INTRODUCTION
Tuberculosis (TB) is an infectious disease, caused by Mycobacterium tuberculosis, with high prevalence in Indonesia. The World Health Organization reported that Indonesia is estimated to be the third country in the world (next to India and China) with the highest TB cases (World Health Organization, 2019). The current treatment of TB requires the administration of oral anti-TB drugs for 4–6 months. However, long-term oral administration of anti-TB drugs may cause severe adverse effects, poor patient compliance toward the regimen, low absorption of anti-TB drugs to the lung, and inadequate anti-TB level in the infected organ (Sotgiu et al., 2015). Those conditions may eventually lead to therapeutic failure, bacterial resistance, or latent TB condition (Ahmad, 2011; Barry et al., 2009). Direct delivery of anti-TB drugs to the lung allows higher drug concentration of anti-TB drugs inside the infected organ, thus preventing bacterial resistance and latent TB condition. Pulmonary delivery of anti-TB drugs would also avoid systemic adverse effects, as well as reduce dose, duration, and cost of therapy (Das et al., 2015; Parumasivam et al., 2016). Therefore, pulmonary-targeted dosage forms would be a strategy to improve the efficiency of TB therapy.
Among other lung-targeted dosage forms, the dry powder inhaler (DPI) provides better stability under storage conditions. The DPI provides noninvasive therapy and allows various formulations and carriers for the intended drug release profile (de Boer et al., 2017). Therefore, in this study, the DPI was formulated to deliver the anti-TB drug into the lung. Rifampicin is one of the first-line anti-TB drugs which makes it a suitable model drug for this study.
Latent tuberculosis infection (LTBI) is defined by the absence of clinical symptoms of TB but carries a risk of subsequent progression to clinical disease (Barry et al., 2009). In a person with LTBI, M. tuberculosis is entrapped inside the macrophages which create a microenvironment that limits the replication and spreading of the infection; thus, the bacteria are in the dormant stage of the infection and are not active. Nonetheless, these dormant cells can be reactivated when the host immune system is suppressed (Lin and Flynn, 2010). Therefore, targeting anti-TB drugs into the alveolar macrophages is considered to be one of the strategies to combat LTBI.
Despite many studies to deliver anti-TB drugs directly into the lung, there are fewer studies targeting alveolar macrophages. Among those studies, the application of single and synthetic polymers (such as poly lactic-co-glycolic acid/ PLGA) is the most explored. Therefore, in this study, we explore the combination of natural polymers from Indonesia as an excipient for formulating the DPI. Chitosan has been proven as an excipient which effectively induces macrophage uptake and thus is considered as a suitable polymer to deliver anti-TB drugs into the alveolar macrophages to treat LTBI (Park et al., 2013). However, several studies showed that chitosan could induce an unintended inflammation response in the lung (Huang et al., 2005; Mori et al., 2005). The combination of chitosan with alginate (Li et al., 2008) is expected to reduce the immune response after pulmonary administration. A previous study showed that the combination of chitosan with xanthan gum could offer a suitable release profile in physiological fluid (Surini et al., 2019). Therefore, in this study, rifampicin DPI was formulated using chitosan (macrophages-targeted polymer) and alginate (controlled-release polymers) to obtain DPI with a suitable release profile in the lung and macrophage fluid
This study aimed to explore the formulation of rifampicin DPI using the combination of chitosan and alginate, to reach adequate rifampicin concentration in both lung tissue and alveolar macrophages, to combat both active and LTBI and improve the efficiency of TB therapy. In this study, rifampicin DPI was prepared by spray drying, using the combination of chitosan and sodium alginate. The physical properties of the DPIs were characterized. Furthermore, a dissolution study in a phosphate buffer of pH 7.4 with sodium lauryl sulfate (SLS) 0.05% and in a phthalate buffer of pH 4.5, as well as a cytotoxicity study towards cell line A549, was performed, in order to evaluate its potency to target lung and alveolar macrophages.
MATERIALS AND METHODS
Materials
Materials used in this research included rifampicin (Luohe Nanjiecun, China), chitosan (Bio Chitosan, Indonesia), sodium alginate (Shandong Jiejing, China), and other chemicals of analytical grade.
Method
Preparation of rifampicin DPI
Rifampicin DPI was prepared by the spray drying technique using a B-290 mini spray dryer with a nozzle size of 0.7 mm (BÜCHI, Germany), using a combination of chitosan and alginate as carrier excipients. The formulation of rifampicin DPI was shown in Table 1. Briefly, chitosan and rifampicin were dissolved separately in 1% acetic acid of pH 2–3 and then mixed. After the mixture was dispersed homogeneously, the pH was adjusted with NaOH 1N to pH 4–5. Sodium alginate was dispersed in deionized water and then dripped into a chitosan-rifampicin solution while stirring until homogenous. The mixture was continuously stirred and sprayed with a spray dryer with an inlet temperature of 150°C and a spraying rate of 25 ml/minute. Dry powders were stored in an airtight container in a desiccator.
Physical characterization
The organoleptic properties of rifampicin DPI were observed, including its shape, color, taste, and smell. Particle morphology was analyzed using a scanning electron microscope (SEM) (SSX-550, Shimadzu, Japan). The moisture content of rifampicin DPI was measured with a moisture balance (Adam Equipment AMB 50, UK) at 105°C. Aerodynamic particle size was analyzed by a cascade impactor (Andersen, Japan) and presented as mass median aerodynamic diameter (MMAD).
Drug content and entrapment efficiency
The drug content of DPI was analyzed by dissolving 50 mg DPI in 25.0 ml methanol. The solution was then diluted in a phosphate buffer of pH 7.4, and its absorbance was measured using a spectrophotometer (UV-1800, Shimadzu, Japan) at 473 nm. Drug content was expressed as rifampicin concentration in each gram DPI. Entrapment efficiency was calculated by comparing the drug amount in DPI and its theoretical amount, as described in the following formula:
Drug release profile
The drug release study was performed on DPI (equal to 25 mg of rifampicin), using a dissolution tester (Electrolab TDT-08L, India) basket type. This study was performed in 900 ml of simulated lung fluid (containing a phosphate buffer of pH 7.4 with 0.05% SLS) and 900 ml of simulated macrophage fluid (containing a phthalate buffer of pH 4.5). The dissolution medium was maintained at 37°C ± 0.5°C with a stirring speed of 100 rpm. Six milliliters of the samples was taken at 5, 15, 30, 45, 60, 75, 90, and 120 minutes. The samples were filtered using a 0.45 µm filter and then measured using a spectrophotometer (UV-1800, Shimadzu, Japan) at the maximum absorbance of rifampicin at 473 nm.
In vitro cytotoxicity
An in vitro cytotoxicity study was carried out toward cell line A549 (epithelial cell carcinoma of the lung). Cells were grown with a concentration of 5,000 cells in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) until reaching 50% confluency. Rifampicin and rifampicin DPI were dispersed in the DMEM/F-12 to obtain a final active ingredient concentration of 0.01, 0.1, 1, 2, and 5 mg/ml and were then added to the cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test was performed by adding 10 µl MTT (5 mg/ml) per well and incubation for 4 hours at 37°C. Formazan crystal was dissolved in ethanol, and the absorbance was measured at a wavelength of 595 nm (Chvatal et al., 2018).
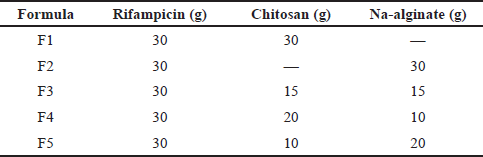 | Table 1. Formulation of rifampicin DPI. [Click here to view] |
RESULTS AND DISCUSSION
Physical properties of rifampicin DPI
As depicted in Figure 1, the spray drying process of rifampicin DPI produced a fine and orange-colored dry powder. Observation of DPI using the SEM showed different shapes and morphologies of the rifampicin powder and rifampicin DPI. As depicted in Figure 2, the rifampicin powder showed various nonspherical shapes within its powder. F1 (RIF-Chi) showed more spherical powder, while F2 (RIF-Alg) had a rough surface. The rough fibrous surface of F2 was due to the alginate which did not perfectly dissolve in the medium; thus, the spray drying process was not optimal. The combination of chitosan and alginate in DPI (F3, F4, and F5) also produced a rough-surfaced particle.
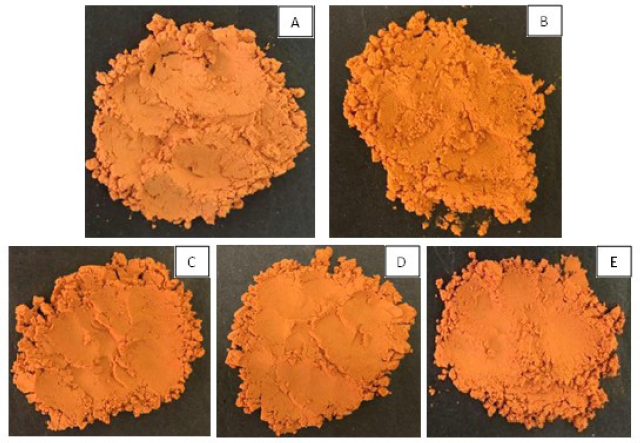 | Figure 1. The physical appearance of rifampicin DPI: F1 (A), F2 (B), F3 (C), F4 (D), and F5 (E). [Click here to view] |
 | Figure 2. Morphology of Rifampicin (A), F1 (B), F2 (C), F3 (D), F4 (E), and F5 (F) with 5,000× magnification. [Click here to view] |
The spray drying process of rifampicin DPI produced a powder with a moisture content of 7.05%–12.97% (Table 2). Alginate-containing DPIs (F3, F4, and F5) showed higher moisture content. DPI F2 which contained alginate only as a carrier showed the highest moisture content. This phenomenon was due to the hygroscopic properties of the alginate and its high ability to absorb water (Rowe, 2009). The higher moisture content of the alginate-containing DPI was also due to the high viscosity of the alginate solution, which caused a challenge in the drying process of these formulas. The drying process was performed with the same parameters for all formulas (inlet temperature of 150°C and a spraying rate of 25 ml/minute). However, with the high viscosity of the alginate solution (in DPIs F2, F3, F4, and F5), it was more difficult for water to be evaporated from the DPIs, thus resulting in a powder with a higher moisture content.
The moisture content of the DPI was further correlated to its particle size. As shown in Table 2, DPI F1 which contains chitosan only as the carrier possessed the lowest moisture content, as well as the smallest aerodynamic particle size. On the other hand, DPIs F2–F5 with a higher moisture content also showed a bigger aerodynamic particle size. As discussed above, the high viscosity of the alginate solution (in DPIs F2, F3, F4, and F5) caused difficulty for water evaporation from the DPIs; thus, more water was still entrapped inside the powder. A high moisture content may also decrease the flowing capacity of powders due to stickiness properties (Lechanteur and Evrard, 2020), thus increasing aerodynamic particle size.
Among those formulas containing the combination of chitosan and alginate, DPI F3 (RIF-Ch-Alg 2:1:1) showed the smallest aerodynamic (MMAD) particle size. Measurement of the aerodynamic particle size of these DPIs was based on the deposition of powders in the cascade impactor (which represent the trachea, bronchus, and bronchioles of the lung), which is also affected by its morphology, density, and moisture content (de Boer et al., 2017).
A particle with MMAD 1–5 µm can be expected to be deposited in the deep lung. Therefore, since the aerodynamic particle size of all formulas, as shown in Table 2, was above 5 µm, it is assumed that these powders were deposited in the upper airway (bronchus or bronchioles). For further study, it is important to optimize the formula and improve the preparation and drying process of the DPIs to produce DPIs with lower moisture content and smaller aerodynamic particle size (5–10 µm) which can deposit in the deep lung.
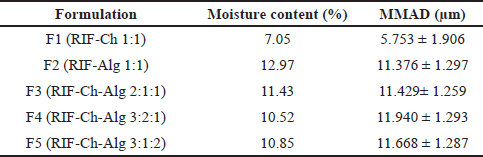 | Table 2. Physical properties of rifampicin DPI. [Click here to view] |
Drug content and entrapment efficiency of rifampicin DPI
Drug content and entrapment efficiency of the DPIs were measured, and the results were presented in Table 3. The drug content of DPIs was 3.227–12.153 mg/g powder. The entrapment efficiency of DPIs was still lower than 50% (12.826%–48.107%). It was due to the suspension form of the polymers, and rifampicin was not perfectly dissolved in it, and thus much of the rifampicin was not well entrapped by the carrier. In the above formula, F3 (RIF-Ch-Alg 2:1:1) showed the lowest drug content and entrapment efficiency. It was probably due to the forming of the polyelectrolyte complexes of chitosan-alginate, which were less dissolved in the solution and thus less capable of entrapping the loaded drug (Huang et al., 2021; Li et al., 2008).
Drug release profile of rifampicin DPI
The drug release profile of rifampicin DPI was studied in the phosphate buffer of pH 7.4 with SLS 0.05% and in the phthalate buffer medium of pH 4.5 to represent simulated lung fluid and simulated macrophage fluid, respectively, to ensure the intended drug release profile in both conditions is achieved. A dissolution test on the rifampicin powder itself was also performed.
As shown in Table 4 and Figure 3, we found no significant difference in the dissolution of the rifampicin powder in simulated lung fluid (medium pH 7.4) and that in simulated macrophage fluid (medium pH 4.5). The rifampicin powder is dissolved more in an acidic medium (19.20 ± 1.57 mg/ml in pH 2.06) than in a basic medium (0.85 ± 0.13 mg/ml in pH 7.06) (Mariappan et al., 2007). However, simulated lung fluid of pH 7.4 contained additional SLS 0.05% to mimic the surfactant in lung fluid and thus can increase the solubility of rifampicin.
The dissolution profiles of the rifampicin DPIs were different compared to the rifampicin powder. This difference was due to the different characteristics of each excipient in the simulated lung or macrophage fluid. Formulating rifampicin in the chitosan carrier (F1) provides a higher dissolution rate in the medium of pH 4.5 (40.246% ± 1.054% in 2 hours) than in the medium of pH 7.4 (34.691% ± 1.086% in 2 hours). On the contrary, the dissolution of rifampicin from the alginate carrier (F2) was higher in the medium of pH 7.4 (98.827% ± 1.164% in 2 hours) than in the medium of pH 4.5 (53.082% ± 0.996% in 2 hours). This phenomenon was related to the solubility of chitosan, which is more soluble in the acid medium, and sodium alginate, which is more soluble in the neutral and base medium (Rowe, 2009).
 | Table 3. Drug content and entrapment efficiency of rifampicin DPI. [Click here to view] |
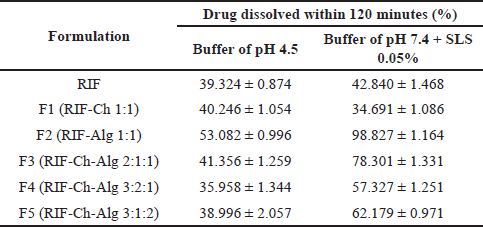 | Table 4. Dissolution of rifampicin DPI. [Click here to view] |
 | Figure 3. Drug release profile of rifampicin powder and rifampicin DPI (F1 – F5) in phthalate buffer medium pH 4.5 (A) and in phosphate buffer medium pH 7.4 + SLS 0.05% (B). Data is presented as average ± standard deviation (n=3). [Click here to view] |
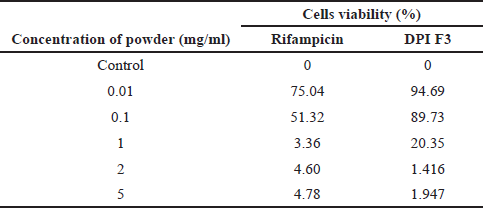 | Table 5. Viability of A549 lung epithelial cell lines after being exposed to the powder. [Click here to view] |
The combination of chitosan and alginate as a carrier for the rifampicin DPIs (F3, F4, and F5) resulted in higher dissolution of rifampicin in the medium of pH 7.4. Among those three formulas, F3 (RIF-Ch-Alg 2:1:1) showed higher rifampicin dissolution in both media (41.356% ± 1.259% in pH 4.5 in 2 hours; 78.301 ± 1.331% in pH 4.5 in 2 hours) as compared to F4 and F5. This result was suggested to be correlated with the formation of chitosan-alginate polyelectrolyte complexes. F3 which contained chitosan-alginate 1:1 formed more polyelectrolyte complexes which facilitate the dissolution of drugs from the DPIs (Huang et al., 2021; Li et al., 2008).
The development of this DPI was aimed at the treatment of active and latent TB. Therefore, among chitosan-alginate-containing DPIs, F3 (RIF-Ch-Alg 2:1:1) was considered as the best formula since it can provide a higher rifampicin concentration in the simulated lung medium (to target active TB) and in the simulated macrophages medium (to target latent TB). However, further studies on macrophage uptake of this DPI should be performed to support the goal. Furthermore, it has potential to be further studied in vivo, to explore its efficiency in targeting active and latent TB.
In vitro cytotoxicity of rifampicin DPI
DPI F3 (RIF-Ch-Alg 2:1:1) was selected for in vitro cytotoxicity toward A549 lung epithelial cells, since it resulted in a satisfactory dissolution profile. A cytotoxicity test on the rifampicin powder was also performed as a comparison to the F3 DPI. Table 5 shows the viability of A549 lung epithelial cell lines after being exposed to the rifampicin DPI. The results revealed that DPI F3 was less toxic than the rifampicin powder. At a concentration of 0.1 mg/ml, the viability of A549 cells was still higher after being exposed to DPI F3 (89.73%) than that after being exposed to the rifampicin powder (51.32%). This might be due to the hydrophilicity of the particle. The particle of DPI F3 was considered more hydrophilic than the rifampicin powder due to the hydroxyl groups of chitosan and alginate as a carrier. The more hydrophilic the particles are, the less the particle would be recognized as a foreign particle; thus, it was less toxic to the lung epithelial cells (Chvatal et al., 2018). This result suggested that the application of chitosan-alginate as a carrier in the DPI is considered beneficial to increase the safety of the powder toward lung cells.
CONCLUSION
The combination of chitosan and alginate in DPI F3 (RIF-Ch-Alg 2:1:1) provides a suitable drug release profile of rifampicin DPI in both simulated lung fluid (78.301% ± 1.332% in 2 hours) and simulated macrophage fluid (41.355% ± 1.259% in 2 hours). DPI F3 possessed an aerodynamic particle size of 11.429 ± 1.259 µm. DPI F3 was also considered safe toward pulmonary epithelial cells (viability 89.73%) in concentrations up to 0.1 mg/ ml. Based on above data, combination of chitosan and alginate can be considered as a prospective carrier to develop dry powder inhaler with suitable characteristics for tuberculosis therapy. Therefore, it has potential to be further studied, to explore its efficiency in targeting active and latent TB in vivo.
ACKNOWLEDGMENTS
The authors would like to acknowledge Universitas Indonesia for funding this research through the “Publikasi Terindeks Internasional (PUTI) Q3 Universitas Indonesia” grant with Contract no. NKB-1812/UN2.RST/HKP.05.00/2020. The authors also would like to thank Dr. Wouter L. J. Hinrichs (University of Groningen, The Netherlands) for his professional advice on the DPI.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHORS’ CONTRIBUTIONS
Kurnia Sari Setio Putri designed the work, performed supervision on laboratory works, performed data analysis and interpretation, and revised the manuscript thoroughly. Laily Syahri Ramdhani and Theodora Rachel performed laboratory work, gained data, and wrote the manuscript. Gatot Suhariyono performed data analysis and interpretation and conducted critical revision of the manuscript. Silvia Surini performed supervision, conducted critical revision of the manuscript, and gave final approval for the manuscript. Kurnia SS Putri, Gatot Suhariyono, and Silvia Surini are the main contributors of this work.
REFERENCES
Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol, 2011; 2011:814943. CrossRef
Barry CE 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol, 2009; 7:845–55. CrossRef
Das S, Tucker I, Stewart P. Inhaled dry powder formulations for treating tuberculosis. Curr Drug Deliv, 2015; 12(1):26–39. CrossRef
Chvatal A, Alzhrani R, Tiwari A, Ambrus R, Szabó-Révész P, Boddu S. Cytotoxicity of inhalable dry powders in A549 human lung cancer cell line. Farmacia, 2018; 66:172–5.
de Boer AH, Hagedoorn P, Hoppentocht M, Buttini F, Grasmeijer F, Frijlink HW. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv, 2017; 14(4):499–512. CrossRef
Huang WT, Zhu LP, Liu DZ, Li JF, Yang SG. Fabrication of alginate/chitosan complex fibers via diffusion controlled in-situ polyelectrolyte complexation. Carbohydr Polym Tech Appl, 2021; 2:100030. CrossRef
Huang YC, Vieira A, Huang KL, Yeh MK, Chiang CH. Pulmonary inflammation caused by chitosan microparticles. Journal of Biomedical Materials Research Part A, 2005; 75(2):283–7. https://doi.org/10.1002/jbm.a.30421 CrossRef
Lechanteur A, Evrard B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: a review. Pharmaceutics, 2020; 12:55. CrossRef
Li P, Dai YN, Zhang JP, Wang AQ, Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci, 2008; 4(3):221–8.
Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol, 2010; 185:15–22. CrossRef
Mariappan TT, Sharda N, Singh S. Atypical log D profile of rifampicin. Indian J Pharm Sci, 2007; 69(2):197–201. CrossRef
Mori T, Murakami M, Okumura M, Kadosawa T, Uede T, Fujinaka T. Mechanism of macrophage activation by chitin derivatives. J Vet Med Sci, 2005; 67(1):51–6. CrossRef
Park, JH, Jin HE, Kim DD, Chung SJ, Shim WS, Shim CK. Chitosan microspheres as an alveolar macrophage delivery system of ofloxacin via pulmonary inhalation. Int J Pharm, 2013; 441(1–2):562–9. CrossRef
Parumasivam T, Yoon R, Chang K, Abdelghany S, Tian T, John W, Chan H. Dry powder inhalable formulations for anti-tubercular therapy. Adv Drug Deliv Rev, 2016; 102:83–101. CrossRef
Rowe RC, Sheskey PJ, Quinn ME . Handbook of pharmaceutical excipients. APhA, (PhP) Pharmaceutical Press, London, UK, 2009.
Sotgiu G, Centis R, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med, 2015; 5(5):a017822. CrossRef
Surini S, Providya R, Putri KSS. Formula optimization of rifampicin dry powder inhalation with chitosan-xanthan carrier using response surface methodology. J Appl Pharm Sci, 2019; 9(01):033–41. CrossRef
World Health Organization. Global tuberculosis report: executive summary. 2019. Available via https://www.who.int/tb/publications/global_report/GraphicExecutiveSummary.pdf