear regression coefficients of calibration curves of the estimated metal standard were 0.99974 (Al), 0.99918 (Cd), 0.99923 (Ni), and 0.99923 (Pb), respectively. In this measurement, the machine-recorded limit of detection (three times the SD of the blank) was 0.0020 ± 0.0014 (Al), 0.0011 ± 0.0009 (Ni), 0.0017 ± 0.0006 (Pb), 0.0004 ± 0.0003 μg/ml (Cd); the limit of quantification (10 times the SD of the blank) were 0.0073 ± 0.0057, 0.0038 ± 0.0032 (Ni), 0.0059 ± 0.0021 (Pb), and 0.0015 ± 0.0013 μg/ml (Cd) (FDA, 2021).
The sample was prepared using a microwave digestion system. The method’s accuracy was determined by analytical recovery of added 10, 25, and 50 μg standards from the sample S10. The recoveries of Al, Cd, Ni, and Pb were in the ranges of 97.10%–100.06%, with %RSD of 1.16%–3.98%. The precision of the analytical method was evaluated by running three different levels of standard solution, such as 0.2, 0.5, and 1.0 μg/ml, and %RSD from the within-day precision was ≤2%. The %RSD from the day-to-day precision data was ≤5%; hence, the analytical method was accurate and the machine’s operating condition was optimal and precise for carrying out the trace toxic metal analysis of herbal supplements. The analytical recovery and precision analysis data are presented in Table 3. The daily intake of Ni, monthly intake of Cd, and weekly intake of Pb and Al from consuming these herbal products were calculated based on the recorded level of metals (μg)/serving unit and their maximum recommended daily doses (USP-NF, 2019).
Aluminum (Al)
The general population is exposed to Al from food, drinking water, pharmaceuticals, and food additives (EFSA, 2008; Yeh et al., 2016). There is no firm indication that ingested Al produces acute toxicity in the human body; however, daily exposure to Al is limited, and the provisional tolerable weekly intake (PTWI) of Al is 2 mg/kg BW (WHO, 2011a) and the limit set by European Food Safety Authority (EFSA, 2008) is 1 mg/kg BW. The present study revealed that all the studied herbal supplements contained Al. The highest amount was observed in S14 (8.7932 μg), which had three fruits, and the lowest amount was recorded in S6 (1.4502 μg), which represented the product containing peppermint leaves. The recorded Al concentration in the studied herbal products is presented in Table 4. About 4–5 μg Al was recorded per capsule in the samples of S4, S7–S9, S13, S15, and S17–S19, and ≈ 2–3 μg Al found in products S1, S2, S3, S5, S10–S12, S16, and S20–S22, respectively. The permitted tolerable weekly intake of Al from the recommended daily dose was calculated and is presented in Table 5, where it can be seen that the maximum intake of Al was 156.8292 μg from consumption of S9; however, the value was lower than the recommended limit, PTWI: 2,000 μg/kg BW (WHO, 2011a). The lowest intake of Al was from S20, and the recommended dose of this product was the lowest, 0.2 g, among the studied products. However, the consumption of certain herbal products for a longer period that contained higher levels of Al may increase the risk of Al toxicity compared to their natural benefit. In conclusion, the level of Al present within the capsules or tablets of herbal supplements complied with the limit set by different regulatory bodies (EFSA, 2008; WHO, 2011a).
Nickel (Ni)
Ni has no nutritional value in terms of human health; however, trace levels are needed as an essential element by certain plants, microorganisms, and animals (Song et al., 2017). Ni is present in many foods, and humans can intake about 100–300 μg/day of Ni based on their dietary habits (Nickel, 1991). Based on the public health statement, Ni is a hazardous substance and it can exert adverse effects on human health; hence, this study addressed the Ni contents of commercial 22 herbal supplements. All the studied products contained a lower level of Ni and ranged from 0.1401 to 3.1681 μg per capsule, and the results are shown in Table 4. Products S1, S4–S6, S16, S18, S20, and S22 contained the lowest level of Ni (≈ <1 μg/capsule), whereas the remaining products such as S2, S3, S7–S15, S17, S19 and S21 contained 1–3 μg Ni/capsule. According to the daily recommended dose of the studied products, the calculated daily intake of Ni was 8.1405–12.6727 μg from consuming the products S2, S8–S10, and S15, whereas 4.1074–6.3363 μg was from consuming S3, S8, S14, S17, and S21, respectively. The lowest level of daily Ni intake, about 0.2803–3.5265 μg, was from consuming the products S1, S2, S4–S5, S7, S11–13, S16, and S18–S22, respectively. The daily intake of Ni from studied products is shown in Table 5. The recommended tolerable upper level of Ni intake (from Ni soluble salts) is 1 mg/day (≡17 μg/kg BW/day) for an adult of 60 kg BW (Institute of Medicine, 2001). The Occupational Safety and Health Administration recommended that the intake of Ni from drinking water should not exceed 0.1 mg/l (ATSRD, 2005). Compared with the recommended tolerable upper daily intake of Ni (17 μg/kg BW), all the studied herbal supplements exhibited lower concentrations of Ni than the regulatory body’s recommended limit. Hence, all the products can be considered safe to consume for a long period.
 | Table 3. Analytical recovery studies, within-day precision, and interday precision data for Al, Cd, Ni, and Pb were analyzed using ICP-AES. [Click here to view] |
 | Table 4. Ni, Cd, Pb, and Al contents of 22 herbal supplements determined by ICP-AES. Results are mean ± SD (n = 3). [Click here to view] |
 | Table 5. Daily intake of Ni, weekly intake of Al and Pb, and monthly intake of Cd from consuming 22 herbal supplements and their maximum recommended daily doses. [Click here to view] |
Lead (Pb)
The medicinal plants, herbs, and spices grown in contaminated soil, water, and a polluted environment lead to the occurrence of Pb in supplements. Acute toxicity from Pb exposure is low; however, long-term exposure to Pb may lead to Pb poisoning (Patrick, 2006, WHO, 2011b). Hence, the Pb content of 22 herbal supplements was determined and revealed that all the studied products contained <1 μg Pb/capsule, and the results are shown in Table 4. The recommended level for PTWI of Pb by the Joint FAO/WHO Expert Committee (WHO, 2011b) was 3 mg/person, which was equivalent to 50 μg/kg BW for an adult. Later, the committee revised the PTWI for Pb to 25 μg/kg BW and applied it to all age groups, including children (WHO, 2011b). The calculated weekly intake of Pb from the recommended daily dose of 22 herbal supplements revealed that the consumer consumes variable amounts of Pb weekly from various products, such as about ≈10–14 μg Pb from S2, S5, and S1, respectively, whereas about ≈7–9 μg Pb is consumed from the products S3, S6, S10, S14, S18, and S21, respectively. The weekly intake of Pb was lowest from S20, which contained raspberry extract. The weekly intake of Pb from the studied herbal products was lower than the recommended PTWI (WHO, 2011b), and the results are presented in Table 5 with their recommended daily doses.
Cadmium (Cd)
Cd has been classified as carcinogenic and has no health benefits in humans (Godt et al., 2006; WHO 2011b). This study revealed that trace levels of Cd present in all the studied herbs ranged from 0.0016 to 0.1166 μg per capsule or tablet (Table 4). At first, the PTWI of Cd was 7 μg/kg BW, and later, this value was withdrawn (WHO, 2011b). Given the longer half-life of Cd, daily exposure health risk is negligible, and monthly exposure will provide more accurate data; hence, the PTMI of Cd is revised to 25 μg/kg BW (WHO, 2011b). Based on the recommended daily doses, the calculated monthly intake of Cd from the studied products was 0.1010–20.9957 μg, and the calculated values are presented in Table 5. Among the studied products, the lowest monthly intake of Cd (<1 μg) was from 12 products such as S3, S4, S6, S7, S11–S13, S16, S19–S21. In contrast, comparatively higher Cd concentrations, such as 10.2714, 13.3804, and 20.9957 μg intake, were from the products S2, S10, and S22, respectively. A moderate level of Cd intake (1–6 μg) was from consuming the products S1, S5, S8, S9, S14, S15, S17, and S18, respectively. Based on the PTMI level of Cd, the studied products contained lower levels of Cd and can be considered safe to consume for a longer period.
CONCLUSION
Twenty-two herbal supplements were analyzed for their free radicals scavenging activities and four nonessential toxic metals content. Plant parts such as flowers, fruits, leaves, seeds, hulls, rhizomes, extracts, and barks were used as raw materials for manufacturing these supplements. The studied products contained various amounts of polyphenols that contributed to their various levels of DPPH? and ABTS?+ radical scavenging activities. Nine products, S1, S5, S8–S10, S13, S14, S16, and S17, were highly potent scavenging ≥80% DPPH? and ≥99% ABTS?+ as they had a higher level of polyphenols content. Four nonessential toxic metals, namely, Al, Cd, Ni, and Pb, were determined using ICP-AES, and all the studied products exhibited positive responses for the studied metals. Based on the recommended daily doses of these products, the daily intake of Ni, weekly intake of Al and Pb, and monthly intake of Cd from consuming these products were lower than the WHO and other regulatory bodies recommended tolerable daily/weekly/monthly intake of toxic metals (Institute of Medicine, 2001; WHO, 2011a, 2011b). In conclusion, the studied products can be considered safe to consume for the duration as instructed by the manufacturer. However, the presence of other toxic metals, such as arsenic and mercury, must be determined for their safe daily intake for long periods.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ansari JA, Ahmad MK, Verma AK, Fatima N, Jilani H. Microwave assisted determination of minerals and toxic metals in traditionally used medicinal plant Zingiber officinale Roscoe by inductively coupled plasma-optical emission spectrometer. Int J Adv Res, 2015; 3(4):879–87.
Araujo J, Beelen AP, Lewis LD, Robinson GG, DeLaurier C, Carbajal M, Ericsson B, Chin Y, Hipkins K, Kales SN, Saper RB, Nordness R, Rabin R, Jeffery N, Cone J, Ramaswamy C, Curry-Johnson P, Gelberg KH, Paquin J, Homa DM, Roscoe RJ. Lead poisoning associated with ayurvedic medications-five states, 2000-2003. MMWR Morb Mortal Wkly Rep, 2004; 53(26):582–4.
ATSRD. Agency for toxic substances and disease registry (ATSDR). Public Health Statement on Nickel. 2005. Available via https://www.atsdr.cdc.gov/ToxProfiles/tp15-c1-b.pdf (Accessed 26 October 2022)
Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res, 2008; 128(4):557–64.
Bondy SC. Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology, 2014; 315:1–7.
Chambial S, Bhardwaj P, Mahdi AA, Sharma P. Lead poisoning due to herbal medications. Indian J Clin Biochem, 2017; 32(2):246–7; doi: 10.1007/s12291-016-0617-2.
Costa M, Zhuang Z, Huang X, Cosentino S, Klein CB, Salnikow K. Molecular mechanisms of nickel carcinogenesis. Sci Total Environ, 1994; 148(2–3):191–9.
Duffus JH. Heavy metals-a meaningless term? Pure Appl Chem, 2002; 74:793–807.
EFSA (European Food Safety Authority). Safety of aluminium from dietary intake, scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J, 2008; 6(7):1–34. Available via https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2008.754 (Accessed September 2022)
Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition, 2002; 18:872–9.
FDA (Food Drug Administration). Elemental analysis manual (EAM) for food and related products, version 3.0. 2021. Available via https://www.fda.gov/food/laboratory-methods-food/elemental-analysis-manual-eam-food-and-related-products. (Accessed 15 March 2023)
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol, 2006; 1:22.
Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med, 1994; 16:845–50.
Hermann JR. Minerals and the body. 2017. Available via https://extension.okstate.edu/fact-sheets/minerals-and-the-body.html (Accessed 26 October 2022)
ICH. (International conference on harmonization of technical requirements for registration of pharmaceuticals for human use) Q2A guidelines. 2005. Available via https://database.ich.org/sites/default/files/Q2_R1__Guideline.pdf (Accessed 10 May 2022).
Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press (US), Washington, DC, 2001. Available via https://www.ncbi.nlm.nih.gov/books/NBK222322/ (Accessed 26 October 2022)
Khan N, Jamil J, Amin F, Masood R, Atlas A. Quantification of macro, micro and trace elements, and antimicrobial activity of medicinal herbs and their products. Arab J Chem, 2021; 14:103055.
Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H. The health effects of aluminum exposure. Dtsch Arztebl Int, 2017; 114:653–9.
Korfali SI, Hawi T, Mroueh M. Evaluation of heavy metals content in dietary supplements in Lebanon. Chem Central J, 2013; 7:10.
Korkina L, Afanasev I. Antioxidant and chelating properties of flavonoids. Adv Pharmacol, 1997; 38:151–63.
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci, 2015; 16:26087–124.
Mustafa S, Mustafa T, Ibrahim N, Hayati S. Comparison of digestion procedures for the determination of trace metal contents in spice samples produced in Turkey. J Food Drug Anal, 2004; 12(3):254–8.
Nessa F, Khan SA, Abu Shawish KYI. Lead, cadmium and nickel contents of some medicinal agents. Indian J Pharm Sci, 2016; 78(1):111–9.
Nessa F, Khan SA, George S. In-vitro evaluation of xanthine oxidase inhibition and antioxidant capacity of water extracts of corn silk (Zea mays L.). Pharm Sci Asia, 2021; 48(5):471–80.
Nickel. Environmental health criteria, no.108. World Health Organization, Geneva, Switzerland, 1991. Available via https://apps.who.int/iris/bitstream/handle/10665/37667/924157108X-eng.pdf?sequence=1. (Accessed 26 October 2022).
Patrick L. Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev, 2006; 11:2–22.
Peng C, Wang X, Chen J, Jiao R, Wang L, Li YM, Zuo Y, Liu Y, Lei L, Ma KY, Huang Y, Chen ZY. Biology of ageing and role of dietary antioxidants. BioMed Res Int, 2014; 2014:831–41.
Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN. Lead, mercury, and arsenic in US-and Indian-manufactured ayurvedic medicines sold via the internet. JAMA, 2008; 300(8):915–23.
Sobolev N, Ellingsen DG, Belova N, Aksenov A, Sorokina T, Trofimova A, Varakina Y, Kotsur D, Grjibovski AM, Chashchin V, Bogolitsyn K, Thomassen Y. Essential and non-essential elements in biological samples of inhabitants residing in Nenets Autonomous Okrug of the Russian Arctic. Environ Int, 2021; 152:106510.
Song X, Kenston SSF, Kong L, Zhao J. Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology, 2017; 392:47–54.
Sunderman Jr FW. Search for molecular mechanisms in the genotoxicity of nickel. Scand J Work Environ Health, 1993; 19(1):75–80.
USP-NF. The United State pharmacopeia 42, National formulary 37. The United States Pharmacopeial Convention, Rockville, MD, 2019.
Wani AL, Anjum Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol, 2015; 8(2):55–64.
WHO Technical Report Series 966. Evaluation of certain food additives and contaminants: seventy-fourth report of the joint FAO/WHO expert Committee on Food Additives. 2011a. Available via http://apps.who.int/iris/bitstream/handle/10665/44788/WHO_TRS_966_eng.pdf (Accessed 26 October 2022)
WHO Technical Report Series No. 960. Evaluation of certain food additives and contaminants: seventy-third report of the joint FAO/WHO expert. 2011b. Available via http://apps.who.int/iris/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf?sequence=1 (Accessed 26 October 2022).
Yeh TS, Liu YT, Liu YT, Liou PJ, Li HP, Chen CC. Investigation of aluminum content of imported candies and snack foods in Taiwan. J Food Drug Anal, 2016; 24(4):771–9.