INTRODUCTION
Cancer is the leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly 1 in 6 deaths. It is estimated that the number of deaths may increase up to 28.4 million in 2040. Female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers (Ferlay et al., 2020; Sung et al., 2021). Packages of effective and resource-sensitive preventative and therapeutic interventions are available for cancer; however, serious side effects, resistance, and long-term sequelae offered by these modern treatments continue to be major problems in cancer therapies. To date, colorectal cancer (CRC) is the third most deadly and fourth most commonly diagnosed cancer in the world. In fact, CRC is the third leading cause of cancer death in both men and women, and the second overall among men and women combined (GLOBOCAN, 2021; Høydahl et al., 2020; Siegel et al., 2021). Because standard treatments for CRC usually involves surgery to remove the cancer tumor, followed by adjuvant chemotherapy or radiation therapy, and because of the high toxicity associated with standard chemotherapy regimens together with drug resistance, high doses, and low tumor-specific selectivity, there is an urgent need to search for novel, more potent, safe, and selective treatment strategies in CRC based on targeted therapies able to interfere with closely related signaling pathways in CRC.
Marketed by Bayer under the trade name Aspirin, acetylsalicylic acid (ASA) used currently for minor pain relief and fever reduction has also demonstrated to have chemopreventive effects in cancer incidence and mortality associated with CRC (Bagheri et al., 2018; Burn et al., 2020; Cuzick et al., 2009; Patrignani and Patrono, 2016; Peleg et al., 1994; Spiegel, 2020; Thun et al., 1991). As for ASA, its metabolite salicylic acid (SA), displayed antiproliferative and proapoptotic effects in CRC in in vitro and in vivo models (Brennan et al., 2021; Deb et al., 2011; Elder et al., 1996; Elder and Paraskeva 1999; Giardina and Inan, 1998; Gökçe et al., 2017; Pathi et al., 2012; Paterson and Lawrence, 2001). Similarly, isoleucine has been revealed to exhibit high potency against several cancer cell lines, including lines of human colorectal carcinoma cells (Ananieva and Wilkinson, 2018; Lieu et al., 2020; Murata et al., 2007; Wakshlag et al., 2006). Furthermore, reports of diagnosed CRC patients suggest that isoleucine is linked to survival after diagnosis. This finding not only suggests isoleucine as a possible prognostic biomarker in CRC patients, but that those patients might need more of this amino acid (Bener et al., 2006; Delphan et al., 2018; Long et al., 2020; Wang et al., 1997). In addition, the antitumoral effectiveness of N-acylhydrazone-containing compounds, especially to prevent proliferation in human colorectal cancerous cells, has been extensively reported (Al-awar et al., 2019; Dasgupta et al., 2020; Huff et al., 2018; Iliev et al., 2019; Song et al., 2011; Thota et al., 2018). In this regard, N-acylhydrazones derivatives have drawn special attention for anticancer treatment, due to their ability for inhibiting the growth of a range of tumor cells including lung, colon, pancreas, and breast cancer, at micromolar levels (Buss et al., 2002; Chen et al., 2018; Chaston et al., 2003).
From a therapeutic point of view, due to the complexity of cancer and the abnormal activation of several signaling pathways in its progression, the multitarget-directed ligands strategy is considered to be an effective way to treat this disease. In an extension to this approach, merging of two (or more than two) antitumoral pharmacophoric features from known bioactive compounds covalently linked to a single molecule (hybrid molecules) offers new avenues for the development of improved chemotherapeutic cancer candidates (Ali et al., 2013; Viegas-Junior et al., 2007). Thus, considering the high antitumoral effectiveness found for SA, isoleucine and the N-acylhydrazone have not been combined into a single drug candidate for anticancer agent, and so we designed for the first time a series of molecular hybrids featuring structurally moieties of SA, N-acylhydrazone, and isoleucine into parent molecules (Fig. 1). To further analyze their structure–activity relationships (SARs), the phenyl ring substitution on N-acylhydrazone framework was used. We hypothesize that these new hybrids would exert a valuable antitumor response, in a synergistic effect, leading to tumor cell apoptosis in human colon carcinoma SW480 cells, which would likely open a new clinical possibility for CRC management. The most promising hybrid was also chosen to be investigated for its pharmacokinetics profile using in silico-derived parameters.
MATERIALS AND METHODS
General information
All chemicals used were obtained from commercial suppliers and used without further purification. Thin-layer chromatography (TLC, silica gel 60 F254) was used to follow reactions and was visualized in an iodine chamber or with a UV lamp (λ = 254 nm). Melting points were determined using a Stuart SMP10 Digital Melting Point instrument. Microwave-assisted reactions were conducted in CEM Focused Microwave. Nuclear Magnetic Spectroscopy (NMR) spectra of compounds measured on a Varian instrument spectrometer (300 MHz 1H NMR and 75 MHz 13C NMR), 1H and 13C NMR spectra were recorded in DMSO-d6, CDCl3, or CD3OD solutions and chemical shift values are in parts per million (ppm) using the Me4Si as internal standard.
Preparation of methylisoleucinate (1)
Thionyl chloride (1.5 mmol) was dissolved into dry methanol (10 ml) cooled to −10°C and the resulting mixture was stirred for 5 minutes. Afterward, the solution was treated with isoleucine (1.0 mmol), stirred for 10 minutes, maintained at −10°C for 2 hours, and then kept at room temperature for another 24 hours. After completion of the reaction, the reaction mixture was poured into ether (100 ml) and cooled for 2 hours collecting the interfacial precipitate which was filtered and oven-dried at 45°C for 24 hours; pure product 1 was obtained in a quantitatively yield (95%) as colorless needles: mp 96°C–98°C. 1H NMR (300 MHz, CDCl3) δ 3.90 (dd, J = 10.2, 4.2 Hz, 1H), 3.74 (s, 1H, CH3O), 1.67–1.39 (m, 1H), 1.34–1.03 (m, 1H), 0.94 (d, J = 6.9 Hz, 3H, CH3), 0.92 (t, J = 8.0 Hz, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 168.9 (C=O), 56.8 (CH-NH), 52.2 (CH3O), 36.4 (CH), 25.3 (CH2), 13.5 (CH3), 10.6 (CH3).
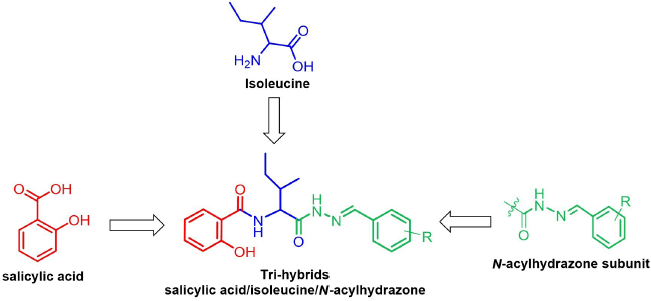 | Figure 1. Design strategy of the novel trihybrids bearing salicylic acid (in red), isoleucine (in blue), and N-acylhydrazone (in green) features as anticancer agents. [Click here to view] |
Synthesis of methyl (2-hydroxybenzoyl)isoleucinate (2)
The present synthetic procedure is similar to a previously reported method (Castrillón et al., 2019). Triethylamine (4 mmol) and SA (1 mmol) in Tetrahydrofuran (THF) (10 ml) were stirred for 15 minutes. 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, Hexafluorophosphate Benzotriazole Tetramethyl Uronium (HBTU) (1.5 mmol) was added to this mixture and stirred for a further 20 minutes. Isoleucine ester 1 (1.2 mmol) was then added and allowed to stir for 24 hours. Upon completion, the reaction mixture solvent was evaporated to dryness under reduced pressure and the residue was purified on a silica gel chromatography using mixtures hexane-ethyl acetate (1:1 ratio) as eluent yielding pure intermediate 2 in a good yield (71%) as a viscous yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 8.2 Hz, 1H, 1-H), 7.40 (t, J = 8.2 Hz, 1H, 3-H), 6.98 (d, J = 8.2 Hz, 1H, 4-H), 6.88 (t, J = 8.2 Hz, 1H, 2-H), 4.78 (dd, J = 8.3, 4.9 Hz, 1H), 3.79 (s, 3H, OCH3), 2.13–1.90 (m, 1H), 1.64–1.36 (m, 1H), 1.39–1.05 (m, 1H), 1.07–0.82 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 172.3 (C=O), 169.6 (C=O), 161.6 (Ar-O), 134.6 (Ar), 125.6 (Ar), 118.8 (Ar), 118.7 (Ar), 114.0 (Ar), 55.4 (CH-NH), 52.6 (CH3O), 38.2 (CH), 25.4 (CH2), 14.8 (CH3), 11.8 (CH3).
Synthesis of N-((2S,3S)-1-hydrazineyl-3-methyl-1-oxopentan-2-yl)-2-hydroxybenzamide (3)
The present synthetic procedure is similar to a previously reported method (Coa et al., 2015; Vergara et al., 2017). Compound 2 (1 mmol) and MeOH (1 ml) were mixed. Subsequently, hydrazine hydrate (5 mmol, 98% solution) was added and the resulting mixture was stirred in a preheated oil bath at 65°C for 24 hours when analysis by TLC indicated the end of the reaction. After being cooled to room temperature, the solvent was evaporated under reduced pressure and the residue was poured onto ice. The resulting precipitate was filtered, washed with ice water, and oven-dried at 45°C for 24 hours, affording the corresponding N-acylhydrazide 3 as a white solid (93%); mp 111°C–113°C. 1H NMR (300 MHz, Methanol-d4) δ 7.90 (td, J = 8.0, 1.8 Hz, 1H), 7.38 (td, J = 8.0, 7.4, 1.8 Hz, 1H), 6.92 (m, 2H), 3.33 (dd, J = 8.1, 3.9 Hz, 1H), 2.10–1.92 (m, 1H), 1.70–1.44 (m, 1H), 1.04–0.92 (m, 6H, 2 × CH3). 13C NMR (75 MHz, Methanol-d4) δ 171.7 (C=O), 168.1 (C=O), 158.3 (Ar-O), 133.4 (Ar), 129.0 (Ar), 119.0 (Ar), 116.7 (Ar), 116.5 (Ar), 56.6 (CH-NH), 36.8 (CH), 24.8 (CH2), 14.5 (CH3), 9.9 (CH3).
General procedure for synthesis of trihybrids (4a–l)
The present synthetic procedure is similar to a previously reported method (Coa et al., 2015; Vergara et al., 2017). The appropriate aromatic aldehyde (1 mmol), acylhydrazide 3 (1 mmol), and a few drops of acetic acid in ethanol were deposited into a CEM microwave reaction vessel. After that, the mixture was irradiated in a CEM Discover microwave synthesizer at 200 W in the open-vessel mode attached to a reflux condenser at 80°C with vigorous stir during 10 minutes. After being cooled to room temperature, the solvent was evaporated under reduced pressure and the oily residue was purified by preparative TLC over silica gel 60 F 254 (Merck) using an mixture of hexane:AcOEt (10:8–1:1) as eluent, furnishing the target hybrids 4a–l in the yields as described below.
2-hydroxy-N-(1-(2-((2-hydroxynaphthalen-1-yl)methylene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)benzamide (4a). Pale yellow solid; yield = 94%; mp = 258°C–260°C; 1H NMR (300 MHz, DMSO-d6) δ 9.22 (s, 1H, N=C-H), 7.93–7.72 (m, 2H), 7.67–7.48 (m, 2H), 7.32 (t, J = 8.1 Hz, 1H), 7.14–7.00 (m, 2H), 6.85–6.66 (m, 2H), 6.42 (t, J = 7.8 Hz, 1H), 4.60 (d, J = 6.9 Hz, 1H), 2.06–1.92 (m, 1H), 1.70–1.43 (m, 2H), 1.06–0.71 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 172.7 (C=O), 168.2 (C=O), 159.2 (Ar-O), 157.2 (Ar-O), 140.6 (C=N), 137.4, 133.6, 132.6, 130.4, 129.1, 127.1, 126.8, 125.9, 124.3, 120.0, 118.8, 118.6, 117.4, 133.5, 56.6, 37.4, 25.1, 16.1 (CH3), 11.4 (CH3).
2-hydroxy-N-(1-(2-(2-hydroxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)benzamide (4b). Pale yellow solid; yield = 75%; mp = 280°C–282°C; 1H NMR (300 MHz, DMSO-d6) δ 8.11 (s, 1H, N=C-H), 7.64 (d, J = 7.8 Hz, 1H), 7.29–6.77 (m, 4H), 6.56 (d, J = 8.2 Hz, 2H), 6.30 (t, J = 7.1 Hz, 1H), 4.40 (d, J = 6.8 Hz, 1H), 2.18–1.75 (m, 1H), 1.42–1.08 (m, 2H), 0.98–0.70 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 172.7 (C=O), 168.7 (C=O), 165.8 (Ar-O), 156.7 (Ar-O), 152.5 (C=N), 149.0, 138.2, 137.9, 133.7, 131.9, 118.3, 114.8, 106.1, 104.6, 102.2, 62.8, 37.7, 26.4, 15.9 (CH3), 11.4 (CH3).
N-(1-(2-(2,3-dihydroxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4c). Pale yellow solid; yield = 58%; mp = 197°C–199°C; 1H NMR (300 MHz, DMSO-d6) δ 8.85 (s, 1H, N = C-H), 8.34 (d, J = 7.5 Hz, 1H), 7.87 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 7.06 (d, J = 8.1 Hz, 1H), 6.85 (d, J = 7.2 Hz, 1H), 6.81 (t, J = 7.2 Hz, 1H), 6.75 (d, J = 8.1 Hz, 1H), 4.48 (d, J = 7.1 Hz, 1H), 2.10–1.78 (m, 1H), 1.68–1.27 (m, 2H), 1.00–0.64 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 168.4 (C=O), 168.2 (C=O), 157.5 (Ar-O), 147.3 (Ar-O), 145.4 (Ar-O), 145.3 (C=N), 134.1, 130.1, 122.7, 121.1, 120.2, 120.0, 119.4, 118.7, 117.5, 56.7, 36.8, 24.9, 15.6 (CH3), 11.2 (CH3).
N-(1-(2-(2,4-dihydroxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4d). Pale yellow solid; yield = 54%; mp = 196°C–198°C; 1H NMR (300 MHz, DMSO-d6) δ 8.00 (s, 1H, N = C-H), 7.67 (dt, J = 7.9, 2.4 Hz, 1H), 7.20 (m, 2H), 7.88 (t, J = 8.2 Hz, 1H, 2-H), 7.01 (d, J = 3.2 Hz, 1H), 6.66 (dd, J = 8.5, 3.2 Hz, 1H), 6.52 (t, J = 7.4 Hz, 1H), 6.09 (d, J = 8.5 Hz, 1H), 4.34 (d, J = 6.8 Hz, 1H), 2.16–1.86 (m, 1H), 1.43–1.14 (m, 2H), 0.93 (d, J = 7.4 Hz, 3H, CH3), 0.83 (t, J = 7.6 Hz, 3H, CH3). 13C NMR (75 MHz, DMSO-d6) δ 171.5 (C=O), 171.0 (C=O), 168.9 (Ar-O), 153.9 (Ar-O), 151.4 (Ar-O), 142.1 (C=N), 135.5, 134.1, 134.1, 129.6, 122.2, 122.1, 117.7, 114.2, 110.5, 57.2, 36.9, 24.7, 15.5 (CH3), 11.2 (CH3).
N-(1-(2-(2,5-dihydroxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4e). Pale yellow solid; yield = 86%; mp = 201°C–203°C; 1H NMR (300 MHz, DMSO-d6) δ 8.24 (s, 1H, N=C-H) 7.73 (td, J = 7.3, 1.7 Hz, 1H), 7.26 (dd, J = 7.3, 2.4 Hz, 1H), 6.89 (d, J = 2.7 Hz, 1H), 6.84 (dd, J = 7.5, 1.7 Hz, 1H), 6.77 (d, J = 8.4 Hz, 1H), 6.73 (dd, J = 8.4, 2.7 Hz, 1H), 6.59 (t, J = 7.5 Hz, 1H), 4.41 (d, J = 6.6 Hz, 0H), 2.24–1.90 (m, 1H), 1.47–1.20 (m, 2H), 1.01 (d, J = 6.9 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 172.8 (C=O), 168.7 (C=O), 165.7 (Ar-O), 155.1 (Ar-O), 150.2 (Ar-O), 145.6 (C=N), 133.0, 130.1, 120.5, 119.3, 118.3, 118.0, 117.7, 116.2, 115.9, 55.9, 37.1, 25.0, 15.9 (CH3), 11.9 (CH3).
N-(1-(2-(3,4-dihydroxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4f). Yellow solid; yield = 64%; mp = 188°C–191°C; 1H NMR (300 MHz, DMSO-d6) δ 7.98 (s, 1H, N=C-H), 7.79 (t, J = 7.6 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.22 (s, 1H), 6.99 (d, J = 7.7 Hz, 1H), 6.96–6.85 (m, 2H), 6.78 (d, J = 7.7 Hz, 1H), 4.34 (d, J = 7.6 Hz, 1H), 1.93–1.79 (m, 1H), 1.36–1.06 (m, 2H), 0.88–0.78 (m, 6H). 13C NMR (75 MHz, DMSO-d6) δ 169.0 (C=O), 167.6 (C=O), 157.2 (Ar-O), 152.5 (Ar-O), 149.9 (Ar-O), 147.8 (C=N), 145.1, 134.4, 130.0, 125.7, 122.1, 117.3, 117.0, 116.0, 113.4, 57.1, 36.8, 24.9, 14.8 (CH3), 10.7 (CH3).
2-hydroxy-N-(3-methyl-1-oxo-1-(2-(2,3,4-trihydroxybenzylidene)hydrazineyl)pentan-2-yl) benzamide (4g). Yellow solid; yield = 68%; mp = 126°C–128°C; 1H NMR (300 MHz, DMSO-d6) δ 8.22 (s, 1H, N=C-H), 7.85 (d, J = 8.5 Hz, 1H), 7.37 (t, J = 7.7 Hz, 1H), 7.06–6.84 (m, 2H), 6.77 (dd, J = 8.5, 2.4 Hz, 1H), 6.40 (d, J = 8.7 Hz, 1H), 4.38 (d, J = 7.2 Hz, 1H), 1.96–1.71 (m, 1H), 1.43–1.07 (m, 2H), 0.94–0.73 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 168.1 (C=O), 168.0 (C=O), 157.4 (Ar-O), 157.1 (Ar-O), 151.1 (Ar-O), 148.8 (Ar-O), 147.2 (C=N), 132.6, 130.2, 122.4, 120.2, 117.5, 117.1, 111.0, 108.2, 56.9, 36.9, 25.0, 15.6 (CH3), 10.9 (CH3).
N-(1-(2-(2,3-dimethoxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4h). Pale yellow solid; yield = 91%; mp = 118°C–120°C; 1H NMR (300 MHz, DMSO-d6) δ 8.55 (s, 1H, N=C-H), 8.32 (d, J = 7.6 Hz, 1H), 7.92 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.27 (m, 2H), 7.11 (m, 2H), 4.44 (d, J = 7.0 Hz, 1H), 3.79 (s, 3H, OMe), 3.78 (s, 3H, OMe), 2.05–1.91 (m, 1H), 1.65–1.40 (m, 2H), 1.02–0.88 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6). δ 166.9 (C=O), 166.7 (C=O), 161.2 (Ar-O), 153.2 (Ar-OMe), 153.1 (Ar-OMe), 148.3 (C=N), 143.0, 133.2, 130.4, 128.1, 124.8, 118.6, 118.2, 117.4, 114.4, 61.6 (CH3O), 57.0 (CH3O), 56.2, 37.1, 27.0, 16.0 (CH3), 11.3 (CH3).
N-(1-(2-(2,4-dimethoxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4i). Pale yellow solid; yield = 61%; mp = 120°C–122°C; 1H NMR (300 MHz, DMSO-d6) δ 8.52 (s, 1H, N=C-H), 7.91 (d, J = 8.1 Hz, 1H), 7.76 (dd, J = 8.6, 2.2 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 6.97 (t, J = 8.1 Hz, 1H), 6.93 (d, J = 7.7 Hz, 1H), 6.75 (d, J = 2.1 Hz, 1H), 6.65 (dd, J = 8.4, 2.1 Hz, 1H), 3.88 (d, J = 6.8 Hz, 1H), 3.85 (s, 3H, OMe), 3.82 (s, 3H OMe), 2.07–1.91 (m, 1H), 1.65–1.38 (m, 2H), 0.94 (d, J = 6.8 Hz, 3H), 0.89 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 167.9 (C=O), 167.4 (C=O), 162.9 (Ar-O), 159.5 (Ar-OMe), 159.4(Ar-OMe), 143.1 (C=N), 133.3, 130.4, 127.1, 118.4, 118.3, 115.5, 115.3, 106.8, 98.7, 56.2 (CH3O), 56.1 (CH3O), 55.9, 37.3, 27.0, 15.9 (CH3), 11.4 (CH3).
N-(1-(2-(2,5-dimethoxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4j). Pale yellow solid; yield = 60%; mp = 127°C–129°C; 1H NMR (300 MHz, DMSO-d6) δ 8.59 (s, 1H, N=C-H), 8.33 (d, J = 7.5 Hz, 1H), 7.89 (d, J = 7.5 Hz, 1H), 7.32 (d, J = 3.1 Hz, 1H), 7.04 (d, J = 8.9 Hz, 2H), 7.00 (td, J = 8.6, 2.9 Hz, 1H), 6.92 (d, J = 8.6 Hz, 1H), 3.81 (s, 3H, OMe), 3.76 (d, J = 6.9 Hz, 1H), 3.74 (s, 3H, OMe), 2.07–1.89 (m, 1H), 1.65–1.39 (m, 2H), 0.97–0.87 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 168.3 (C=O), 167.4 (C=O), 153.7 (Ar-O), 152.7 (Ar-OMe), 152.4 (Ar-OMe), 142.9 (C=N), 133.1, 130.2, 123.2, 118.7, 118.4, 118.2, 117.4, 113.8, 109.4, 56.7 (CH3O), 55.9 (CH3O), 55.7, 37.2, 25.2, 16.0 (CH3), 11.4 (CH3).
N-(1-(2-(3,4-dimethoxybenzylidene)hydrazineyl)-3-methyl-1-oxopentan-2-yl)-2-hydroxy benzamide (4k). White solid; yield = 60%; mp = 108°C–110°C; 1H NMR (300 MHz, DMSO-d6) δ 8.18 (s, 1H, N=C-H), 7.96 (dd, J = 8.3, 1.4 Hz, 1H), 7.39–7.25 (m, 1H), 7.17 (dd, J = 7.5, 2.1 Hz, 1H), 7.01 (t, J = 8.3 Hz, 1H), 6.96 (dd, J = 7.5, 2.1 Hz, 1H), 6.83 (t, J = 7.5 Hz, 1H), 4.44 (d, J = 7.4 Hz, 1H), 3.79 (s, 6H, 2 × OCH3), 2.07–1.90 (m, 1H), 1.67–1.34 (m, 1H), 1.02–0.74 (m, 6H, 2 × CH3). 13C NMR (75 MHz, DMSO-d6) δ 173.1 (C=O), 173.0 (C=O), 168.0 (Ar-O), 167.4 (Ar-OMe), 166.5 (Ar-OMe), 151.1 (C=N), 149.4, 147.9, 127.3, 122.4, 118.5, 118.0, 117.4, 111.8, 108.6, 57.0 (CH3O), 56.0 (CH3O), 55.9, 37.0, 25.2, 15.9 (CH3), 11.3 (CH3).
2-hydroxy-N-(1-(2-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene) hydrazineyl)-3-methyl-1-oxopentan-2-yl)benzamide (4l). Yellow solid; yield = 86%; mp = 198°C–200°C; 1H NMR (300 MHz, DMSO-d6) δ 8.64 (s, 1H, N=C-H), 7.77 (d, J = 7.8 Hz, 1H), 7.19 (s, 1H, H pyridine), 7.09 (t, J = 8.0 Hz, 1H), 6.75 (d, J = 8.1 Hz, 1H), 6.43 (d, J = 8.0 Hz, 1H), 4.46 (s, 2H, CH2O), 4.44 (d, J = 6.9 Hz, 1H), 2.29 (s, 3H, CH3-Py), 2.01–1.87 (m, 1H), 1.54–1.25 (m, 2H), 0.99 (d, J = 6.5 Hz, 3H), 0.93 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 168.0 (C=O), 166.2 (C=O), 158.3 (Ar-O), 154.1 (Py-O), 150.2 (Py-CH3), 146.8 (C=N), 139.3 (C=Npy), 133.8, 132.6, 130.4, 128.9, 120.2, 118.5, 118.2, 68.6 (-CH2O-Py), 60.2, 37.5, 25.1, 16.0 (CH3-Py), 15.5 (CH3), 11.3 (CH3).
Biological activity assays
Cell lines and culture medium
Nonmalignant cells (CHO-K1) along with the adenocarcinoma colon cancer cell line (SW480) were used for biological experiments. Cultures were obtained from The European Collection of Authenticated Cell Cultures (England), cultivated with horse serum, 1% nonessential amino acids (Gibco Invitrogen, Carlsbad, CA), 10% heat-inactivated (56°C), and maintained in Dulbecco’s Modified Eagle Medium. Serum was reduced to 3% and the medium was supplemented with insulin (10 mg/ml), selenium (5 ng/ml), and transferrin (5 mg/ml) (Herrera et al., 2018).
Cytotoxic activity
The colorimetric sulforhodamine B (SRB) assay was used to evaluate the cytotoxicity activity of the synthesized trihybrids as well as the reference compounds. Using 96-well tissue culture plates, cells were plated at a seeding density of 20,000 cells/well at 37°C and incubated in a humidified atmosphere containing 5% CO2. All cultures were allowed to grow for 24 hours, and then treated with vehicle control containing 1% (v/v) DMSO or increasing concentrations (0.01–200 μM) of the target trihybrids, as well 5-FU (the standard drug), pyridoxal isonicotinoyl hydrazone (PIH), parental compounds (SA and isoleucine), and the equimolar mixture of these. After cell fixation with trichloroacetic acid (50% v/v, 1 hour at 4°C) (Merck), cell proteins were determined by staining with 0.4% (w/v) SRB (Sigma-Aldrich, United States), and afterward unbound SRB was removed washed with 1% acetic acid and left for air-drying. After that, 10 mM Tris base was used to dissolve protein-bound SRB. The absorbance measured at 492 nm in a microplate reader (Mindray MR-96A) (Perez et al., 2014). All experiments were conducted at least three times.
Antiproliferative ativity
The assessment of antiproliferative activity of the most promising compound was also tested through SRB assay. Typically, after the cells were seeded at an initial density of 2,500 cells/well in 96-well tissue culture plates, they were incubated in the same conditions described earlier for cytotoxicity. Afterward, cultures were allowed to grow for a period of 24 hours and then treated with DMSO (vehicle control, 1%) or increasing concentrations of the hit-trihybrid 4j [6.25–100 μM, ranges dependent on the inhibitory concentration (IC50) values] for 0, 2, 4, 6, and 8 days. The culture medium is changed every 48 hours. After the incubation time, cells were fixed, stained, and read as previously reported (Castrillón et al., 2019).
Statistical analysis
Data obtained from three independent experiments was reported as mean ± standard error (SE). One-way analysis of variance and Dunnett’s test were used to evaluated statistical differences between treated cells and control group (nontreated). p-values lesser than 0.05 (typically ≤ 0.05) were considered statistically significant. To analyze experimental data, GraphPad Prism software V.7 for Windows was used.
Theoretical drug-likeness and pharmacokinetic indices
Ten biopharmaceutical relevant properties were screened for the most promising trihybrid 4j. To that, MW, topographical polar surface area (TPSA), and rotatable bonds descriptors were calculated by using Molinspiration Chemoinfomatics Software. Meanwhile, human serum albumin (LogKHSA), apparent predicted intestinal permeability (app. Caco-2 and MDCK models), and gastrointestinal uptake and absorption (%GI) were estimated using the online PreADMET 2.0 program. In addition, the ALOGPS 2.1 algorithm was used to calculate the lipophilicity parameter, log Po/w.
RESULTS AND DISCUSSION
Chemistry
Novel salicylic acid/isoleucine/N-acylhydrazone (SA-Ile-NAH) hybrids were synthetized through a four-step (i–iv) divergent sequence of reactions shown in Scheme 1. To summarize, commercially available isoleucine as starting material was esterified to the corresponding methyl ester using thionyl chloride (SOCl2) in methanol solvent by a modified Fischer esterification mechanism (Castrillón et al., 2019) to give ester 1 in quantitative yields, which was subjected to one-pot coupling procedure with available SA using HBTU as amide bond promoter and triethylamine (Et3N) as a Lewis base to obtain 2 in good yields (71%) (Castrillón et al., 2019; Herrera et al., 2021). Subsequently, this intermediate reacted with hydrazine hydrate via nucleophilic substitution to obtain in high yield (93%) the desired N-acylhydrazide 3 (Coa et al., 2015; Vergara et al., 2017). Finally, the target SA-Ile-NAH hybrids 4a–l were obtained in moderate to excellent yields (54%–94%) via Brønsted acid-catalyzed imine formation between the hydrazide 3 with the corresponding aromatic aldehydes (ArCHO) in ethanol under conditions of microwave heating, as described in Scheme 1 (Coa et al., 2015; Vergara et al., 2017). The structures of all compounds have been fully characterized by a combined 1H-NMR and 13C-NMR study with carbon atom types (C, CH, CH2, and CH3), determined by using the distortionless enhancement by polarization transfer or attached proton test pulse sequence. The signals were assigned using two-dimensional heteronuclear correlations (correlation spectroscopy and heteronuclear single quantum correlation). The 1H-NMR spectra of hybrids 4a–l dissolved in DMSO-d6 showed a characteristic singlet peak within the 7.98–9.22 ppm range which is assigned to the proton of the azomethine group (-CH=N-). The isoleucine moiety was recognized by their two methyl groups which give a characteristic row of double signals within the 0.64–1.06 ppm range, while the signals corresponding to α-CH (~4.5 ppm) and CH2 aliphatic protons were registered in the range of δ = 1.4–2.0 ppm. 1H-NMR spectra also confirm the presence of aromatic protons which were located in the downfield region (6.70–8.60 ppm). Analysis of the 13C NMR data for the 4a–l hybrids showed a peak in the range of 140–152 ppm which confirmed the presence of azomethine carbon in the hybrid structure. Moreover, the 13C-NMR spectrum shows 5 signals in the 10–60 ppm upfield region, which was assigned to the carbon atoms of the aliphatic chain in the isoleucine portion. In addition, 13C-NMR spectra of hybrids show two picks around 170 and 167 ppm, respectively, which confirms the presence of both carbonyl groups (C=O). Finally, aromatic carbons appear between 120 and 170 ppm. (see Supplementary Online Material for further details)
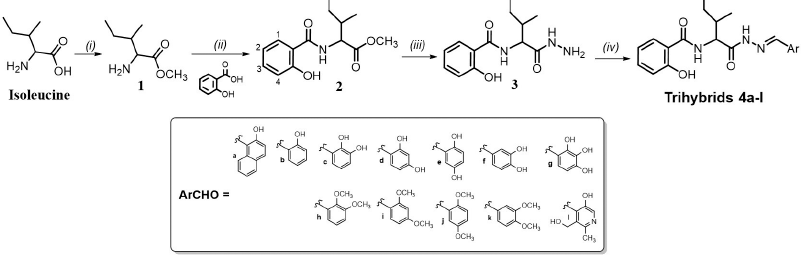 | Scheme 1. Reagents and conditions: (i) SOCl2, MeOH, 0°C; then rt, 48 hours, 95%. (ii) HBTU, Et3N, THF, rt, 24 hours, 71%. (iii) NH2NH2.H2O, 98%, MeOH, reflux, 24 hours, 93%. (iv) AcOH (cat), EtOH, reflux, 10 minutes, (μw), 54%–94%. [Click here to view] |
Biological activity
Cytotoxic effect of SA-Ile-NAH hybrids on SW480 and CHO-K1 cell lines
The cytotoxic effect of the synthesized SA-Ile-NAH hybrids using an in vitro model of human colorectal adenocarcinoma (SW480) and the nonmalignant CHO-K1 cell line were measured by the SRB colorimetric assay. In addition to the trihybrid compounds, 5-FU and the promising PIH were also included as positive controls, as well as the parent scaffolds SA and isoleucine and their equimolar mixture. Cytotoxicity data for the compounds are reported as half-maximal IC50 values and listed in Table 1.
In general, most trihybrids exhibited comparable cytotoxic activity to the reference drug 5-FU and PIH, being significantly more active than the parental subunits (SA and isoleucine) and their respective unlinked equimolar mixture, displaying promising in vitro cytotoxic effects in a concentration range of 20.43 ± 1.88 and 168.2 ± 20.33 μM after 48 hours of treatment. Furthermore, as can be seen in Table 1, most of those compounds increased the cytotoxic response over time as evidenced by the reduction in the IC50 values at 48 hours (see values at 24 vs. 48 hours). It is worth noting that the proposed molecular design allowed obtaining molecules with improved cytotoxic properties with respect to their respective parental compounds (SA and isoleucine), considerably higher activity than the equimolar mixture, and exhibited cytotoxic potency similar to that of 5-FU and PIH, thereby demonstrating a remarkable advantage of the molecular hybridization strategy.
Despite trihybrids 4a-c, 4f, 4h, 4i, and 4l improving the efficacy over malignant cells (SW480), a decrease in selectivity due to the high toxicity on nonmalignant cells (CHO-K1) was also observed after 48 hours of treatment. It was, however, observed that the 2,5-dimethoxy-substituited trihybrid 4j highlighted not only for its good cytotoxic effect (IC50 = 78.15 ± 6.73 μM) but has no significant toxicity to nontumor cells (IS = 1.31). More importantly, a microscopic inspection (Fig. 2A and B) revealed that hybrid 4j induced marked cell morphology changes related to the size and shape in SW480 colorectal cell line which is highly associated with cell death process, while a typical and healthy shape was observed after treatment with DMSO as control vehicle. Moreover, hybrid 4j was able to induce a visible reduction in the total number of cells in a dose-dependent manner suggesting that this compound is able to induce a short-term loss of cell viability by either cytostatic or cytotoxic effect on tumor cells.
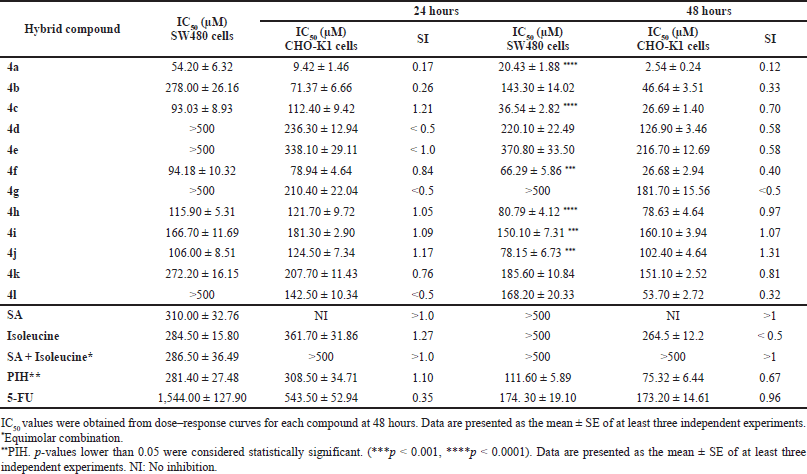 | Table 1. Cytotoxic effect of SA-Ile-NAH hybrids against SW480 and CHO-K1 cell lines after 24 and 48 hours of treatment. [Click here to view] |
Studies of the SAR were conducted at 48 hours after incubation. Results revealed a synergistic action of the parent subunits when they are linked to form a single structure in the hybrid. Hybrid 4a exhibited better cytotoxicity than compound 4b, which shows the importance of the naphthyl group over the phenyl group for activity. The change of the phenyl group by the pyridyl group causes a slight decrease in the activity (4b vs. 4l). The presence of a hydroxyl group at the C-3 position of the aromatic ring increased the activity (4b vs. 4c). However, when this group changes position, the activity decreases (4c vs. 4d and 4e). Hybrid 4g, a combination of compounds 4b and 4f, was not active, showing that there was no synergistic effect in SW480 cells, which was reflected in decreased activity compared to the initial hybrids (Fig. 3).
The methylation effect is not clear, because when the hybrids 4c and 4h are compared, it is possible to observe a decrease in activity. However, an opposite behavior is observed when the other hybrids are compared (4d vs. 4i and 4e vs. 4j); in these cases, the activity increases. These findings are in good agreement with other reports, where compounds combining structural moieties of SA and hydrazone displayed a promising anticancer potential (Alam et al., 2017; Jongstra et al., 2006; Misko et al., 2019). Moreover, similar results were reported by Herrera et al. (2021) who also measured good cytotoxic and selective activity of hybrids of SA incorporating an S-allylcysteine fragment, using human colorectal adenocarcinoma SW480 cell lines as model. We hypothesized that the cytotoxic effect on SW480 cells observed after SA-Ile-NAH hybrids exposure could be associated to the fact that these compounds would be able to target essential bioreceptors through formation of intermolecular hydrogen bonds, oxidative stress triggered by reactive free-radical species formation, and/or the ability of metal complexation. However, further experimental and computational investigations need to be conducted in order to elucidate the most plausible antiproliferative mechanism of these promising trihybrids. According to the aforementioned findings, merging SA, isoleucine, and hydrazone moieties into a unique structural core provides promising compounds that could be considered a novel interesting therapeutical scaffold for developing new antitumoral compounds, particularly in CRC. In addition, further investigations by introducing electron-withdrawing substituents and electron-donating core combinations in the aromatic ring attached to the hydrazone moiety will be conducted aiming at improving the selectivity of most of the tested compounds. Studies of how these electronics effects can affect the antiproliferative potency, not only in CRC but other most commonly diagnosed cancers at even higher risk, are also needed.
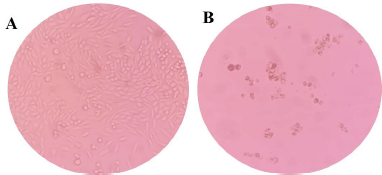 | Figure 2. Images of growth control of SW480 cancer cell line (magnification: 20×). (A). Vehicle control. (DMSO) treatment. (B). After hybrid 4j exposure (48 hours posttreatment) [Click here to view] |
Antiproliferative effect of 4j on SW480 cells
The antiproliferative effect of the most promising compound 4j highlighted by the selectivity and cytotoxic effect was further evaluated to know if it can preserve its activity against SW480 cells after periods of time ranging from 2 to 8 days of treatment (Table 2).
To achieve this goal, SW480 adenocarcinoma cells were treated with increasing concentrations of 6.25, 12.5, 25, 50, and 100 μM of 4j for 8 days and the cell viability was measured by the colorimetric SRB assay. As shown in Figure 4, our results in comparison with the control showed that trihybrid 4j inhibited the proliferation of human colorectal adenocarcinoma cells (SW480), in dose- and time-dependent manner. Thus, treatment of tumor cells with 4j reduced the number of viable cells and prevented the exponential growth of the primary tumor cell line examined. Particularly, after 4 days of treatment with 25–100 μM, this hybrid significantly reduced the viability of the SW480 cells compared to untreated control cells, with p-values lesser than 0.001. Notably, when SW480 cells were treated with a low concentration of 4j (12.5 μM), the number of tumor cell colonies was also significantly reduced within 4 days of treatment. All these findings demonstrated that hit compound 4j caused a time- and dose-dependent loss of SW480 cell viability confirming the previous results of viability and suggesting that trihybrid 4j may in vitro induce either cytostatic/cytotoxic response on the colon cancer cell line model. We conclude here that, the synthetized SA-Ile-NAH hybrids compounds, particularly compound 4j, emerge as attractive building blocks in the search of new anti-CRC agents.
 | Figure 3. SAR for the cytotoxic activity of the novel SA-Ile-NAH hybrid. [Click here to view] |
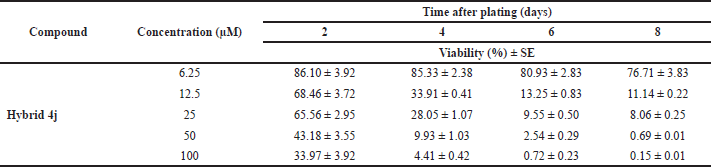 | Table 2. Data viability (%) of the five concentrations evaluated against SW480 cell. [Click here to view] |
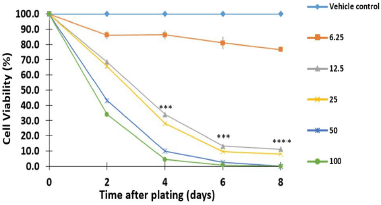 | Figure 4. Antiproliferative effect of hybrid 4j against SW480 cell line; Data are presented as the mean ± SE of at least three independent experiments (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). Vehicle control was assumed as 100% viable. [Click here to view] |
Theoretical pharmacokinetic and drug-likeness properties of promising trihybrid 4j
Drug-likeness and pharmacokinetics inspections represent a valuable strategy to complete the journey from initial discovery to the marketplace of novel drug candidates in pharmaceutical research, particularly in assessing the quality of novel antitumor scaffolds. Here, for the most promising trihybrid 4j, we have predicted 10 biopharmaceutical indices that have the most profound influence on drug-like properties of a molecule, such as molecular weight, polar surface area, number of hydrogen bond acceptors/donors, octanol–water partition coefficient, binding-serum albumin, human intestinal permeation (Caco-2 and MDCK cells models), and a number of rotatable bonds. The drug-likeness and pharmacokinetic predictions provided by the SwissADME and pre-ADMET software are listed in Table 3. Our results show that a favorable pharmacokinetics profile was found for the examined hybrid when compared to 95% of oral U.S. Food and Drug Administration-marketed drugs. 4j did not violate any of Lipinski’s rule of five (Lipinski et al., 1997). Moreover, the degree of lipophilicity (expressed as LogPo/w) was in good agreement with the optimal quality parameters for oral lipid-based formulations (–2.0 to 6.0) (Ditzinger et al., 2019). Furthermore, 4j displayed an optimal human intestinal absorption (%GI) number larger than 80%, and good predicted permeability data in the range of 536–1,077 nm/second when traditional Caco-2 and MDCK cells models were used, respectively (Broccatelli et al., 2016; Pham-The et al., 2018; Press and Di Grandi, 2008). Meanwhile, the compound exhibits a computational TPSA value of 106.75 Å2 within the ideal value of less than 140 Å2 (Ertl et al., 2000). We predict that 4j would bind favorably to human serum albumin (expressed as logKHSA), within the recommended therapeutic range suggested for potential drug candidates (–1.5 to 1.5) (Colmenarejo, 2003; Zhivkova, 2015). Finally, the pan-assay interference compounds (PAINS) filter, which provides early alerts for potential toxicity as part of successful drug discovery, showed that the promising 4j hybrid can be regarded as valid starting points in cancer drug discovery.
Taken altogether, merging salicylic acid, N-acylhydrazone, and isoleucine scaffolds into a new single chemical structure provides bioactive compounds with an optimal pharmacokinetic profile. Accordingly, novel trihybrid scaffolds based on SA-Ile-NAH should be taken into account for further investigations focused on the design of new oral antitumoral drug candidates to combat CRC.
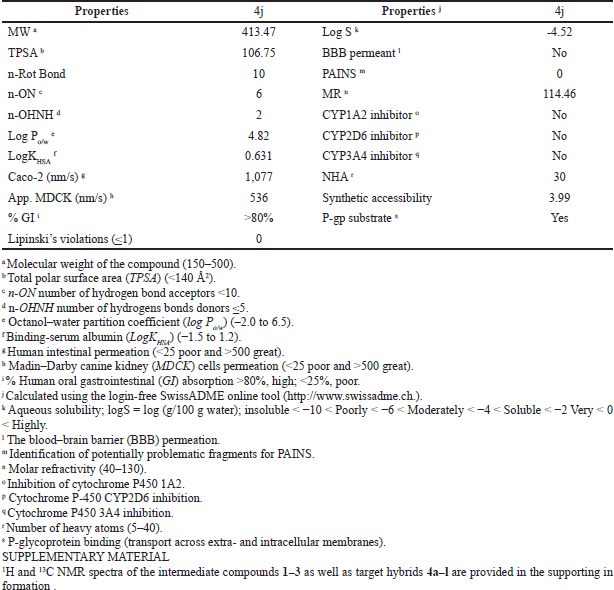 | Table 3. Lipinski’s rule and pharmacokinetic indices for the hit compound 4j. [Click here to view] |
CONCLUSION
A novel series of SA-Ile-NAH trihybrids was designed, synthesized, and screened for cytotoxic effect on SW480 human colon adenocarcinoma cells. Most of these hybrids were more potent than the reference drug (5-FU) and the equimolar mixture as well as than the parental subunits (SA and isoleucine). In particular, the 2,5-dimethoxy-substituited trihybrid 4j was the most promising compound because it not only displayed good cytotoxic potency against SW480 human colon cancer cells, but also expressed remarkable antiproliferative activity at lower concentrations, and no significant toxic effects on nontumorigenic cells. Moreover, 4j showed an optimal drug-likeness/pharmacokinetic profile. Considering all these results, trihybrids based on SA-Ile-NAH emerge as a promising therapeutic opportunity against CRC in the forthcoming years.