INTRODUCTION
Photoaging is premature skin aging caused by chronic sunlight exposure and characterized by rough wrinkles, rough texture, and loss of elasticity in the skin (Cavinato and Jansen-Dürr, 2017; Le Digabel et al., 2018). UV is also reported to be responsible for 80% of facial skin aging (Zhang and Duan, 2018). In addition to photoaging, DNA damage, caused by short-wavelength ultraviolet B (UVB) exposure, can be the forerunner of skin cancer (Garg et al., 2017; Pandel et al., 2013). UVB that reaches the skin excites the skin chromophore and stimulates the production of reactive oxygen species (ROS). Namely, ROS will then activate factors such as the transcription factors nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1). The transcription factor NF-κB will transcribe proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1. However, the transcription factor AP-1 will transcribe matrix metalloproteinases (MMPs), which play an important role in collagen degradation, whereby it is deemed a hallmark of photoaging (Cavinato et al., 2017; Garg et al., 2017; Panich et al., 2016; Rittié and Fisher, 2015; Sanches Silveira and Myaki Pedroso, 2014; Young et al., 2017). Thus, for skin that is exposed to UVB, TNF-α expression will increase; however, type 1 collagen expression will decrease (Poon et al., 2015; Zhi et al., 2019).
Nowadays, the use of herbal products in preventing photoaging has increased substantially inasmuch as plant extracts are considered natural and safe skincare products. In this context, turmeric (Curcuma longa) is a rhizomatous herb that is cultivated and used as a kitchen spice in tropical regions such as India, China, Indonesia, Thailand, and Africa (Gopinath and Karthikeyan, 2018). A substance called curcumin in turmeric is known to have anti-inflammatory properties (Dunaway et al., 2018).
The topical turmeric extracted in this study was applied immediately after UVB exposure. Thus, the topical turmeric extract is presumed to act as an adjuvant for sunscreens and a compound that shields the skin from the photoaging UVA rays besides being for therapy. The benefits of turmeric as a photoprotector in various dosage forms have been investigated by several previous studies. These studies report that turmeric is a potent ingredient used as a photoprotector, especially when administered topically (Li et al., 2016; Phillips et al., 2013; Sumiyoshi and Kimura, 2009). Surprisingly, although there has been a lot of research about the effect of turmeric on the skin, the effect of a single long-term application of turmeric on collagen expression in skin exposed to UVB is still scant. Hence, this study aimed to examine the role of turmeric extract in preventing photoaging by analyzing the expression of TNF-α and type 1 collagen in BALB/c mice exposed to UVB (in vivo).
MATERIALS AND METHODS
Research design
This research was pure experimental research with a posttest-only control group design conducted in the Faculty of Pharmacy, Universitas Airlangga, Indonesia (Ethical Clearance Certificate Number: 447/HRECC.FODM/X/2020). Thirty-two mice were randomly divided into 2 control groups and 2 different treatment groups: normal control group (NC), sick control/UVB-exposed group (SC), UVB-exposed + vehicle group (VT), and UVB-exposed + topical turmeric extract group (TT). Groups SC, VT, and TT were exposed to UVB 24 times for 4 weeks (6 times/week). Still, immediately after exposure, groups VT and TT received topical vehicle (Vaseline) and 16% turmeric extract, respectively. Thereafter, mice were then evaluated on day 27 after treatment.
UVB exposure
The radiation process for mice was carried out by exposing mice to UVB light from a UVB lamp (Philips PL-S 9W/01/2P), which had been installed in the mice irradiation box. Before radiation, the dorsum area of the mice was shaved using a shaver to eliminate any resistance that blocks UVB from reaching the skin’s surface. The dorsum of the mice was shaved with an area of 2.5 × 4 cm2. Thereafter, the mice were exposed to a narrow spectrum of UVB (305–315 nm, peak 311 nm) at a dose of 1 minimal erythemal dose (MED), where 1 MED was 240 mJ/cm2 (obtained from a preliminary study), at a distance of 18 cm above the dorsal mice, six times per week for 4 weeks, with an exposure duration of 35 minutes/exposure. The UVB radiation exposure was repeated 24 times with a total exposure dose of 5,760 mJ/cm2.
Ointment application to mice
The turmeric extract ointment was only applied to the group of mice TT. Immediately, the vehicle and the turmeric extract were applied after UVB irradiation six times a week for 4 weeks (Agrawal and Kaur, 2010). On the mice’s skin with an area of 2.5 × 4 cm2, 1 g (two fingertip units) of turmeric extract ointment was applied (Oakley, 2016).
Skin tissue sampling
The skin tissue of the mice was taken on the 27th day after all treatments were completed. The mice were anesthetized using ketamine at a dose of 50 mg/kg. Posteriorly, the dorsal skin tissue of the mice that had been exposed to UVB was excised to the depth of the dermis. After skin sampling, the mice were executed by cervical dislocation and buried. All skin tissue that had been obtained was then stored in a 10% formalin solution (formaldehyde 37%–40%). Subsequently, for examination, they were embedded in paraffin (wax) to create paraffin-embedded tissue blocks from TNF-α expression and type 1 collagen. The paraffin blocks were created using the immunohistochemical method at the Anatomical Pathology Laboratory, Faculty of Medicine, Airlangga University.
Immunohistochemistry
Immunohistochemical staining was performed using an anti-TNF-α monoclonal antibody [TNF-α (52B83): sc-52746, Santa Cruz Biotechnology, Inc.] and type 1 anti-collagen monoclonal antibody [COL1A1 (3G3): sc-293182, Santa Cruz Biotechnology, Inc.]. TNF-α expression was measured by counting the number of macrophage cells in the dermis that expressed TNF-α using a light microscope with 400× magnification in five fields of view. Type 1 collagen expression was measured by measuring the thickness of the collagen layer in the dermis (in micrometers) in five fields of view.
Statistical analysis
The research data obtained were then processed and statistically examined using Statistical Package for the Social Sciences for Windows Release 25.0. The statistical test chosen was the one-way analysis of variance (ANOVA) test and continued with the post hoc test [least significant difference (LSD)] to determine the difference between the undertaken treatments.
RESULTS AND DISCUSSION
Immunohistochemistry of TNF-α
The results demonstrated that the lowest expression of TNF-α was found in NC. Nevertheless, the highest expression was found in the group that was exposed to UVB and given the vehicle (VT). The results of TNF-α expression from the lowest to the highest, respectively, were the groups including NC, TT, SC, and VT (Figs. 1 and 2).
Additionally, the results of the one-way ANOVA test eliminated a significant value of p < 0.001. Hence, it can be concluded that there was a significant difference in the mean TNF-α expression between the treatment groups (p < 0.05). Furthermore, based on the results of the post hoc LSD test, it was found that there was a significant difference in almost all groups, including the groups NC and SC (p = 0.000), NC and VT (p = 0.00000), NC and TT (p = 0.000), and SC and TT (p = 0.000). However, contingent on the SC and VT groups, the results were not significantly different (p = 0.238).
Accordingly, it was derived that there was a significant difference between the means of TNF-α expression in SC and the NC (p < 0). Fortunately, it was found that all the findings coincided with a previous report that addressed that synergistic upregulation in TNF-α is counted as a key early response to UVB exposure (Bashir et al., 2009a, 2009b). Bashir et al. (2009a, 2009b) showed that UVB exposure induced an increase in TNF-α expression mainly in epidermal keratinocytes. However, in this study, TNF-α expression could only be read and analyzed in macrophages. Besides, it was assumed that the unclear TNF-α expression in epidermal keratinocytes was likely caused by the peroxidation reaction of immunohistochemical staining.
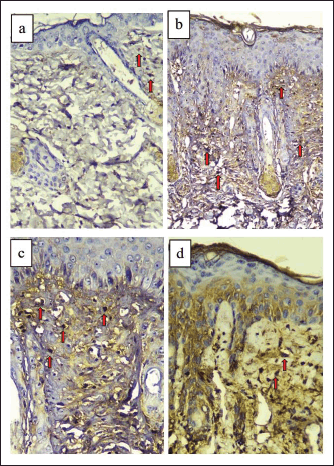 | Figure 1. Dorsal skin tissue of BALB/c mice stained with TNF-α immunohistochemistry (400× magnification). (a) Normal control group (NC). (b) Sick control/UVB-exposed group (SC). (c) UVB-exposed + vehicle group (VT). (d) UVB-exposed + topical turmeric extract group (TT). Macrophage cells that express TNF-α are stained brown (red arrows), while cells that do not express TNF-α are stained blue/purple. [Click here to view] |
Furthermore, Bashir et al. (2009a, 2009b) reported that UVB exposure to the skin induces the formation of ROS. This increase in ROS can then cause damage to cell structures due to oxidative stress. Nucleic acids, lipids, and proteins that are modified by ROS or UVB exposure act as damage-associated molecular patterns that can activate sensors/receptors such as toll-like receptor (TLR) or cyclic guanosine monophosphate-adenosine monophosphate synthase on cell membranes and cytosols resulting in a signaling cascade that induces the transcription of proinflammatory cytokines such as TNF-α (Gallo and Bernard, 2014).
The results also showed that there was a significant difference in the mean TNF-α expression in TT compared to SC (p < 0.05). These results were in line with a previous report that stated that curcumin in turmeric is a strong intracellular antioxidant (Barzegar and Moosavi-Movahedi, 2011). Curcumin is also known to inhibit TNF-α promoters by methylation and can also inhibit TLR 2 and 4 signaling by lipopolysaccharide, which is responsible for TNF-α induction. In addition, curcumin can also directly bind to the TNF-α receptor binding site through covalent and noncovalent interactions, thereby blocking the TNF-α-dependent activation of NF-κB (Vollono et al., 2019).
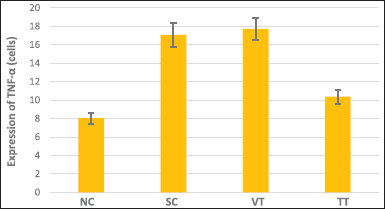 | Figure 2. Graph of TNF-α expression in all groups. [Click here to view] |
Immunochemistry of type 1 collagen
The results showed that the highest expression of type 1 collagen was found in the group exposed to UVB and given 16% turmeric extract ointment (TT), while the lowest expression was found in the group that was exposed to UVB only (SC). The results of the highest to lowest type 1 collagen expression, respectively, were TT, NC, VT, and SC (Figs. 3 and 4).
The results of the one-way ANOVA test showed a significant value of p < 0.001; hence, it can be concluded that there is a significant difference in the mean type 1 collagen expression between the treatment groups (p < 0.05). Furthermore, based on the results of the post hoc LSD test, it was found that there was a significant difference in the mean in groups NC and SC (p < 0.001), groups NC and VT (p = 0.017), groups SC and VT (p = 0.007), and groups SC and TT (p < 0.001). Meanwhile, the mean difference which was not significant was only found in groups NC and TT (p = 0.443).
In the results of the study above, the lowest thickness of type 1 collagen expression was found in SC with a significant difference in average compared to the NC (p < 0.05). These results are in line with the photoaging theory that states that long-term UVB exposure can cause degradation or a decrease in the amount of type 1 collagen. The photoaging process begins with the formation of ROS due to UVB exposure. Increased ROS will activate the MAPK pathway in macrophage cells and fibroblast cells. Activation of the MAPK pathway will induce the transcription factor AP-1, resulting in MMP-1 transcription. It is this MMP-1 that acts as the main enzyme for degrading type 1 collagen (Pandel et al., 2013; Pittayapruek et al., 2016).
In addition to inducing the degradation of type 1 collagen, UVB light can also reduce the expression of type 1 collagen by inhibiting its synthesis. ROS as a result of UVB exposure can cause a decrease in the transforming growth factor-β (TGF-β) receptor type 2, which is a receptor required by TGF-β to induce procollagen synthesis through the SMAD signaling pathway. ROS has also been reported to inhibit Smad2/Smad3 phosphorylation and nuclear translocation in procollagen synthesis (Ansary et al., 2021; Mcdaniel et al., 2018; Pittayapruek et al., 2016). UVB-induced ROS can also directly reduce the expression of TGF-β (Yin et al., 2015). Therefore, UVB exposure can decrease the expression of type 1 collagen by inducing its degradation and inhibiting its synthesis. A decrease in the amount of type 1 collagen is a hallmark of external aging due to UV exposure (photoaging) (Poon et al., 2015).
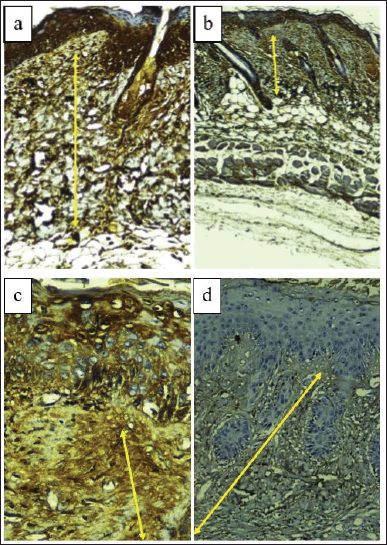 | Figure 3. Dorsal skin tissue of BALB/c mice stained with type 1 collagen immunohistochemistry (400× magnification). (a) Normal control group (NC). (b) Sick control/UVB-exposed group (SC). (c) UVB-exposed + vehicle group (VT). (d) UVB-exposed + topical turmeric extract group (TT). Type 1 collagen expression was measured by measuring the thickness of the collagen layer in the dermis (μm) indicated by the yellow arrow. [Click here to view] |
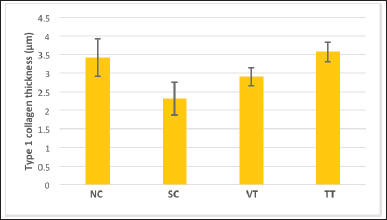 | Figure 4. Graph of type 1 collagen expression in all groups. [Click here to view] |
The mean expression of type 1 collagen in VT was higher than in SC (p < 0.05). This indicates that there is a possibility that the vehicle, in this case, Vaseline, has a protective effect against UVB exposure. However, the difference in the mean between VT and TT was significant (p < 0.05). Hence, it can be concluded that the protective effect of topical turmeric extract is still significantly better than the protective effect of the vehicle (Vaseline) alone. No reference explains the protective effect of Vaseline in photoaging, so further research on this needs to be done.
If the lowest type 1 collagen expression was found in SC, the highest type 1 collagen expression was found in the UVB exposure group given topical turmeric extract (TT). The results of the different tests showed that there was a significant difference in the average expression of type 1 collagen between TT and SC (p < 0.05). As previously explained in the discussion of TNF-α expression, curcumin in turmeric has photoprotective properties and acts as a potent antioxidant. ROS neutralized by curcumin will not activate the MAPK pathway, so type 1 collagen will not be degraded (Barzegar and Moosavi-Movahedi, 2011). In addition, curcumin is known to reduce MMP-1 expression by blocking the phosphorylation of JNK and p38 in the MAPK signaling pathway (Dunaway et al., 2018; Hwang et al., 2013).
One point to note is that the expression of type 1 collagen in the group given topical turmeric (TT) had a higher mean than the normal control group (NC), although it was not significantly different (p > 0.05). It indicates that topical turmeric extract can not only protect the dermis layer from the degradation of type 1 collagen but also increase the amount of type 1 collagen in the dermis. This result is in line with previous studies that reported that turmeric could induce collagen deposition and inhibit collagen accumulation disorders (Li et al., 2016; Phillips et al., 2013). More specific studies using various doses of topical turmeric extract need to be done in the future to see which topical doses are most effective in protecting the skin against UVB radiation exposure.
CONCLUSION
From the results, it could be concluded that there was a significant decrease in TNF-α expression in the skin tissue of BALB/c mice that were irradiated with UVB and given the topical C. longa extract compared to those not given the topical C. longa extract. There was also a significant increase in the expression of type 1 collagen in the skin tissue of BALB/c mice that were irradiated with UVB and given the topical C. longa extract compared to those not given the topical C. longa extract.
AUTHORS’ CONTRIBUTIONS
Aletheia Threskeia contributed to the concept and design, data acquisition, drafting of the manuscript, statistical analysis, and funding. Willy Sandhika contributed to the concept and design, data analysis, critical revision of the manuscript, funding, supervision, and final approval. Retno Pudji Rahayu contributed to the concept and design, technical support, and supervision.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors of this study declare that there are no conflicts of interest.
ETHICAL APPROVALS
This research was pure experimental research conducted on mice in the Faculty of Pharmacy, Universitas Airlangga, Indonesia (Ethical Clearance Certificate Number: 447/HRECC.FODM/X/2020).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agrawal R, Kaur IP. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res, 2010; 13(4):397–410; doi:10.1089/rej.2009.0906
Ansary TM, Hossain MR, Kamiya K, Komine M, Ohtsuki M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int J Mol Sci, 2021; 22(8):3974; doi:10.3390/ijms22083974
Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One, 2011; 6(10):e26012; doi:10.1371/journal.pone.0026012
Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res, 2009a; 301(1):87–91; doi:10.1007/s00403-008-0893-7
Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol, 2009b; 129(4):994–1001; doi:10.1038/jid.2008.332
Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol, 2017; 94:78–82; doi:10.1016/j.exger.2017.01.009
Cavinato M, Waltenberger B, Baraldo G, Grade CVC, Stuppner H, Jansen-Dürr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology, 2017; 18(4):499–516; doi:10.1007/s10522-017-9715-7
Dunaway S, Odin R, Zhou L, Ji L, Zhang Y, Kadekaro AL. Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front Pharmacol, 2018; 9:392; doi:10.3389/fphar.2018.00392
Gallo RL, Bernard JJ. Innate immune sensors stimulate inflammatory and immunosuppressive responses to UVB radiation. J Invest Dermatol, 2014; 134(6):1508–11; doi:10.1038/jid.2014.32
Garg C, Khurana P, Garg M. Molecular mechanisms of skin photoaging and plant inhibitors. Int J Green Pharm, 2017; 11(2):S217–32.
Gopinath H, Karthikeyan K. Turmeric: a condiment, cosmetic and cure. Indian J Dermatol Venerol Leprol, 2018; 84:16–21.
Hwang BM, Noh EM, Kim JS, Kim JM, You YO, Hwang JK, Kwon KB, Lee YR. Curcumin inhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Exp Dermatol, 2013; 22(5):371–4; doi:10.1111/exd.12137
Le Digabel J, Houriez-Gombaud-Saintonge S, Filiol J, Lauze C, Josse G. Dermal fiber structures and photoaging. J Biomed Opt, 2018; 23(9):1–12; doi:10.1117/1.JBO.23.9.096501
Li H, Gao A, Jiang N, Liu Q, Liang B, Li R, Zhang E, Li Z, Zhu H. Protective effect of curcumin against acute ultraviolet B irradiation-induced photo-damage. Photochem Photobiol, 2016; 92(6):808–15; doi:10.1111/php.12628
McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol, 2018; 17(2):124–37; doi:10.1111/jocd.12518
Oakley A. Topical formulation. 2016. Available via https://dermnetnz.org/topics/topical-formulations/ (Accessed 5 June 2021).
Pandel R, Poljšak B, Godic A, Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol, 2013; 2013:930164; doi:10.1155/2013/930164
Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int, 2016; 2016:7370642; doi:10.1155/2016/7370642
Phillips J, Moore-Medlin T, Sonavane K, Ekshyyan O, McLarty J, Nathan CA. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol Head Neck Surg, 2013; 148(5):797–803; doi:10.1177/0194599813476845
Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci, 2016; 17(6):868; doi:10.3390/ijms17060868
Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed, 2015; 31(2):65–74; doi:10.1111/phpp.12145
Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med, 2015; 5(1):a015370; doi:10.1101/cshperspect.a015370
Sanches Silveira JE, Myaki Pedroso DM. UV light and skin aging. Rev Environ Health, 2014; 29(3):243–54; doi:10.1515/reveh-2014-0058
Sumiyoshi M, Kimura Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine, 2009; 16(12):1137–43; doi:10.1016/j.phymed.2009.06.003
Vollono L, Falconi M, Gaziano R, Iacovelli F, Dika E, Terracciano C, Bianchi L, Campione E. Potential of curcumin in skin disorders. Nutrients, 2019; 11(9):2169; doi:10.3390/nu11092169
Yin R, Chen Q, Hamblin MR. Skin aging and photoaging. Skin photoaging. Morgan & Claypool Publishers, 2015.
Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol, 2017; 76(3S1):S100–9; doi:10.1016/j.jaad.2016.09.038
Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant, 2018; 27(5):729–38.
Zhi Q, Lei L, Li F, Zhao J, Yin R, Ming J. The anthocyanin extracts from purple-fleshed sweet potato exhibited anti-photoaging effects on ultraviolent B-irradiated BALB/c-nu mouse skin. J Funct Foods, 2019; 64:103640; doi:10.1016/j.jff.2019.103640