INTRODUCTION
Infectious diseases are contagious illnesses or diseases brought on by pathogenic microorganisms, such as bacteria, fungi, viruses, protozoans, and helminthes (Shukla et al., 2014). In low-income societies, it is one of the primary causes of fatalities and morbidity (WHO, 2018a, 2018b). The diseases can cause suffering and death of the people but also have significant economic impacts that are not often recognized (Lindahl and Grace, 2015). The emergence and increasing rates of antimicrobial resistance to modern antibiotics are the main challenges to eradicating microbial infections, and the worst aspect is the development of antimicrobial resistance as a natural protective process among microorganisms; even rational use of antibiotics provides for antimicrobial resistance development (Review on Antimicrobial Resistance, 2016). The World Health Organization (WHO) has identified several priority pathogens against which newer antimicrobials should be developed to simplify the search for appropriate antimicrobials; these include Mycobacterium tuberculosis, Escherichia coli, Candida albicans, Streptococcus pneumoniae, Enterobacter spp., Staphylococcus aureus, and Streptococcus pneumonia and others (WHO, 2017). Most of these microorganisms have the capacity to produce biofilms, which are mostly made of DNA, proteins and polysaccharides. The biofilms created by these pathogenic bacteria and fungi are of serious concern because they give the underlying microbes a broad range of resistance (Bakkiyaraj et al., 2013). Novel agents are therefore required to combat these drug-resistant pathogens. Utilizing natural sources is one of many strategies for finding novel antimicrobial agents in developing nations, including India. About 70–90% of the population still uses traditional medicines made of plant extracts (WHO, 2013). The largest biochemical reserves are found in plants, which can produce several compounds with medicinal properties, including antibacterial, antifungal, anticancer, antioxidant, antimycobacterial, and anti-inflammatory effects (Abdallah, 2011; Katiyar et al., 2012; Savoia, 2012). According to the WHO, plants are the most abundant source of numerous pharmaceuticals, and both microbial and non-microbial diseases can be treated with these traditional medicines (Gupta et al., 2016). An estimated 7,500 species of medicinal plants serve as the primary source of medicine for the rural population in India and are utilized for preventative, promotional, and therapeutic purposes (Patil, 2016).
Stemodia verticillata (Mill.) Hassl is an annual herbaceous plant belonging to the family Plantaginaceae; it grows on the banks of rivers and streams (Liang et al., 2011). In India, the plant species are widely distributed in Maharashtra, Kerala, Arunachal Pradesh, Karnataka, and Tamil Nadu state (Sharma et al., 2016). The plant species reported from various states as an agricultural weed used by rural peoples in the Western Ghats of India as a sedative and for managing and treating various infections and their symptoms (Thomas, 2020). The plant species is famously known as Dhangar by the local community of Northern Western Ghats; they use it to treat inflammation and breast cancer (Pers. Communication). However, despite its widespread use, antimicrobial, antioxidant activity and cytotoxicity effects were uninvestigated. Therefore, the study aimed to evaluate antimicrobial, cytotoxicity effects and antioxidant activity of S. verticillata extracts. Furthermore, chemical profiling and quantification of phytochemicals were studied (Awalekar et al., 2021).
MATERIALS AND METHODS
Solvent and chemicals
Ethanol, ascorbic acid, acetone, phenol from Folin-Ciocalteu, dichloromethane (DCM), ethyl acetate, methanol, sodium nitrite, 2, 2-Diphenyl picrylhydrazyl (DPPH), aluminum chloride, dimethylsulphoxide (DMSO), distilled water, sodium carbonate, gallic acid are the reagents and chemicals used. The analytical grade chemicals were all purchased from Loba Chem Pvt. Ltd. in Kolhapur, Maharashtra, India.
Plant sample collection and preparation
The whole plant species of S. verticillata was collected from the Kolhapur District in December 2021. The qualified taxonomist identified the plant species, and the verified plant materials were submitted for herbarium sheets and voucher specimens (VBS 6312); they were then placed in The New College’s herbarium in Kolhapur, Maharashtra, India. The materials were delivered to the Medicinal Chemistry Research Laboratory at Shivaji University’s Chemistry Department for the study. Distilled water was used to clean the plant materials, which were then shade-dried. The dried materials were then ground into powder and stored in a clean labeled container at room temperature until extraction.
Extraction
Cold maceration method using 80% ethanol, aqueous, acetone, ethyl acetate, and DCM separately was used to extract dry powdered plant materials (200 g per solvent) for 48 hours with occasional agitation. The filter paper (Whatman No. 1, England) was used for filtration; the resulting filtrate was concentrated between 40°C and 50°C in a rotary evaporator. The dry extracts were packed in airtight containers and kept in a refrigerator (5°C–8°C) until when required for biological and chemical experimentation.
Antimicrobial activities evaluation
Test microorganism used
A total of six pathogen strains were used, including two Gram-positive bacteria (Bacillus subtilis MTCC 1687 and Staphylococcus aureus MTCC 96), two Gram-negative bacteria (Escherichia coli MTCC 1687 and Pseudomonas aeruginosa MTC 1628), and two fungal strain (Candida albicans ATCC 10231 and Aspergillus niger ATCC 9029), all were purchased from the NCCS, Pune, Maharashtra, India.
Agar-well diffusion assay
With slight modifications, the 80% ethanolic of S. verticallata was first screened for antimicrobial activity using the agar well diffusion method (Mohamed, 2017). Growth mediums were incorporated into the experimental design, and the standard methods for sample preparation and zone of inhibition assessment were used (Gupta et al., 2016). In potato dextrose and nutritional agar media, the microorganisms were grown overnight at 37°C, and 100 μl of suspension containing 106 CFU ml−1 of microbes was dispersed at the top of the surface of prepared agar plates. Wells was created with a cork borer and filled with 100 μl of plant extracts with a 100 mg concentration; kanamycin and surfactin drugs were used as a positive control for comparison. After that, 24 hours were spent incubating the plates at 37°C; the diameters of the inhibition zones (mm) on the surface of the agar surrounding the well were measured using a vernier caliper to determine the antibacterial activity.
Determination of minimum inhibitory concentration (MIC)
Using 96-well microplates, the broth microdilution technique was used to determine the MIC of the 80% ethanolic extracts of S. verticillata (Romulo et al., 2018). Briefly, in 1% DMSO, plant extracts were dissolved and placed into the plate in two-fold repeated dilutions (100 μl each) and diluted in Sabouraud Dextrose or Mueller Hinton Broth (MHB), resulting in concentrations of 500 to 128,000 μg/ml. The positive control (Streptomycin/ Miconazole, MHB, and test organism) and negative control (MHB, test organism, and 1% DMSO) were maintained. Afterward, the appropriate microorganisms were suspended and inoculated to the plates, resulting in a final density of 5 × 105 CFU/ml for bacteria and 1.5 × 103 CFU/ml for fungi, respectively. Plates were then incubated for 24 hours at 37°C (48 hours for C. albicans). ELISA microplate reader was used to measure the turbidity of microorganism growth at 500 nm (Naz et al., 2017). The MIC was defined as the lowest concentration that inhibits the growth of microorganisms greater than 50%. The experiment was done in triplicate.
Minimum bactericidal/fungicidal concentration (MBC/MFC) determination
MBC/MFC was determined by taking 100 μl of culture medium from each well not showing growth and inoculating into Mueller Hinton or Sabouraud Dextrose agar plates and incubating for 24 hours at 37°C, after that, the growth of microorganisms was examined. The extract concentration found to kill 99.9% of bacteria was found to be MBC/MFC.
Evaluation of anti-biofilm formation
Crystal violet assay was used to evaluate the anti-biofilm potential of 80% ethanolic extract of S. verticillata as described by O’toole and Kolter (1998) with some modifications (Fathi et al., 2022). Briefly, biofilms were grown in 96-well plates; 200 μl of TSB was added in each well of the microplate, followed by 100 μl of each selected bacteria (1 × 106 cells/ml). 100 μl of plant extract of various concentrations (1,000, 3,000, 5,000, 7,000 and 10,000 μg/ml) was added in each test wells. As a positive control, 100 ml of standard streptomycin (500 μg/ml) solution was applied to standard test wells for bacteria, Micanozole for fungi, and three wells without test extract or Streptomycin/Micanozole were used as a negative control. The plates were incubated at 37°C for 24 hours. After incubation, the supernatant was taken out. Each well was thoroughly cleaned with 200 μl of sterile saline to remove bacteria/fungi floating around, plates were then dried in the air for 30 minutes. The biofilms created by adhering cells in the plate were stained for 20 minutes at room temperature using 0.1% crystal violet. After incubation, the excess stain was removed, and any unabsorbed stain was eliminated by washing the plate three times with sterile saline. Finally, 200 μl of 96% ethanol was added to solubilize the dye attached to the cells. Using ELISA microplate reader, the optical densities (OD) of stained adhering bacteria/fungi were measured at 630 nm. Each assay was performed in triplicate. The mean absorbance of the samples was determined. The percentage inhibition was calculated as per the formula below,
Determination of antioxidant activity
Ferric reducing power
With a minor modification, the method reported by Alavi and Karimi (2018) was used to assess the reducing power of crude extracts. 2.6 ml of [K3 Fe (CN)6] (1% W/v) and buffer phosphate buffer (0.2 M, pH 6.6) were mixed with 1.0 ml of extracts (5, 10, 15, 20, 25, and 30 μg/ml). The mixed solution was placed in an incubator at 50°C for 20 minutes. 2.6 ml of Trichloroacetic acid (10%) was added to the mixture to stop the chemical reaction and then centrifuged for 15 minutes at 3,000 rpm. The top layer (2.6 ml) of the centrifuged solution was removed and combined with 2.6 ml of water and 0.6 ml (0.1%) Iron (III) chloride. Using ms-Vis Spectrophotometer, the absorbance was recorded at 700 nm in triplicate versus blank solution and expressed as mean ± standard deviation SD. Ascorbic acid was used as standard.
DPPH radical scavenging assay
Using a slightly modified of Shekhar et al. (2014)’s DPPH free radical scavenging test, the sample’s antioxidant activity was examined (Tailor Chandra Shekhar and Goyal Anju, 2014). The 3.0 ml of 1.0 M solutions of DPPH in methanol were added to 3.0 ml of different extracts in methanol and ascorbic acid in water (5, 10, 15, 20, 25, and 30 μg/ml). After shaking, the mixture was let to stand for 30 minutes, and the absorbance was taken at 517 nm. The DPPH solution served as control, methanol was used as a blank, and three times the experiment was repeated (n = 3). The percentage radical scavenging was calculated using the equation below,
Cytotoxicity assay
According to Mosmann (1983), with minor modifications, the MTT colorimetric assay was employed to screen the cytotoxic activities of plant extracts (Ogbole et al., 2017). The normal kidney cell line NRK52E and colon cancer cell line COLO205 were incubated at 1 × 104 cells/ml in culture medium for 24 hours at 37°C and 5% CO2. At a density of (100 μl) 104 cells/well) in 100 μl culture medium, the cells were seeded in microplates and then treated to solutions containing 80% ethanolic plant extracts (20, 40, 60, 80, and 100 μg/ml) (tissue culture grade and 96 wells). DMSO [0.2% in phosphate buffer solution (PBS)] and the cell line were used as the controls in the wells; the samples were subsequently incubated in duplicate. In order to determine the control cell survival and the percentage of live cells after culture, controls were maintained. Cell cultures were incubated for 24 hours at 37°C and 5% CO2 in a CO2 incubator (Thermo Scientific BB150); after that, the medium was removed and replaced with 20 μl of the MTT reagent (5 mg/minute PBS). After MTT was added, cells were incubated for 4 hours at 37°C in a CO2 incubator; forming formazan crystals in the wells was seen under a microscope. Only live cells could convert the yellowish MTT into a dark formazan. After completely removing the medium, 200 μl of DMSO was added, kept for 10 minutes, and then incubated at 37°C. (Wrapped with aluminium foil). Using a microplate reader (Benesphera E21), each sample’s absorbance at 550 nm was measured. Standard 5- Fluorouracil of different concentrations (20, 40, 60, 80, 100 μg) was used as a positive control. The % cell inhibition was determined by using the formula below. By using a linear approximation regression of the percentage of inhibition versus the concentration of the test extract, IC50 values were calculated.
% Cell Inhibition = 100 − [(A1 – A2) / (A3 – A2)] × 100%
where A1 = absorbance of the test extract, A2 = blank absorbance, A3 = absorbance of the control.
Qualitative phytochemical analysis
To determine whether different secondary metabolites were present in S. verticillata, the chemical techniques described by other researchers were used, and the extracts were submitted to conventional preliminary phytochemical examination (Akinyemi et al., 2006; Rao et al., 2016).
Quantification of phytochemical constituents
Quantification of protein content
With some modifications, the traditional Lowry’s method was used to determine total protein content (Sarkar et al., 2020). Briefly, six different concentrations of 5, 10, 15, 20, 25, and 30 μg/ml of standard Bovine serum albumin (BSA) were prepared. 4 ml of an alkaline copper sulphate solution was added to 1,000 μl of diluted sample/standard and was thoroughly mixed. 500 μl of Folin Ciocalteu Reagent was added and mixed at room temperature; the mixture was left for 30 minutes, and the absorbance was taken at 500 nm against a blank made by water and the reagents without test extract. All samples were run three times (n = 3). The total protein concentration was measured using the BSA calibration curve, which was then reported in terms of μg Bovine serum albumin Equivalents (BSAE)/mg dry weight (DW) extracts.
Flavonoids content determination
The samples’ flavonoid quantities were assessed using an aluminum chloride spectrophotometric assay with slight modification (Tabasum et al., 2016). Briefly, distilled water (2 ml) and 0.15 mL of 5% NaNO2 solution were added to 0.5 ml aliquots of each extract (3,400 μg/ml) and quercetin solution (5,000–200,000 μg/ml) separately and thoroughly mixed. After the mixture had been left for 6 minutes, 0.15ml of aluminum chloride (10% AlCl3 w/v) solution was added, and the mixture was kept for 6 minutes. A 2 ml solution of 4% NaOH was added to the mixture, adding more distilled water until the mixture had a total volume of 5 ml for 15 minutes; the mixture was kept. The absorbance of each mixture was recorded at 500 nm and compared to one that didn’t contain quercetin or plant extract. All experiment was made in triplicate (n = 3). The amount of flavonoid was estimated and reported from the quercetin calibration curve as μg of QE/mg of DW extract.
Quantification of tannin content
The vanillin/HCl method was slightly modified to determine the amount of tannin (Medini et al., 2014). Six different concentration of catechin was prepared. 4 ml of 4% vanillin dissolved in methanol and 1.6 ml of strong HCl were mixed separately with 500 μl of diluted sample and catechin. After that, the mixture was left to stand for 15 minutes, and the absorbance was measured at 500 nm using methanol as a blank. All experiment was done in triplicate (n = 3), and μg of catechin equivalent (QE)/mg DW extract was used to express the amount of tannin.
Phenolic content determination
As per the method described by Singleton et al. (1965), with a few minor alterations, the number of phenolics was determined using the Folin-Ciocalteu method (Tabasum et al., 2016). The 1.5 mL (2,000 μg/ml) of each extract solution and 3.5 ml of Folin Ciocalteu diluted 10 times, and 4.5 ml of Na2CO3 (7.5%) solution were mixed. The mixtures were held at room temperature for 30 minutes, with absorbance at 765 nm being measured. All the experiments were done in triplicate (n = 3). Gallic acid’s calibration curve was used to quantify the total phenolic content, which was then represented as gallic acid equivalents (GAE)/mg DW extracts.
Identification of bioactive compounds
At the Common Facility Center USIC, Shivaji University, Kolhapur, the 80% ethanolic extract was subjected to a gas chromatography-mass spectrometer (GC-MS) analysis using a Shimadzu, Japan, model TQ8050 plus with HS20 equipment. A silica capillary column SH-Rxi-5silMS (dimensions 30.0 m 0.25 mm, film thickness 0.25 m) was coupled to the instrument and used as the stationary phase, while the Helium was used as the carrier gas with 54.1 ml/minute flow rate. The 70 eV was used for electron ionization with a 200°C ion source temperature. The oven temperature was kept at 80°C, held for 2 minutes, and ramped to 250°C at the rate of 3.5°C/minute and held for 6 minutes. A 1 μg sample was injected into the column at 300°C; the entire run time for GC was 53.33 minutes, with the splitting ratio being 50:050. The samples’ phytochemical content was identified based on a comparison of the mass spectral patterns and retention times of the test samples with the spectral databases of real compounds kept in the library (Jagadhane et al., 2022a, 2022b).
Statistical analysis
Each experiment was carried out in duplicate and at least three times. Means ± SD were used to express the results; all the experiments were subject to statistical analysis by t-test. The levels of significance were set at p ? 0.05.
RESULTS AND DISCUSSION
The extraction yield obtained in this study ranged from 24% to 36%, and the type of solvent used significantly impacted the extraction yield recorded. It has been seen that the extraction yield of 80% ethanol is higher (36%) than that of other solvents, it was followed by acetone (30.5%), aqueous (28.5%), ethyl acetate (25.5%), and DCM (24%). The higher extraction yield recorded in 80% ethanol is caused by the combination of organic solvent and water that facilitates the extraction of all soluble compounds in both water and organic solvents. In the preliminary phytochemicals analysis of S. verticillata extracts, the results demonstrated the presence of tannins, glycosides, flavonoids, amino acids/proteins, phenolics, saponnins, and terpenoids; however, alkaloids were absent in all extracts Table 1, these phytochemicals reported to have a diversity of biological activity such as anti-biofilm, antifungal, anticancer, anti-inflammatory, anti-oxidative, and antibacterial (Akinyemi et al., 2006).
In the determination of phytochemicals, standard curves shown in Figure 1 was used, comparing the phenolic content of the different extracts; acetone extract had the highest amount (89.314 ± 0.001 μg GAE/mg DW), followed by 80% ethanol (42.9715 ± 0.012 μg GAE/mg DW), aqueous (40.6011 ± 0.003 μg GAE/mg DW), ethyl acetate (24.1451 ± 0.002 μg GAE/mg DW) and DCM (19.9585 ± 0.001μg GAE/mg DW; Table 2). In the determination of flavonoids content, the acetone extract also contained more flavonoids than the other extracts, with a concentration of 589.46 ± 0.010 μg QE/mg DW followed by ethyl acetate (464.65 ± 0.014 μg QE/mg DW), DCM (286.47 ± 0.097 μg QE/mg DW), 80% ethanolic (111.62 ± 0.055 μg QE/mg DW) and aqueous (71.47 ± 0.030 μg QE/mg DW; Table 2). In the case of tannin, the ethyl acetate extract had the highest level of total tannin (44.959 ± 0.001 μg CE/mg DW), followed by DCM (44.838 ± 0.003 μg CE/mg DW), acetone (33.379 ± 0.001 μg CE/mg DW), 80% ethanolic (11.689 ± 0.001 μg CE/mg DW), and aqueous (3.900 ± 0.002 μg CE/mg DW; Table 2). While in protein, the acetone extract continued to exhibit higher protein content (30.025 ± 0.002 μg BSAE/mg DW), followed by that 80% ethanolic (24.038 ± 0.000 μg BSAE/mg DW), ethyl acetate (21.638 ± 0.001 g BSAE/mg DW), aqueous (12.342 ± 0.003 μg BSAE/mg DW), and DCM (4.060 ± 0.004 μg BSAE/mg DW; Table 2). These results demonstrate unequivocally that the polarity of the solvent has a bigger impact on the extraction of secondary metabolites.
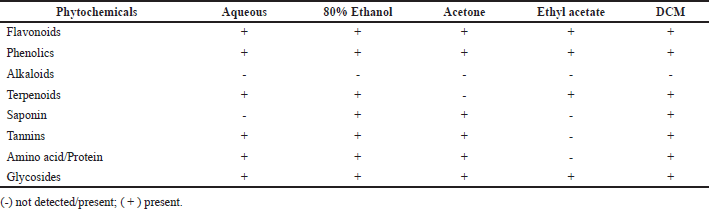 | Table 1. Qualitative phytochemical analysis of S. verticillata extracts. [Click here to view] |
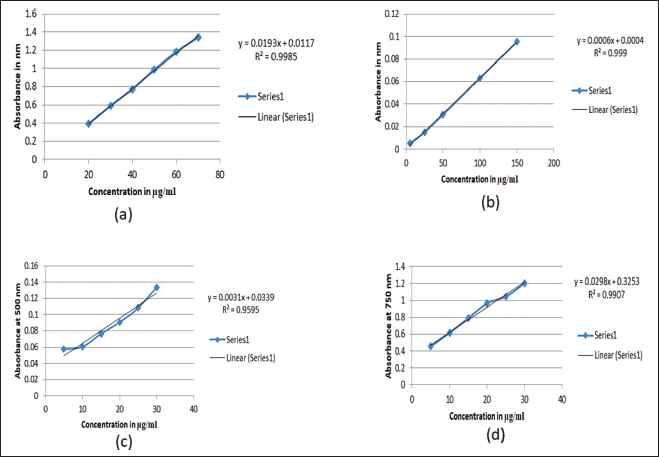 | Figure 1. Standard curves used for quantification of phytochemicals; a) curve of gallic acid for quantification of phenolics, b) curve of quercetin used to quantify flavonoids, c) curve of catechin used to quantify tannin, d) curve of BSA used to quantify protein. [Click here to view] |
In DPPH analysis, the acetone extract exhibited greater antioxidant activity than other solvent extracts with IC50 of 29.112 μg/ml, followed by 80% ethanolic (34.72 μg/ml), aqueous (35.92 μg/ml), ethyl acetate (49.17 μg/ml) and DCM (112 μg/ml; Table 2). It was observed that the percentage scavenging of different solvent extracts increases with the increase of concentration, as displayed in Figure 3b; therefore, free radical scavenging activity is concentration dependent. While in the reducing power assay, the acetone extract exhibited higher reducing power than other solvent extracts (Table 5). The reducing power of test extracts increases as sample concentration increases, as shown in Figure 3a. The greater antioxidant activity of acetone extract may be explained by the ability of acetone solvent to extract more phenolic and flavonoid components, and these chemical compounds are believed to possess reducing and chelating capabilities; it is thought that they have more potent antioxidant properties (Tabasum et al., 2016); therefore, acetone solvent is effective for extracting antioxidant compounds. According to other research, higher phenolic content is correlated with stronger DPPH scavenging ability (Bakchiche, 2017). Antioxidant compounds are essential in the fight against several diseases, including diabetes, cancer, hypertension, and cerebral cardiovascular disease (Rebaya et al., 2015). Flavonoids, which serve as anti-inflammatory and anti-allergenic substances, are significant health-protective molecules, and they lower the risk of developing chronic illnesses, such as cancer and heart disease (Rebaya et al., 2015). Also, it is reported that phenolic substances can prevent human mutagenesis and carcinogenesis, while polyphenols play an important role in protecting against free radicals which attack cells (Ghadigaonkar et al., 2021). Previous research has demonstrated that phenols and flavonoids have potent antioxidant properties and work as powerful anticancer agents, inhibiting angiogenesis and promoting apoptosis (Nguyen et al., 2020).
 | Table 2. DPPH radical scavenging activity total phenolic, flavonoid, tannin and protein content of the different solvent extracts of S. verticillata solvent. [Click here to view] |
Regarding antimicrobial activity, the agar well diffusion method was used to screen the antibacterial activity of an 80% ethanolic extract of S. verticillata against a variety of microorganisms, the extract demonstrated good antimicrobial activity, and the results are presented in Table 3 and illustrated in Figure 2a. The findings showed that plant extract was effective in preventing bacteria growth; E. coli showed the highest antimicrobial activity with a zone of inhibition of 26 ± 0.75 mm, followed by S. aureus (12 ± 0.85), P. aeruginosa (11 ± 1.05 mm), C. albicans (10 ± 0.61 mm), B. subtilis (9 ± 0.86 mm), and A. niger (8 ± 0.45 mm); however, also it was observed that bacterial strains were more affected by plant extract than fungal strains. The findings of the evaluation of the extract’s minimum bactericidal/fungicidal concentration and MIC are displayed in Table 3. The MIC of S. verticillata extract against all tested microorganisms varied and ranged from 4,000 to 16,000 μg/ml, while the MBC/MFC was between 16,000 and 64,000 μg/ml. The highest activity was observed against S. aureus with MIC and MBC of 4,000 and 16,000 μg/mL, respectively, but A. niger showed the least activity, with MIC and MBC values of 16,000 and 64,000 μg/ml, respectively. Compared with a zone of inhibition results, the S. verticillata extract appeared more effective in suppressing bacteria growth than fungi. These results strongly support the presence of bioactive components with antibacterial and antifungal effects in the S. verticillata 80% ethanolic extract and can be used to treat and manage infectious diseases. The different activity levels observed could result from the microbes’ unique intrinsic characteristics and the substances found in the extracts (Aldakheel et al., 2020). An earlier study showed that phenolic compounds have stronger antibacterial activity because of their chelating characteristics and capacity to trap substances required for bacterial development (Kamdem et al., 2022). Due to their hydrophobic properties, phenolic compounds have a high affinity for lipids, which is related to how their antibacterial actions work; they are directly involved in rupturing the bacterial cell membrane, which inhibits cell metabolism and allows for the loss of cellular contents (Aldakheel et al., 2020). Inhibition of activities connected to the cell membrane, such as phosphorylation, electron transport, and protein translocation, is caused by phenolic compounds (Nizio et al., 2018). The antimicrobial properties of phytochemical constituents present in S. verticillata extracts are the main cause of the activity observed and may engage in multiple modes of action; the disruption of bacteria’s cell membrane is reportedly caused directly by phenolic compounds, resulting in the inhibition of cell metabolism and, ultimately, emptying the cellular content (Andal et al., 2018), flavonoids compounds involves direct in the inhibition of nucleic acid synthesis, cytoplasmic membrane damage caused by process of perforation, and a decrease in membrane fluidity (Slobodníková et al., 2016). Proteins and amino acids may contribute to the antibacterial activity of S. verticillata; peptides can directly affect microorganisms and restrict their growth by interfering with the production of vital enzymes in the cell membrane (Andal et al., 2018).
Anti-biofilm activities of 80% ethanolic extract of S. verticillata were evaluated against fungi, Gram-negative and Gram-positive bacteria; the extract showed good anti-biofilm activity in a concentration-dependent manner against both Gram-negative and Gram-positive and fungal strains tested as illustrated in Figure 2c and d. At 10,000 μg/ml, S. aureus had the best anti-biofilm activity, followed by B. subtilis, P. aeruginosa, E. coli, C. albicans, and A. niger (Table 4). The result shows that Gram-positive bacteria were most affected, followed by Gram-negative bacteria, while fungi were least affected. The same study from other literature reported that Gram-positive strains are more sensitive than Gram-negative strains, where S. aureus was most sensitive (Lai et al., 2014). Our findings demonstrated that the plant extract had a greater impact on biofilm formation inhibition on Gram-positive bacteria than Gram-negative bacteria, but in comparison between bacteria and fungi, the findings from this study showed that the extract has greater anti-biofilm activity against bacteria than fungi. In other studies evaluating the anti-biofilm formation of methanolic pomegranate extract, the results revealed that C. albicans was less susceptible to the extract than S. aureus and E. coli (Bakkiyaraj et al., 2013). A high quantity of flavonoids, phenolics, tannins compounds, and other phytochemicals in S. verticillata extract may be the source and responsible for the activity observed. A previous study reported that flavonoids like quercetin, apigenin, and naringenin inhibit the formation of biofilms by suppressing the communications between cells in the body (Kim et al., 2016). Polyphenols compounds play a crucial role as anti-biofilm agents; a previous study reported that these compounds inhibit biofilm production in E. coli, S. aureus, and E. faecalis (Slobodníková et al., 2016). Some literature suggested that tannins induce astringency, which could be involved in disabling the biofilm (Bakkiyaraj et al., 2013).
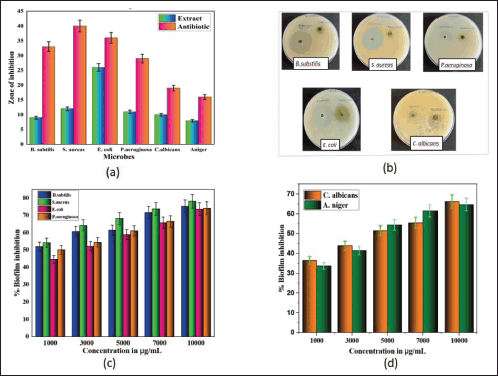 | Figure 2. The effects of 80% ethanolic extract of S. verticillata on zone of inhibition and biofilm formation of microorganisms; a) Zone of inhibition shown by extract (100,000 μg/ml) in comparison with antibiotic against six microbes, b) plates showing effects of extract in terms of zone of inhibition for some microorganisms, c) biofilm inhibition effects shown by extract against four bacterial strains, d) effects of extract of biofilm formation against two fungal strains. [Click here to view] |
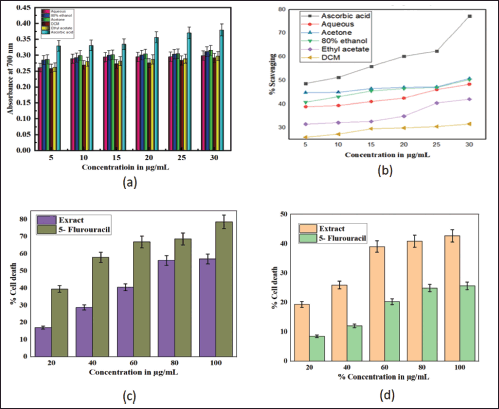 | Figure 3. Antioxidant and cytotoxicity effects of S. verticillata extract; a) reducing power effect of different solvent extracts (aqueous, 80% ethanol, acetone, dichloromethane, ethyl acetate) in comparison with ascorbic acid, b) scavenging capacity of different solvent extracts (aqueous, 80% ethanol, acetone, dichloromethane, ethyl acetate) in comparison with ascorbic acid, c) effects of 80% ethanolic extract and standard (5- Flurouracil) on colon cancer cell lines, d) effects of 80% ethanolic extract and standard (5- Flurouracil) on normal kidney cancer cell lines. [Click here to view] |
 | Table 3. Zone of inhibition, MIC and MBC/MFC of S. verticillata on microorganisms. [Click here to view] |
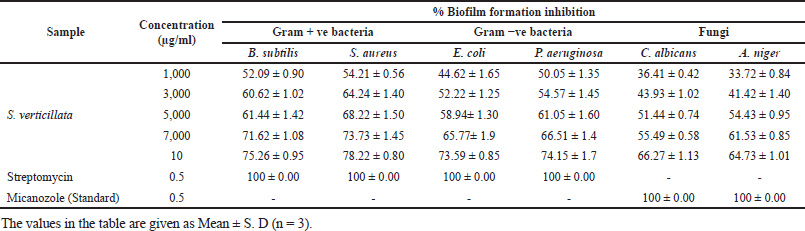 | Table 4. Anti-biofilm activity of S. verticillata extracts against pathogenic bacterial and fungal strains. [Click here to view] |
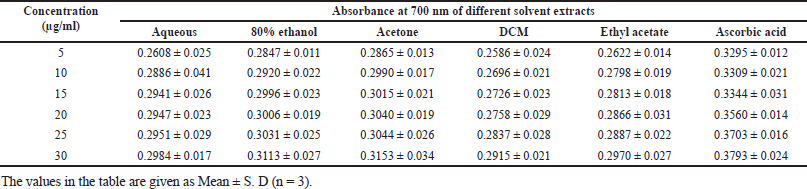 | Table 5. Reducing power capacity of S. verticillata. [Click here to view] |
In cytotoxicity activity, the COLO205 colon Cancer cell lines and NRK52E normal kidney cell lines were used to assess 80% ethanolic extract of S. verticillata cytotoxicity effects by MTT assay, and their results are shown in Table 6. The extract showed a more significant inhibition effect on COLO205 cancer cell line a than NRK52E normal kidney cell line, as illustrated in Figure 3c and d, with IC50 values of 79.03 μg/ml for COLO205 colon cancer cell line and 112.58 μg/ml for NRK52E normal kidney cell line. Since human cell lines can more easily predict probable consequences and produce data that is more useful to humans, they were chosen for our investigation (Erhirhie et al., 2018). The effective comparison of cytotoxicity effects of chemicals and plant extracts can be done using the IC50, which assesses a substance’s ability to stop the growth of 50% of cells. Chemicals and extracts are categorized as follows in accordance with the National Cancer Institute’s and Geran Protocol’s standards, IC50 ≤ 20 μg/ml = highly toxic to cells, IC50 21–200 μg/ ml= moderately toxic to cells, IC50 201–500 μg/ml = weakly toxic to cells, and IC50 > 501 μg/ml = no toxic to cells (Ogbole et al., 2017). The IC50 of 80% ethanolic extract of S. verticillata in NRK52E normal kidney cell line and COLO205 Colon Cancer cell line were 112.58 and 79.03 μg/ml, respectively, since the IC50 values for the two cell lines examined ranged from 21 to 200 g/ ml, the results clearly show that the 80% ethanolic extract of S. verticillata displayed moderate cytotoxic action against them. Still, the extract had stronger activity against colon cancer and little activity in normal kidney cell lines. This cytotoxic potential may result from the plant extract’s phytochemicals’ capacity to trigger apoptosis and inhibit the cell cycle (Kamdem et al., 2022).
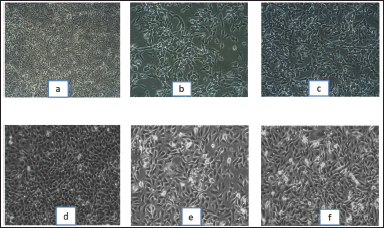 | Figure 4. Microscopic images of colon cancer cell lines and normal kidney cell lines under treatment of 80% ethanolic extract of S. verticillata, control (lactic acid) and standard (5-Flurouracil); a) Colon cancer cell lines treated with control (lactic acid), b) colon cancer cell lines treated with standard (5-Flurouracil), c) colon cancer cell lines treated with S. verticillata extract, d) normal kidney cell lines treated with control (lactic acid), e) normal kidney cell lines treated with standard (5-Flurouracil), f) normal kidney cell lines treated with S. verticillata extract. [Click here to view] |
Bioactive compounds identification, the GC-MS chromatograms of the 80% ethanolic extract of S. verticillata revealed 5 compounds (Supplementary Material). The most abundant compounds in the 80% ethanolic extract included Butylated Hydroxytoluene (1), Hexadecanoic acid, ethyl ester (2), Linoleic acid ethyl ester (3), Ethyl 9,12,15-octadecatrienoate (4), Octadecanoic acid, ethyl ester (5) all compounds are represented in Figure 5. A thorough literature search in online databases such as PubChem, Scopus, Web of Science, and PubMed was conducted to assess whether any bioactivity has been reported regarding the identified compounds. The biological actions of certain identified compounds have been described in several studies; Butylated hydroxytoluene is reported to be a powerful antioxidant that is safe to use in the pharmaceutical and food industries (Yehye et al., 2015). Hexadecanoic acid-ethyl ester has antioxidant, antimicrobial, nematicide, hemolytic, hypocholesterolemic, and anti-androgenic properties (Aldakheel et al., 2020). The compound linoleic acid ethyl ester was reported to have the following properties: anti-arthritic, anti-inflammatory, nematicide, anti-coronary, hypocholesterolemic, hepatoprotective, anti-androgenic, antihistaminic, insectifuge, and anti- eczematic (Tyagi and Agarwal, 2017). Ethyl 9,12,15-octadecatrienoate was reported to possess antioxidant, antimicrobial, anti-inflammatory, and pesticide activity (Aldakheel et al., 2020). Since S. verticillata has been reported to be a sedative and treatment for unspecified medicinal infections/ disorders and associated symptoms (Thomas, 2020). With all the above justification, it can be concluded that S. verticillata is the potential for the treatment and management of a variety of non-infectious and infectious diseases and their symptoms.
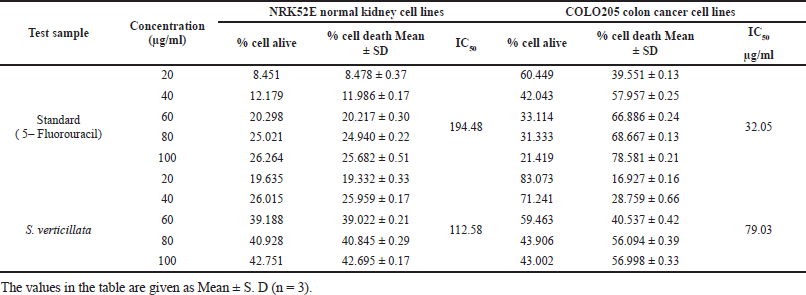 | Table 6. Effect of S. verticillata extract against NRK52E normal kidney cell line and COLO205 colon cancer cell line. [Click here to view] |
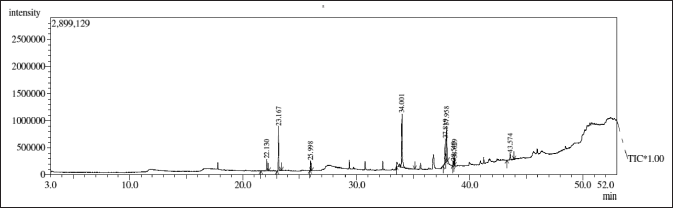 | Figure 5. GC-MS chromatogram of 80% ethanolic extract of S. verticillata, five major bioactive compounds were identified. [Click here to view] |
CONCLUSION
The efficiency of the S. verticillata extracts as antimicrobials, anti-biofilm, antioxidants, and cytotoxicants was assessed. The plant extracts demonstrated good antimicrobial activity; the highest activity was observed against S. aureus with MIC value of 4000 μg/mL and MBC of 16000 μg/ml. The cytotoxicity activity was observed against COLO205 colon cancer cell lines with an IC50 of 79.03 μg/ml. Moreover, the plant extract exhibited strong antioxidant activity, and various bioactive substances with antimicrobial, anticancer, and antioxidant were identified. Based on those findings, this study points out that the plant species could be considered a new natural source of antimicrobial, antioxidant, and anticancer molecules with therapeutic potential. Therefore, isolation and characterization of bioactive compounds should be done; additionally, sub-acute and sub-chronic toxicity investigation of extracts and isolated bioactive compounds should be conducted to assess the side effects, biochemical, and hematological parameters.
ACKNOWLEDGMENTS
We would like to express our gratitude to the Institute of Research and Analytics Sangli, Maharashtra, India’s management for allowing us access to their facility so we could perform MTT cytotoxicity assays.
LIST OF ABBREVIATIONS
BSA, Bovine serum albumin; BSAE, Bovine serum albumin equivalents; CE, Catechin equivalents; CFU, colony forming unit; DCM, Dichloromethane; DMSO, Dimethyl sulphoxide; DNA, Deoxyribonucleic acid; DPPH, 2, 2- diphenyl picrylhydrazyl; DW, Dry weight; GAE, Gallic acid equivalents; GC, Gas chromatography; GC-MS, Gas chromatography mass spectrometer; HCl, Hydrochloric acid; IC50, Inhibitory concentration of 50%; ICCR, Indian Council for Cultural Relations; MHB, Mueller Hinton broth; μg, microgram; MTT, 3-(4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide; MIC, Minimum inhibitory concentration; MBC, Minimum bactericidal concentration; MFC, Minimum fungicidal concentration; mg, milligram; NCCS, National Centre for Cell Science; OD, Optic density; PBS, Phosphate buffer solution; QE, Quercetin equivalent; rpm, revolution per minute; SD, Standard deviation; TSB, Trypticase soy broth; UV, Ultraviolet light; WHO, World Health Organization.
AUTHORSHIP CONTRIBUTIONS
Alfredi A. Moyo: Developed the idea, conducted the study and experimentation, gathered the information, and wrote the initial manuscript. Kishor S. Jagadhane: prepared all the figures. Sneha R. Bhosale: performed the data analysis and created all the tables. Sachin B. Shinde: Data analysis/interpretation. Vinod B. Shimpale: Identifying plants, editing, and reviewing manuscripts. Prashant V. Anbhule: Editing, reviewing, and finishing the manuscript.
FUNDING
This study was fully funded by ICCR through the MEA-Africa Scholarship Program (G0179/ 2021).
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdallah EM. Plants: an alternative source for antimicrobials. J Appl Pharm Sci, 2011; 1(6):16–20.
Akinyemi KO, Oluwa OK, Omomigbehin EO. Antimicrobial activity of crude extracts of the three medicinal plants used in South-West Nigerian folk medicine on some food borne bacterial pathogens. Afr J Tradit, Complement Alternat Med, 2006; 3(4):13–22; doi10.4314/ajtcam.v3i4.31173 CrossRef
Alavi M, Karimi N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif Cells Nanomed Biotechnol, 46(8), 2066–81; doi10.1080/21691401.2017.1408121 CrossRef
Aldakheel RK, Rehman S, Almessiere MA, Khan FA, Gondal MA, Mostafa, A, Baykal, A. (2020). Bactericidal and in vitro cytotoxicity of moringa oleifera seed extract and its elemental analysis using laser-induced breakdown spectroscopy. Pharmaceuticals, 2020; 13(8):1–8; doi10.3390/ph13080193 CrossRef
Andal P, Tamilselvy S, Indra Priyatharesini P. Green synthesis of silver nanoparticles from carrot. Res J Pharm Technol, 2018; 11(7):2757–60; doi10.5958/0974-360X.2018.00509.7 CrossRef
Awalekar R, Jagadhane K, Usmani S, Salunkhe S, Jamale D, Hangirgekar S, Kolekar G, Anbhule P. . Stereospecific Synthesis of (4E,10Z)-4,10-tetradecadienyl acetate, the major sex pheromone of apple laf miner moth, phyllonorycter ringoniella. Letters in Organic Chemistry, 2021; 18(8):588–93; doi10.2174/1570178617999200922145900 CrossRef
Bakchiche B. Total phenolic, flavonoid contents and antioxidant activities of honey and propolis collected from the region of laghouat (South of Algeria). Int J Pharm Chinese Med, 2017; 1(2); doi10.23880/ipcm-16000110 CrossRef
Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling, 2013; 29(8), 929–37; doi10.1080/08927014.2013.820825 CrossRef
Erhirhie EO, Ihekwereme CP, Ilodigwe EE. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdisciplinar Toxicol, 2018; 11(1), 5–12; doi10.2478/intox-2018-0001 CrossRef
Evans WC. Trease Evans Pharm. In: Evans WC, Evans D (Eds.). 16th edition, Elsevier.
Fathi M, Ghane M, Pishkar L. Phytochemical Composition, antibacterial, and antibiofilm activity of malva sylvestris against human pathogenic bacteria. Jundishapur J Nat Pharm Prod, 2022; 17(1):1–9; doi10.5812/jjnpp.114164 CrossRef
Ghadigaonkar S, Reddy AG, Kalakumar B, Lakshman M, Rajkumar U. Quantification of total phenolic content, total flavonoid content and evaluation of in vitro free radical scavenging activities in Ficus religiosa Linn. 2021; 2–7.
Gupta D, Dubey J, Kumar M. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected common human pathogenic microorganisms. Asia Pac J Trop Dis, 2016; 6(1):15–20; doi10.1016/S2222-1808(15)60978-1 CrossRef
Jagadhane KS, Bhosale SR, Gunjal DB, Nille OS, Kolekar GB, Kolekar SS, Dongale, TD, Anbhule PV. Tetraphenylethene-based fluorescent chemosensor with mechanochromic and aggregation-induced emission (aie) properties for the Selective and sensitive detection of hg 2+ and ag + ions in aqueous media: application to environmental analysis ACS Omega, 2022a; 7:34900; doi10.1021/ACSOMEGA.2C03437/ASSET/IMAGES/LARGE/AO2C03437_0018.JPEG CrossRef
Jagadhane KS, Bhosale S. R, Moyo AA, Kolekar GB, Sharma KK, Yadav HM, Anbhule PV. A tetraphenylethene-based aggregation-induced emission luminogen (aiegen) with mechanochromic phenomena for highly selective naked-eye detection of mno4− directly in aqueous media. ChemistrySelect, 2022b; 7(43):e202203185; doi10.1002/SLCT.202203185 CrossRef
Kamdem MHK, Makoni GP, Silihe KK, Jiyane P, Tonga JL, Mmutlane EM, Tata CM, Krause RWM, Ndinteh DT. Cytotoxic and antimicrobial activities of new phytosteroids from the leaves of Anonidium mannii (Oliv.) Engl. & Diels (Annonaceae). S Afr J Bot, 2022; 147:628–35; doi10.1016/j.sajb.2022.02.027 CrossRef
Katiyar CKanjilal S, Gupta A, Katiyar S. Drug discovery from plant sources: an integrated approach. AYU (An Int Quart J Res Ayurved), 2012; 33(1):10; doi10.4103/0974-8520.100295 CrossRef
Kim KJ, Liu X, Komabayashi T, Jeong SIl Selli S. Natural products for infectious diseases. Evid-Based Complement Altern Med, 2016; doi10.1155/2016/9459047 CrossRef
Lai CC, Lee K, Xiao Y, Ahmad N, Veeraraghavan B, Thamlikitkul V, Tambyah PA, Nelwan RHH, Shibl AM, Wu JJ, Seto WH, Hsueh PR. High burden of antimicrobial drug resistance in Asia. J Glob Antimicrob Resist, 2014; 2(3):141–7; doi10.1016/j.jgar.2014.02.007 CrossRef
Liang YS, Jung MJ, Wu, SC, Kao YC, Wang JC. Stemodia L. (Scrophulariaceae), a newly naturalized genus in Taiwan. Taiwania, 2011; 56(1):62–5; doi10.6165/tai.2011.56(1).62 CrossRef
Lindahl JF, Grace, D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol 2015; 5(1):30048; doi10.3402/iee.v5.30048 CrossRef
Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah University Sci, 2014; 8(3):216–24; doi10.1016/j.jtusci.2014.01.003 CrossRef
Mohamed A. Evaluation of antimicrobial activity of different solvent extracts of Saussurea Lappa. World J Pharm Pharm Sci 2017; 4(2):12–8; doi10.20959/wjpps20179-9868 CrossRef
Naz R, Ayub H, Nawaz S, Islam ZU, Yasmin T, Bano A, Wakeel A, Zia S, Roberts TH. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complement Altern Med, 2017; 17(1):1–3; doi10.1186/s12906-017-1815-z CrossRef
Nguyen, NH, Hoai Ta QT, Pham QT, Han Luong TN, Phung VT, Duong TH, Vo VG. Anticancer activity of novel plant extracts and compounds from Adenosma bracteosum (Bonati) in human lung and liver cancer cells. Molecules, 2020; 25(12) https://doi.org/10.3390/molecules25122912 CrossRef
Nizio Z, Furman-toczek D, Zagórska-dziok M. Antioxidant activity and cytotoxicity of jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. 2018; 8–12.
Ogbole OO, Segun PA, Adeniji AJ. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement Altern Med, 2017; 17(1):1–0; doi10.1186/s12906-017-2005-8 CrossRef
Patil SS. Traditional medicine knowledge and diversity of medicinal plants in Sharavathi valley region of central western ghats. ~ 124 ~ International J Herbal Med, 2016; 4(6):124–130.
Rao USM, Abdurrazak M, Mohd KS. Phytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiaca. Malaysia J Anal Sci, 2016; 20(5):1181–90.
Rebaya A, Belghith SI, Baghdikian B, Leddet VM, Mabrouki F, Olivier E, CherifJ. K, Ayadi MT. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of halimium halimifolium (Cistaceae). J Appl Pharm Sci, 2015; 5(1):052–7; doi10.7324/JAPS.2015.50110 CrossRef
Review on Antimicrobial Resistance. Tackling drug resistant infections globally: final report and recommendations. In Rev Antimicrob Resist 2016; 136(1); doi10.1016/j.jpha.2015.11.005 CrossRef
Romulo A, Zuhud EAM, Rondevaldova J, Kokoska L. Screening of in vitro antimicrobial activity of plants used in traditional indonesian medicine. Pharm Biol, 2018; 56(1):287–93; doi10.1080/13880209.2018.1462834 CrossRef
Sarkar S, Mondal M, Ghosh P, Saha M. (2020). Quantification of total protein content from some traditionally used edible plant leaves?: a comparative study quantification of total protein content from some traditionally used edible plant leaves?: acomparative study. August; doi10.22271/plants.2020.v8.i4c.1164 CrossRef
Savoia, D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Fut Microbiol, 2012; 7(8):979–90; doi10.2217/fmb.12.68 CrossRef
Sharma G, Kumar PG, Gupta RK, BlockM, Alipore N. (2016). Bio Bulletin. Bio Bull, 2(1), 43–51.
Shukla AN, Srivastava S, Rawat AKS. A survey of traditional medicinal plants of Uttar Pradesh ( India ) - used in treatment of infectious diseases. Nat Sci, 2014; 11(9):24–36.
Slobodníková L, Fialová S, Rendeková K, Ková? J, Mu?aji P. Antibiofilm activity of plant polyphenols. Molecules, 2016; 21(12), 1–5; doi10.3390/molecules21121717 CrossRef
Tabasum S, Khare S, Jain K. Spectrophotometric quantification of total phenolic, flavonoid, and alkaloid contents of abrus precatorius L. Seeds. Asia J Pharm Clin Res, 2016; 9(2):371–4.
Tailor Chandra Shekhar and Goyal Anju. Antioxidant activity by DPPH radical scavenging method of ageratum conyzoides. Orient, 2014; 1(4):244–9.
Thomas B. Documentation of medico-potential plants in the riparian zone of Chaliyar River in Malabar region of Kerala , India Documentation of medico-potential plants in the riparian zone of Chaliyar River in Malabar region of kerala , India. December.
Tyagi T, Agarwal M. Phytochemical screening and GC-MS analysis of ethanol ACN extract. J Pharm Phytochem, 2017; 6(1):195–206.
WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva swizerland, pp5–7, 2017
WHO. Global Health Estimates 2016: disease burden by cause, age, sex, by country and by region, 2000-2016. World Health Organization, Geneva Swizerland, 2018
WHO. World health statistics 2018: monitoring health for the SDGs, sustainable development goals. World Health Organization, Geneva Swizerland, 2018
World Health Organization (WHO). WHO traditional medicine strategy 2014-2023. (WHO), 2013; 1–76; doi2013 CrossRef
Yehye WA, Rahman NA, Ariffin A, Abd Hamid SB, Alhadi, AA, Kadir FA, Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review. Eur J Med Chem, 2015; 101:295–312; doi10.1016/j.ejmech.2015.06.026 CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website